ABSTRACT
Helicobacter pylori colonization is prevalent throughout the world, and is predominantly acquired during childhood. In developing countries, >70% of adult populations are colonized with H. pylori and >50% of children become colonized before the age of 10 years. However, the exact timing of acquisition is unknown. We assessed detection of H. pylori acquisition among a birth cohort of 105 children in Mirzapur, Bangladesh. Blood samples collected at time 0 (cord blood), and at 6, 12, 18, and 24 months of life were examined for the presence of IgG and IgA antibodies to whole cell H. pylori antigen and for IgG antibodies to the CagA antigen using specific ELISAs and immunoblotting. Breast milk samples were analyzed for H. pylori-specific IgA antibodies. Cord blood was used to establish maternal colonization status. H. pylori seroprevalence in the mothers was 92.8%. At the end of the two-year follow-up period, 50 (47.6%) of the 105 children were positive for H. pylori in more than one assay. Among the colonized children, CagA prevalence was 78.0%. A total of 58 children seroconverted: 50 children showed persistent colonization and 8 (7.6%) children showed transient seroconversion, but immunoblot analysis suggested that the transient seroconversion observed by ELISA may represent falsely positive results. Acquisition of H. pylori was not influenced by the mother H. pylori status in serum or breastmilk. In this population with high H. pylori prevalence, we confirmed that H. pylori in developing countries is detectable mainly after the first year of life.
Introduction
Helicobacter pylori is most prevalent in developing countriesCitation1-3 since the incidence of its acquisition has fallen dramatically in developed countries.Citation4-9 Colonization of adults with H. pylori is persistent and usually asymptomatic.Citation5,Citation10 In contrast, both transient and persistent colonization has been observed in children.Citation11-13 Acquisition of H. pylori occurs predominantly in childhoodCitation11,Citation14–16 and intra-familial clustering has also been described.Citation17-19 However, the exact timing of acquisition is still not well-understood.
Our prior prospective study in Apache children showed that detection of H. pylori acquisition occurs mainly in the second and third year of life.Citation20 A parallel trend was observed in Bangladeshi children in whom there was 33% H. pylori prevalence at age 10–15 month and 84% at age 5 to 8 years.Citation21 Similarly, in Gambian children, H. pylori prevalence was 19% at 3 months of age and 84% in those older than 30 months.Citation22 In Madagascar, prevalence was 18.1% in children <1 year, but 61.5% for children >10 years.Citation23 In developed countries, although overall prevalence is lower, in German children, prevalence was 8.9%, 36.4% and 31.9% in 1-, 2-, and 4-year olds.Citation24 In Japanese children, prevalence was 1.9%, 7.1%, 6.7%, and 7.6% at <1, 1–3, 4–6, and 7–9 years.Citation25 While all of these studies indicate that acquisition of H. pylori is detected mostly after the first year of life, they did not determine maternal H. pylori status at time of birth, nor were the specific bacterial antigens that are the target of the immune response identified. The humoral immune responses against H. pylori have been used as markers of acquisition as well as a diagnostic tool in humans,Citation26,Citation27 mice,Citation28 and rhesus macaques.Citation29 However, there is no indication that the specific humoral responses to H. pylori are protectiveCitation30 and reinfection following anti-microbial treatment is not unusual.Citation31 Pepsinogen I (PGI) is a proenzyme of pepsin secreted by chief cells in the gastric fundus.Citation32 With H. pylori acquisition, serum PG1 levels in adults rise, remain high and can predict later changes in the gastric mucosa.Citation33 In children, PG1 levels display a sharp but transient increase.Citation20 Breastfeeding is important for the development of the infant microbiota and immune systemCitation34 and is known to be protective against many organisms.Citation35 There are indications that nursing provides protection for H. pylori colonization in both developedCitation36,Citation37 and developing countries.Citation38
The purpose of the present study was to assess when H. pylori acquisition can be detected among children in developing countries, to characterize antigenic targets that are potentially associated with early seroconversion, to confirm whether PGI levels reflect gastric physiologic changes associated with H. pylori acquisition, and to determine the effect of breastfeeding on H. pylori acquisition.
Results
Maternal antibodies in the birth cohort
We first estimated the prevalence of H. pylori in the maternal population by assessing the immune response to H. pylori antigens in the cord blood samples, as an indicator of the mother's status (). As in previous studiesCitation39,Citation40 we used the presence of either H. pylori specific IgG or CagA specific IgG as our gold standard for H. pylori positivity. Of 98 cord blood samples examined, the prevalence of IgG to water-extracted whole cell antigen (WEWCA) (70.4%) and CagA (78. 6%) were similar. In total, 85 (86.7%) of the 98 mothers were H. pylori-positive when defining positivity as the presence of serum antibodies to either antigen. Assuming that H. pylori-positive mothers would display H. pylori or CagA-pecific IgA antibodies in their milk, we also used the presence of IgA in the mother's breast milk as an indication of maternal status (). Of 43 mothers who had detectable breast milk antibodies to H. pylori antigens, 33 (76.7%) also had serum antibodies to either the H. pylori or CagA antigen. Subsequently, we considered the presence of serum or breast milk antibodies as evidence of H. pylori positivity in mothersCitation41 to exclude the possibility of false negative results based on the serum IgG, which has been reported.Citation40,Citation42–44 Thus, 91 (92.8%) of the mothers had evidence for carriage of H. pylori; of these mothers, 93.4% were CagA positive. Thus, the mothers in this study represented a population with very high H. pylori prevalence, as reported from other developing countries.Citation45
Table 1. ELISA results to assess H. pylori status in mothers using cord blood from their children or breastmilk.
Final H. pylori status of children
Next, we determined the H. pylori status of the children at the end of the study period (24 month of age). Again, we considered presence of either H. pylori- or CagA-specific IgG as the standard for H. pylori positivity and then combined the results of the serum IgA determinations in order to exclude falsely negative results. Of the 105 children, 55 (52.4%) were negative in all ELISA tests determinations, whereas 50 children (47.6%) were positive for H. pylori in one or more of the ELISAs during months 12 to 24 (). The sero-prevalence of H. pylori (47.6%) was substantially lower in the 2–year old children than in their mothers (86.7%). However, as with the mothers, CagA sero-positivity [39 (37.1%) of 105] exceeded H. pylori IgG sero-positivity [33 (31.4%) of 105]. Of the 50 H. pylori positive children, 39 (78%) were CagA-positive, similar to the proportion of their mothers who were CagA seropositive (78.6%).
Table 2. Serologic markers of H. pylori status in 105 birth-cohort children at 24 months of age.
The relationship of maternal H. pylori status to the child's status
Next, we evaluated the role of maternal status in the child's early life acquisition of H. pylori (). We expected that children from H. pylori-positive mothers would have a higher likelihood of being positive, therefore; we compared the final maternal status with the final status of their children. Of the 98 mothers, only 7 were H. pylori-negative and four (57.1%) of their children also were negative but the other three (42.9%) showed positive ELISA results. However, despite the high H. pylori prevalence in mothers, only 47 (51.6%) children of the 91 H. pylori+ mothers were sero-positive. Thus, despite low power for calculations (only 7 negative mothers) the H. pylori sero-status of the mother seems not to be a predictor for the acquisition and maintenance of H. pylori in 24-month old children (OR 1.05, 95% CI 0.22-4.94, p = 0.96).
Table 3. Relationship between maternal H. pylori status and child's H. pylori status at 24 months of age.
To extend this observation, we repeated the analysis examining serum IgA to H. pylori in the mothers. However, only 8 (8.2%) of the 98 mothers were positive for H. pylori-specific serum IgA. Among these children, 3 (37.5%) were sero-positive and 5 (62.5%) sero-negative; this was similar to the sero-positivity in the 90 IgA-negative mothers (48.9%; p = NS).
Next, we evaluated the utility of H. pylori-specific IgA in maternal breast milk as a predictor of childhood outcome. We hypothesized that H. pylori-specific IgA in breast milk would protect children from H. pylori acquisition. Of 104 mothers (), 43 (41.3%) were positive for H. pylori- or CagA-specific IgA in their breast milk. Among these mothers, 20 (46.5%) of their children were H. pylori-sero-positive at age 2. Of the 61 mothers who did not have H. pylori-specific IgA-detectable in their breast milk, 29 (47.5%) had sero-positive children. The presence of IgA in the breast milk was not a predictor for the H. pylori status of children (OR 0.96, 95% CI 0.44-2.10, p = 0.92); thus, H. pylori-specific IgA in breast milk does not appear to protect breast-fed children from H. pylori colonization.
H. pylori-seroconversion in children
We assessed the number of children who seroconverted to H. pylori positivity during the first two-years of life (). The majority [50 (86.2%)] of the 58 children who seroconverted to H. pylori antigens remained sero-positive at age 2 years which is in line with other studies.Citation16 For the 8 children who had transient seroconversion, the mean number of positive tests was significantly lower than in the children who were sero-positive at age 24 months (Student's t-test; p = 0.05) ().
Table 4. Response to H. pylori antigens in 105 Bangladeshi birth-cohort children, at 24 months of age.
Next, using these assays we determined when H. pylori seroconversion and presumably acquisition occurred (). Since we were unable to ascertain H. pylori status based on IgG antibodies in the first 6 months of life, we asked whether there was IgA positivity. During the first 6 months of life, we found that only 1 (0.95%) of the children became positive, and we found evidence for continued seropositivity at later times in that child.
Table 5. Calculated seroconversion rates for H. pylori persistence in 105 Bangladeshi birth-cohort children during the first two years of life.
Between 6–11 months of life, 14.3% of the 98 children analyzed became sero-positive; for an annualized rate of 28.6%. During the next 6 months of life, 19.8% of those who had remained sero-negative showed evidence of positivity (annualized rate 39.6%). For the final 6 months of follow-up, 29.9% of those remaining sero-negative showed evidence of H. pylori acquisition (annualized rate of 59.8%). Thus, despite the high H. pylori prevalence in mothers, we detected most [36/50 (72%)] H. pylori acquisition after the first year of life, which is in line with our prior study in Native American children.Citation20
Identification of H. pylori antigens by immunoblotting
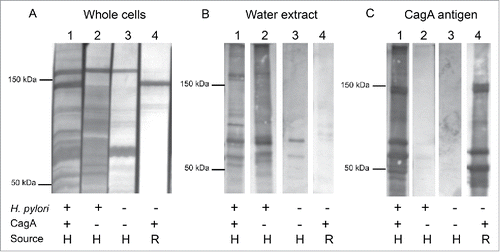
Next, we assessed the type and number of H. pylori antigens that children recognize as result of seroconversion. Using immune human serum or rabbit serum to detect the specific antigens present, we first characterized three antigen preparations, including an H. pylori whole cell (WC) preparation, an H. pylori water-extracted whole cell antigen (WEWCA), and a recombinant CagA protein (). When the H. pylori WC preparation was used as antigen, high background signal was obtained even with the serum from an H. pylori-negative human (, panel A, lane 3). In particular, two intense bands – at ∼150 kDa and ∼66 kDa – were observed. When strips with the same antigen preparation were incubated with an anti-urease rabbit serumCitation46 (not shown), these two bands also were present, suggesting that the cross-reactivity is for the HSP60 chaperones that are part of the urease complex.Citation47 The water-extracted preparation (WEWCA) does not contain the CagA antigen since the rabbit α-CagA serum did not detect a band. This observation helps explain why sera from some persons carrying a CagA+ strain, may not react with sufficient intensity to be considered as positive (, panel B, lanes 1 and 4). However, the CagA antigen was recognized in the WC preparation of H. pylori 26695 (, panel A, lanes 1 and 4) and when purified truncated CagA protein (, panels C, lanes 1 and 4) was used in immunoblotting (at ∼120 kDa, as well as possible degradation products). The nonspecific and low molecular weight bands in the CagA preparation are most likely due to cross reactivity with Escherichia coli proteinsCitation47 since most of those bands disappear if serum is absorbed with E. coli whole-cells as describedCitation48 or represent degradation products of the recombinant CagA.Citation49 These results indicate the utility of using the WEWCA H. pylori and CagA antigens for serological testing, but not the whole cell antigen, which lacks specificity, which confirms a wide body of prior studies.Citation40,Citation50
Figure 1. Western blot of H. pylori antigen preparations with human and rabbit serum samples. Panel A. Whole cell preparation of H. pylori 26695; Panel B. Water extracted whole cell antigen (WEWCA), as described.Citation20 Panel C. Recombinant CagA protein, as described.Citation83 Sera are: Lane 1, H. pylori+/CagA+ patient (H, human serum), lane 2, H. pylori+/CagA− patient. Lane 3, H. pylori−/CagA− patient, and Lane 4, serum from rabbit immunized with purified H. pylori CagA antigen (R, rabbit).
Analysis of H. pylori acquisition, using immunoblotting
We next assessed the type and number of antigens that children recognized as a result of seroconversion, using the three antigen preparations mentioned above. We originally included the WC antigen preparation; however, since this showed very high background, and the recognition of CagA was not consistent (data not shown), we did not use it for further studies, focusing on the WEWCA and CagA preparations, which showed only minor background. To understand the range of responses, we examined serum from 10 of the persistently colonized children. For each assay, all serum specimens including cord blood samples were tested. Supplementary Figure 1, panel A shows three positive children with the most commonly observed band pattern at seroconversion. The immunoblot results obtained in all children tested who had persistent seroconversion correlated with their ELISA results, since most of the H. pylori-positive sera showed recognition of a cluster of protein bands between 95 and 50 kDa, and we also confirmed seroconversion to the CagA antigen (panel A).
We then assessed the serum samples of 3 children who showed transient seroconversion (panel B). No particular band was correlated with the transient seroconversions, although there was an increase in non-specific band recognition, particularly around 60 kDa, suggesting that this finding may be an indicator of a falsely positive result. There was no recognition of the band associated with the CagA antigen (panel B). For 6 persistently H. pylori-negative children, only bands around 60 kDa were observed, without reactivity to the CagA antigen (panel C). Performing further immunoblots using a purified VacA antigen, we did not observe recognition in any of the serum samples tested (data not shown). These results differentiate the nature of the antigenic recognition in those subjects with persistent and transient serologic responses.
Physiological changes as a result of H. pylori acquisition
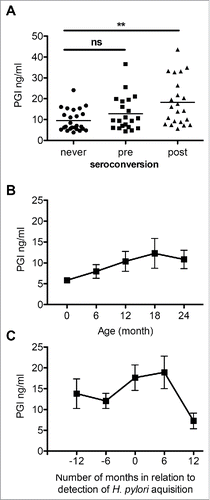
We determined serum PG1 levels as an indication of gastric physiology in relation to H. pylori acquisition (, panel A). The baseline levels of the uninfected children (months 6–18; mean ± SEM 9.12 ± 1.20 ng/ml) were significantly lower compared to the levels of children after H. pylori acquisition (months 12–18; mean±SEM 18.24 ± 2.38 ng/ml) (Mann-Whitney test, p = 0.001). Values of these children before seroconversion (6-18 months, mean ± SEM 12.83 ± 1.80) were not significantly different from the values of the non-seroconverters. For children who did not show H. pylori seroconversion, the serum PGI levels progressively increased over the first year of life, but remained relatively stable after 12 months (, panel B), similar to a prior report.Citation20 In contrast, in serum samples from children who had persistent seroconversion, there was a transient increase in the PGI level that correlated with the time of seroconversion (, panel C)
Figure 2. Serum pepsinogen I (PGI) levels of 18 birth-cohort children. Panel A. PGI levels of samples (n = 20) from children who never seroconverted compared to children with seroconversion (split into samples from children before (n = 21) and after (n = 23) seroconversion). Horizontal bars indicate the mean.**p = 0.001; Panel B. Serum PGI levels over time (mean±SEM ng/ml) in five children who were persistently H. pylori sero-negative and were followed from birth; n = 5 per time point. Panel C. Changes in PGI levels (mean±SEM ng/ml) associated with H. pylori seroconversion in children who became persistently sero-positive. n = 9, 12, 13, 9, 3 for the indicated time points respectively;
Discussion
To identify when H. pylori acquisition occurs in human children, the optimal approach is to perform longitudinal birth cohort studies that can document the time point of acquisition. Such studies are not common, and studies of recent years mostly utilize stool antigen detection as the sole diagnostic tool, which lacks accuracy, especially in young populations.Citation51 Moreover, such studies did not study children starting from birth and mostly do not assess the maternal H. pylori status, as recently reviewed.Citation16 The current study provides unique insights into when H. pylori is acquired in children from developing countries by following a cohort of children from birth from mothers of known H. pylori status. Moreover, there is controversy about which method to use to confirm H. pylori acquisition in infants and children.Citation52-55 This question is relevant because there is need for a reliable, reproducible, and technically feasible non-invasive method for detecting H. pylori acquisition.
Serum antibody tests are reliable for adults based on the fact that seroconversion occurs in less than one month,Citation27,Citation56 but may not be reliable in children because immune responses to H. pylori infection may be delayed.Citation22,Citation57 Other studies have used as gold standards the urea breath test or stool H. pylori antigen detection, but these too also have been reported to have limited reliability in early life,Citation58,Citation59 and breath tests are difficult to do with very young children.Citation60
However, measurement of IgA and IgG serum antibodies by ELISA is a reliable method with more than 95% sensitivity and specificity both in adults and in children.Citation44,Citation61,Citation62 We combine these results to minimize the rate of false-negative events. Moreover, we have previously used this method to show that both transient and persistent H. pylori colonization can be determined by serological methods in both human childrenCitation20 and rhesus monkeys.Citation63,64
In the present report, in a birth-cohort of 105 infants to 24 months of age, H. pylori prevalence gradually increased with subject age. On the basis of persistently positive H. pylori results, this study confirmed that acquisition of H. pylori can be detected mainly after the first year of life.Citation20,Citation22,Citation24,Citation25 We also avoided measuring the transplacental transfer of IgG antibodies that others have reported,Citation65-67 since we used the serum sample from each infant at six-months of age, and not before, as our baseline specimen.
An unresolved issue in the assessment of H. pylori acquisition is the significance of transient responses, and the difficulties determining whether the transiency represents true colonization or false positive results.Citation12,Citation20,Citation67 In this study, we observed a low incidence (7.6%) of transient responses, but in testing the serum samples by immunoblot to identify which antigens are the main targets in those transient responses, no specific antigens were detected. Although the bands observed may represent heat shock protein (Hsp60) or urease,Citation46,Citation47 their identity was not confirmed. In total, these transient antibody responses may represent false positive events due to cross-reactivity from other infections. Alternatively, the transience may reflect H. pylori suppression or elimination after antibiotic treatment for other infections, or the acquisition of other competing organisms, before a full serological response is formed.
Intra-familial spread of H. pylori appears important, with mothers playing the pivotal role in H. pylori transmission.Citation9,Citation17,Citation68 Our finding that maternal H. pylori status did not predict H. pylori status of the infant reflects the low power of this study since nearly all mothers were positive. In another study performed in Bangladesh, isolates from mothers and their youngest children were strongly related, with weaker associations with other siblings,Citation69 which could reflect on-going genomic changes in H. pylori during colonization.Citation70 Another hypothesis for the lack of association between maternal and infant H. pylori status, is that in the developing world, horizontal rather that familial H. pylori transmission may be favored.Citation71 In the population we studied, frequent care-giving by grandmothers or aunts may introduce other sources for the organism. Although we assessed acquisition until the age of 2 years, this may not be a sufficient period for the totality of childhood transmission.Citation17,Citation69 Detectable H. pylori gastric colonization may perturb gastric physiology,Citation72,Citation73 including effects on PGI levelsCitation20 and we confirm the sharp but transient increase in PGI associated with detecting H. pylori acquisition.Citation20 Confirmation of this finding is important and might be of value for detection of H. pylori acquisition, and also might provide an indicator of the future health or disease in colonized children.
Infants may be protected from early H. pylori colonization by breastfeeding,Citation36,Citation37 and by the presence of specific human milk antibodies to H. pylori.Citation65 However, our results showed no protection against H. pylori colonization in extensively (∼1 year) breast-fed infants by mothers with high breast milk antibody levels against H. pylori antigens. A major limitation in this study for assessing potential protective roles of breastfeeding against H. pylori acquisition is that extended breastfeeding (two or more years) is a universal practice in this community. However, that in few of the infants was H. pylori detected earlier than one year after birth suggests that either specific or total IgA antibodies or dietary components in milk could suppress H. pylori colonizationCitation74-78 which reduces the host immunological responses that we use to detect the organism's presence. Another scenario could be that breastfeeding delays the introduction of solid food and weaning, or pre-masticated foods from the mother subsequently reducing frequent horizontal H. pylori exposure.Citation79 A third scenario is that breastfeeding selects for microbiota, e.g. lactobacilliCitation74-77 hat may inhibit H. pylori.
If H. pylori is truly acquired mostly after age 1, is it that the stomach is an inhospitable niche in the first year of life, and that transmission from the mother and other sources is ineffective until the stomach can appropriately mature? An alternative possibility is that H. pylori is transmitted from the mother at birth, but remains in very small populations until the stomach becomes sufficiently hospitable. At that point, the H. pylori population blooms, and then is detected by the host immune response, detection of its urease activity, and other markers of the presence of large numbers of organisms. This study could not distinguish between these possibilities, but both remain feasible.
Conclusions
In conclusion, this study confirms that in developing countries in which H. pylori prevalence is very high, the organisms are not commonly detected in the first year of life, followed by very high seroconversion rates. Detection of transient responses may reflect true transience due to lack of adaptation of the organism or loss due to collateral antibiotic use, which is widespread in this population.Citation80 Future studies should focus on when the organisms are actually acquired. Longitudinal studies as well as studies that determine the H. pylori status of the mother are not common.Citation16 Since this study utilized samples obtained 20 years ago, the results provide an excellent basis for comparisons to present-day cohorts.
Materials and methods
Study group
A prospective birth-cohort study was conducted from October 1993 through September 1994 in Mirzapur Upazila, a rural community located 63 kilometers from Dhaka. Initially, 288 children were enrolled but 36 were excluded because of death or out-migration, and 252 children were enrolled in a study of the cause of diarrhea.Citation81 Of those children, 105 who completed the follow-up and from whom more than one serum specimen was collected were included in the present study. For 79 (75.2%) of those children, five samples were collected: birth (cord blood), 6, 12, 18, and 24 months of life. We tested a total of 491 serum samples (mean±SD number of samples per child = 4.7±0.7). Written informed consent was obtained from the parents of the children before enrollment. The study was approved by the Research Review Committee (RRC) and the Ethical Review Committee (ERC) of the International Centre for Diarrhea Disease Research (ICDDR, B).Citation81,Citation82
Serum antibodies to H. pylori antigens
Antigen-specific enzyme-linked immunosorbent assays (ELISAs) were used to determine antibody responses in the study group. Each serum sample was tested for the presence of IgG and IgA antibodies to a water-extracted whole cell antigen (WEWCA) preparation of H. pylori, as reported.Citation20 In brief, ELISA plates were coated with WEWAC, washed and incubated with serum samples diluted 1:200 and 1:800. After removal of the secondary antibody (goat anti-human IgG/IgA alkaline phosphatase conjugated antibodies) and ELISA development, optical density (OD) values were calculated from the mean absorbance of two assays per samples. On each plate, the positive control was used to express the results as OD-ratios (ODRs) for normalization between the plates. Sensitivity and specificity of this ELISA is >95%. The presence of IgG antibodies to a recombinant CagA antigen was determined as previously reported.Citation5,Citation39,Citation83 In brief, ELISA plates were coated with recombinant CagA, washed, incubated with human serum (1:100 diluted), and developed and analyzed as described above. The ELISA has a sensitivity of 94.4% and a specificity of 92.5%. The cut-off values used to determine a positive or negative serology (for WEWAC and CagA) were based on our previous work with a birth-cohort of Native American children.Citation20 The pre-defined cut-off values were 0.7 for IgG WEWAC, 0.4 for IgA WEWAC and 0.3 for IgG CagA.Citation20
Immunoblot assays
To confirm the H. pylori status determined by ELISA serology in a subset of children, immunoblots were performed using 3 different antigen preparations including: H. pylori strain 26695 whole cell (WC) preparation, the WEWCA, or the recombinant CagA preparation,Citation83 to determine which proteins are recognized by the children's sera (1:100 dilutions). SDS-PAGE gels and nitrocellulose strips were prepared as described.Citation84 Using the WEWCA, we examined serum samples of 23 children that included 10 from the birth cohort study who had persistent H. pylori seroconversion, 7 who were transient seroconverters and 6 children who did not seroconvert to H. pylori antigens during the study period. Since multiple sera from each child were available, a total of 104 serum samples were tested. Sera of 3 children from each group also were tested against the CagA and the 26695 WC antigen preparations.
Determination of serum pepsinogen I
We tested PGI levels in 84 serum samples from 18 children. Five children who did not seroconvert to H. pylori were used to determine the baseline levels of PGI and we studied 13 children (mean±SD 9.2±3.9 number of samples per time point) who persistently seroconverted to assess the PGI level as an indicator of perturbation on the gastric mucosa. All determinations were assessed at least in duplicate using the BioHit ELISA kit (BioHit, Helsinki Finland) following the manufacturer's recommendations. One outlier specimen was excluded from the statistical analysis (seroconverter group at 12 months, value: 76.2 ng/ml, p = 0.018, Dixon´s test).
Breast milk ELISA
Breast milk samples were obtained from nursing mothers (n = 104) at 0, 3, 6, and 9 months (Mean = 3.0±0.9 times per mother). We tested for specific IgA antibodies to H. pylori WEWAC and CagA antigen preparations as described above by using 1:100 dilutions of breast milk. The thresholds for positivity were estimated from breast milk samples from 12 mothers that were negative in both H. pylori serological assays (WEWAC and CagA) and whose children were persistently negative for H. pylori. The thresholds were 0.47 for IgA WEWAC and 0.13 for IgA CagA, which represent the mean plus two SD of the values from the negative mothers. Because of natural variation in breast milk concentrations, specific IgA titers were adjusted to total IgA, as reported.Citation67 Total secretory IgA was calculated in each sample by ELISA as reportedCitation85 by using an rabbit polyclonal anti-IgA antibody as coating antibody.
Study definitions
We have previously reported that maternal antibodies to H. pylori that pass via the placenta remain in the child's circulation until 6 months after birth.Citation20 Therefore, the following criteria were used in the characterization of H. pylori status of the subject children: Never positive: all serum samples at 12, 18, and 24 months were negative; Transient positive: any serum sample positive at 12 and/or 18 months of life but negative at 24 months. Persistent-positive was defined as a child with positivity remaining at 24 months of life.
Contributors
GIPP and MJB designed the study. SK, GIPP, AZP, PB, SAS, KZH, and RBS collected samples and performed experiments. SK and GIPP analyzed the data. SK, GIPP, and MJB wrote the paper. All authors have read and were involved in critical revisions of the final paper.
Competing interest
None declared.
Ethics approval.
Each reporting center gave local approval as mentioned in text.
1421887-f03_Supp_-bw.pdf
Download PDF (414.8 KB)Acknowledgments
We dedicate this paper to the memory of our colleague, R. Bradley Sack, M.D., Ph.D., who sadly did not survive until its publication. We remember Brad as a dedicated scientist and a great friend.
Additional information
Funding
References
- Megraud F, Brassens-Rabbe MP, Denis F, Belbouri A, Hoa DQ. Seroepidemiology of Campylobacter pylori infection in various populations. J Clin Microbiol. 1989;27:1870–3.
- Torres J, Perez-Perez G, Goodman KJ, Atherton JC, Gold BD, Harris PR, la Garza AM, Guarner J, Munoz O. A comprehensive review of the natural history of Helicobacter pylori infection in children. Arch Med Res. 2000;31:431–69. doi:S0188-4409(00)00099-0
- Rowland M, Daly L, Vaughan M, Higgins A, Bourke B, Drumm B. Age-specific incidence of Helicobacter pylori. Gastroenterology. 2006;130:65–72; quiz 211. doi:S0016-5085(05)02264-X
- Miendje Deyi VY, Vanderpas J, Bontems P, Van den Borre C, De Koster E, Cadranel S, Burette A. Marching cohort of Helicobacter pylori infection over two decades (1988-2007): combined effects of secular trend and population migration. Epidemiol Infect. 2011;139:572–80. doi:10.1017/S095026881000110X
- Perez-Perez GI, Salomaa A, Kosunen TU, Daverman B, Rautelin H, Aromaa A, Knekt P, Blaser MJ. Evidence that cagA(+) Helicobacter pylori strains are disappearing more rapidly than cagA(-) strains. Gut. 2002;50:295–8.
- den Hoed CM, Vila AJ, Holster IL, Perez-Perez GI, Blaser MJ, de Jongste JC, Kuipers EJ. Helicobacter pylori and the birth cohort effect: evidence for stabilized colonization rates in childhood. Helicobacter. 2011;16:405–9. doi:10.1111/j.1523-5378.2011.00854.x
- Nakajima S, Nishiyama Y, Yamaoka M, Yasuoka T, Cho E. Changes in the prevalence of Helicobacter pylori infection and gastrointestinal diseases in the past 17 years. J Gastroenterol Hepatol. 2010;25 Suppl 1:S99–S110. doi:10.1111/j.1440-1746.2009.06214.x
- Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis. 2008;198:553–60. doi:10.1086/590158
- den Hollander WJ, Holster IL, van Gilst B, van Vuuren AJ, Jaddoe VW, Hofman A, Perez-Perez GI, Kuipers EJ, Moll HA, Blaser MJ. Intergenerational reduction in Helicobacter pylori prevalence is similar between different ethnic groups living in a Western city. Gut. 2015;64:1200–8. doi:10.1136/gutjnl-2014-307689
- Kosunen TU, Aromaa A, Knekt P, Salomaa A, Rautelin H, Lohi P, Heinonen OP. Helicobacter antibodies in 1973 and 1994 in the adult population of Vammala, Finland. Epidemiol Infect 1997;119:29–34.
- Granstrom M, Tindberg Y, Blennow M. Seroepidemiology of Helicobacter pylori infection in a cohort of children monitored from 6 months to 11 years of age. J Clin Microbiol 1997;35:468–70.
- Okuda M, Miyashiro E, Booka M, Tsuji T, Nakazawa T. Helicobacter pylori colonization in the first 3 years of life in Japanese children. Helicobacter. 2007;12:324–7. doi:10.1111/j.1523-5378.2007.00510.x
- O'Ryan ML, Lucero Y, Rabello M, Mamani N, Salinas AM, Pena A, Torres-Torreti JP, Mejias A, Ramilo O, Suarez N, et al. Persistent and transient Helicobacter pylori infections in early childhood. Clin Infect Dis. 2015;61:211–8. doi:10.1093/cid/civ256
- Mitchell HM, Li YY, Hu PJ, Liu Q, Chen M, Du GG, Wang ZJ, Lee A, Hazell SL. Epidemiology of Helicobacter pylori in southern China: identification of early childhood as the critical period for acquisition. J Infect Dis 1992;166:149–53.
- Sarker SA, Rahman MM, Mahalanabis D, Bardhan PK, Hildebrand P, Beglinger C, Gyr K. Prevalence of Helicobacter pylori infection in infants and family contacts in a poor Bangladesh community. Dig Dis Sci 1995;40:2669–72.
- Zabala Torrres B, Lucero Y, Lagomarcino AJ, Orellana-Manzano A, George S, Torres JP, O'Ryan M. Review: Prevalence and dynamics of Helicobacter pylori infection during childhood. Helicobacter. 2012;22:e12399. doi:10.1111/hel.12399.
- Goodman KJ, Correa P. Transmission of Helicobacter pylori among siblings. Lancet. 2000;355:358–62. doi:S0140-6736(99)05273-3
- Tindberg Y, Bengtsson C, Granath F, Blennow M, Nyren O, Granstrom M. Helicobacter pylori infection in Swedish school children: lack of evidence of child-to-child transmission outside the family. Gastroenterology. 2001;121:310–6. doi:S0016508501726856
- Urita Y, Watanabe T, Kawagoe N, Takemoto I, Tanaka H, Kijima S, Kido H, Maeda T, Sugasawa Y, Miyazaki T, et al. Role of infected grandmothers in transmission of Helicobacter pylori to children in a Japanese rural town. J Paediatr Child Health. 2013;49:394–8. doi:10.1111/jpc.12191
- Perez-Perez GI, Sack RB, Reid R, Santosham M, Croll J, Blaser MJ. Transient and persistent Helicobacter pylori colonization in Native American children. J Clin Microbiol. 2003;41:2401–7.
- Sarker SA, Mahalanabis D, Hildebrand P, Rahaman MM, Bardhan PK, Fuchs G, Beglinger C, Gyr K. Helicobacter pylori: prevalence, transmission, and serum pepsinogen II concentrations in children of a poor periurban community in Bangladesh. Clin Infect Dis. 1997;25:990–5.
- Thomas JE, Dale A, Harding M, Coward WA, Cole TJ, Weaver LT. Helicobacter pylori colonization in early life. Pediatr Res. 1999;45:218–23. doi:10.1203/00006450-199902000-00010
- Ravelomanana L, Imbert P, Kalach N, Ramarovavy G, Richard V, Carod JF, Ravelomanana N, Al Nakib M, Langue J, Avenell C, et al. Helicobacter pylori infection in children in Madagascar: risk factors for acquisition. Trop Gastroenterol. 2013;34:244–51.
- Rothenbacher D, Inceoglu J, Bode G, Brenner H. Acquisition of Helicobacter pylori infection in a high-risk population occurs within the first 2 years of life. J Pediatr. 2000;136:744–8. doi:S0022-3476(00)77103-4
- Okuda M, Miyashiro E, Koike M, Okuda S, Minami K, Yoshikawa N. Breast-feeding prevents Helicobacter pylori infection in early childhood. Pediatr Int. 2001;43:714–5. doi:1481
- Perez-Perez GI, Dworkin BM, Chodos JE, Blaser MJ. Campylobacter pylori antibodies in humans. Ann Intern Med 1988;109:11–7.
- Morris AJ, Ali MR, Nicholson GI, Perez-Perez GI, Blaser MJ. Long-term follow-up of voluntary ingestion of Helicobacter pylori. Ann Intern Med 1991;114:662–3.
- Kienesberger S, Cox LM, Livanos A, Zhang XS, Chung J, Perez-Perez GI, Gorkiewicz G, Zechner EL, Blaser MJ. Gastric Helicobacter pylori infection affects local and distant microbial populations and host responses. Cell Rep. 2016;14:1395–407. doi:10.1016/j.celrep.2016.01.017
- Kienesberger S, Perez-Perez GI, Rivera-Correa JL, Tosado-Acevedo R, Li H, Dubois A, Gonzalez-Martinez JA, Dominguez-Bello MG, Blaser MJ. Serologic host response to Helicobacter pylori and Campylobacter jejuni in socially housed Rhesus macaques (Macaca mulatta). Gut Pathogens. 2012;4:9. doi:10.1186/1757-4749-4-9
- Zevering Y, Jacob L, Meyer TF. Naturally acquired human immune responses against Helicobacter pylori and implications for vaccine development. Gut 1999;45:465–74.
- Stenstrom B, Windsor HM, Fulurija A, Benghezal M, Kumarasinghe MP, Kimura K, Tay CY, Viiala CH, Ee HC, Lu W, et al. Helicobacter pylori overcomes natural immunity in repeated infections. Clin Case Rep. 2016;4:1026–33. doi:10.1002/ccr3.687
- Samloff IM. Cellular localization of group I pepsinogens in human gastric mucosa by immunofluorescence. Gastroenterology 1971;61:185–8.
- Kim N, Jung HC. The role of serum pepsinogen in the detection of gastric cancer. Gut Liver. 2010;4:307–19. doi:10.5009/gnl.2010.4.3.307
- Guaraldi F, Salvatori G. Effect of breast and formula feeding on gut microbiota shaping in newborns. Front Cell Infect Microbiol. 2012;2:94. doi:10.3389/fcimb.2012.00094
- Stordal K, Lundeby KM, Brantsaeter AL, Haugen M, Nakstad B, Lund-Blix NA, Stene LC. Breast-feeding and Infant Hospitalization for Infections: Large Cohort and Sibling Analysis. J Pediatr Gastroenterol Nutr. 2017;65:225–31. doi:10.1097/MPG.0000000000001539
- Malaty HM, Logan ND, Graham DY, Ramchatesingh JE. Helicobacter pylori infection in preschool and school-aged minority children: effect of socioeconomic indicators and breast-feeding practices. Clin Infect Dis. 2001;32:1387–92. doi:CID000410
- Thomas JE, Austin S, Dale A, McClean P, Harding M, Coward WA, Weaver LT. Protection by human milk IgA against Helicobacter pylori infection in infancy. Lancet 1993;342:121.
- Carreira H, Bastos A, Peleteiro B, Lunet N. Breast-feeding and Helicobacter pylori infection: systematic review and meta-analysis. Public Health Nutr. 2015;18:500–20. doi:10.1017/S1368980014000500
- Perez-Perez GI, Bhat N, Gaensbauer J, Fraser A, Taylor DN, Kuipers EJ, Zhang L, You WC, Blaser MJ. Country-specific constancy by age in cagA+ proportion of Helicobacter pylori infections. Int J Cancer 1997;72:453–6. doi:10.1002/(SICI)1097-0215(19970729)
- Romero-Gallo J, Perez-Perez GI, Novick RP, Kamath P, Norbu T, Blaser MJ. Responses of endoscopy patients in Ladakh, India, to Helicobacter pylori whole-cell and Cag A antigens. Clin Diagn Lab Immunol. 2002;9:1313–7.
- Tanriverdi HA, Acun C, Ustundag G, Barut A, Tekin IO, Ustundag Y. Investigation of human colostrum Helicobacter pylori IgA content in lactating women. Eur J Obstet Gynecol Reprod Biol. 2006;124:58–60. doi:S0301-2115(05)00227-7
- Bhat N, Gaensbauer J, Peek RM, Bloch K, Tham KT, Blaser MJ, Perez-Perez G. Local and systemic immune and inflammatory responses to Helicobacter pylori strains. Clin Diagn Lab Immunol. 2005;12:1393–400. doi:12/12/1393
- Suerbaum S, Thiberge JM, Kansau I, Ferrero RL, Labigne A. Helicobacter pylori hspA-hspB heat-shock gene cluster: nucleotide sequence, expression, putative function and immunogenicity. Mol Microbiol 1994;14:959–74.
- Talley NJ, Newell DG, Ormand JE, Carpenter HA, Wilson WR, Zinsmeister AR, Perez-Perez GI, Blaser MJ. Serodiagnosis of Helicobacter pylori: comparison of enzyme-linked immunosorbent assays. J Clin Microbiol 1991;29:1635–9.
- Eusebi LH, Zagari RM, Bazzoli F. Epidemiology of Helicobacter pylori infection. Helicobacter. 2014;19 Suppl 1:1–5. doi:10.1111/hel.12165
- Perez-Perez GI, Olivares AZ, Cover TL, Blaser MJ. Characteristics of Helicobacter pylori variants selected for urease deficiency. Infect Immun 1992;60:3658–63.
- Dunn BE, Roop RM, 2nd, Sung CC, Sharma SA, Perez-Perez GI, Blaser MJ. Identification and purification of a cpn60 heat shock protein homolog from Helicobacter pylori. Infect Immun 1992;60:1946–51. doi:0019-9567/92/051946-06$02.00/0
- Tummuru MK, Cover TL, Blaser MJ. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun 1993;61:1799–809.
- Angelini A, Tosi T, Mas P, Acajjaoui S, Zanotti G, Terradot L, Hart DJ. Expression of Helicobacter pylori CagA domains by library-based construct screening. FEBS J. 2009;276:816–24. doi:10.1111/j.1742-4658.2008.06826.x
- Koshiol J, Flores R, Lam TK, Taylor PR, Weinstein SJ, Virtamo J, Albanes D, Perez-Perez G, Caporaso NE, Blaser MJ. Helicobacter pylori seropositivity and risk of lung cancer. PLoS One. 2012;7:e32106. doi:10.1371/journal.pone.0032106
- Ritchie B, Brewster D, Tran CD, McNeil Y, Zacharakis B, Davidson GP, Butler RN. Lack of diagnostic accuracy of the monoclonal stool antigen test for detection of Helicobacter pylori infection in young Australian aboriginal children. Pediatr Infect Dis J. 2009;28:287–9. doi:10.1097/INF.0b013e31818e039b
- Leal YA, Cedillo-Rivera R, Simon JA, Velazquez JR, Flores LL, Torres J. Utility of stool sample-based tests for the diagnosis of Helicobacter pylori infection in children. J Pediatr Gastroenterol Nutr. 2011;52:718–28. doi:10.1097/MPG.0b013e3182077d33
- Guarner J, Kalach N, Elitsur Y, Koletzko S. Helicobacter pylori diagnostic tests in children: review of the literature from 1999 to 2009. Eur J Pediatr. 2010;169:15–25. doi:10.1007/s00431-009-1033-x
- Yang HR. Updates on the Diagnosis of Helicobacter pylori Infection in Children: What Are the Differences between Adults and Children? Pediatr Gastroenterol Hepatol Nutr. 2016;19:96–103. doi:10.5223/pghn.2016.19.2.96
- Seo JH, Park JS, Rhee KH, Youn HS. Limitations of urease test in diagnosis of pediatric Helicobacter pylori infection. World J Clin Pediatr. 2015;4:143–7. doi:10.5409/wjcp.v4.i4.143
- Malfertheiner P, Schultze V, Rosenkranz B, Kaufmann SH, Ulrichs T, Novicki D, Norelli F, Contorni M, Peppoloni S, Berti D, et al. Safety and immunogenicity of an intramuscular Helicobacter pylori vaccine in noninfected volunteers: a phase I study. Gastroenterology. 2008;135:787–95. doi:10.1053/j.gastro.2008.05.054
- Okuda M, Miyashiro E, Koike M, Tanaka T, Bouoka M, Okuda S, Yoshikawa N. Serodiagnosis of Helicobacter pylori infection is not accurate for children aged below 10. Pediatr Int. 2002;44:387–90. doi:1585
- Mittal SK, Mathew JL. Helicobacter pylori infection in children: a review. Trop Gastroenterol. 2003;24:106–15.
- Megraud F. Comparison of non-invasive tests to detect Helicobacter pylori infection in children and adolescents: results of a multicenter European study. J Pediatr. 2005;146:198–203. doi:S0022347604009825
- Imrie C, Rowland M, Bourke B, Drumm B. Limitations to carbon 13-labeled urea breath testing for Helicobacter pylori in infants. J Pediatr. 2001;139:734–7. doi:S0022-3476(01)11505-2
- Camorlinga-Ponce M, Torres J, Perez-Perez G, Leal-Herrera Y, Gonzalez-Ortiz B, Madrazo de la Garza A, Gomez A, Munoz O. Validation of a serologic test for the diagnosis of Helicobacter pylori infection and the immune response to urease and CagA in children. Am J Gastroenterol 1998;93:1264–70. doi:S0002-9270(98)00288-3
- Glassman MS, Dallal S, Berezin SH, Bostwick HE, Newman LJ, Perez-Perez GI, Blaser MJ. Helicobacter pylori-related gastroduodenal disease in children. Diagnostic utility of enzyme-linked immunosorbent assay. Dig Dis Sci 1990;35:993–7.
- Dubois A, Berg DE, Incecik ET, Fiala N, Heman-Ackah LM, Perez-Perez GI, Blaser MJ. Transient and persistent experimental infection of nonhuman primates with Helicobacter pylori: implications for human disease. Infect Immun 1996;64:2885–91. doi:0019-9567/96/$04.0010
- Kienesberger S, Perez-Perez GI, Rivera-Correa JL, Tosado-Acevedo R, Li H, Dubois A, Gonzalez-Martinez JA, Dominguez-Bello MG, Blaser MJ. Serologic host response to Helicobacter pylori and Campylobacter jejuni in socially housed Rhesus macaques (Macaca mulatta). Gut Pathog. 2012;4:9. doi:10.1186/1757-4749-4-91757-4749-4-9 [pii]
- Thomas JE, Bunn JE, Kleanthous H, Monath TP, Harding M, Coward WA, Weaver LT. Specific immunoglobulin A antibodies in maternal milk and delayed Helicobacter pylori colonization in Gambian infants. Clin Infect Dis. 2004;39:1155–60. doi:10.1086/424514
- Bunn JE, Thomas JE, Harding M, Coward WA, Weaver LT. Placental acquisition of maternal specific IgG and Helicobacter pylori colonization in infancy. Helicobacter. 2003;8:568–72. doi:10.1046/j.1523-5378.2003.00178.x
- Bhuiyan TR, Saha A, Lundgren A, Qadri F, Svennerholm AM. Immune responses to Helicobacter pylori infection in Bangladeshi children during their first two years of life and the association between maternal antibodies and onset of infection. J Infect Dis. 2010;202:1676–84. doi:10.1086/657085
- Rothenbacher D, Winkler M, Gonser T, Adler G, Brenner H. Role of infected parents in transmission of Helicobacter pylori to their children. Pediatr Infect Dis J. 2002;21:674–9. doi:10.1097/01.inf.0000021081.69738.42
- Nahar S, Kibria KM, Hossain ME, Sultana J, Sarker SA, Engstrand L, Bardhan PK, Rahman M, Endtz HP. Evidence of intra-familial transmission of Helicobacter pylori by PCR-based RAPD fingerprinting in Bangladesh. Eur J Clin Microbiol Infect Dis. 2009;28:767–73. doi:10.1007/s10096-008-0699-8
- Kennemann L, Didelot X, Aebischer T, Kuhn S, Drescher B, Droege M, Reinhardt R, Correa P, Meyer TF, Josenhans C, et al. Helicobacter pylori genome evolution during human infection. Proc Natl Acad Sci U S A. 2011;108:5033–8. doi:10.1073/pnas.1018444108
- Schwarz S, Morelli G, Kusecek B, Manica A, Balloux F, Owen RJ, Graham DY, van der Merwe S, Achtman M, Suerbaum S. Horizontal versus familial transmission of Helicobacter pylori. PLoS Pathog. 2008;4:e1000180. doi:10.1371/journal.ppat.1000180
- Marshall BJ, Armstrong JA, McGechie DB, Glancy RJ. Attempt to fulfil Koch's postulates for pyloric Campylobacter. Med J Aust 1985;142:436–9.
- Graham DY, Opekun AR, Osato MS, El-Zimaity HM, Lee CK, Yamaoka Y, Qureshi WA, Cadoz M, Monath TP. Challenge model for Helicobacter pylori infection in human volunteers. Gut. 2004;53:1235–43. doi:10.1136/gut.2003.037499
- Stromqvist M, Falk P, Bergstrom S, Hansson L, Lonnerdal B, Normark S, Hernell O. Human milk kappa-casein and inhibition of Helicobacter pylori adhesion to human gastric mucosa. J Pediatr Gastroenterol Nutr. 1995;21:288–96.
- Sakamoto I, Igarashi M, Kimura K, Takagi A, Miwa T, Koga Y. Suppressive effect of Lactobacillus gasseri OLL 2716 (LG21) on Helicobacter pylori infection in humans. J Antimicrob Chemother. 2001;47:709–10.
- Ryan KA, Daly P, Li Y, Hooton C, O'Toole PW. Strain-specific inhibition of Helicobacter pylori by Lactobacillus salivarius and other lactobacilli. J Antimicrob Chemother. 2008;61:831–4. doi:10.1093/jac/dkn040
- de Klerk N, Maudsdotter L, Gebreegziabher H, Saroj SD, Eriksson B, Eriksson OS, Roos S, Linden S, Sjolinder H, Jonsson AB. Lactobacilli reduce Helicobacter pylori attachment to host gastric epithelial cells by inhibiting adhesion gene expression. Infect Immun. 2016;84:1526–35. doi:10.1128/IAI.00163-16
- Arnold IC, Lee JY, Amieva MR, Roers A, Flavell RA, Sparwasser T, Muller A. Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology. 2011;140:199–209. doi:10.1053/j.gastro.2010.06.047
- Sinha SK, Martin B, Gold BD, Song Q, Sargent M, Bernstein CN. The incidence of Helicobacter pylori acquisition in children of a Canadian First Nations community and the potential for parent-to-child transmission. Helicobacter. 2004;9:59–68.
- Biswas M, Roy DN, Tajmim A, Rajib SS, Hossain M, Farzana F, Yasmen N. Prescription antibiotics for outpatients in Bangladesh: a cross-sectional health survey conducted in three cities. Ann Clin Microbiol Antimicrob. 2014;13:15. doi:10.1186/1476-0711-13-15
- Hasan K, Jolly P, Marquis G, Roy E, Podder G, Alam K, Huq F, Sack R. Viral etiology of pneumonia in a cohort of newborns till 24 months of age in Rural Mirzapur, Bangladesh. Scand J Infect Dis. 2006;38:690–5. doi:N04747837306445P
- Pathela P, Hasan KZ, Roy E, Alam K, Huq F, Siddique AK, Sack RB. Enterotoxigenic Bacteroides fragilis-associated diarrhea in children 0–2 years of age in rural Bangladesh. J Infect Dis. 2005;191:1245–52. doi:10.1086/428947
- Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, Stemmermann GN, Nomura A. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res 1995;55:2111–5.
- Hook-Nikanne J, Perez-Perez GI, Blaser MJ. Antigenic characterization of Helicobacter pylori strains from different parts of the world. Clin Diagn Lab Immunol 1997;4:592–7.
- Filteau SM, Rice AL, Ball JJ, Chakraborty J, Stoltzfus R, de Francisco A, Willumsen JF. Breast milk immune factors in Bangladeshi women supplemented postpartum with retinol or beta-carotene. Am J Clin Nutr. 1999;69:953–8.
