ABSTRACT
The intestinal tract is inhabited by a large and diverse community of bacteria, collectively referred to as the gut microbiota. Composed of 500–1000 distinct species, the intestinal microbiota plays an important role in immunity and metabolism. However, alterations in its composition are associated with a variety of inflammatory diseases including obesity, diabetes, and inflammatory bowel disease (IBD). Among many other factors, our diet impacts microbiota composition and function, in either beneficial or detrimental ways. In this addendum, we will discuss our recent findings on how dietary emulsifying agents can directly and detrimentally impact the microbiota, leading to inflammatory diseases and cancer.
From correlation to observation: Dietary emulsifiers induce deleterious health effect
The intestinal microbiota is a vast and complex community of microorganisms that includes 1014 bacteria per intestine and about 1000 different species. Among its various functions, the gut microbiota is essential to promote maturation of the intestinal immune system, help digestion and favor calorie extraction. Besides its essential beneficial roles, the gut microbiota can also turn out to be detrimental and, if not well managed, lead to the development of inflammatory diseases, such as Inflammatory Bowel Disease (IBD)Citation1 and metabolic syndrome.Citation2 Inflammatory bowel diseases are severe, debilitating, and lead to a significantly increased risk to develop colon cancer that affects millions of people worldwide. Metabolic syndrome is a cluster of obesity-related disorders (high blood pressure, hyperglycemia, abnormal triglyceride and cholesterol levels) that together, substantially increase the risk of developing type-2 diabetes, cardiovascular, and/or liver diseases.
Amidst constant genetics a dramatic increase in the incidence of IBD and metabolic syndromes occurred during the last 50 years. This rapid evolution suggested that factors related to life styleCitation3,Citation4 and/or the use of antibioticsCitation5 could be involved. We and others previously hypothesized that emulsifiers, which are added to most processed foods to aid texture and extend shelf-life, could play a role in the rapid increase in the incidence of these diseases.Citation6–8 We recently demonstrated that emulsifiers induced a chronic intestinal inflammation that promotes development of chronic colitis in susceptible mice and metabolic syndrome in wild-type mice.Citation9 By treating mice with two commonly used emulsifiers, namely polysorbate 80 (P80) and carboxymethylcellulose (CMC), at doses seeking to model the broad consumption of the numerous emulsifiers that are incorporated into a large variety of processed foods, we observed changes in species composition of the gut microbiota and in its pro-inflammatory potential. Specifically, this altered microbiota had enhanced capacity to infiltrate the dense mucus layer that lines the intestine, and which is normally devoid of bacteria. Moreover, the alterations of the microbiota were characterized by an increased expression of bacterial inflammatory molecules, such as flagellin and lipopolysaccharide, which can in turn activate the expression of pro-inflammatory genes by the immune system. Such functional changes of the microbiota triggered chronic colitis in mice genetically prone to this disorder, due to abnormal immune systems. In contrast, in wild-type mice with normal immune systems, emulsifiers induced low-grade (i.e. mild) intestinal inflammation and metabolic syndrome, characterized by increased adiposity and hyperglycemia. Furthermore, we identified that emulsifiers affected the gut microbiota, creating a favorable niche that lead to an exacerbation of tumor development in mouse models of colorectal cancer.Citation10 Overall, these findings support the concept that disturbance of the host-microbiota relationship can cause inflammation that will manifest as chronic inflammatory disorders in genetically susceptible individuals, or insulin resistance and associated metabolic syndrome in unimpaired host.Citation11
From observation to mechanism: Effects of dietary emulsifier on intestinal microbiota
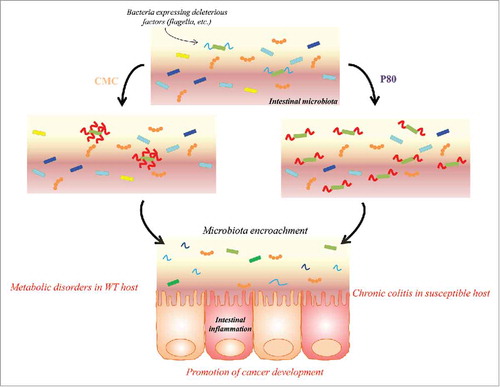
When we aimed to understand how mechanistically, emulsifiers could detrimentally impact health and promote inflammation, we identified that the effects of their consumption were eliminated in mice lacking a microbiota (germ-free). Importantly, transplantation of microbiota from emulsifier-treated mice to wild type germ-free recipient mice was sufficient to transfer some parameters of low-grade inflammation and metabolic syndrome, indicating a central role played by the microbiota in mediating the effects. We then demonstrated that a complex microbiota community is required for emulsifier-mediated detrimental effects. Indeed, we recently observed that emulsifier consumption by gnotobiotic mice colonized with a highly-restricted microbiota comprised of only 8 bacteria, namely “Altered Schaedler Flora” (ASF),Citation12 was not sufficient to induce microbiota encroachment, intestinal inflammation, or altered metabolism.Citation13 These findings suggest that a complex microbiota, containing specific species, is required for the detrimental effects of emulsifiers. Moreover, they suggest that dietary emulsifiers directly target the microbiota, and seem to have little to no effect on the host itself. In order to demonstrate this hypothesis, we recently collaborated with Pr. Tom Van de Wiele, from Ghent University (Ghent, Belgium), who has developed a state-of-the-art in vitro microbiota model, namely the mucosal-simulated human intestinal microbiota ecosystem (M-SHIME), a dynamic model that simulates the lumen- and mucus-associated human intestinal microbial ecosystem.Citation14–16 Using this in vitro microbiota model, we demonstrated that both P80 and CMC acted directly on a single human microbiota to alter microbiota composition and/or gene expression (measured by meta-transcriptomic approach). This result suggests that microbiota is a direct target of these commonly used food additives.Citation13 Specifically, this study revealed that both P80 and CMC increased the pro-inflammatory potential of the human microbiota as revealed by increased levels of bioactive flagellin. The CMC-induced increase in flagellin was rapid (1 day) and driven by altered microbiota transcriptome. In contrast, the P80-induced flagellin increase occurred more gradually and was closely associated with altered microbiota composition. Importantly, when transferred to germfree recipient animals, both emulsifier-treated M-SHIME microbiotas induced most of the host and microbial alterations observed in mice directly treated by emulsifiers (). Hence, these results demonstrate a novel paradigm of deconstructing host-microbiota interactions and indicate that the microbiota can be directly impacted by these commonly used food additives, in a manner that subsequently drives intestinal inflammation.
Figure 1. Dietary emulsifiers directly impact the intestinal microbiota, increasing their pro-inflammatory potential. Carboxymethylcellulose (CMC) is directly impacting bacterial gene expression, leading to an increase expression of molecules with pro-inflammatory potential. Polysorbate 80 (P80) is altering microbiota composition, favoring the expansion of bacteria with pro-inflammatory potential. In both cases, such altered microbiotas are able to penetrate the normally sterile mucus layer, leading to intestinal inflammation that manifest as chronic inflammatory disorders in genetically susceptible individuals or insulin resistance and associated metabolic syndrome in unimpaired host. Moreover, altered microbiota following emulsifier exposure predispose to colon cancer development.
Conclusions and future directions
To summarize, we demonstrated that dietary emulsifiers directly target the intestinal microbiota, increasing its ability to penetrate the normally sterile mucus layer, leading to intestinal inflammation and metabolic syndrome in wild-type host. While our research does not disagree with the commonly held assumption that over-eating is a central cause of obesity and metabolic syndrome, those findings reinforce the concept suggested by earlier work that low-grade inflammation resulting from an altered microbiota can be an underlying cause of excess eating.Citation17,Citation18
In the coming years, the molecular mechanisms by which emulsifiers affect bacteria should be investigated, as it will be crucial to understand how such compounds can be sensed by the intestinal microbiota and lead to altered composition and gene expression. While we previously used only one donor in the M-SHIME model, comparing emulsifier effect (variable = emulsifier, constant = donor), further experimentations should analyze inter-individual variations in response to emulsifier treatment by using multiple donors (variable = donor, constant = emulsifier). Such approach should allow the identification of “susceptible” and “resistant” microbiota, with the identification of key bacterial species driving subsequent inflammation. With those specific bacteria identified, it will then be important to understand the mechanism by which they can induce inflammation. Are those bacteria able to adhere to and/or to invade intestinal epithelial cells in the presence of emulsifiers? Which bacterial organelles / secreted factors are impacting host inflammatory response? Which host pathways are activated?
While we previously reported that switching mice off from emulsifier exposure was sufficient to reverse most of the detrimental effects, it will be important to decipher how a normal host-microbiota relationship can be restored. It remains, for example, unclear if emulsifier removal is sufficient for the microbiota to recover a healthy state that associates with a decreased risk of colon cancer development. Additionally, and given that soluble fiber such as inulin or the prebiotic Akkermansia muciniphila are both known to favor mucus secretion,Citation19,Citation20 the use of prebiotic or probiotic on emulsifier-treated animals should be explored as therapeutic approaches.
Furthermore, the exact mechanism by which the altered microbiota is driving inflammation remains unclear and requires further investigations. Moreover, the direct effects these compounds have toward the microbiota do not exclude the possibility that they also have direct effects on mucus layer integrity and/or on the intestinal mucosa. Concomitantly, it remains important to identify key bacterial species that drive intestinal inflammation and its associated disorders. To this endeavor, bacteria infiltrating the mucus layer (“mucus invaders”), the mechanism by which they can do so, and the mechanism by which they will subsequently lead to intestinal inflammation and altered metabolism, should be identified. Finally, translating our findings from mice to human is required, for example by performing dietary intervention and investigating the impact of such compound on human microbiota, intestinal inflammation, and metabolism.
Abbreviations
| ASF | = | Altered Schaedler Flora |
| CMC | = | carboxymethylcellulose |
| IBD | = | inflammatory bowel disease |
| M-SHIME | = | mucosal-simulated human intestinal microbiota ecosystem |
| P80 | = | polysorbate 80. |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
E.V. is a recipient of the Career Development Award from the Crohn's and Colitis Foundation. B.C. is a recipient of the Career Development Award from the Crohn's and Colitis Foundation and an Innovator Award from the Rainin Foundation. We thank Samantha Spencer (Georgia State University) for manuscript editing.
Additional information
Funding
References
- Chassaing B, Darfeuille-Michaud A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011; 140:1720–28.
- Chassaing B, Aitken JD, Gewirtz AT, Vijay-Kumar M. Gut microbiota drives metabolic disease in immunologically altered mice. Adv Immunol. 2012; 116:93–112.
- Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014; 514:181–6.
- Devkota S, Chang EB. Diet-induced expansion of pathobionts in experimental colitis: implications for tailored therapies. Gut Microbes. 2013; 4:172–4.
- Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014; 158:705–21.
- Roberts CL, Rushworth SL, Richman E, Rhodes JM. Hypothesis: Increased consumption of emulsifiers as an explanation for the rising incidence of Crohn's disease. J Crohns Colitis. 2013; 7:338–41.
- Roberts CL, Keita AV, Duncan SH, O'Kennedy N, Soderholm JD, Rhodes JM, et al. Translocation of Crohn's disease Escherichia coli across M-cells: contrasting effects of soluble plant fibres and emulsifiers. Gut. 2010; 59:1331–9.
- Swidsinski A, Ung V, Sydora BC, Loening-Baucke V, Doerffel Y, Verstraelen H, et al. Bacterial overgrowth and inflammation of small intestine after carboxymethylcellulose ingestion in genetically susceptible mice. Inflamm Bowel Dis. 2009; 15:359–64.
- Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015; 519:92–6.
- Viennois E, Merlin D, Gewirtz AT, Chassaing B. Dietary emulsifier-induced low-grade inflammation promotes colon carcinogenesis. Cancer Res. 2016.
- Blander JM, Longman RS, Iliev ID, Sonnenberg GF, Artis D. Regulation of inflammation by microbiota interactions with the host. Nat Immunol. 2017; 18:851–60.
- Dewhirst FE, Chien CC, Paster BJ, Ericson RL, Orcutt RP, Schauer DB, et al. Phylogeny of the defined murine microbiota: altered Schaedler flora. Appl Environ Microbiol. 1999; 65:3287–92.
- Chassaing B, Van de Wiele T, De Bodt J, Marzorati M, Gewirtz AT. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut. 2017; 66:1414–27.
- Van den Abbeele P, Roos S, Eeckhaut V, MacKenzie DA, Derde M, Verstraete W, et al. Incorporating a mucosal environment in a dynamic gut model results in a more representative colonization by lactobacilli. Microb Biotechnol. 2012; 5:106–15.
- Van den Abbeele P, Belzer C, Goossens M, Kleerebezem M, De Vos WM, Thas O, et al. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013; 7:949–61.
- Geirnaert A, Wang J, Tinck M, Steyaert A, Van den Abbeele P, Eeckhaut V, et al. Interindividual differences in response to treatment with butyrate-producing Butyricicoccus pullicaecorum 25-3T studied in an in vitro gut model. FEMS Microbiol Ecol. 2015; 91.
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006; 444:860–7.
- Hotamisligil GS. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int J Obes. 2008; 32(Suppl 7):S52–4.
- Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013; 110:9066–71.
- Zou J, Chassaing B, Singh V, Pellizzon M, Ulman E, Ricci M, et al. Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health Cell Host Microbe 2017; in press.
