ABSTRACT
The murine cecum is a major site of fermentation of dietary materials, and production of short chain fatty acids (SCFAs). To examine the role that the cecum plays in acute bacterial infection in mice, the cecum was surgically removed, and changes in bacterial communities and production of SCFAs were analyzed relative to surgical sham animals. To incite bacterial colitis, mice were orally challenged with Citrobacter rodentium. The impact of butyrate administered directly into the colon was also examined. Concentrations of SCFAs in feces were substantially lower in mice with an excised cecum. Bacterial communities were also less diverse in cecectomized mice, and densities of major SCFA-producing taxa including bacteria within the Ruminococcaceae and Lachnospiraceae families were reduced. Colonization of the intestine by C. rodentium was not affected by removal of the cecum, and the bacterium equally incited acute colitis in mice with and without a cecum. However, cecectomized mice exhibited lower body weights at later stages of infection indicating an impaired ability to recover following challenge with C. rodentium. Furthermore, removal of the cecum altered immune and inflammatory responses to infection including increased inflammatory markers in the proximal colon (Tnfα, Il10, βd1), and heightened inflammatory response in the proximal and distal colon (Ifnγ, Tnfα, Relmβ). Exogenous administration of butyrate was insufficient to normalize responses to C. rodentium in cecectomized mice. The murine cecum plays a critical role in maintaining intestinal health, and the murine cecectomy model may be a useful tool in elucidating key aspects of intestine-pathogen-microbiota interactions.
Introduction
THE CECUM is an intraperitoneal pouch located at the most cranial aspect of the large intestine. While the function of the cecum is not fully understood, it is thought to play a role in production of short chain fatty acids in many mammals,Citation1 and it has been proposed to serve as a reservoir of anaerobic bacteria that populate the colon. The composition of the bacterial community that colonizes intestines are thought to be critical in mitigating responses to infection,Citation2 yet mechanisms of this ‘colonization resistance’ are enigmatic at present.
Short-chain fatty acids (SCFAs) are bi-products of bacterial fermentation of dietary fermentable materials.Citation3 The cecum is the main site of fermentation in mice and SCFA production decreases along the colon as a function of distance from the cecum due to absorption by colonocytes (i.e. epithelial cells lining the colon) for energy (e.g. butyrate), or for use in cholesterol, fat, and sugar metabolism (e.g. acetate and propionate).Citation4 As the cecum has been identified as a primary site of colonic fermentationCitation1 and a reservoir of anaerobic bacteria, we hypothesize that the removal of the cecum will be detrimental to intestinal health (e.g. inflammation) as a result of decreased SCFA availability to colonocytes and a greatly decreased richness of the ‘commensal’ colonic bacterial community. Our recent investigation demonstrated that colonic infusion of butyrate can ameliorate intestinal inflammation in mice.Citation5 Others also have indicated that butyrate modulates bacterial communities within the colon of mice during periods of aberrant inflammation (e.g. colitis)Citation6,Citation7; however, the mechanisms by which butyrate affects intestinal health (e.g. amelioration of enteric inflammation) remain poorly understood.
In 1987, Voravuthikunchai and LeeCitation8 reported that the removal of the cecum in mice severely compromised production of a SCFAs and lowered resistance to infection by Salmonella enterica serovar Enteritidis. This pathogen typically incites typhus in mice,Citation9 and the mechanisms involved in loss of colonization resistance including changes in host responses (i.e. enteritis) and commensal bacterial communities that were associated with reduced SCFA production and pathologic changes were not determined.Citation8 In the current study, cecectomy surgery was performed in mice, and fecal SCFA and changes in colon bacterial communities were characterized; Illumina sequencing was employed. The implications of cecectomy surgery and associated changes in response to enteric inflammation were determined by inciting acute colitis using C. rodentium and measuring histological and molecular indicators of inflammation. Finally, butyrate was administered directly to the colon to ascertain whether exogenous butyrate can compensate for the low concentrations of butyrate within the colon (i.e. as result of the inability of cecectomized mice to ferment carbohydrates).
Results
Cecum removal decreases fecal short chain fatty acids in feces
Concentrations of total SCFAs, acetic acid, butyric acid, isobutyric acid, valeric acid, isovaleric acid, and propionic acid were substantially lower (P < 0.001) in feces from cecectomized mice following surgery and post-inoculation (p.i.) with C. rodentium (). Cecum removal had no effect (P = 0.375) on concentrations of caproic acid in feces (). Inoculation with C. rodentium increased production of total SCFAs (P < 0.001), particularly acetic (P < 0.001), butyric (P < 0.001), and propionic (P < 0.001) acids in feces from cecectomy or sham mice. Colonic administration of butyrate did not increase (P = 0.714) concentrations of this SCFA within the cecum (Figure S1) or excreted in feces (Figure S2).
Cecum removal alters bacterial communities in the colon ± colitis
A lower diversity of bacteria associated with mucosa (P ≤ 0.001) was observed in both the proximal and distal colon of cecectomized relative to sham mice regardless of colitis (). The lower diversity was reflected in Simpson's metric of species richness, as well as Shannon's diversity index, which also accounts for species evenness. Furthermore, the composition of the microbiota differed conspicuously (P ≤ 0.002) in cecectomized mice in both the proximal () and distal () colon. At both sites there was an increase in the abundance of Enterobacteriaceae and Erysipelotrichaceae, and decreased abundance in Lachnospiraceae and Ruminococcaceae families in cecectomized mice (). Within the Lachnospiraceae family, an increase in abundance of Clostridium cluster XIVb and Dorea spp. was noted in the distal colon, while decreases in abundance of Lachnospira, Marvinbryantia and Roseburia spp. were observed in both the proximal and distal colon (). Within the Ruminococcaceae family, an increase in abundance of Anaerotruncus was observed in the distal colon, and decreases in abundance of Butyricicoccus, Clostridium cluster IV, Flavonifractor, Oscillobacter, Ruminococcus, and Sporobacter spp. occurred in the proximal and distal colon (). No differences in alpha diversity (P ≥ 0.520), beta diversity (P ≥ 0.202), or relative abundance of individual taxa were observed as a result of butyrate administration to mice with or without a cecum (Figure S3; Figure S4).
Infection by C. rodentium may have differentially affected the abundance of several bacterial taxa, such as Clostridium cluster XIVb and Anaerotruncus spp. in the proximal colon of Cec+ mice, and Oscillibacter spp. in the proximal and distal colon of Cec- mice ().
Cecum removal does not affect intestinal colonization by Citrobacter rodentium
To incite colitis, we inoculated mice with C. rodentium. In cecectomized mice inoculated with the pathogen (± colonic butyrate administration) there was no difference (P ≥ 0.247) in densities of C. rodentium cells shed in feces over the duration of the experiment (). Furthermore, examination of the colonic tissues of mice inoculated with the pathogen by fluorescence in situ hybridization (FISH) showed an equal abundance and distribution of C. rodentium cells associated with the colonic mucosa (). No C. rodentium was isolated from feces, nor was the bacterium visualized in the colons of mice not inoculated with the pathogen by FISH.
Cecum removal alters host responses to Citrobacter rodentium infection
While body weights were similar (P ≥ 0.137) between cecectomized and sham mice at the early and peak stages of infection (), at late infection, mice without a cecum had lower (P ≤ 0.031) body weights (). It is noteworthy that 13 days following inoculation with C. rodentium, sham mice had recovered to the same body weight as uninfected mice; however, inoculated mice with no cecum exhibited lower body weights relative to the infected sham control mice (). Colonic administration of butyrate had no effect (P = 0.269) on relative weight loss following C. rodentium inoculation in either cecectomized or sham mice.
Cecum removal altered inflammatory response to Citrobacter rodentium infection
Indicators of inflammation within the spleen and intestine were analyzed to determine whether the presence of the cecum within mice (and associated effects) alters host responses to infection. In mice that received sham and cecectomy surgery, equally prominent responses to infection at peak infection (i.e. 14 days p.i.) were observed including increased size and number of germinal centres, increased numbers of apoptotic and necrotic lymphocytes, and increased infiltration of inflammatory cells into the spleen (P = 0.010). At early infection (i.e. 7 days p.i.) in contrast, expression of markers of inflammation in splenic tissue were observed in cecectomized mice (P = 0.001) but not in sham treated mice (P = 0.215) (). Colonic injury was determined by histopathologic scoring for epithelial cell hyperplasia, crypt height, epithelial injury, inflammatory cell infiltration, mitotic activity, and goblet cell depletion. In the distal colon, higher (P ≤ 0.013) total histopathologic scores were observed in infected versus non-infected mice at both 7 and 14 days p.i. (). In the proximal colon, higher total scores were found only in infected mice that had their cecum removed at 14 days p.i. (P = 0.031); however, a similar trend for higher histopathologic scores was observed in infected mice at 7 days p.i. and for infected sham mice at 14 days p.i. (). There was no difference (P ≥ 0.141) in the severity of histopathologic changes in infected cecectomized versus infected sham mice, nor were there changes in histopathological scores as a result of butyrate administration (P ≥ 0.243).
To assess molecular mechanisms involved in inflammation within the intestines, expression of mRNA of genes encoding proteins involved in the detection of SCFA, intestinal inflammation, and intestinal repair and recovery were quantified. Several markers were differentially expressed in non-infected mice that had their cecum removed, including Tnfα (P = 0.025), Il17a (P = 0.022), Il10 (P = 0.023), and βd1 (P ≤ 0.028) in the proximal colon (), and Tnfα (P = 0.017) and Relmβ (P ≤ 0.048) in the distal colon (). Infection with C. rodentium affected expression of several markers in the proximal colon, including Ifnγ (P ≤ 0.043), Tnfα (P = 0.036), Il17 (P < 0.043), Tgfβ (P ≤ 0.050), and Ffar3 (P = 0.021) (), and in the distal colon, including Ifnγ (P < 0.001), Tnfα (P ≤ 0.002), Il17a (P < 0.001), Relmβ (P < 0.001), Muc2 (P ≤ 0.009), and Ffar3 (P < 0.001) (). Differential responses to infection by C. rodentium were also observed in cecectomized mice. In this regard, in the proximal colon of infected mice, higher levels of expression of Ifnγ were observed at 7 (P = 0.002) and 14 (P = 0.009) days p.i., and higher expression of Tnfα (P = 0.041) and Tgfβ (P = 0.041) was observed at 7 days p.i. (). Furthermore, expression of Ffar2 was higher (P = 0.012) in the proximal colon of infected mice without a cecum at 7 days p.i. In the distal colon, intestinal inflammation was generally more severe but appeared less affected by the removal of the cecum although higher levels of expression of both Ifnγ (P = 0.032) and Relmβ (P = 0.007) was observed in infected mice without a cecum ().
Exogenous administration of butyrate did not affect bacterial communities or colitis
Concentrations of butyric acid in feces ranged from 0.28 ± 0.05 to 2.32 ± 0.16 mM, and the colonic administration of butyrate did not increase (P = 0.714) the concentration of butyric acid excreted in feces (Figure S1). Administration of butyrate also had no effect on the concentration of SCFAs in cecal contents at the time of animal euthanization (Figure S3) Moreover, no effects of butyrate administration on expression of inflammatory markers in the proximal (P ≥ 0.222) or distal (P ≥ 0.280) colon were observed (). In the distal colon, a trend for lower total histopathologic scores was observed in mice rectally administered butyrate with colitis (i.e. Cec- mice at 7 days p.i., and Cec+ mice at 14 days p.i.), suggesting that butyrate may have imparted a mild protective effect within the colon.
Discussion
The cecum is considered the primary site of SCFA production in mice, and as such, surgical removal of the cecum may provide insights into colonization resistance within the colon and factors that contribute to intestinal health. In the current investigation, we removed the cecum in mice using a surgical procedure described previously,Citation8 and we applied modern analytical tools not available to Voravuthikunchai and LeeCitation8 to obtain insight into the possibility of using the murine ‘cecectomy model’ for elucidating key aspects of the host-pathogen-microbiota interaction. Furthermore, we used a pathogen that produces localized colitis in mice (i.e. C. rodentium)Citation10 in contrast to S. Enterditis.Citation8
The intestine is colonized by a complex community of bacteria, which varies in both density and complexity along its length.Citation11,Citation12 In mice, the cecum represents a junction between a lower diversity of bacterial communities within the small intestine and a higher diversity of bacterial communities within the colon.Citation11 The variations in bacterial populations cranial and distal to the ileal-cecal junction, in addition to high diversity of bacteria within the blind-ended cecum, have led to the hypothesis that the cecum functions as a bacterial reservoir from which the colon microbiota is populated. At present, however, there is a paucity of conclusive evidence to support this hypothesis. In our study we demonstrated that removal of the cecum resulted in a conspicuous decrease in both richness and evenness of bacterial communities of the colon, as well as a pronounced change in the composition of the bacterial community structure. A more diverse microbiota is considered to provide beneficial effects to the host, whereas a reduced diversity community is often associated with intestinal disease.Citation13 Thus, it is plausible that the cecum may play an important role in maintaining intestinal homeostasis and overall health of the host by influencing both the amount and diversity of bacteria within the large intestine.
Bacteria that contain SCFA-producing taxa (e.g. members of the Ruminococcaceae and Lachnospiraceae families)Citation14 were conspicuously reduced in relative abundance as a result of cecal removal. Within the Ruminococcaceae family, Butyricoccus, Ruminococcus, Flavonifractor and Sporobacter were among the genera that were decreased in abundance as a result of cecum removal. Within the Lachnospiraceae family, a decrease in Marvinbryantia and Roseburia spp. was observed in mice without a cecum. Little is known about the functional capacity of these bacteria individually; however, bacteria within these groups have been found to be positi vely correlated with SCFA-associated improvement in health outcomes including reduced severity of disease in Crohn's disease patientsCitation15 and protection from colorectal cancer in rats.Citation16 In concert with the observation of reduced abundances of SCFA-producing taxa, we found that prominent SCFAs in feces, including acetic, propionic, butyric, isobutyric, isovaleric, and valeric acids were markedly lower in mice with excised cecums. Significant decreases in concentration of acetic, propionic, and butyric acid in the colon of cecectomized mice were noted in a previous cecectomy study with a similar trend of reduction in isobutyric, isovaleric, and valeric acids.Citation8 We observed that the concentration of caproic acid was unaffected by cecum removal. Short chain fatty acids are thought to provide several beneficial effects on host tissues within the intestine and in extra-intestinal tissues of the body.Citation17 For example, higher concentrations of SCFAs decrease luminal pH, which inhibits the growth of pathogenic organisms and increases nutrient absorption.Citation18 Furthermore, butyrate is an energy source for colonocytes, and this SCFA may enhance intestinal health by increasing mucin productionCitation19 and improve epithelial integrity.Citation20 Additionally, decreased abundance of SCFA-producing bacteria and lower concentrations of fecal SCFA have been observed in individuals with inflammatory diseases of the intestine.Citation21 Given our expanding knowledge on the role of individual SCFAs on intestinal health,Citation22 the information obtained in the current study supports the emerging hypothesis that the murine cecum is an essential organ for maintaining a diverse and physiologically beneficial microbiota in the colon. Furthermore, the cecectomy model may prove useful to elucidate other key aspects of the microbiota-host interactions not measured in the current investigation (e.g. colonization resistance) in mice and other monogastric mammals (e.g. pigs).
To determine how these cecum-derived changes in the intestinal environment alter host responses to enteric stress, we incited intestinal inflammation with the murine pathogen C. rodentium. It has been reported that lymphoid tissue within the cecum (i.e. the cecal patch) is the location within the intestinal tract where C. rodentium initially colonizes during infection.Citation23 We observed that the densities of live C. rodentium in feces was the same in mice that received cecectomy and sham surgeries and thus, our data indicates that the cecum is not required for the initiation of C. rodentium infection. Despite equivalent patterns of colonization with C. rodentium within the colon, differences were noted in the colonic responses to infection by C. rodentium in cecectomized mice. Mice with an excised cecum failed to recover from C. rodentium infection, as underscored by an inability to return to normal body weight at later stages of infection. In general, non-surgically manipulated mice infected by C. rodentium commenced gaining weight by the peak to late stages of colitis.Citation24 In contrast, we observed that infected mice without a cecum weighed less at late infection than they did before inoculation with C. rodentium. These observations suggest that the cecum or cecum-derived microbiota is directly or indirectly important to the ability of mice to recover from C. rodentium infection.
To assess whether removal of the cecum alters immune and inflammatory responses to infection with C. rodentium, intestinal tissues were examined for histopathological changes as well as molecular markers of intestinal health. As well, effects on the bacterial community structure were examined. Histopathological effects of C. rodentium infection were similar in both the proximal and distal colon; however, several molecular markers of inflammation incited by the pathogen were differentially expressed in cecectomized mice. In general, removal of the cecum had a greater effect on tissues harvested from the proximal colon, as there was a decrease in expression of defensins (βd1), and an increase in expression of both pro-inflammatory (Tnfα and Ifnγ) and anti-inflammatory (Il10) genes. There was also a more pronounced response to C. rodentium inoculation in cecectomized mice as indicated by increased expression of immune regulators, Relmβ, Ifnγ, and Tgfβ, and the free fatty acid receptor, Ffar2. Although colitis incited by C. rodentium did not appreciably affect bacterial community composition in the colon, Clostridium cluster XIVb, Anaerotruncus, Oscillibacter spp. may have been differentially affected as a result of colitis in cecetomized or sham surgery mice. There are some reports of these taxa being affected by enteric inflammation or barrier function,Citation25-29 but little is currently known about their impacts on host health.
In our investigation, we used C. rodentium as an incitant of self-limiting colitis. In contrast, Voravuthikunchai and LeeCitation8 examined the effects of S. Enteritidis on weight gain and mortality in normal, cecectomized, and sham mice. They observed that cecectomized mice inoculated with S. Enteritidis exhibited conspicuous weight loss, and possibly higher mortality (89% to 100%) relative to normal (50% to 78% mortality) and sham (67% to 83% mortality) mice. This was proposed to be due to cecectomy-induced dysbiosis of the colonic microbiota, characterized by an increase in coliforms, and a decrease in fusiform bacteria and total anaerobes. However, their characterizations of the colonic microbiota relied on culture-based enumeration methods, and beyond weight gain and mortality the authors did not report any indicators of disease. Moreover, S. Enteritidis incites typhoid-like systemic disease in mice with high rates of mortality.Citation9 Thus, this model is of limited value to study physiologic mechanisms of enteritis or colonization resistance within the intestine of mice. In this regard, S. enterica serovar Typhimurium would be a better choice since this pathogen is capable of inciting enterocolitis in mice with similar pathology to that observed in human beings.Citation30,Citation31 However, the current murine model of enteritis incited by S. Typhimurium requires pre-treatment of the animals with streptomycin,Citation31 which is a broad-spectrum antibiotic. Antibiotics, including streptomycin, can impart both direct and indirect impacts on the host,Citation32,Citation33 and it is possible that the murine cecectomy model will prove to be a useful alternative to the streptomycin dysbiosis model to explore the mechanisms of pathogenesis of enteric salmonellosis and colonization resistance. This may prove particularly useful considering emerging evidence which indicates that several classes of antibiotics modulate inflammatory responses in host tissues within the intestine.Citation34,Citation35
To determine if of butyrate could compensate for the loss of endogenous production of this SCFA by the enteric microbiota in cecectomized mice, butyrate was administered to mice by colonic infusion. Bacteria-derived butyrate is known to promote the differentiation of T regulatory cells in mice,Citation36,Citation37 and direct administration of butyrate to the intestine has shown promising beneficial results in some clinical trials. For example, 2 week treatment with 100 mmol/L sodium butyrate decreased symptoms of ulcerative colitis,Citation38 although these results have proved inconsistent and difficult to replicate,Citation39 potentially due to variability in intestinal transit time and inconsistency in contact time of butyrate with the intestinal mucosa. Moreover, Jiminez et al.Citation5 showed that colonic administration of butyrate increased feed consumption and weight gain, ameliorated C. rodentium-induced cell injury via enhanced mucus production and tissue repair mechanisms (i.e. Relmβ, Tff3, Myd88), and increased the abundance of butyrate-producing bacteria in mice with enteritis. Furthermore, butyrate concentrations measured in feces from mice with enteritis were higher than control miceCitation5 suggesting that the SCFA was more rapidly absorbed by colonocytes of animals without enteritis.Citation40,Citation41 In contrast to the above studies, we found that colonic administration of butyrate at a concentration of 100 mM had no effect on the structure of the bacterial communities in the colon, nor did it increase the concentration of butyric acid in the feces or reduce the severity of colitis in C. rodentium challenged mice. Concentrations of total SCFAs are higher in the cecum (131 ± 9 mmol/kg), but similar in the descending colon (80 ± 11mmol/kg) and feces (77.6 ± 4.5 mmol/kg) of human beings,Citation42,Citation43 with similar trends observed in mice.Citation5 Although we did not measure SCFAs in ingesta or tissues within the colon, as it requires the euthanization of additional mice, our results suggest that direct administration of SCFAs to the colon using a rigid gavage needle may not be the most effective technique to deliver butyrate to the colon. Others have administered butyrate via colonic infusions to rodents.Citation44,Citation45 However, the use of flexible infusion tubes for direct colonic delivery of butyrate may be a superior strategy in future studies in mice. Alternatively, per os or intra-gastric administration of butyrate could be used.Citation46,Citation47 We chose to deliver butyrate directly to the colon to ensure equal targeting between cecectomized and sham treatment mice; however, effective and equal passage of butyrate through the proximal alimentary canal to the colon, especially in mice ± a cecum may be problematic. The per os administration of phenylalanine-butyramide to overcome the poor palatability of sodium butyrate is an option in subsequent studies.Citation48
In conclusion, we observed that the removal of the murine cecum disrupted bacteria fermentation as evidenced by greatly reduced SCFA production. Furthermore, cecal removal resulted in a conspicuous dysbiosis in the colonic microbiota, and acute inflammation incited by C. rodentium was enhanced in cecectomized mice. However, the administration of butyrate directly to the colon did not ameliorate inflammation. With respect to our hypothesis, we showed that the removal of the cecum decreased richness of the ‘commensal’ colonic bacterial community and resulted in shifts in bacterial communities including decreased SCFA-associated bacteria. While the changes in SCFA were insufficient to modulate enteric inflammation resulting froma C. rodentium infection, our study indicates that the cecum in mice, and possibly other mammals, is a critical organ for maintaining bacterial diversity and SCFAs in the colon. Importantly, the murine cecectomy model may prove to be an effective tool to study the impact of dysbioses and SCFAs on various aspects of intestinal physiology and host well-being, including mechanisms of colonization resistance.
Materials and methods
Ethics statement
The study was carried out in strict accordance with the recommendations specified in the Canadian Council on Animal Care Guidelines. The project was reviewed and approved by the Lethbridge Research and Development Centre (LRDC) Animal Care Committee (Animal Use Protocol Review 1423), and the LRDC Biosafety and Biosecurity Committee before commencement of the research.
Surgical protocol
The surgical procedure used to remove the cecum was described previously.Citation8 Mice were anesthetized with isoflurane and placed in dorsal recumbency while receiving continuous anaesthetic. The abdomen was shaved and scrubbed twice with a chlorhexidine surgical solution, rinsed with 70% ethanol, and a final scrub of prepodyne solution was applied just prior to surgery. A surgical drape was placed on the abdomen and a 1.5 to 2.0 cm incision was made along the lower abdomen. The cecum was gently exteriorized, and a sterile barrier drape rinsed in phosphate buffered saline (PBS; pH 7.2) was placed under the cecum, the distal ileum, and the proximal colon. The cecum was ligated at the ileocecal junction, and the cecum was excised. Any remaining excess cecal tissue was trimmed. Care was taken to ensure that cecal contents were not released into the peritoneal cavity, and any residual ingesta on mucosal surfaces was irrigated with sterile PBS. The intestine was kept moist throughout the procedure and following the cecectomy, the intestine was returned into the abdomen cavity. The abdomen muscle layers were then closed with 4-0 or 5-0 Vicryl sutures and skin was closed with Michel suture clips (7.5mm x 1.75 mm). Clips were removed 7 to 10 days post-surgery. Each surgery lasted ≈10 min. For sham control mice, the surgical procedure described is above; the cecum was exteriorized and left outside the abdominal cavity for 2 min, and the cecum was then replaced in the peritoneal cavity and the abdomen and skin closed. During surgical induction, mice were provided meloxicam and buprenorphine subcutaneously while under general anaesthesia. A second dose of buprenorphine was administered 2 to 3 hr after surgery based on the level of discomfort exhibited by individual mice. Upon recovery from anesthesia, mice were administered a subcutaneous injection of warmed saline. Meloxicam was administered once daily to all mice for 2 days post-surgery. Mice were fed a conventional low fiber diet (Prolab RMH 3500, Canadian Lab Diets, Leduc, AB), and allowed to drink ad libitum. The surgical incision sites were monitored daily until fully healed, and animals were examined daily for changes in body temperature and behavioural manifestations of post-surgical distress.
Experimental design and treatment administrations
The experiment was conducted as a two (cecectomization) by two (inflammation) by two (butyrate administration) by two (sample time, 7 and 14 days p.i.) factorial experiment arranged as a completely randomized design (). Four time-independent replicates were conducted on separate occasions including a total of 64 mice. Mice were individually housed within individually ventilated cages attached to a HEPA filter unit operated in containment mode (Techniplast, Montreal, QC). Twenty-one days after surgery, cecectomized and sham control mice were colonically administered butyrate in PBS or PBS alone. Butyric acid (Sigma-Aldrich, Oakville, ON) was diluted with PBS to attain final concentrations of 100 mM butyrate; the pH was adjusted to 7.4 ± 0.2 with sodium hydroxide. The butyrate solution was prepared the day prior to administration, and stored at 4°C until used. Solutions were warmed to room temperature (RT) for 30 min before administration. Butyrate and PBS were administered via enemas (200 µL) at 2-day intervals for the duration of the experiment. To administer enemas, mice were anesthetized with isoflurane, inverted at a 45° angle, a 22G × 2.5 cm-long rigid gavage ‘needle’ with a 1.25 mm ball tip was gently inserted into the colon, the liquid was slowly injected, and mice were maintained in an inverted position for 30 sec after administration of the enema.Citation5 Animals were monitored for discomfort/pain for 4 hr after the enemas were administered. On the same day of butyrate administration, mice were orally gavaged with C. rodentium (ATCC 51459; 2 × 109 cells/ml) suspended in PBS or PBS alone on two consecutive days according to an established protocol.Citation24
Figure 1. The experiment was designed as four factor factorial experiment with two levels of time (7 and 14 days post-inoculation [p.i.]), two levels of cecum (i.e. mice that underwent sham [Cec-] or cecectomy [Cec+] surgery), two levels of inflammation (mice that were orally administered phosphate buffered saline [CR-] or Citrobacter rodentium [CR+]), and two levels of butyrate (i.e. mice were administered phosphate buffered saline [But-] or butyrate [But+] via enemas every second day p.i.). Thus, 16 mice were included in each replicate, and four replicates were conducted on separate occasions equaling 64 mice in total.
![Figure 1. The experiment was designed as four factor factorial experiment with two levels of time (7 and 14 days post-inoculation [p.i.]), two levels of cecum (i.e. mice that underwent sham [Cec-] or cecectomy [Cec+] surgery), two levels of inflammation (mice that were orally administered phosphate buffered saline [CR-] or Citrobacter rodentium [CR+]), and two levels of butyrate (i.e. mice were administered phosphate buffered saline [But-] or butyrate [But+] via enemas every second day p.i.). Thus, 16 mice were included in each replicate, and four replicates were conducted on separate occasions equaling 64 mice in total.](/cms/asset/5a7dd665-92d7-4ee2-b403-85ce95edfa13/kgmi_a_1408763_f0001_b.gif)
Figure 2. Concentration of: (A) total short chain fatty acids; (B) acetic acid; (C) butyric acid; (D) isobutyric acid; (E) valeric acid; (F) isovaleric acid; (G) propionic acid; and (H) caproic acid in feces from mice that received sham (Cec-) or cecectomy (Cec+) surgery and were orally administered PBS (CR-) or Citrobacter rodentium (CR+). Fatty acids were measured in feces collected prior to surgery (pre-surgery), between surgery and inoculation (post-surgery), and after administration of C. rodentium or PBS. Vertical lines associated with histogram bars represent standard error of the means (n = 10 to 15 mice/treatment). *P ≤ 0.050, ***P ≤ 0.001.

Figure 3. Alpha diversity of bacterial communities in the proximal (A) and distal (B) colon of mice that received sham (Cec-) or cecectomy (Cec+) surgeries and were orally administered PBS (CR-) or Citrobacter rodentium (CR+) 14 days post-inoculation. Vertical lines associated with histogram bars represent standard error of the means (n = six to eight mice/treatment).
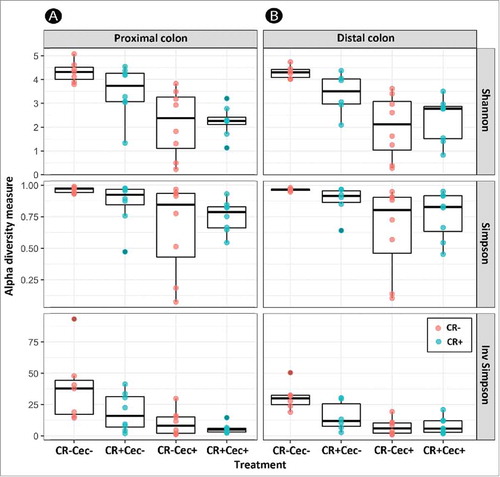
Figure 4. Principal component analysis based on Bray-Curtis dissimilarity in bacterial communities in the proximal (A) and distal (B) colon of mice that received sham (Cec-) or cecectomy (Cec+) surgeries 14 days post-inoculation.
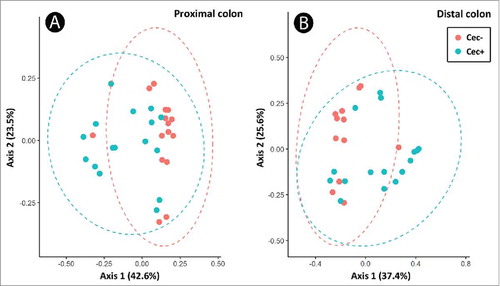
Figure 5. Relative abundance of bacteria genera within different families in the proximal (A) and distal (B) colon of mice that received sham (Cec-) or cecectomy (Cec+) surgeries and were orally adminstered PBS (CR-) or Citrobacter rodentium (CR+) 14 days post-inoculation. Bars indicate the mean (n = four mice/treatment).
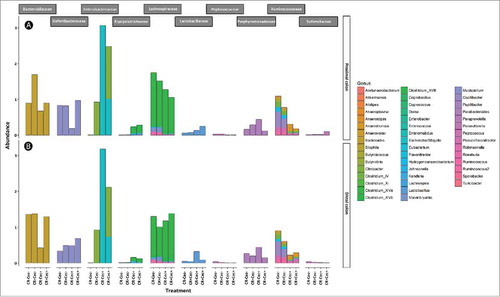
Figure 6. Relative abundance of genera within the Lachnospiraceae family in the proximal (A) and distal (B) colon of mice that received sham (Cec-) or cecectomy (Cec+) surgeries and were orally administered PBS (CR-) or Citrobacter rodentium (CR+) 14 days post-inoculation. Data represents the mean (n = four mice/treatment).
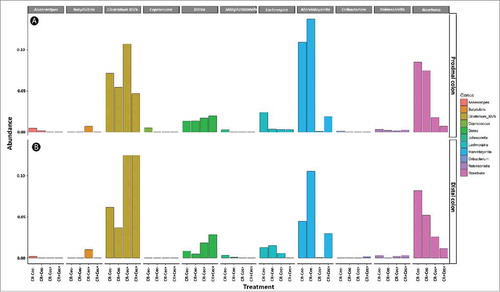
Figure 7. Relative abundance of genera within the Ruminococcaceae family in the proximal (A) and distal (B) colon of mice that received sham (Cec -) or cecectomy (Cec+) surgeries and were orally administered PBS (CR-) or Citrobacter rodentium (CR+) 14 days post-inoculation. Data represents the mean (n = four mice/treatment).
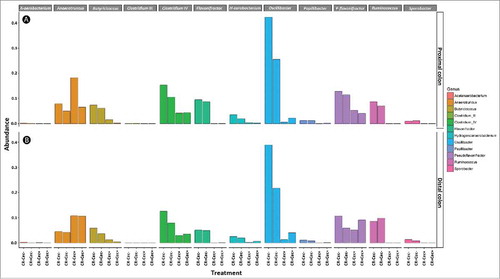
Figure 8. (A) Densities of viable C. rodentium (log CFU/g) in feces as determined by dilution plating for mice that received sham (Cec -) or cecectomy (Cec +) surgeries, were orally administered Citrobacter rodentium (CR+), and received enemas containing PBS (But-) or butyrate (But+). Vertical lines associated with markers indicate standard error of the means (n = four mice/treatment). (B-E) Visualization of C. rodentium in distal colon tissues at peak infection (i.e. 7 days post-inoculation); (B) Cec- and But-; (C) Cec- and But+; (D) Cec+ and But-; (E) Cec+ and But+. Cell nuclei are stained with DAPI (blue), total bacteria are stained with Alexa-488 (green) and γ-Proteobacteria (C. rodentium) are stained with Alexa-594 (red). Scale bar = 50 µm.
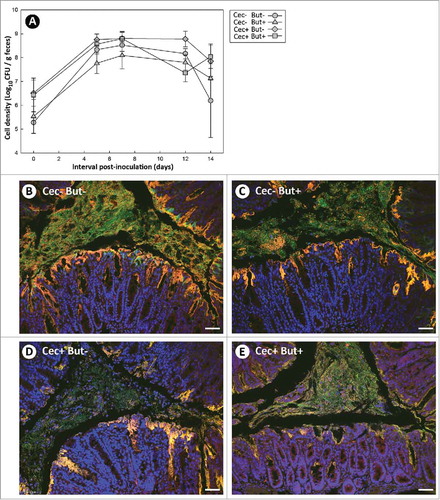
Figure 9. (A) Temporal change in body weight, and (B) change in body weight at 13 days after administration of Citrobacter rodentium or PBS. Mice that received sham (Cec-) or cecectomy (Cec+) surgeries, were orally administered PBS (CR-) or C. rodentium (CR+), and received enemas containing PBS (But-) or butyrate (But+). Vertical lines associated with markers or histogram bars represent standard error of the means (n = three to five mice/treatment). *P ≤ 0.050.
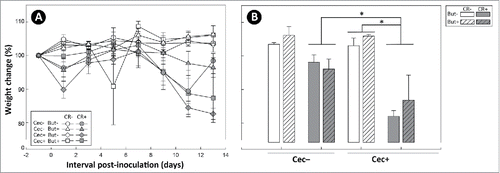
Figure 10. Histopathological scores of spleen tissues from mice that received sham (Cec-) or cecectomy (Cec+) surgeries, were orally administered PBS (CR-) or Citrobacter rodentium (CR+), and received enemas containing PBS (But-) or butyrate (But+). Data is shown 7 and 14 days after administration of C. rodentium or PBS. Vertical lines associated with histogram bars represent standard error of the means (n = three to five mice/treatment). *P ≤ 0.050, **P ≤ 0.010. The maximum possible score is 12.
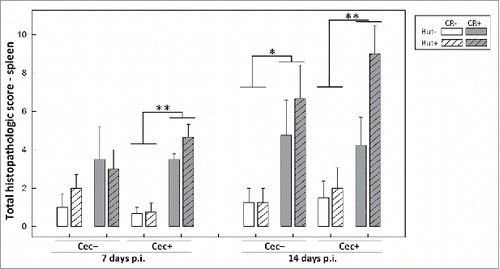
Figure 11. Histopathological scores of tissue sections from the (A) proximal and (B) distal colon of mice that received sham (Cec-) or cecectomy (Cec+) surgeries, were orally administered PBS (CR-) or Citrobacter rodentium (CR+), and received enemas containing PBS (But-) or butyrate (But+). Data is shown 7 and 14 days after administration of C. rodentium or PBS. Vertical lines associated with histogram bars represent standard error of the means (n = three to five mice/treatment). *P ≤ 0.050, **P ≤ 0.010.
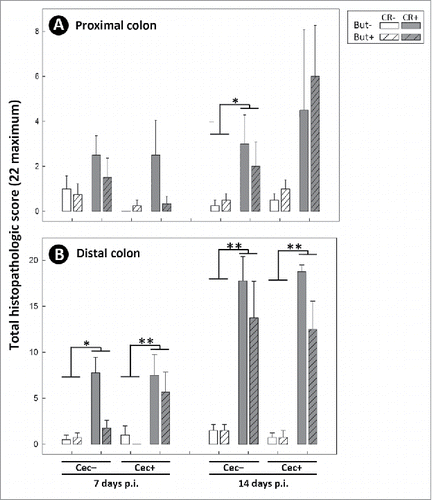
Figure 12. Expression of mRNA transcripts which code for: (A) Ifnγ; (B) Tnfα; (C) Il17a; (D) Il10; (E) Tgfβ; (F) Relmβ; (G) Muc2; (H) βd1; (I) Ffar2; and (J) Ffar3 in proximal colonic tissues of mice that received sham (Cec-) or cecectomy (Cec+) surgeries, were orally administered PBS (CR-) or Citrobacter rodentium (CR+), and received enemas containing PBS (But-) or butyrate (But+). Data is shown 7 and 14 days after administration of C. rodentium or PBS (p.i.). Vertical lines associated with histogram bars represent standard error of the means (n = three to five mice/treatment). *P ≤ 0.050, **P ≤ 0.010.
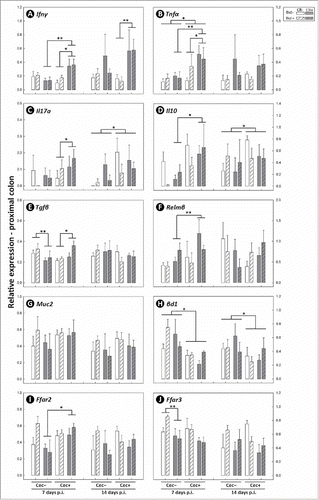
Figure 13. Expression of mRNA transcripts which code for: (A) Ifnγ; (B) Tnfα; (C) Il17a; (D) Il10; (E) Tgfβ; (F) Relmβ; (G) Muc2; (H) βd1; (I) Ffar2; and (J) Ffar3 in distal colonic tissues of mice that received sham (Cec-) or cecectomy (Cec+) surgeries, were orally administered PBS (CR-) or Citrobacter rodentium (CR+), and received enemas containing PBS (But-) or butyrate (But+). Data is shown 7 and 14 days after administration of C. rodentium or PBS (p.i.). Vertical lines associated with histogram bars represent standard error of the means (n = three to five mice/treatment). *P ≤ 0.050, **P ≤ 0.010, ***P ≤ 0.001.
Data acquisition and tissue collection
Mice were monitored daily for changes in health status and these included changes in body temperature, food consumption, behavioral and the presence of diarrhea. Recently voided feces were collected at intervals before and after inoculation of C. rodentium to measure the presence of C. rodentium and amounts of SCFAs. Seven and 14 days p.i. (i.e. corresponding to peak and late stages of disease, respectively), animals were anaesthetized with isofluorane and humanely euthanized by cervical dislocation. The intestine, liver and spleen were aseptically harvested, and segments of the intestine and spleen were frozen at -80°C for analysis of bacterial communities and fixed in 10% neutral buffered formalin for histopathological analysis. Tissue samples (liver, ileum, cecum, proximal colon, and distal colon) were stored in RNAlater® (Sigma-Aldrich, Oakville, ON) for quantitative PCR analysis.
Enumeration of C. rodentium
Densities of C. rodentium cells were determined by homogenizing fecal or colonic mucosal samples in Columbia broth (Oxoid, Nepean, ON), and spreading serial dilutions of the homogenate onto MacConkey agar (Becton, Dickinson and Company, Mississauga, ON). Cultures were incubated overnight at 37°C, enumerated at the dilution yielding 30–300 colony forming units per culture, and adjusted by initial sample weight.
Histopathological scoring
Tissue samples from the cecum, proximal colon, distal colon, and spleen were fixed in 10% buffered formalin for 12–24 hr, dehydrated, embedded in paraffin, sectioned (≈4 μm thick), and stained with hematoxylin and eosin (H&E) according to a standard procedure.Citation24 Scoring of tissues for histopathologic changes was performed by a veterinary pathologist (RREU) blinded to the treatments, with scoring criteria adapted from previously described methods.Citation24 Briefly, colonic sections were graded 0 to 4 for epithelial cell hyperplasia, crypt height, epithelial injury, extent of inflammatory infiltrates, and 0 to 3 for mitotic activity of epithelial cells, and goblet cell depletion. Spleen tissues were graded 0 to 3 for germinal centre number, germinal centre size, cell infiltration, and apoptotic and necrotic cells. The total histopathologic score was obtained by calculating the sum of scores for all categories for each mouse; the maximum total scores were 22 and 12 for colonic and splenic tissues, respectively.
Quantification of mRNA expression
Total RNA was extracted from proximal and distal colon sections using the RNeasy Mini Kit (Qiagen Inc., Toronto, ON) according to manufacturer`s instructions with the addition of a DNase step to remove genomic DNA. RNA quality and quantity was determined using Bioanalyzer RNA 6000 Nano kit (Agilent, Mississauga, ON) and cDNA was reverse transcribed using Quantitect Reverse Transcription Kit (Qiagen Inc.). Quantitative PCR was performed using Quantitect SYBR Green Master Mix (Qiagen Inc.) with primers specific to genes encoding cytokines, chemokines, defensins, and mucins. Relative expression was calculated using qBase software (Biogazelle, Gent, Belgium) relative to reference genes Peptidylprolyl isomerase A, hypoxanthine-guanine phosphoribosyltransferase, and beta-glucuronidase.
Analysis of bacterial communities
DNA from mucosal plugs (3-mm in diameter) from the proximal and distal colon of mice in the 14 days after administration of C. rodentium or PBS was extracted using DNeasy Tissue Kit (Qiagen Inc.) according to manufacturer's instructions. Standard protocols for library preparation were used according to the manufacturer's recommendations (Illumina Canada, Victoria, BC), including primers which flank the V3/V4 region.Citation49 Sequencing was performed on a Miseq instrument (Illumina Canada) using a MiSeq Regent Kit v3 (600-cycle) (Illumina Inc.). Primer removal and quality trimming was performed in CutadaptCitation50 with a threshold quality score of 18, and analysis of the sequencing reads was performed using dada2Citation51 and phyloseqCitation52 packages within R.Citation53 Within dada2, forward reads were trimmed to 240 base pairs and reverse reads to 210 base pairs, dereplicated sequences were merged, and chimeras identified and removed using the removeBimeraDenovo function. Taxonomy was assigned based on the RDP database and a neighbor joining phylogenetic tree was generated using the Phangorn package.Citation54 Sequence depth varied between ≈2000 to ≈50,000 sequences (Table S1) and depth was rarefied to 9000 sequences per sample which captured bacterial diversity within the samples (Figure S5). Within sample diversity was calculated using Shannon, Simpson, and Inverse Simpson metrics. Beta diversity was calculated using the weighted UNIFRAC metricCitation55 and differences among groups was identified using permutational analysis of variance (PERMANOVA).Citation56
Short chain fatty acid quantification
Fecal samples were collected, weighed, and homogenized in PBS (1 mg/ml). Meta-phosphoric acid (Sigma Aldrich, Oakville, ON) was added to the homogenate at a concentration of 20% v/w and samples were incubated at room temperature (RT) for 30 min. Samples were centrifuged at RT for 75 min at 16000 x g, and the supernatants were collected and stored at -20°C until further processing. Concentrations of total SCFAs, acetic acid, butyric acid, isobutyric acid, valeric acid, isovaleric acid, propionic acid, and caproic acid were quantified using a gas chromatograph (Agilent Technologies, Model 6890N with 7683 Series Injector) according to established protocols.Citation57,Citation58
Fluorescence in situ hybridization
Formalin fixed and paraffin embedded colonic tissue sections were deparaffinized with xylene and subsequently rehydrated. An Alexa-fluor 594 labelled probe for Gammaproteobacteria (GAM42a: 5′-GCC TTC CCA CAT CGT TT-3′) and an Alexa-fluor labelled 488 probe for Eubacteria (EUB338: 5′-GCT GCC TCC CGT AGG AGT-3′) prepared at a concentration of 2.5 ng/µl in hybridization solution (52.6 g NaCl, 12.2 g Trizma base, 300 mL formamide, 1 g SDS in 1 L; pH 7.2) was incubated with the tissues at 37°C for ≈18 hr in the dark. Slides were washed for 15 min in hybridization solution, then washed for 15 min in a wash buffer (52.6 g NaCl, 12.1 g Tris base in 1 L; pH 7.2) then placed in deionized water. Slides were mounted with Fluoroshield Tm with DAPI (Sigma Aldrich) and visualized and images using a T81X confocal microscope (Olympus Canada Inc., Toronto, ON).
Statistical analysis
All parametric statistical analyses were performed using Statistical Analysis Software (SAS Institute Inc. Cary, NC). Continuous data was checked for normality and analyzed using the MIXED procedure of SAS. Where applicable (i.e. fecal C. rodentium densities and body weights), collection time was treated as a repeated measure; the appropriate covariance structure was utilized according to the lowest Akaike's Information criterion. In the event of a main treatment event effect (P ≤ 0.050), the least squares means test was used to compare treatments within factors. Histopathologic measurement data were analysed using the Chi squared (NPAR1WAY) procedure of SAS. Data is represented by mean ± standard error of the means (SEM), and asterisks represent the following probability values: *P ≤ 0.050; **P ≤ 0.010; and ***P ≤ 0.001.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Authors’ contributions
G.D.I. conceived the research; K.B., R.R.E.U, and G.D.I. designed the research; G.D.I. obtained ethics approvals; K.B., R.R.E.U., and G.D.I. performed experiments; K.B. and G.D.I. analyzed data; K.B., R.R.E.U, and G.D.I interpreted results of experiments; K.B. and G.D.I. prepared figures; K.B. and G.D.I. generated the initial draft of the manuscript; K.B., D.W.A., R.R.E.U, and G.D.I. edited the manuscript and approved final version of manuscript
1408763_supp.pdf
Download PDF (396.7 KB)Acknowledgments
The authors would like to thank the following individuals at Agriculture and Agri-Food Canada Lethbridge Research and Development Centre: Tara Shelton for overseeing, conducting, or assisting with all aspects of the animal component of the study; Angela Bamra, Paige Fletcher and Kaylie Graham for their assistance with sample collection and animal husbandry; and Toby Entz for experimental design and statistical advice.
Additional information
Funding
References
- den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–40. doi:10.1194/jlr.R036012.
- Ghosh S, Dai C, Brown K, Rajendiran E, Makarenko S, Baker J, Ma C, Halder S, Montero M, Ionescu VA, et al. Colonic microbiota alters host susceptibility to infectious colitis by modulating inflammation, redox status, and ion transporter gene expression. Am J Physiol Gastrointest Liver Physiol. 2011;301:G39–49. doi:10.1152/ajpgi.00509.2010.
- Health Canada. Policy for labelling and advertising of dietary fibre-containing food products. 2012. https://www.canada.ca/en/health-canada/services/food-nutrition/legislation-guidelines/policies/policy-labelling-advertising-dietary-fibre-containing-food-products-2012.html. Accessed January 10, 2018.
- Tremaroli V, Backhed F Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–9. doi:10.1038/nature11552.
- Jiminez JA, Uwiera TC, Abbott DW, Uwiera RRE, Inglis GD Butyrate supplementation at high concentrations alters enteric bacterial communities and reduces intestinal inflammation in mice infected with Citrobacter rodentium. mSphere. 2017;2:e00243–17. doi:10.1128/mSphere.00243-17.
- Flint HJ, Duncan SH, Scott KP, Louis P Interactions and competition within the microbial community of the human colon: links between diet and health. Environ Microbiol. 2007;9:1101–11. doi:10.1111/j.1462-2920.2007.01281.x.
- Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–6. doi:10.1038/nature08530.
- Voravuthikunchai SP, Lee A Cecectomy causes long-term reduction of colonization resistance in the mouse gastrointestinal tract. Infect Immun. 1987;55:995–9.
- Silva CA, Blondel CJ, Quezada CP, Porwollik S, Andrews-Polymenis HL, Toro CS, Zaldívar M, Contreras I, McClelland M, Santiviago CA Infection of mice by Salmonella enterica serovar Enteritidis involves additional genes that are absent in the genome of serovar Typhimurium. Infect Immun. 2012;80:839–49. doi:10.1128/IAI.05497-11.
- Borenshtein D, McBee ME, Schauer DB Utility of the Citrobacter rodentium infection model in laboratory mice. Curr Opin Gastroenterol. 2008;24:32–7. doi:10.1097/MOG.0b013e3282f2b0fb.
- Gu S, Chen D, Zhang JN, Lv X, Wang K, Duan LP, Nie Y, Wu XL Bacterial community mapping of the mouse gastrointestinal tract. PLoS One. 2013;8:e74957. doi:10.1371/journal.pone.0074957.
- Stearns JC, Lynch MD, Senadheera DB, Tenenbaum HC, Goldberg MB, Cvitkovitch DG, Croitoru K, Moreno-Hagelsieb G, Neufeld JD Bacterial biogeography of the human digestive tract. Sci Rep. 2011;1:170. doi:10.1038/srep00170.
- Brown K, DeCoffe D, Molcan E, Gibson DL Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012;4:1095–119. doi:10.3390/nu4081095.
- Scott KP, Martin JC, Duncan SH, Flint HJ Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol Ecol. 2014;87:30–40. doi:10.1111/1574-6941.12186.
- Takahashi K, Nishida A, Fujimoto T, Fujii M, Shioya M, Imaeda H, Inatomi O, Bamba S, Sugimoto M, Andoh A Reduced abundance of butyrate-producing bacteria species in the fecal microbial community in Crohn's disease. Digestion. 2016;93:59–65. doi:10.1159/000441768.
- Hu Y, Le Leu RK, Christophersen CT, Somashekar R, Conlon MA, Meng XQ, Winter JM, Woodman RJ, McKinnon R, Young GP Manipulation of the gut microbiota using resistant starch is associated with protection against colitis-associated colorectal cancer in rats. Carcinogenesis. 2016;37:366–75. doi:10.1093/carcin/bgw019.
- Rios-Covian D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilan CG, Salazar N Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. 2016;7:185. doi:10.3389/fmicb.2016.00185.
- Macfarlane GT, Macfarlane S Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. 2012;95:50–60. doi:10.5740/jaoacint.SGE_Macfarlane.
- Hatayama H, Iwashita J, Kuwajima A, Abe T The short chain fatty acid, butyrate, stimulates MUC2 mucin production in the human colon cancer cell line, LS174T. Biochem Biophys Res Comm. 2007;356:599–603. doi:10.1016/j.bbrc.2007.03.025.
- Wang HB, Wang PY, Wang X, Wan YL, Liu YC Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci. 2012;57:3126–35. doi:10.1007/s10620-012-2259-4.
- Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63:1275–83. doi:10.1136/gutjnl-2013-304833.
- Russell WR, Hoyles L, Flint HJ, Dumas ME Colonic bacterial metabolites and human health. Curr Opin Microbiol. 2013;16:246–54. doi:10.1016/j.mib.2013.07.002.
- Wiles S, Clare S, Harker J, Huett A, Young D, Dougan G, Frankel G Organ specificity, colonization and clearance dynamics in vivo following oral challenges with the murine pathogen Citrobacter rodentium. Cell Microbiol. 2004;6:963–72. doi:10.1111/j.1462-5822.2004.00414.x.
- Costa E, Uwiera RR, Kastelic JP, Selinger LB, Inglis GD Non-therapeutic administration of a model antimicrobial growth promoter modulates intestinal immune responses. Gut Pathog. 2011;3:14. doi:10.1186/1757-4749-3-14.
- Lam YY, Ha CW, Campbell CR, Mitchell AJ, Dinudom A, Oscarsson J, Cook DI, Hunt NH, Caterson ID, Holmes AJ, et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS One. 2012;7:e34233. doi:10.1371/journal.pone.0034233.
- Vermeire S, Joossens M, Verbeke K, Wang J, Machiels K, Sabino J, Ferrante M, Van Assche G, Rutgeerts P, Raes J Donor species richness determines faecal microbiota transplantation success in inflammatory bowel disease. J Crohns Colitis. 2016;10:387–94. doi:10.1093/ecco-jcc/jjv203.
- Satokari R, Fuentes S, Mattila E, Jalanka J, de Vos WM, Arkkila P Fecal transplantation treatment of antibiotic-induced, noninfectious colitis and long-term microbiota follow-up. Case Rep Med. 2014;2014:913867. doi:10.1155/2014/913867.
- Huang C, Chen J, Wang J, Zhou H, Lu Y, Lou L, Zheng J, Tian L, Wang X, Cao Z, et al. Dysbiosis of intestinal microbiota and decreased antimicrobial peptide level in Paneth cells during hypertriglyceridemia-related acute necrotizing pancreatitis in rats. Front Microbiol. 2017;8:776. doi:10.3389/fmicb.2017.00776.
- Ling Z, Jin C, Xie T, Cheng Y, Li L, Wu N Alterations in the fecal microbiota of patients with HIV-1 infection: an observational study in a Chinese population. Sci Rep. 2016;6:30673. doi:10.1038/srep30673.
- Santos RL, Zhang S, Tsolis RM, Kingsley RA, Adams LG, Baumler AJ Animal models of Salmonella infections: enteritis versus typhoid fever. Microbes Infect. 2001;3:1335–44. doi:10.1016/S1286-4579(01)01495-2.
- Hapfelmeier S, Hardt WD A mouse model for S. typhimurium-induced enterocolitis. Trends Microbiol. 2005;13:497–503. doi:10.1016/j.tim.2005.08.008.
- Barnhill AE, Brewer MT, Carlson SA Adverse effects of antimicrobials via predictable or idiosyncratic inhibition of host mitochondrial components. Antimicrob Agents Chemother. 2012;56:4046–51. doi:10.1128/AAC.00678-12.
- Rubin BK, Tamaoki J Antibiotics as anti-inflammatory and immunomodulatory agents. Basel, Switzerland: Birkhauser Verlag; 2005.
- Brown K, Zaytsoff SJ, Uwiera RR, Inglis GD Antimicrobial growth promoters modulate host responses in mice with a defined intestinal microbiota. Sci Rep. 2016;6:38377. doi:10.1038/srep38377.
- Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–6. doi:10.1038/nature11400.
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–41. doi:10.1126/science.1198469.
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–5. doi:10.1038/nature12726.
- Scheppach W, Sommer H, Kirchner T, Paganelli GM, Bartram P, Christl S, Richter F, Dusel G, Kasper H Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterol. 1992;103:51–6. doi:10.1016/0016-5085(92)91094-K.
- Breuer RI, Soergel KH, Lashner BA, Christ ML, Hanauer SB, Vanagunas A, Harig JM, Keshavarzian A, Robinson M, Sellin JH, et al. Short chain fatty acid rectal irrigation for left-sided ulcerative colitis: a randomised, placebo controlled trial. Gut. 1997;40:485–91. doi:10.1136/gut.40.4.485.
- Cummings JH Colonic absorption: the importance of short chain fatty acids in man. Scand J Gastroenterol Suppl. 1984;93:89–99.
- Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217:133–9. doi:10.1111/j.1574-6968.2002.tb11467.x.
- Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–7. doi:10.1136/gut.28.10.1221.
- Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TM, Comelli EM Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes. 2014;4:e121. doi:10.1038/nutd.2014.23.
- Bosmans JW, Jongen AC, Boonen BT, van Rijn S, Scognamiglio F, Stucchi L, Gijbels MJ, Marsich E, Bouvy ND Comparison of three different application routes of butyrate to improve colonic anastomotic strength in rats. Int J Colorectal Dis. 2017;32:305–13. doi:10.1007/s00384-016-2718-z.
- Goulart Pacheco R, Costa Esposito C, Müller LCM, Castelo-Branco MTL, Pereira Quintella L, Chagas VLA, de Souza HSP, Schanaider A Use of butyrate or glutamine in enema solution reduces inflammation and fibrosis in experimental diversion colitis. World J Gastroenterol. 2012;18:4278–87. doi:10.3748/wjg.v18.i32.4278.
- Zhou D, Pan Q, Xin FZ, Zhang RN, He CX, Chen GY, Liu C, Chen YW, Fan JG Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J Gastroenterol. 2017;23:60–75. doi:10.3748/wjg.v23.i1.60.
- Henagan TM, Stefanska B, Fang Z, Navard AM, Ye J, Lenard NR, Devarshi PP Sodium butyrate epigenetically modulates high-fat diet-induced skeletal muscle mitochondrial adaptation, obesity and insulin resistance through nucleosome positioning. Br J Pharmacol. 2015;172:2782–98. doi:10.1111/bph.13058.
- Mattace Raso G, Simeoli R, Russo R, Iacono A, Santoro A, Paciello O, Ferrante MC, Canani RB, Calignano A, Meli R Effects of sodium butyrate and its synthetic amide derivative on liver inflammation and glucose tolerance in an animal model of steatosis induced by high fat diet. PLoS One. 2013;8:e68626. doi:10.1371/journal.pone.0068626.
- Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glöckner FO Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41:e1. doi:10.1093/nar/gks808.
- Martin M Cutadapt removes adaptor sequences form high throughput sequencing reads. EMBnetjournal. 2011;17:10–2.
- Callahan BJ, Sankaran K, Fukuyama JA, McMurdie PJ, Holmes SP Bioconductor workflow for microbiome data analysis: from raw reads to community analyses. F1000Res. 2016;5:1492. doi:10.12688/f1000research.8986.2.
- McMurdie PJ, Holmes S phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8:e61217. doi:10.1371/journal.pone.0061217.
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013.
- Schliep KP phangorn: phylogenetic analysis in R. Bioinformatics. 2011;27:592–3. doi:10.1093/bioinformatics/btq706.
- Lozupone C, Knight R UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71:8228–35. doi:10.1128/AEM.71.12.8228-8235.2005.
- Anderson MJ A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26:32–46.
- Cottyn B, GaB CV Rapid method for the gas-chromatographic determination of volatile fatty acids in rumen fluid. J Agric Food Chem. 1968;16:105–7. doi:10.1021/jf60155a002.
- Playne MJ Determination of ethanol, volatile fatty acids, lactic and succinic acids in fermentation liquids by gas chromatography. J Sci Food Agric. 1985;36:638–44. doi:10.1002/jsfa.2740360803.
