ABSTRACT
Over the past decade, application of culture-independent, next generation DNA sequencing has dramatically enhanced our understanding of the composition of the gut microbiome and its association with human states of health and disease. Host genetics, age, and environmental factors such as where and who you live with, use of pre-, pro- and antibiotics, exercise and diet influence the short- and long-term composition of the microbiome. Dietary intake is a key determinant of microbiome composition and diversity and studies to date have linked long-term dietary patterns as well as short-term dietary interventions to the composition and diversity of the gut microbiome. The goal of this special focus issue was to review the role of diet in regulating the composition and function of the gut microbiota across the lifespan, from pregnancy to old age. Overall dietary patterns, as well as perturbations such as undernutrition and obesity, as well as the effects of dietary fiber/prebiotics and fat composition are explored.
KEYWORDS:
Introduction
Over the past decade, application of culture-independent, next generation DNA sequencing has dramatically enhanced our understanding of the composition of the gut microbiome and its association with human states of health and disease.Citation1-3 Our understanding of human gut microbe metabolic function, microbe-microbe interactions and host-microbe interactions is less well developed, but is rapidly emerging due to the application of multiple omic technologies, including metagenomics, transcriptomics, metabolomics, proteomics and glycomics, applied to both the microbiota and the host.Citation4-6
Host genetics, age, and environmental factors such as where and who you live with, use of pre-, pro- and antibiotics, exercise and diet influence the short- and long-term composition of the microbiome.Citation7-15 Dietary intake is a key determinant of microbiome composition and diversity and studies to date have linked long-term dietary patterns and well as short-term dietary interventions to the composition and diversity of the gut microbiome.Citation16-18 While studies in rodent models have shown that diet may be more impactful than genetics in shaping the gut microbiota,Citation19 Deblius and colleagues recently estimated the relative effect of the long-term dietary patterns as similar to genetics (medium to small effect), but noted that is difficult to rank.Citation7 Short term dietary interventions were assessed to have a small impact on the microbiome, whereas host species, age and lifestyle were the biologic covariates noted to have the largest impact.Citation7 However, it is likely that diet contributes in large part to the large differences observed between species,Citation20 across the lifespanCitation9 and with Western lifestyle.Citation21
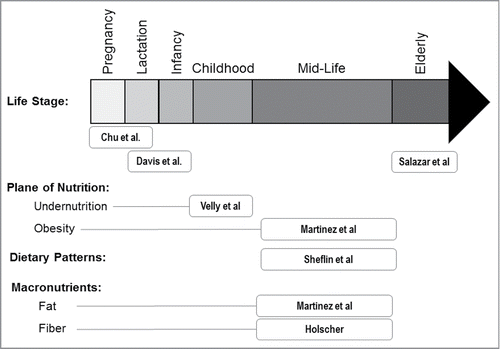
Therefore, the goal of this special focus issue was to review the role of diet in regulating the composition and function of the gut microbiota across the lifespan, from pregnancy to old age. Overall dietary patterns, as well as perturbations such undernutrition and obesity, as well as the effects of dietary fiber/prebiotics and fat composition are explored ().
Across the life-span
Intestinal microbiota development is a complex process that begins in utero and continues for the first 2–3 y of life.Citation22 Delineating this process in diverse populations of infants and how it can be manipulated through diet is clinically important since current evidence suggest that the microbiota acquired in early life can program gastrointestinal, immune and neural development.23-26 Furthermore, perturbations to this developmental trajectory, as evidenced by reduced microbial diversity or dysbiosis during early life has been linked to disorders in infancy and childhood.Citation26,27
Pregnancy and lactation
In the first article, Chu and colleagues from the laboratory of Kjersti Aagaard reviewed how maternal nutrition during pregnancy and lactation affect the composition and function of the gut microbiota of the offspring.Citation28 They discuss the concept that gut microbes should be considered as a mediating factor in the relationship between early life nutrition and later life disease as a component of the Developmental Origins of Health and Disease (DOHaD) hypothesis. Recent work from her laboratory and others have shown that the intrauterine environment of healthy pregnancies is not sterile, supporting the potential for the microbiome to influence fetal developmental processes in utero as well as the maternal-fetal transmission of microbiota during pregnancy. Although additional preclinical and clinical research is needed in this area, the potential for nutrition to influence the maternal-fetal microbiota axis to influence neonatal development prenatally and during breastfeeding is compelling.
Infancy
In the next article, Davis and coauthors from the laboratory of Sharon Donovan reviewed the development of the infant gut microbiome in the first year of life and how that process is influenced by breast- versus formula-feeding, prebiotic and probiotic addition to infant formula, and the addition of solid foods.Citation29 Despite the fact that the infant diet is much more homogenous than that of adult populations, geographical variation exists - even among infants in economically developed countries in Europe. The authors proposed that to more fully understand the ontogeny of the infant microbiota, a collaborative study that obtains stool samples “from a large cohort of infants located in various sites around the globe in which detailed records of dietary intake and other environmental factors are collected is needed to provide a more complete understanding of the development of the microbiota in infants who are breast- vs. formula-fed.”Citation29
Older age
The next paper in the supplement from the laboratory of Clara de los Reyes-Gavilán focuses on the other end of the age spectrum by reviewing how changes in physiologic function, dietary intake and nutritional status affect the microbiome in aged populations.Citation30 Aging is associated with a lower microbial diversity and alterations in the composition, which was associated with a change in diet that occurs upon entering a long-term residential facility.Citation31 These microbiota changes are clinically relevant as they were correlated with increased frailty and markers for inflammation and that the associations between the microbiota and health outcomes was strongest in long-stay subjects.Citation31 More recently, the same group showed that elderly subjects who were placed in residential facilities exhibited a change in their microbiota composition from one associated with health and youth to an elderly-associated microbiota.Citation32 Salazar and colleagues proposed that strategies directed at restoring the microbiota in the elderly must be addressed from a global perspective and nutritional strategies can use probiotics, prebiotics or specific nutrients to counterbalance alterations associated with aging.Citation30
Effects of nutritional status
Our gut microbial consortia provide nutrients and energy to the host via the fermentation of non-digestible dietary components.Citation33 In the healthy state, détente is maintained between the microbiome and the metabolism and immune system of the host. However, dysbioses can lead to inflammation and infection and possible contributions to diabetes mellitus and obesity.Citation33 Indeed, alterations in the gut microbiota diversity and composition have been reported in states of under-,Citation34,35 and over-nutrition, obesity and its co-morbidities, including type 2 diabetes.Citation21,34,36 A number of potential mechanisms have been proposed to describe how the gut microbiota drives obesity and its metabolic complications, including interaction with metabolic processes and the immune system of the host.Citation36,37 The next 2 papers in the supplement describe the relationships between diet and the microbiome in undernourishedCitation38 and obeseCitation39 individuals, respectively.
Undernutrition
Velly and coauthors from the laboratory of Geoffrey Preidis provide an overview of the mechanisms of cross-talk between the gut microbiota, diet and the undernourished host.Citation38 Undernutrition contributes to approximately half of all deaths in children under 5 y of age. Malnutrition arises from a combination of inadequate dietary intake, which is exacerbated by enteric infections and inflammation. However, there is a growing appreciation for the concept that an abnormal gut microbial population associated with malnutrition can perpetuate the vicious cycle of pathophysiology, which can lead to persistent growth impairment in children.Citation38 Velly and colleagues highlight the differences in the composition and function of gut microbiota between undernourished and healthy children and review the factors specific to the host and the environment (including diet) that produce this altered microbiome and the consequences to the host physiology. Finally, they suggest opportunities for microbiome-targeted interventions to implement in the treatment of child undernutrition.Citation38
Overnutrition
At the opposite end of the spectrum is obesity, which is estimated by the World Health Organization to affect 600 million individuals world-wide in both developed and developing countries.Citation40 Western style diets, which are typically high in fat and simple sugars influence microbiome structure and function.Citation21 In their review, Martinez and coauthors from the Eugene Chang's laboratory highlight the impact of obesity and Western diets on the gut microbiota and focus on the effect of high fat diets, including the level and type of fat consumed, and how rapidly diet-induced shifts in the microbiome occur. Lastly, they outline potential avenues for further research and propose strategies for microbiome-based interventions to prevent or treat diet-induced obesity.
Dietary patterns and specific macronutrients
As noted in the above, both short-term dietary interventions and long-term dietary patterns influence the composition and diversity of the gut microbiome.Citation16-20 Of the dietary components, non-digestible carbohydrates, or fiber, have received the most attention as it is the primary energy source for most gut microbes and influences bacterial fermentation, total bacterial numbers and species composition.Citation41,42 The final 2 papers in the supplement review the evidence linking dietary patterns with gut microbial composition and functionCitation43 and how dietary fiber and prebiotics impact the microbiota.Citation44
Dietary patterns
Sheflin and coauthors from the laboratory of Tiffany Weir review the current literature on diet effects on the microbiome and the generation of microbial metabolites of dietary constituents that may influence human health.Citation43 A particular focus of the review is on how animal- vs. plant-based diets impact the microbiome. They conclude that diet-induced differences in microbiome composition and microbial metabolites, such as volatile fatty acids, secondary bile acids, and products of protein degradation, have the potential to modulate the host environment for disease prevention or promotion.Citation43 Given that metabolic effects of plant and animal-based diets are likely driven by differences in macronutrient and phytochemical intake, these components are also discussed in the review.Citation43 The authors summarize areas where further research is required to realize the potential of exploiting diet-microbiota interactions for improved health, which includes a better understanding of the effects of non-nutrient dietary components, such as artificial sweeteners, and emerging novel food ingredients on the gut microbiota.Citation43
Dietary fiber and prebiotics
The human host does not contain digestive enzymes that are capable of breaking the molecular linkages contained in most complex carbohydrates and plant polysaccharides. Thus, these polysaccharides pass through the upper gastrointestinal tract and are metabolized by microbes in the colon. This metabolism generates water and gases, including carbon dioxide and methane, and metabolites, including the volatile fatty acids. In her review, Hannah Holscher summarizes the current evidence linking dietary fiber and prebiotic consumption to human gastrointestinal microbiota composition and function, including the effects of physiochemical properties of the complex carbohydrates, adequate intake and treatment dosages, and the phenotypic composition of the human microbiota.Citation44 In addition to human trials, animal, in vitro, and computational research are all needed to advance our understanding of the physiologic effects of dietary fibers and prebiotics in both healthy and disease states and to delineate mechanisms of action.
Future opportunities for nutritional modulation of the gut microbiome to improve human health
The gut microbiome, also referred to as our second genome, integrates external signals to exert nutritional, metabolic, and immunomodulatory functions that are relevant to health and well-being of the host.Citation45-47 There are immense opportunities to use personalized nutritional interventions to prevent and/or treat acute and chronic diseases including gastrointestinalCitation48 and fatty liver diseases,Citation49 obesity,Citation36,50 cardiovascular diseaseCitation51 and even neuropsychiatric disorders.Citation52,53 In addition to modulating microbial composition and/or richness, diet composition can impact the microbial metabolome,Citation47 which could increase or decrease the risk of disease. As reviewed by Sheflin et al.Citation43 in this supplement, the microbiota is a potentially important link between dietary choline and atherosclerosis via the catabolism of choline to form the gas TMA (trimethylamine) that is metabolized by the liver to form TMA oxide (TMAO). Foods rich in choline, include eggs, liver, and peanuts. Another example could be to increase the intake of fermentable fiber or prebiotics in patients with Irritable Bowel Disease (IBD) to promote the production of short chain fatty acids, which in turn stimulate intestinal hormone secretion, and modify immune function to reduce inflammation.Citation47,48,54
Despite some promising clinical data, many limitations and challenges currently limit our ability to integrate patient-specific microbial data into the clinical realm.Citation45-47 These range from our ability to implement low cost, high throughput sequencing methods to enable rapid characterization of the microbiome. In addition, due to limitations in the depth of sequencing and the completeness of databases for bioinformatics, species-level identification of microbes associated with disease states is incomplete. Emerging sequencing technologies may enable identification of genera and species involved in clinically relevant disease states.Citation55 In addition to characterizing a patient's microbiome composition and function to assist in personalized diagnostic assessment, disease prevention, and treatment,Citation45 it will likely be important to more fully characterize the microbiome metabolites in feces and serumCitation47 and potentially host genetic risk of disease to assess disease susceptibility.
As noted by Hannah Holscher in her review,Citation44 multidisciplinary research approaches are needed to holistically advance our understanding of mechanisms underlying host-microbe interactions on host physiology. In addition, physicians and registered dietitian nutritionists (RDN) have the opportunity to plan a key role in translating evidence on the role that diet plays in shaping the microbiome to clinical recommendations that help patients make healthy choices.Citation48,49 However, to incorporate findings from the gut microbiome into clinical practice, RDNs must understand the data that are being generated by specific patient cohorts through microbiome analysis. As summarized by Harvie and colleagues,Citation56 “the RDN's role in collaborations investigating the microbiome will be to design dietary interventions, provide the dietary education to patients, ensure food composition data are available for the nutrient of interest, and measure dietary intake and adherence to the interventions.”
Lastly, there are vast opportunities to develop more effective pre-, pro- and synbiotics that are targeted toward specific disease states. Fecal microbiota transplantation has been shown to be a highly effective method in the treatment of refractory Clostridium difficile infection and there is expanding interest in using FMT in the prevention and management of gastrointestinal and non-gastrointestinal diseases.Citation57 However, the potential of modifying dietary intake of the donor or the recipient to optimize engraftment of the FMG has been largely unexplored.
A recent round table identified areas where knowledge of the gut microbiome could fuel innovation in physiologic and therapeutic outcomes.Citation58 They identified 4 domains of innovation that could derive from ongoing efforts in deciphering the interactions between human cells and intestinal microbiome as a central component of human health, namely:Citation1 development of stratification and monitoring tools;Citation2 identification of new target and drug discovery, as a part of our supra-genome;Citation3 exploitation of microbiota as a therapeutic target that can be modulated;Citation4 and finally as a source of live biotherapeutics and adjuvants.Citation58 The Experts in nutrition and clinical dietetics could actively contribute to several of these areas, particularly 3 and 4, and I encourage greater cross-disciplinary collaboration to achieve the full potential of diet to modify the intestinal microbiome to improve human health.
Disclosure of potential conflicts of interest
The author reports no conflicts of interest.
References
- Robles-Alonso V, Guarner F. From basic to applied research: lessons from the human microbiome projects. J Clin Gastroenterol 2014; 48 Suppl 1:S3-4; PMID: 25291122; http://dx.doi.org/10.1097/MCG.0000000000000242
- O'Toole PW, Flemer B. Studying the microbiome: “Omics” made accessible. Semin Liver Dis 2016; 36(4):306-311; PMID:27997969; http://dx.doi.org/10.1055/s-0036-1594008
- Cénit MC, Matzaraki V, Tigchelaar EF, Zhernakova A. Rapidly expanding knowledge on the role of the gut microbiome in health and disease. Biochim Biophys Acta 2014; 1842(10):1981-1992; PMID:24882755; http://dx.doi.org/10.1016/j.bbadis.2014.05.023
- Schwartz S, Friedberg I, Ivanov IV, Davidson LA, Goldsby JS, Dahl DB, Herman D, Wang M, Donovan SM, Chapkin RS. A metagenomic study of diet-dependent interaction between gut microbiota and host in infants reveals differences in immune response. Genome Biol 2012; 13(4):r32; PMID:22546241; http://dx.doi.org/10.1186/gb-2012-13-4-r32
- Morgan XC, Huttenhower C. Meta'omic analytic techniques for studying the intestinal microbiome. Gastroenterology 2014; 146(6):1437-1448.e1; PMID:24486053; http://dx.doi.org/10.1053/j.gastro.2014.01.049
- Arnold JW, Roach J, Azcarate-Peril MA. Emerging technologies for gut microbiome research. Trends Microbiol 2016; 24(11):887-901; http://dx.doi.org/10.1016/j.tim.2016.06.008
- Debelius J, Song SJ, Vazquez-Baeza Y, Xu ZZ, Gonzalez A, Knight R. Tiny microbes, enormous impacts: what matters in gut microbiome studies? Genome Biol. 2016 17(1):217; PMID:27760558; http://dx.doi.org/10.1186/s13059-016-1086-x
- Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JT, et al. Human genetics shape the gut microbiome. Cell 2014; 159:789-99; http://dx.doi.org/10.1016/j.cell.2014.09.053
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222-7; http://dx.doi.org/10.1038/nature11053
- Grześkowiak Ł, Collado MC, Mangani C, Maleta K, Laitinen K, Ashorn P, Isolauri E, Salminen S. Distinct gut microbiota in southeastern African and northern European infants. J Pediatr Gastroenterol Nutr. 2012;54(6):812-6; http://dx.doi.org/10.1097/MPG.0b013e318249039c
- Adair KL, Douglas AE. Making a microbiome: the many determinants of host-associated microbial community composition. Curr Opin Microbiol. 2016;35:23-29; http://dx.doi.org/10.1016/j.mib.2016.11.002
- Clarke SF, Murphy EF, O'Sullivan O, Lucey AJ, Humphreys M, Hogan A, Hayes P, O'Reilly M, Jeffery IB, Wood-Martin R, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014;63:1913-20; PMID:25021423; http://dx.doi.org/10.1136/gutjnl-2013-306541
- Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108(Suppl):4554-61; http://dx.doi.org/10.1073/pnas.1000087107
- Song SJ, Lauber C, Costello EK, Lozupone CA, Humphrey G, Berg-Lyons D, Caporaso JG, Knights D, Clemente JC, Nakielny S, et al. Cohabiting family members share microbiota with one another and with their dogs. Elife. 2013;2:e00458; PMID:23599893; http://dx.doi.org/10.7554/eLife.00458
- Dunn RR, Fierer N, Henley JB, Leff JW, Menninger HL. Home life: factors structuring the bacterial diversity found within and between homes. PLoS One 2013;8(5). http://dx.doi.org/10.1371/journal.pone.0064133
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, Keilbaugh SA, Bewtra M, Knights D, Walters WA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105-8; http://dx.doi.org/10.1126/science.1208344
- Wu GD, Compher C, Chen EZ, Smith SA, Shah RD, Bittinger K, Chehoud C, Albenberg LG, Nessel L, Gilroy E, et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut. 2016;65(1):63-72; PMID:25431456; http://dx.doi.org/10.1136/gutjnl-2014-308209
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505(7484):559-63; PMID:24336217; http://dx.doi.org/10.1038/nature12820
- Carmody RN, Gerber GK, Luevano JM Jr, Gatti DM, Somes L, Svenson KL, Turnbaugh PJ. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe 2015; 17(1):72-84; http://dx.doi.org/10.1016/j.chom.2014.11.010
- Muegge BD, Kuczynski J, Knights D, Clemente JC, González A, Fontana L, Henrissat B, Knight R, Gordon JI. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science 2011; 332(6032):970-4; http://dx.doi.org/10.1126/science.1198719
- Broussard JL, Devkota S. The changing microbial landscape of Western society: Diet, dwellings and discordance. Molec Metab. 2016;5:737e742; http://dx.doi.org/10.1016/j.molmet.2016.07.007
- Wopereis H, Oozeer R, Knipping K, Belzer C, Knol J. The first thousand days - intestinal microbiology of early life: establishing a symbiosis. Pediatr Allergy Immunol 2014; 25(5):428-38; PMID:24899389; http://dx.doi.org/10.1111/pai.12232
- Donovan S. Role of human milk components in gastrointestinal development: Current knowledge and future needs. J Pediatr 2006;149(5):S49-S61; http://dx.doi.org/10.1016/j.jpeds.2006.06.052
- Li M, Wang M, Donovan SM. Early development of the gut microbiome and immune-mediated childhood disorders. Semin Reprod Med. 2014;32(1):74-86; http://dx.doi.org/10.1055/s-0033-1361825
- Wang M, Monaco MH, Donovan SM. Impact of early gut microbiota on immune and metabolic development and function. Semin Fetal Neonatal Med. 2016;21(6):380-387; http://dx.doi.org/10.1016/j.siny.2016.04.004
- Rautava S, Isolauri E. The development of gut immune responses and gut microbiota: effects of probiotics in prevention and treatment of allergic disease. Curr Issues Intest Microbiol 2002; 3(1):15-22
- Weber TK, Polanco I. Gastrointestinal microbiota and some children diseases: a review. Gastroenterol Res Pract 2012; 2012:676585; http://dx.doi.org/10.1155/2012/676585
- Chu DM, Meyer KM, Prince AL, Aagaard KM. Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes. 2016;7(6):459-70; http://dx.doi.org/10.1080/19490976.2016.1241357
- Davis EC, Wang M, Donovan SM. The role of early life nutrition in the establishment of gastrointestinal microbial composition and function. Gut Microbes 2017; Jan 9:1-29; [Epub ahead of print]; http://dx.doi.org/10.1080/19490976.2016.1278104
- Salazar N, Valdés-Varela L, González S, Gueimonde M, de los Reyes-Gavilán CG. Nutrition and the gut microbiome in the elderly. Gut Microbes 2016 3:1-16; [Epub ahead of print]; http://dx.doi.org/10.1080/19490976.2016.1256525
- Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S Harris HM, Coakley M, Lakshminarayanan B, O'Sullivan O. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012; 488:178-84; PMID:22797518; http://dx.doi.org/10.1038/nature11319
- Jeffery IB, Lynch DB, O'Toole PW. Composition and temporal stability of the gut microbiota in older persons. ISME J. 2016;10(1):170-82; http://dx.doi.org/10.1038/ismej.2015.88
- Flint HJ, Scott KP, Petra L, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012;9:577-89; http://dx.doi.org/10.1038/nrgastro.2012.156
- de Clercq NC, Groen AK, Romijn JA, Nieuwdorp M. Gut microbiota in obesity and undernutrition. Adv Nutr. 2016;7(6):1080-9; PMID:28140325; http://dx.doi.org/10.3945/an.116.012914
- Blanton LV, Barratt MJ, Charbonneau MR, Ahmed T, Gordon JI. Childhood undernutrition, the gut microbiota, and microbiota-directed therapeutics. Science 2016; 352(6293):1533; PMID:27339978; http://dx.doi.org/10.1126/science.aad9359
- Bouter KE, van Raalte DH, Groen AK, Nieuwdorp M. Role of the gut microbiome in the pathogenesis of obesity and obesity-related metabolic dysfunction. Gastroenterology 2017; pii: S0016-5085(17)30141-5; http://dx.doi.org/10.1053/j.gastro.2016.12.048. [Epub ahead of print].
- Bleau C, Karelis AD, St-Pierre DH, Lamontagne L. Crosstalk between intestinal microbiota, adipose tissue and skeletal muscle as an early event in systemic low-grade inflammation and the development of obesity and diabetes. Diabetes Metab Res Rev 2015; 31(6):545-61; PMID:25352002; http://dx.doi.org/10.1002/dmrr.2617
- Velly H, Britton RA, Preidis GA. Mechanisms of cross-talk between the diet, the intestinal microbiome, and the undernourished host. 2016; 5:1-15; PMID:27918230; http://dx.doi.org/10.1080/19490976.2016.1267888; [Epub ahead of print]
- Martinez KB, Leone V, Chang EB. Dietary fat promotes gut microbiota dysbiosis and metabolic disease. Gut Microbes. 2017; 6:1-13; [Epub ahead of print]; http://dx.doi.org/10.1080/19490976.2016.1270811
- World Health Organization. Obesity and Overweight Fact Sheet. Geneva, Switzerland; 2016 June [accessed 2017 Feb 19]. http://www.who.int/mediacentre/factsheets/fs311/en/
- Sawicki CM, Livingston KA, Obin M, Roberts SB, Chung M, McKeown NM. Dietary fiber and the human gut microbiota: Application of evidence mapping methodology. Nutrients 2017 9(2):125; doi:10.3390/nu9020125
- Flint HJ. The impact of nutrition on the human microbiome. Nutr Rev 2012; 70(Suppl. 1):S10-3; http://dx.doi.org/10.1111/j.1753-4887.2012.00499.x
- Sheflin AM, Melby CL, Carbonero F, Weir TL. Linking dietary patterns with gut microbial composition and function. Gut Microbes. 2016 Dec 14:1-17; [Epub ahead of print]; http://dx.doi.org/10.1080/19490976.2016.1270809
- Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017; Feb 6:0; [Epub ahead of print]; PMID:28165863;http://dx.doi.org/10.1080/19490976.2017.1290756
- Zmora N, Zeevi D, Korem T, Segal E, Elinav E. Taking it personally: personalized utilization of the human microbiome in health and disease. Cell Host Microbe 2016; 19(1):12-20; http://dx.doi.org/10.1016/j.chom.2015.12.016
- Murtaza N, Ó Cuív P, Morrison M. Diet and the microbiome. Gastroenterol Clin North Am 2017; 46(1):49-60; http://dx.doi.org/10.1016/j.gtc.2016.09.005
- Wu GD. The gut microbiome, its metabolome, and their relationship to health and disease. Nestle Nutr Inst Workshop Ser 2016; 84:103-10; http://dx.doi.org/10.1159/000436993
- Harvie R, Walmsley R, Schultz M. New Zealand Society of Gastroenterology “We are what our bacteria eat:” The role of bacteria in personalizing nutrition therapy in gastrointestinal conditions. J Gastroenterol Hepatol. 2016; 32(2):352-357; PMID:27248703; http://dx.doi.org/10.1111/jgh.13462
- Bluemel S, Williams B, Knight R, Schnabl B. Precision medicine in alcoholic and nonalcoholic fatty liver disease via modulating the gut microbiota. Am J Physiol Gastrointest Liver Physiol. 2016;311(6):G1018-36; PMID:27686615; http://dx.doi.org/10.1152/ajpgi.00245.2016
- Stenman LK, Lehtinen MJ, Meland N, Christensen JE, Yeung N, Saarinen MT, Courtney M, Burcelin R, Lähdeaho ML, Linros J, et al. Probiotic with or without fiber controls body fat mass, associated with serum zonulin, in overweight and obese adults-randomized controlled trial. EBioMedicine. 2016;13:190-200; http://dx.doi.org/10.1016/j.ebiom.2016.10.036
- Tuohy KM, Fava F, Viola R. ‘The way to a man's heart is through his gut microbiota’–dietary pro- and prebiotics for the management of cardiovascular risk. Proc Nutr Soc. 2014;73(2):172-85; PMID:24495527; http://dx.doi.org/10.1017/S0029665113003911
- Sandhu KV, Sherwin E, Schellekens H, Stanton C, Dinan TG, Cryan JF. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res. 2017;179:223-244; Epub 2016 Oct 21; http://dx.doi.org/10.1016/j.trsl.2016.10.002
- Saulnier DM, Ringel Y, Heyman MB, Foster JA, Bercik P, Shulman RJ, Versalovic J, Verdu EF, Dinan TG, Hecht G, et al. The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes 2013; 4(1):17-27; http://dx.doi.org/10.4161/gmic.22973
- Albenberg LG, Wu GD. Diet and the intestinal microbiome: associations, functions, and implications for health and disease. Gastroenterology 2014; 146(6):1564-72; . http://dx.doi.org/10.1053/j.gastro.2014.01.058
- Sadowsky MJ, Staley C, Heiner C, Hall R, Kelly CR, Brandt L, Khoruts A. Analysis of gut microbiota - An ever changing landscape. Gut Microbes 2017 Jan 4:1-8; [Epub ahead of print]; http://dx.doi.org/10.1080/19490976.2016.1277313
- Harvie R, Chanyi RM, Burton JP, Schultz M. Using the human gastrointestinal microbiome to personalize nutrition advice: Are registered dietitian nutritionists ready for the opportunities and challenges? J Acad Nutr Diet 2016 Dec 13. pii: S2212-2672(16)31325-9; PMID:27986518; http://dx.doi.org/10.1016/j.jand.2016.10.020 [Epub ahead of print]
- Konturek PC, Haziri D, Brzozowski T, Hess T, Heyman S, Kwiecien S, Konturek SJ, Koziel J. Emerging role of fecal microbiota therapy in the treatment of gastrointestinal and extra-gastrointestinal diseases. J Physiol Pharmacol 2015; 66(4):483-91
- Doré J, Multon MC, Béhier JM; participants of Giens XXXII, Round Table No. 2.The human gut microbiome as source of innovation for health: Which physiological and therapeutic outcomes could we expect? Therapie 2017 Jan 3; pii: S0040-5957(16)31280-X; http://dx.doi.org/10.1016/j.therap.2016.12.007 [Epub ahead of print]