ABSTRACT
Strains of Eggerthella lenta are capable of oxidation-reduction reactions capable of oxidizing and epimerizing bile acid hydroxyl groups. Several genes encoding these enzymes, known as hydroxysteroid dehydrogenases (HSDH) have yet to be identified. It is also uncertain whether the products of E. lenta bile acid metabolism are further metabolized by other members of the gut microbiota. We characterized a novel human fecal isolate identified as E. lenta strain C592. The complete genome of E. lenta strain C592 was sequenced and comparative genomics with the type strain (DSM 2243) revealed high conservation, but some notable differences. E. lenta strain C592 falls into group III, possessing 3α, 3β, 7α, and 12α-hydroxysteroid dehydrogenase (HSDH) activity, as determined by mass spectrometry of thin layer chromatography (TLC) separated metabolites of primary and secondary bile acids. Incubation of E. lenta oxo-bile acid and iso-bile acid metabolites with whole-cells of the high-activity bile acid 7α-dehydroxylating bacterium, Clostridium scindens VPI 12708, resulted in minimal conversion of oxo-derivatives to lithocholic acid (LCA). Further, Iso-chenodeoxycholic acid (iso-CDCA; 3β,7α-dihydroxy-5β-cholan-24-oic acid) was not metabolized by C. scindens. We then located a gene encoding a novel 12α-HSDH in E. lenta DSM 2243, also encoded by strain C592, and the recombinant purified enzyme was characterized and substrate-specificity determined. Genomic analysis revealed genes encoding an Rnf complex (rnfABCDEG), an energy conserving hydrogenase (echABCDEF) complex, as well as what appears to be a complete Wood-Ljungdahl pathway. Our prediction that by changing the gas atmosphere from nitrogen to hydrogen, bile acid oxidation would be inhibited, was confirmed. These results suggest that E. lenta is an important bile acid metabolizing gut microbe and that the gas atmosphere may be an important and overlooked regulator of bile acid metabolism in the gut.
Introduction
Once considered mere detergent molecules aiding and abetting digestion of dietary lipids and cholesterol, bile acids are now regarded as important digestive hormones that regulate numerous physiological processes in the host.Citation1-Citation3 Bile acids are C-24 steroids generated from cholesterol in the liver via side-chain modification, and hydroxylation at the C-3, C-7 position, and in the case of cholic acid (CA; 3α,7α,12α-trihydroxy-5β-cholan-24-oic acid), also the C-12 position. Several grams of bile acids, conjugated to glycine or taurine, are released from the gallbladder daily where they aid in lipid absorption in the small bowel. Once reaching the terminal ileum, bile salts are actively transported into enterocytes, and after being transported across the basolateral membrane into the portal vein, bile salts are returned to the liver and recycled in a process known as the enterohepatic circulation. Several hundred milligrams of bile salts escape the high affinity active transporters in the terminal ileum, entering the large bowel.Citation4,Citation5 Bile salts act as important top-down selective forces, regulating microbial colonization and microbiome structureCitation6 in the small and large intestines due to their detergent properties, and through induction of farnesoid-X-receptor (FXR)-dependent anti-microbial peptide synthesis in the ileum.Citation7
In response, microbes have evolved a suite of genes, that we refer to as the human gut sterolbiome, which encode enzymes that modify bile acids and other steroid molecules.Citation8 These genes include the “gateway” bile salt hydrolase (BSH), a reaction which hydrolyzes the amide bond linking the bile acid side-chain to glycine or taurine.Citation9 Deconjugation is a pre-requisite for the conversion of CA to deoxycholic acid (DCA; 3α,12α-dihydroxy-5β-cholan-24-oic acid) or chenodeoxycholic acid (CDCA; 3α,7α-dihydroxy-5β-cholan-24-oic acid) to lithocholic acid (LCA; 3α-monohydroxy-5β-cholan-24-oic acid) in the gut by as yet only a few intestinal anaerobes in Clostridium clusters IV, XI, and XIVa that encode the multi-step bile acid-inducible (bai) 7α-dehydroxylation regulon.Citation10,Citation11 Additionally, a wide variety of intestinal bacteria are capable of the reversible oxidation and epimerize of bile acid hydroxyl groups.Citation12,Citation13 Enzymes that catalyze the stereo- and regio-specific pyridine nucleotide-dependent oxidation/reduction of bile acids and steroids are known as hydroxysteroid dehydrogenases (HSDH). HSDHs act as reversible “switches” that modulate the physicochemical properties of bile acids,Citation12,Citation14 and in doing so determine their downstream biotransformations by other gut microbes. Many HSDHs are in the short-chain dehydrogenase/reductase (SDR) family, a group of functionally diverse enzymes composed of >300,000 Uniprot entries currently,Citation15 which makes sequence-based comparison challenging, and necessitates functional biochemical characterization.
Important prior bacteriological and biochemical work identified bacterial taxa that produce oxidized or “oxo” bile acids.Citation16-Citation19 The physiological importance of oxo-bile acid formation by gut bacteria still remains largely unknown. Citing the low redox-potential in the gut, two renowned steroid microbiologists expressed puzzlement decades ago regarding oxo-bile acid formation: “What benefit obligate anaerobes derive from this oxidation reaction is hard to understand”.Citation18 Here, we characterize bile acid oxidation by a human gut isolate identified as E. lenta strain C592. We show that E. lenta bile acid metabolites of CDCA are not readily converted to LCA by Clostridium scindens VPI 12708, a bile acid 7α-dehydroxylating gut bacterium. We identified and biochemically characterized a newly identified 12α-HSDH shared by E. lenta DSM 2243 and strain C592 that participates in bile acid oxidation. Finally, we report that the gas atmosphere affects bile acid oxidation by E. lenta strains DSM 2243T and C592.
Results
Characterization of bile acid oxidation by anaerobic fecal isolate, strain C592
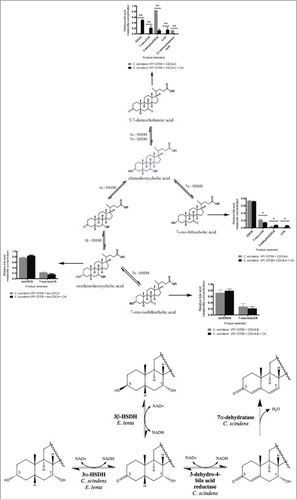
Strain C592 is a gram-positive, obligate anaerobe originally isolated from a centenarian stool sample at the University of Ryukyus in Okinawa, Japan. Based on visual examination of the relative migration of bile acid metabolites on thin layer chromatography (TLC), strain C592 appeared to convert CA to allodeoxycholic acid (ADCA), a 5α-epimer of DCA; an activity previously demonstrated in bile acid 7α-dehydroxylating bacteriaCitation10,Citation20 (). However, this metabolite ran higher than ADCA on TLC plates. The pattern of metabolites suggested oxidation of bile acid hydroxyl groups rather than 7α-dehydroxylation. To simplify our analysis, we chose two dihydroxy bile acids to further evaluate bile acid metabolism by strain C592. We then incubated the dihydroxyl primary bile acid CDCA (3α, 7α-dihydroxyl) or the secondary bile acid DCA (3α, 12α-dihydroxyl) with strain C592 and determined the identity of the reaction products. Formation of mono- and dioxo-bile acid products was evident when incubated with either CDCA or DCA ( & ). We did not observe the 7α-dehydroxylation of CDCA by C592, suggesting this strain is not a bile acid 7α-dehydroxylating bacterium. Incubation of [24-14C] CDCA resulted in the formation of three products, CDCA-A (Rf 0.50), CDCA-B (Rf 0.62), and CDCA-C (Rf 0.98). We also ran non-labeled CDCA under identical conditions and scraped these metabolites from the TLC for UPLC-IT-TOF-MS under negative ion mode. CDCA-A (389.2701 m/z) and CDCA-B (389.2702 m/z) were 2 amu less than the parent compound CDCA (MW 392). CDCA-A, but not CDCA-B, could be oxidized by commercial 3α-HSDH in the presence of NAD+ (data not shown), which co-migrated with CDCA-C (387.2539 m/z). These results suggest that CDCA-A is 7-oxoLCA (3α-hydroxy-7-oxo-5β-cholan-24-oic acid) and that CDCA-B is 7-oxo-isoLCA (3β-hydroxy-7-oxo-5β-cholan-24-oic acid). The loss of 4 amu from CDCA-C, and the conversion of CDCA-B to CDCA-C by oxidation of a 3α-hydroxyl group suggested that CDCA-C is 3,7-dioxocholanoic acid (3,7-dioxo-5β-cholan-24-oic acid) ().
Figure 1. Conversion of [24-14C]-cholic acid by putative bile acid 7α-dehydroxylating strains. First section of autoradiograph of TLC plate includes cholic acid (CA), deoxycholic acid (DCA), and allodeoxycholic acid (ADCA) TLC standards. The second autoradiographic section contains [24-14C]-cholic acid metabolites from several bile acid metabolizing strains, including strain C592. Each isolate was grown anaerobically (N2) in Balch tubes in the presence of 25 µM cholic acid along with 1 µCi [24-14C]-cholic acid. Cultures were extracted with ethyl acetate and separated on TLC as described in the Materials and Methods.
![Figure 1. Conversion of [24-14C]-cholic acid by putative bile acid 7α-dehydroxylating strains. First section of autoradiograph of TLC plate includes cholic acid (CA), deoxycholic acid (DCA), and allodeoxycholic acid (ADCA) TLC standards. The second autoradiographic section contains [24-14C]-cholic acid metabolites from several bile acid metabolizing strains, including strain C592. Each isolate was grown anaerobically (N2) in Balch tubes in the presence of 25 µM cholic acid along with 1 µCi [24-14C]-cholic acid. Cultures were extracted with ethyl acetate and separated on TLC as described in the Materials and Methods.](/cms/asset/00331e41-3206-4285-9257-5523a7049aeb/kgmi_a_1458180_f0001_b.gif)
Figure 2. Characterization of bile acid metabolism and metabolites by Eggerthella lenta strain C592. (Upper Panel) Representative TLC showing formation of oxo-bile acids and iso-bile acid derivatives of CDCA as identified by LC-MS following TLC separation. CDCA metabolites were separated as previously described on TLC both with and without [24-14C]-radiolabel. Isolated unlabeled substrates corresponding to CDCA-A, CDCA-B, and CDCA-C then underwent MS analysis as described in the Materials and Methods. (Lower Panel). Identification of metabolites formed following growth of C592 in the presence of [24-14C] DCA. Autoradiograph of representative TLC shows relative migration of DCA control and formation of DCA-A and DCA-B by strain C592. Regions of the TLC corresponding to DCA-A and DCA-B were subjected to MS analysis resulting in identification of DCA-B and possible products of DCA-A. Three biological replicates of this experiment were performed.
![Figure 2. Characterization of bile acid metabolism and metabolites by Eggerthella lenta strain C592. (Upper Panel) Representative TLC showing formation of oxo-bile acids and iso-bile acid derivatives of CDCA as identified by LC-MS following TLC separation. CDCA metabolites were separated as previously described on TLC both with and without [24-14C]-radiolabel. Isolated unlabeled substrates corresponding to CDCA-A, CDCA-B, and CDCA-C then underwent MS analysis as described in the Materials and Methods. (Lower Panel). Identification of metabolites formed following growth of C592 in the presence of [24-14C] DCA. Autoradiograph of representative TLC shows relative migration of DCA control and formation of DCA-A and DCA-B by strain C592. Regions of the TLC corresponding to DCA-A and DCA-B were subjected to MS analysis resulting in identification of DCA-B and possible products of DCA-A. Three biological replicates of this experiment were performed.](/cms/asset/0739073c-0f9a-4067-bd9b-1a8de75e94e8/kgmi_a_1458180_f0002_b.gif)
To determine the ability of strain C592 to metabolize the 12α-hydroxyl of DCA, we separated DCA metabolites after 24 hr incubation in brain heart infusion broth (BHI). Two major metabolites were detected on TLC and characterized, DCA-A (Rf 0.6) and DCA-B (Rf 0.95) (). Compared with DCA (MW 392), the products DCA-B lost 4 amu (387.2528 m/z) suggesting oxidation of the 3α-hydroxyl and 12α-hydroxyl forming 3,12-dioxocholanoic acid (3,12-dioxo-5β-cholan-24-oic acid). DCA-A (389.2687 m/z) lost 2 amu, forming either 3-dehydroDCA or 12-oxoLCA. Because these results confirmed oxidation of the 12α-hydroxyl, we did not further pursue differentiation of these two possibilities.
Taken together, these results indicated that strain C592 expresses 3α-HSDH, 7α-HSDH, and 12α-HSDH enzymes. 3β-hydroxyl metabolites (so called “iso-bile acids”) were also identified, indicating the expression of 3β-HSDH, and confirmed previously reported activities in strains of Eggerthella lenta and Ruminococcus gnavus.Citation12 Indeed, 16S rDNA gene sequencing placed the taxonomic identity of strain C592 as Eggerthella lenta (99% sequence ID), clustering closely with the type strain (DSM 2243). Sequencing of the 16S rDNA of the other strains represented in resulted in identification of several strains of C. scindens reported previously.Citation21
E. lenta CDCA end-product metabolism by Clostridium scindens VPI 12708
Bile acid 7α-dehydroxylation is quantitatively the most important bile acid biotransformation in the gut, and is implicated in disease of the GI tract including cholesterol gallstone disease and colorectal cancer.Citation3 Therefore, we wanted to determine whether bile acid 7α-dehydroxylating bacterium Clostridium scindens VPI 12708 is capable of converting oxo- and iso-derivatives of CDCA generated by E. lenta strain C592 to the secondary bile acid LCA. We purified and quantified [24-14C] CDCA metabolites from E. lenta C592 culture by TLC and incubated each metabolite in cultures of C. scindens VPI 12708 grown in BHI.
Two major observations were gathered from these experiments. First, only metabolites with 3-oxo or 3α-hydroxyl groups were 7α-dehydroxylated, while iso-CDCA metabolites were not converted to LCA derivatives (). This observation can be explained by the sequence of reactions in the bile acid 7α-dehydroxylation pathway, particularly the step that requires oxidation of the 3α-hydroxyl groupCitation27,Citation28 prior to formation of a C4-C5 double bondCitation22 which is then 7α-dehydroxylated, forming a stable 3-dehydro-4,6-intermediate.Citation23,Citation24 3β-HSDH activity has not been observed in C. scindens VPI 12708. Thus, isoCDCA or isoCA formation is expected to preclude formation of LCA and DCA, respectively (). The iso-bile acid pathway may therefore be useful in shifting the bile acid pool from hydrophobic, and thus toxic, to hydrophilic and health-promoting. Iso-bile acids returning to the liver during enterohepatic circulation will then be converted to the 3α-hydroxyl orientation and re-secreted into bile.Citation25 Second, oxo-bile acid metabolites are only 7α-dehydroxylated by C. scindens VPI 12708 if pre-cultured in the presence of CA, which induces the bai regulon.Citation26
Figure 3. Metabolism of E. lenta CDCA metabolites by CA-induced vs. un-induced cells of Clostridium scindens VPI 12708. (Upper Panel) Conversion of E. lenta bile acid metabolites by induced (+ CA) and uninduced cultures of lithocholic acid (LCA)-forming gut bacterium, C. scindens VPI 12708. Experiments were repeated in triplicate ± standard errors and data were analyzed by two-tailed T-test *p < 0.01, **p < 0.001, ***p < 0.0001. (Lower Panel) Schematic representation of alternative 7α-dehydroxylation and isobile acid (3α-hydroxyl ⇔ 3-oxo ⇔ 3β-hydroxyl) pathways between C. scindens and E. lenta, respectively.
Characterization of a recombinant gene product encoded by E. lenta DSM 2243 involved in bile acid oxidation
Early studiesCitation14,Citation17,Citation19 demonstrated that strains of E. lenta vary with respect to the expression of HSDHs capable of metabolizing bile acids at the 3α, 7α, and 12α-hydroxyl groups, with some strains capable of epimerizing the 3α-hydroxyl to 3β-hydroxyl group, forming iso-bile acids. E. lenta strain C592 falls into group III, possessing 3α, 3β, 7α, and 12α-HSDH activity, along with the type strain, E. lenta DSM 2243.Citation12 Genes encoding 3α-HSDH and 7α-HSDH have been characterized in gut bacteriaCitation10,Citation29-Citation32 and soil isolatesCitation33; however, there is a paucity of characterized 12α-HSDH genes. 12α-HSDH is an enzyme important in industrial synthesis of therapeutic ursodeoxycholic acid from cholic acid,Citation34 and the first step in conversion of host bile acids to 12β-hydroxyl bile acid epimers.Citation35 The only bacterial gene demonstrated to encode a 12α-HSDH (ERJ00208.1) was reported in Clostridium sp. ATCC 29733.Citation36,Citation37 In order to locate a 12α-HSDH in E. lenta strains, we searched the deduced amino acid sequence of the 12α-HSDH reported in Clostridium sp. ATCC 29733 (ERJ00208.1)Citation37 against the E. lenta DSM 2243 genome. The 12α-HSDH from Clostridium sp. ATCC 29733 is a member of the SDR-family with conserved canonical pyridine-nucleotide binding site (GGGX5GXG) and conserved catalytic triad (S160, Y173, K177).Citation13 A tBlastn of ERJ00208.1 against the E. lenta DSM 2243 genome resulted in identification of Elen_2515 as a probable candidate, sharing 56% amino acid sequence identity (70% similarity; E value 2e−81). Elen_2515 was previously overexpressed in E. coli and screened for metabolism of DCA; however, bile acid metabolites were not detected, perhaps due to screening against undialyzed clarified cell lysate without the addition of oxidized pyridine nucleotide.Citation12
We cloned, overexpressed and Talon-affinity purified recombinant N-terminal hexa-histidine tagged enzyme (rElen_2515) (27.57 ± 1.0 kDa) (). The pH optimum for rElen_2515 in the oxidative direction with NAD+ as cofactor was 9.0, with a broad optimum between 7.0-7.5 in the reductive direction with NADH as co-factor (Figure S1). Purified rElen_2515 converted 12-oxoLCA to DCA, which was confirmed by mass spectrometry (). rElen_2515 shows greater activity in the oxidative direction as evidenced by a higher Vmax , lower Km , higher Kcat , and a catalytic efficiency (Kcat/ Km ) two orders of magnitude greater in the oxidative than the reductive direction at optimal pH (). In the oxidative direction with NAD+ as co-factor, rElen_2515 had similar substrate-specificity for DCA and CA (100 ± 2.53% vs. 98.50 ± 1.32%, respectively) and glycine and taurine conjugates were also substrates ().
Figure 4. Characterization of novel 12α-hydroxysteroid dehydrogenase identified in E. lenta DSM 2243 and strain C592. (a). Gene organization of a novel 12α-HSDH in E. lenta C592 and E. lenta DSM 2243. (b). Reaction catalyzed by 12α-HSDH with 12-oxoLCA/DCA as substrates and pyridine nucleotide co-factors. (c). SDS-PAGE of purified recombinant N-terminal histidine-tagged rElen_2515. (d). ESI-MS of DCA standard and rElen_2515 reaction product after 24 hr reaction with NADH as cofactor and 12-oxoLCA substrate. Three biological replicates of TLC and MS analysis were generated.
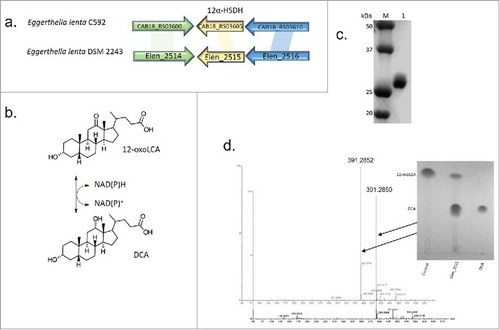
Table 1. Enzyme Kinetic Data for Elen_2515.
Table 2. Substrate-specificity of bile acid and pyridine nucleotides for rElen_2515.
Previous reports suggest E. lenta strains have bile salt hydrolase activity,Citation38 suggesting that oxidation of bile acid hydroxyl groups may precede conversion of conjugated to free bile acids. Lacking a 12α-hydroxyl group, CDCA and its glycine and taurine conjugates were not substrates. NAD+ is the preferred cofactor, as oxidation of DCA in the presence of NADP+ resulted in only 3.14 ± 0.66% relative maximal activity. Reduction of 12-oxoLCA to DCA in the presence of NADH occurred at only 19.60 ± 1.39% maximal activity relative to the oxidation of DCA. Interestingly, activity with 12-oxoCDCA was not detected in the reductive direction. As expected, reduction of 3-dehydroDCA or 3-dehydroCA in the presence of NADH was not detected, demonstrating regio-specificity of this enzyme for C-12.
We attempted to locate a gene encoding 7α-HSDH by cloning into pET46 and overexpressing 7 additional SDR family enzymes (Figure S2) predicted in the genome of E. lenta DSM 2243, but excluded Elen_0198, Elen_0690, and Elen_1325, which were previously shown to recognize the C3 position.Citation12 Surprisingly, we did not detect 7α-HSDH activity in any of the TALON®-purified enzymes, perhaps suggesting that E. lenta DSM 2243 expresses a novel 7α-HSDH in a different family of pyridine nucleotide-dependent enzymes (See Table S3 for E. lenta dehydrogenases). Further studies will be necessary to identify 7α-HSDH(s) from E. lenta.
Identification of Rnf complex, Energy-conserving hydrogenase complex (Ech), and Wood-Ljundahl Pathway genes
Because we observed some notable steroid metabolic potential in E. lenta strain C592, such as the presence of steroid 17β-HSDH activity (Figure S3), we sequenced the complete 3,593,230 bp genome of E. lenta strain C592. (; Tables S1 & S2). Mauve alignments () between E. lenta strain C592 and E. lenta DSM 2243 reveal substantial synteny; however, we observed additional notable differences including the absence of the cgr locus predicted to be involved in metabolism of cardiac glycosides such as digoxinCitation39 as well as the absence of a “baiE” homolog in a “bai-like operon” of unknown function.Citation12 Consistent with the formation of 3-dehydro and 3β-hydroxyl bile acid products (), we identified an ORF in strain C592 corresponding to 3α-HSDH Elen_0690 (CAB18_RS04320) from E. lenta DSM 2243, as well as ORFs corresponding to 3β-HSDHs Elen_0198 (CAB18_RS014915) and Elen_1325 (CAB18_RS09005).Citation12 A homolog of new characterized 12α-HSDH, Elen_2515, was also identified in the genome of E. lenta strain C592 (CAB18_RS03605).
Figure 5. Comparative genomic analysis between Eggerthella lenta strain C592 and Eggerthella lenta DSM 2243 (type strain). Circular and linear Mauve alignment representation are displayed. BlastKOALA pie chart depicts abundances within color-coded functional gene categories.
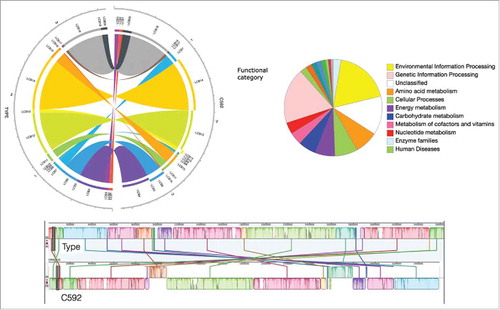
In order to try to elucidate the role of bile acid oxidation in the physiology of E. lenta, we searched the genome of E. lenta strain C592 for metabolic pathways that may suggest the fate of reducing equivalents generated during bile acid oxidation. Genetic information obtained for E. lenta strain C592 was used to populate KEGG maps using Blast KOALA and pathway mapping tools. Of the entirety of predicted protein-encoding sequences in C592, only 1340 (42%) matched KEGG annotations. When carbon metabolism potential was analyzed, genes involved in the Wood-Ljungdahl pathway were found and putative clusters encoding the hydrogenase subunits were determined in both E. lenta strains C592 and DSM 2243.
Prior surveys of the gut of wood-feeding cockroachesCitation40 and acidic fenCitation41 resulted in identification of Eggerthella as encoding a formyl-tetrahydrofolate synthase (fhs) gene, suggesting that Eggerthella may have acetogenic potential. Common to all acetogens are genes encoding acetyl-CoA synthase (ACS)/carbon monoxide dehydrogenase (CODH).Citation42,Citation43 We located a conserved cluster of genes (Elen_3026-3030; CAB18_RS02000-2010) in both E. lenta DSM 2243 and E. lenta C592 which encode 4Fe-4S hybrid cluster proteins which include ACS and CODH. Eggerthella sp. strain YY7918 was found to harbor a different gene cluster, annotated as encoding acsA (EGGYY_24090), ascB/cdhC (EGYY_24100), acsF (BAK45480) providing further genomic evidence that Eggerthella isolates encode WLP genes.
Genes encoding enzymes in the methyl branch of the WLP were also identified in the genomes of E. lenta DSM 2243 and E. lenta sp. strain C592 including formate dehydrogenase (fdh) (Elen_3031; CAB18_RS01995), which flanks the ACS/CODH cluster, formyl-tetrahydrofolate synthase (fsh) (Elen_2864; CAB18_RS01970), methylene-tetrahydrofolate dehydrogenase/cyclohydrolase (folD) (Elen_2861; CAB18_RS01985), bifunctional homocysteine S-methyltransferase/5,10-methylene-tetrahydrofolate reductase (yitJ) (Elen_2573; CAB18_RS03325). These results provide a plausible explanation for why E. lenta oxidizes bile acids in a reducing environment: oxidation of bile acids provides reducing equivalents for the fixation of CO2 to acetate. Genes encoding enzymes involved in the energy conservation by substrate-level phosphorylation resulting in the conversion of acetyl-CoA to acetyl-PO4 (eutD) (Elen_1728, and acetate kinase (ackA) (Elen_1729; CAB18_RS07170) were also identified. We also located pyruvate:ferrodoxin oxidoreductase (PFOR) in both E. lenta DSM 2243 (Elen_2140) and several annotated genes for PFOR in E. lenta C592 (CAB18_RS00220; CAB18_RS05160; CAB18_RS07295; CAB18_RS07920; CAB18_RS13765). PFOR links the Wood-Ljungdahl pathway to the reductive tricarboxylic acid cycle, allowing autotrophic biosynthesis of complex macromolecules.Citation44
Additional gene clusters encoded by model acetogens, including membrane-spanning electron transport chains such as the Clostridium ljungdahlii proton-translocating ferrodoxin:NAD+ oxidoreductase (Rnf)Citation45 were found in both E. lenta DSM 2243 (Elen_0694-0698) and E. lenta strain C592 (CAB18_RS4340-4360). We located Elen_0690 (3α-HSDH) immediately downstream and within the same operon of the Rnf complex in E. lenta DSM 2243 previously determined to have bile acid 3α-HSDH activity.Citation12 An energy-conserving hydrogenase (Ech)Citation46 was also located in E. lenta DSM 2243 (Elen_1570-1575) and E. lenta strain C592 (CAB18_RS07980-7960). In addition, both strains harbor ATP synthase transmembrane complexes, able to utilize this proton gradient generated from Rnf and Ech complexes to generate ATP from ADP.
Molecular hydrogen inhibits bile acid oxidation by E. lenta strains
The Rnf complex, as well as other electron-bifurcating enzymes, can shuttle electrons from reduced ferredoxin to oxidized NAD+, thereby generating reduced NADH, which we predict might ultimately inhibit bile acid oxidation.Citation47 Both E. lenta strain C592 and E. lenta DSM 2243 encode NiFe Group 4e (Ech complex; WP_01576064.1), as well as NiFe Group 1a (WP_009394598.1)Citation48,Citation49 predicted to utilize hydrogen to reduce oxidized ferredoxin, we tested the effect of gas atmosphere on bile acid oxidation.
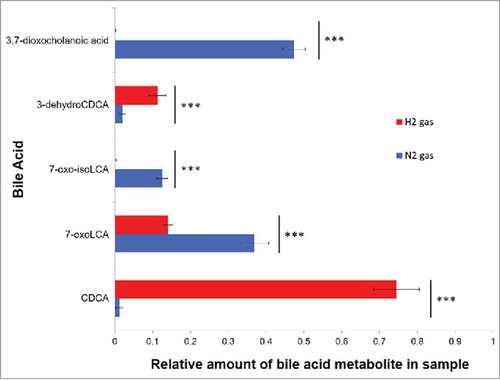
When CDCA was added to an E. lenta sp. strain C592 growth culture, CDCA accounted for only 1.62 ± 0.11% of the total remaining bile acids after 24 hours growth under a N2 atmosphere (0.68 atm) vs. 74.4 ± 2.02% under a H2 atmosphere (0.68 atm) (p < 0.001), indicating that H2 strongly inhibits bile acid oxidation. A similar observation was made with E. lenta DSM 2243 (). N2 atmosphere favored the formation of the most oxidized bile acid metabolite, 3,7-dioxocholanoic acid, which represented 47.55 ± 1.71% of the CDCA metabolites in E. lenta C592 vs. 0.38 ± 0.33% under H2 atmosphere (p < 0.001). 7-oxoLCA was the second most abundant metabolite in both strains representing 36.82 ± 0.24% from E. lenta C592 and 52.12 ± 1.92% under N2, compared with 14.03 ± 1.04% and 12.75 ± 0.99%, respectively, under H2 atmosphere. The formation of isoCDCA derivatives (3β-hydroxyl), which requires oxidation of 3α-hydroxyl forming a stable 3-dehydro-intermediate followed by reduction, was essentially ablated under H2. Similar experiments with C. scindens VPI 12708 demonstrate that bile acid 7α-dehydroxylation is not affected by the gas atmosphere (Figure S4). These findings may indicate that E. lenta can utilize hydrogenases and reverse electron flow to generate NADH from H2 and that the gas atmosphere in the gut may be an important regulator of bile acid metabolism by particular intestinal bacteria ().
Figure 6. Effect of gas atmosphere on the formation of bile acid metabolites by E. lenta strain C592. Relative amount of CDCA metabolites formed by cultures of E. lenta C592 inoculated in Balch tubes in which headspace contained either H2 (red) or N2 (blue) (0.68 Atm). Bile acids were extracted after 24hrs growth in BHI + 1% arginine at 37°C. Experiments were repeated in triplicate ± standard errors and data were analyzed by two-tailed T-test ***p < 0.0001.
Discussion
The current work is a key step toward understanding the physiological role for bile acid oxidation by E. lenta. Our initial screening of E. lenta strain C592, among a number of bile acid 7α-dehydroxylating bacteria such as C. scindens sp. strain I10, C. scindens sp. strain SO96, and C. scindens sp. strain SO77Citation21 revealed a distinct pattern of bile acid metabolites (). Characterization of C592 metabolites of dihydroxyl bile acids such as CDCA (3α-, 7α-hydroxyl), and DCA (3α-, 12α-hydroxyl), resulted in identification of oxo-derivatives and iso-bile acid metabolites (). Sequencing of the 16S rDNA gene and WGS (Oxford nanopore & Illumina) resulted in identification of strain C592 as a novel strain of Eggerthella lenta (). Taken together, E. lenta sp. strain C592 is categorized as a Type III strainCitation17 because it expressed 3α-, 3β-, 7α-, and 12α-HSDHs (), but it lacks the cgr locusCitation35 and a “baiE” homolog in the “bai-like” cluster identified previously.Citation10
Previous work characterizing dozens of E. lenta strains, most of which were not deposited in culture collections, demonstrated 12α-HSDH activity.Citation17,Citation19,Citation50 Here, we present a novel gene encoding 12α-HSDH in E. lenta (). Purified rElen_2515 converted 12-oxoLCA to a product co-migrating with DCA, whose major mass ion was identical to authentic DCA (). Kinetic analysis of the enzyme clearly shows that the oxidative direction is favored with a Km in the oxidative direction ∼100 μM lower than the reductive direction, and a turnover number (Kcat ) an order of magnitude higher in the oxidative direction (). This is consistent with the observed complete oxidation of hydroxyl groups of CA, CDCA and DCA under a nitrogen atmosphere by E. lenta whole cells ( & ). rElen_2515 was NAD(H)-dependent () with broad specificity, recognizing unconjugated primary (CA) and secondary bile acids (DCA), as well as taurine-conjugated (TCA, TDCA) and glycine-conjugated bile acids (GDCA, GCA) possessing a 12α-hydroxyl group. CDCA, which lacks a 12α-hydroxyl group was not a substrate (). This is in contrast to the only other gene reported that encodes 12α-HSDH from Clostridium group P strain C48-50/ATCC 29733, which had greatest specificity for CA and required NADP(H) as co-enzyme.Citation32
The germinant receptor (CspC) in C. difficile was reported to specifically recognizes 12α-hydroxylated bile acids.Citation47 A complex relationship exists between bile acid structure and C. difficile growth and sporulation, and oxidation or epimerization of the 12α-hydroxyl group may thus affect C. difficile germination.Citation51 Nosocomial antibiotic-associated diarrhea by C. difficile results in nearly $5 billion in annual health care costs, with approximately 500,000 cases resulting in 29,000 deaths.Citation52 Future experimentation should look to the effects that 12-oxoLCA has on both C. difficile growth and spore germination, as well as its ability to be recognized by the CspC receptor. The effects these oxo-bile acid metabolites have are not limited to other members of the gut microbiome.
The extent of epimerization and the accumulation of oxo-bile acids appears to be influenced by the oxidation/reduction potential of the local cellular environment. For example, the formation of oxo-bile acids in the gut may be more favorable in bacteria associated closer to the mucosal edges, where there is a higher redox potential than further inside the lumen of the intestines.Citation3 In culture, anaerobic bacteria that reduce bile acids under strict anaerobic conditions, shift to oxidation of bile acid hydroxyl groups when introduced to aerobic conditions, as is the case with C. hiranonis.Citation53 Oxidized or epimerized bile acids have differing effects on host physiology. 3-dehydroLCA has been shown to be the most potent agonist for the vitamin D receptor (VDR).Citation54 Epimerization of the 7α-hydroxyl group on CDCA yields a much more hydrophilic and therefore less toxic metabolite ursodeoxycholic acid,Citation55 which has been shown to be protective against CRC-inducing effects of DCA.Citation3 Recent studies reported that 7-oxoLCA acts as a competitive inhibitor of human hepatic 11β-HSDH-1.Citation56 11β-HSDH-1 is responsible for converting 7-oxoLCA back to CDCA; however, it also catalyzes the activation of cortisol from cortisone.Citation57 When 7-oxoLCA is in high enough concentrations, it acts as a competitive inhibitor preventing production of active cortisol. 7-oxoLCA and ursodeoxycholic acid are both less potent agonists of FXR than the endogenous bile acid they are formed from, CDCA.Citation58 Since the expression of the antimicrobial peptide cathelicidin is controlled by FXR in enterocytes, it follows that by lessening the affinity of bile acids for FXR, an otherwise susceptible microbe could increase its fitness in the lumen of the large intestine. The full extent to which alteration in bile acid hydroxyl oxidation or epimerization effects host metabolism is a field that requires more significant study.
Bile acids are known to activate host G-protein-coupled and nuclear receptors to varying extents based on the regio- and stereo-positioning of the functional groups attached to their steroid cores and side chains.Citation1-Citation3 3-dehydroLCA, the most likely product of E. lenta C592 LCA metabolism, has been shown to be the most potent vitamin D receptor agonist.Citation54 Additionally, 7-oxoLCA and 3,7-dioxocholanoic acid have both been shown to be less potent agonists for FXR than their α-reduced counterparts, but more potent than β-reduced epimers.Citation58 Oxo-bile acids, therefore, already have an established role in host physiology unique from their α- and β-reduced counterparts. However, the full spectrum of primary and secondary oxo-bile acid derivatives has not been tested for agonist properties on various bile acid-sensitive receptors. Of particular interest would be to determine the ability of an oxo-bile to activate TGR-5, as this is implicated in significantly altering host metabolism.Citation59
Intriguingly, genomic analysis of E. lenta strains suggests that this gut microbial species encodes the Wood-Ljungdahl pathway, consistent with previous end-product analysis demonstrating that E. lenta generates acetate and succinate.Citation60 In addition, the partial pressures of molecular hydrogen may be a previously unrecognized factor in colonic bile acid metabolism. E. lenta strains, through generation of dioxo- and trioxo-bile acids, can be thought of as representing a bile acid hydroxyl “reset” affording the opportunity to drive bile acid metabolism in particular directions. However, hydrogen metabolism appears to be an important aspect of regulating bile acid oxidation by E. lenta and requires further study ().Citation48,Citation49,Citation61 At present, we cannot say that bile acid oxidation is linked to reductive acetogenesis. Development of a minimal medium capable of supporting growth of E. lenta in addition to genetic tools to knock out particular genes is currently lacking.
The production of oxo-bile acids, while substrates for intestinal microbial species expressing various HSDHs, may reduce the rate of 7α-dehydroxylation in the intestine. (). Additionally, iso-bile acids are not substrates for early key enzymatic steps prior to rate-limiting 7α-dehydration (). Thus, our data suggests that oxo-bile acid and iso-bile acid primary bile acid formation by E. lenta may reduce formation of toxic, cancer-promoting secondary bile acids such as DCA.
Materials and methods
Bacterial strains, culture conditions, materials
Clostridium scindens VPI 12708 obtained from Virginia Polytechnic Institute, and human fecal isolates C592, I10, SA14, 19BHI, K511, SO96, SO77 from collaborators at Ryukyus University in Okinawa, Japan are maintained as −80°C glycerol stocks in our laboratory. Eggerthella lenta DSM 2243 was acquired commercially (DSMZ). Before further analysis, strains were propagated on brain heart infusion (BHI) agar plates and grown under anaerobic conditions in Brewer jar with AnaeroPack (Mitsubishi) for 48 hours at 37°C, and colonies were picked and grown individually. Unless otherwise noted, bacterial strains were grown in liquid BHI broth (Becton, Dickinson) in round bottom flasks anaerobically under 100% N2 gas atmosphere (Airgas), supplemented with 5g L−1 yeast extract (Becton, Dickinson), 1g L−1 cysteine HCl (Sigma) and 40mL/L of a salt solution containing 0.2g CaCl2, 0.2g MgSO4, 1g K2HPO4, 1g KH2PO4, 10g NaHCO3 per liter. When arginine (Sigma) was used, it was added separately to the media to a final concentration of 5g L−1 (0.5% wt/volume) or 10g L−1 (1% wt/volume). For gas atmosphere experiments, BHI tubes were flushed with either H2 or N2 at 0.68 atm to replace the atmosphere, using a double-needle system in which sterile needles were inserted with input gas filtered with 0.2 μM filter. Oxygen was removed from commercial gases by running through a heated manifold containing reduced copper shavings. Escherichia coli DH5α and E. coli BL21-CodonPlus(DE3) RIPL competent cells were used for cloning and overexpression, respectively. The pET-46 Ek/LIC vector kit was obtained from Novagen (San Diego, CA). The QIAprep Spin Miniprep kit was obtained from Qiagen (Valencia, CA). Isopropyl β-D-1-thiogalactopyranoside (IPTG) was purchased from Gold Biotechnology (St. Louis, MO). Bile acid substrates were purchased from Steraloids, Inc (Newport, RI, USA). Before addition to culture, bile acids were suspended in methanol to a concentration of 10 mM before being diluted to their final concentration in culture media.
Whole cell bile acid conversion assays
Screening of putative bile acid 7α-dehydroxylating bacteria was carried out by cultivation in BHI containing 25 μM CA (0.1μCi/μmole [24-14C] CA) for 24 hrs. [24-14C] allodeoxycholic acid (ADCA; 3α,12α-dihydroxy-5α-cholan-24-oic acid) was produced as previously reported.Citation10 Generation of metabolites for whole-cell bile acid conversion assay were generated by cultivating E. lenta C592 in BHI + 1% arginine containing 25 μM CDCA (0.1μCi/μmole [24-14C] CDCA) for 24 hrs. Metabolites were extracted twice with two volumes ethyl acetate and separated on BakerFlex Silica B gel TLC plates (J.T. Baker, LLC) with solvent system of 75:20:2 (v/v/v) toluene:dioxane:glacial acetic acid. Kodak Biomax MS film was exposed to the TLC for 24 hrs and developed. Metabolites were then extract from the TLC plate and quantified by liquid scintillation spectrometry. Metabolites were identified by mass spectrometry and compared to mass spectra from standards. C. scindens VPI 12708 cells cultured in BHI without bile acids, representing un-induced cells (UI) or BHI + 25 μM CA to induce (I) expression of the bai regulon. After washing centrifuged cell pellets of UI and I cells, the pellets were resuspended in 1 ml fresh BHI under N2 gas and added to vials containing 25 μM (0.1μCi/μmole [24-14C]) of CDCA-A, CDCA-B, or CDCA-C radiolabeled metabolite and incubated 24 hrs at 37°C. Metabolites were extracted and analyzed as described above.
HPLC and mass spectrometry of bile acid metabolites
For MS analysis, 100 mL E. lenta strain C592 cultures were grown to stationary phase as stated above in the presence of 25 μM CDCA or DCA with and without [24-14C] radiolabel. Bile acid metabolites were extracted, separated, and isolated as stated above and then underwent LC-MS analysis. LC-MS analysis was run on a Shimadzu UPLC coupled with a Shimadzu LCMS-IT-TOF System (Shimadzu Corporation, Kyoto, Japan). The LC operating conditions were as follows: LC column, C-18 analytical column (Capcell Pak C18, Shiseido, Japan), 250 mm × 2 mm i.d., particle size – 3 µm (C18 (RP18, ODS, Octadecyl); mobile phase, H2O containing 0.1% formic acid (A), and acetonitrile containing 0.1% formic acid (B); total flow rate of mobile phase, 0.2 ml/min; total run time including equilibration, 41 minutes. The initial mobile phase composition was 70% mobile phase A and 30% mobile phase B. The percentage of mobile phase B was changed linearly over the next 5 minutes until 35%. Over the next 25 minutes, the percentage was increased to 98% linearly. After that the percentage was maintained for 5 minutes, the mobile phase composition was allowed to return to the initial conditions and allowed to equilibrate for 5 minutes. The injection volume was 10 µL. The mass spectrometer (LCMS-IT-TOF) was operated with an electrospray ionization (ESI) source in both positive and negative ion mode. The nebulizer gas pressure was set at 150kPa with the source temperature of 200°C and the gas flow at 1.5L/min. The detector voltage was 1.65kV. High-purity nitrogen gas was used as collision cell gas. The raw chromatograph and mass spectrogram data were processed with the LC solution Workstation software (Shimadzu).
E. lenta strain C592 genomic sequencing and comparative genomics
Generic 16S primers (16s357F, 16s1392R) and ExTaq polymerase kit (Takara-Bio, Kusatsu, Japan) were used for initial 16S screening of E. lenta strain C592. Genomic DNA (1.5 µg) was sheared in a gTube (Covaris, Woburn, MA) for 1 minute at 6,000 rpm in an Eppendorf MiniSpin plus microcentrifuge (Eppendorf, Hauppauge, NY). The sheared DNA was converted into a Nanopore library with the Nanopore Sequencing kit SQK-NSK007 (Oxford Nanopore, UK). The library was sequenced on a SpotON Flowcell MK I (R9) flowcell for 48 hours, using a MinION MK 1B sequencer. Basecalling was performed in real time with the software Metrichor version 2.40.17. Poretools v-0.5.1 softwareCitation62 was used to extract sequences from Oxford Nanopore MinION output file folder, and then converted to fastq format. FastQC v-0.11.2 software was also used to further access quality scores and other attributes of the data set. A Perl script was then used to trim adaptors from the raw nanopore reads. The adapter trimmed reads were used to blast against NCBI Ecoli_strK12_MG1655 genome. Reads with greater than 95% alignment to this genome were removed. 2,113,230 reads from the Illumina paired end MiSeq run and 14,023 reads from Oxford Nanopore sequencing platform were used for de novo hybrid assembly with SPAdes-v3.9.0.Citation63 The assembly produced 245 contigs, five of which were 500 base pairs and longer. The top five contigs were selected to blast NCBI NT database. Nucleotide level comparisons between E. lenta DSM 2243 genome and the longest contig from the assembly were done with the dnadiff program from MUMmer v-3.23.Citation64 Annotation comparisons between E. lenta DSM 2243 genome and the longest contig were made with Prokka v-1.11.Citation65 Annotated CDS file for the longest E. lenta strain C592 contig were then imported into Geneious v9.1.3 for Mauve alignment and further analysis, as well as utilized to form KEGG maps via BlastKOALA.Citation66 The genome data was deposited to NCBI (Reference Sequence NZ_CP021140.1).
Cloning, expression and purification of recombinant proteins
Sequences corresponding to E. lenta genomic DNA regions was synthesized by Integrated DNA Technologies (IDT) (Coralville, IA, USA). Gene fragments were amplified with primers synthesized by IDT (), using the Phusion high fidelity polymerase (Stratagene, La Jolla, CA) and cloned into pET-46b vector (Novagen), as described by the manufacturer's protocol. Cultivation, plasmid isolation and sequence confirmation were as previously described.Citation67 12α-HSDH were expressed as previously described.Citation67 The recombinant proteins were then purified using TALON® Metal Affinity Resin (Clontech Laboratories, Mountain View, CA) as per manufacturing protocol. The recombinant protein was eluted using an elution buffer composed of 20 mM Tris-HCl, 150 mM NaCl, 20% glycerol, 10 mM 2-mercaptoethanol pH 7.9 and 250 mM imidazole. The protein purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and protein bands were visualized by staining with Coomassie brilliant blue G-250. Recombinant protein concentration was calculated based on their molecular mass and extinction coefficients. Subunit molecular mass was calculated using three independent SDS-PAGE gels with Biorad Precision Plus ProteinTm Kaleidoscope Prestained Protein Standards (Bio Rad Laboratories, Inc, Hercules, CA) using the image processing software ImageJ (https://imagej.nih.gov/ij/index.html). Recombinant protein concentrations were calculated based on their molecular mass and extinction coefficients. Deduced amino acid sequence was used as input for Expasy's ProtParam tool (https://web.expasy.org/protparam/) and subunit mass and extinction coefficient (mM−1 cm−1) are utilized to determine enzyme concentration (mg ml−1) in a Nanodrop 2000c with 10 mm pathlength cuvette at 280 nm.
Table 3. Cloning primers used in this study.
Enzyme assays
12α-HSDH enzyme pH optima in both reductive and oxidative direction was determined essentially as described previouslyCitation67 with bile acid substrates DCA and 12-oxoLCA and pyridine-nucleotide co-factor. The reactions were initiated by addition of enzyme. Linearity of enzyme activity with respect to time and enzyme concentration was determined aerobically by monitoring the oxidation/reduction of NAD(P)(H) at 340 nM (ϵ = 6,220 M−1.cm−1) in the presence of bile acid and steroid substrates. Kinetic parameters were estimated by fitting the data to the Michaelis-Menten equation by non-linear regression method using the enzyme kinetics module in GraphPad Prism (GraphPad Software, La Jolla, CA). Substrate-specificity was determined with 25 nM enzyme, 50 µM bile acid substrates, and 150 µM co-factor.
Statistics
Descriptive statistics are provided, where appropriate, in the figure legends. Three biological replicates from bile acid biotransformation experiments were analyzed for significance by GraphPad Prism using two-tailed student T-test.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
2018GUTMICROBES0001R1-file005.docx
Download MS Word (8.2 MB)Acknowledgments
We gratefully acknowledge the financial support provided to J.M.R. for new faculty startup through the Department of Animal Sciences at the University of Illinois at Urbana-Champaign (Hatch ILLU-538-916) and through the Microbial Metabolic Engineering Theme directed by I.C. P.B.H. is supported through Veterans Affairs Merit Grant BX001328. We thank Dr. Fusae Takamine for providing the bacterial strain C592 from which this work follows.
Additional information
Funding
References
- Zhou H, Hylemon PB. Bile acids are nutrient signaling hormones. Steroids. 2014;86:62–68. doi:10.1016/j.steroids.2014.04.016. PMID:24819989.
- Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016;24(1):41–50. doi:10.1016/j.cmet.2016.05.005. PMID:27320064.
- Ridlon JM, Harris SC, Bhowmik S, Kang DJ, Hylemon PB. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. 2016;7(1):22–39. doi:10.1080/19490976.2015.1127483. PMID:26939849.
- Hofmann AF. Chemistry and enterohepatic circulation of bile acids. Hepatology. 1984;4(5 Suppl):4S–14S. doi:10.1002/hep.1840040803. PMID:6384004.
- Hofmann AF, Hagey LR. Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades. J Lipid Res. 2014;55(8):1553–95. doi:10.1194/jlr.R049437. PMID:24838141.
- Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 2011;141(5):1773–81. doi:10.1053/j.gastro.2011.07.046. PMID:21839040.
- Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, Mangelsdorf DJ, Kliewer SA. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A. 2006;103(10):3920–25. doi:10.1073/pnas.0509592103. PMID:16473946.
- Ridlon JM, Bajaj JS. The human gut sterolbiome: bile acid-microbiome endocrine aspects and therapeutics. Acta Pharm Sin B. 2015;5(2):99–105. doi:10.1016/j.apsb.2015.01.006. PMID:26579434.
- Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47(2):241–59. doi:10.1194/jlr.R500013-JLR200. PMID:16299351.
- Ridlon JM, Kang DJ, Hylemon PB. Isolation and characterization of a bile acid inducible 7alpha-dehydroxylating operon in Clostridium hylemonae TN271. Anaerobe. 2010;16(2):137–46. doi:10.1016/j.anaerobe.2009.05.004. PMID:19464381.
- Wells JE, Hylemon PB. Identification and characterization of a bile acid 7alpha-dehydroxylation operon in Clostridium sp. strain TO-931, a highly active 7alpha-dehydroxylating strain isolated from human feces. Appl Environ Microbiol. 2000;66(3):1107–13. doi:10.1128/AEM.66.3.1107-1113.2000. PMID:10698778.
- Devlin AS, Fischbach MA. A biosynthetic pathway for a prominent class of microbiota-derived bile acids. Nat Chem Biol. 2015;11(9):658–90. doi:10.1038/nchembio.1864.
- Kisiela M, Skarka A, Ebert B, Maser E. Hydroxysteroid dehydrogenases (HSDs) in bacteria: a bioinformatic perspective. J Steroid Biochem Mol Biol. 2012;129(1-2):31–46. doi:10.1016/j.jsbmb.2011.08.002. PMID:21884790.
- Hofmann AF, Roda A. Physicochemical properties of bile acids and their relationship to biological properties: an overview of the problem. J Lipid Res. 1984;25(13):1477–89. PMID:6397555.
- Kallberg Y, Opperman U, Persson B. Classification of the short-chain dehydrogenase/reductase superfamily using hidden Markov models. FEBS J. 2010;277(10):2375–86. doi:10.1111/j.1742-4658.2010.07656.x. PMID:20423462.
- Midtvedt T, Norman A. Bile acid transformations by microbial strains belonging to genera found in intestinal contents. Acta Path Microbiol Scandinav. 1967;71:629–38. doi:10.1111/j.1699-0463.1967.tb05183.x.
- Edenharder R, Mielek K. Epimerization, oxidation and reduction of bile acids by Eubacterium lentum. System Appl Microbiol. 1984;5:287–98. doi:10.1016/S0723-2020(84)80031-4.
- Bokkenheuser VD, Winter J. Biotransformation of steroid hormones by gut bacteria. Am J Clin Nutr. 1980;33:2502–6. doi:10.1093/ajcn/33.11.2502. PMID:7001886.
- Hirano S, Masuda N. Transformation of bile acids by Eubacterium lentum. Appl Environ Microbiol. 1981;42(5):912–15. PMID:6947718.
- Hylemon PB, Melone PD, Franklund CV, Lund E, Bjorkhem I. Mechanism of intestinal 7alpha-dehydroxylation of cholic acid: evidence that allo-deoxycholic acid is an inducible side-product. J Lipid Res. 1991;32:89–95. PMID:2010697.
- Ridlon JM, Ikegawa S, Alves JM, Zhou B, Kobayashi A, Iida T, Mitamura K, Tanabe G, Serrano M, De Guzman A, et al. Clostridium scindens: a human gut microbe with a high potential to convert glucocorticoids into androgens. J Lipid Res. 2013;54(9):2437–49. doi:10.1194/jlr.M038869. PMID:23772041.
- Kang DJ, Ridlon JM, Moore DR, Barnes S2nd, Hylemon PB. Clostridium scindens baiCD and baiH genes encode stereo-specific 7alpha/7beta-hydroxy-3-oxo-delta4-cholenoic acid oxidoreductases. Biochim Biophys Acta. 2008;1781(1-2):16–25. doi:10.1016/j.bbalip.2007.10.008. PMID:18047844.
- Dawson JA, Mallonee DH, Björkhem I, Hylemon PB. Expression and characterization of a C24 bile acid 7alpha-dehydratase from Eubacterium sp. strain VPI 12708 in Escherichia coli. J Lipid Res. 1996;37(6):1258–67. PMID:8808760.
- Bhowmik S, Chiu HP, Jones DH, Chiu HJ, Miller MD, Xu Q, Farr CL, Ridlon JM, Wells JE, Elsliger MA, et al. Structure and functional characterization of a bile acid 7α dehydratase BaiE in secondary bile acid synthesis. Proteins. 2016;84(3):316–31. doi:10.1002/prot.24971. PMID:26650892.
- Marschall HU, Oppermann UC, Svensson S, Nordling E, Persson B, Hoog JO, Jornvall H. Human liver class I alcohol dehydrogenase gamma isozyme: the sole cytosolic 3beta-hydroxysteroid dehydrogenase of iso bile acids. Hepatology. 2002;31:990–96. doi:10.1053/he.2000.5720.
- Mallonee DH, White WB, Hylemon PB. Cloning and sequencing of a bile acid-inducible operon from Eubacterium sp. strain VPI 12708. J Bacteriol. 1990;172(12):7011–19. doi:10.1128/jb.172.12.7011-7019.1990. PMID:2254270.
- Mallonee DH, Lijewski MA, Hylemon PB. Expression in Escherichia coli and characterization of a bile acid-inducible 3alpha-hydroxysteroid dehydrogenase from Eubacterium sp. strain VPI 12708. Curr Microbiol. 1995;30(5):259–63. doi:10.1007/BF00295498. PMID:7766153.
- Bhowmik S, Jones DH, Chiu HP, Park IH, Chiu HJ, Axelrod HL, Farr CL, Tien HJ, Agarwalla S, Lesley SA. Structural and functional characterization of BaiA, an enzyme involved in secondary bile acid synthesis in human gut microbe. Proteins. 2014;82(2):216–29. doi:10.1002/prot.24353. PMID:23836456.
- Hwang CC, Chang YH, Hsu CN, Hsu HH, Li CW, Pon HI. Mechanistic roles of Ser-114, Tyr-155, and Lys-159 in 3alpha-hydroxysteroid dehydrogenase/ carbonyl reductase from Comamonas testosteroni. J Biol Chem. 2005;280(5):3522–28. doi:10.1074/jbc.M411751200. PMID:15572373.
- Bennett MJ, McKnight SL, Coleman JP. Cloning and characterization of the NAD-dependent 7alpha-Hydroxysteroid dehydrogenase from Bacteroides fragilis. Curr Microbiol. 2003;47(6):475–84. doi:10.1007/s00284-003-4079-4. PMID:14756531.
- Tanaka N, Nonaka T, Tanabe T, Yoshimoto T, Tsuru D, Mitsui Y. Crystal structures of the binary and ternary complexes of 7alpha-hydroxysteroid dehydrogenase from Escherichia coli. Biochemistry. 1996;35(24):7715–30. doi:10.1021/bi951904d. PMID:8672472.
- Baron SF, Franklund CV, Hylemon PB. Cloning, sequencing, and expression of the gene coding for bile acid 7 alpha-hydroxysteroid dehydrogenase from Eubacterium sp. strain VPI 12708. J Bacteriol. 1991;173(15):4558–69. doi:10.1128/jb.173.15.4558-4569.1991. PMID:1856160.
- Ferrandi EE, Bertolesi GM, Polentini F, Negri A, Riva S, Monti D. In search of sustainable chemical processes: cloning, recombinant expression, and functional characterization of the 7α- and 7β-hydroxysteroid dehydrogenases from Clostridium absonum. Appl Microbiol Biotechnol. 2012;95(5):1221–33. doi:10.1007/s00253-011-3798-x. PMID:22198717.
- Eggert T, Bakonyl D, Hummel W. Enzymatic routes for the synthesis of ursodeoxycholic acid. J Biotechnol. 2014;191:11–21. doi:10.1016/j.jbiotec.2014.08.006. PMID:25131646.
- Edenharder R, Schneider J. 12β-dehydrogenation of bile acids by Clostridium paraputrificum, C. tertium, and C. difficile and epimerization at carbon-12 of deoxycholic acid by co-cultivation with 12α-dehydrogenating Eubacterium lentum. Appl Environ Microbiol. 1985;49(4):964–68. PMID:4004226.
- MacDonald IA, Jellett JF, Mahony DE. 12alpha-hydroxysteroid dehydrogenase from Clostridium group P strain C48-50 ATCC No. 29733: partial purification and characterization. J Lipid Res. 1979;20(2):234–9. PMID:438663.
- Aigner A, Gross R, Schmid R, Braun M, Mauer S. Novel 12alpha-hydroxysteroid dehydrogenases, production and use thereof. US Patent 20110091921A1. Filed Mar 25, 2009;Issued Apr. 21, 2011.
- Wegner K, Just S, Gau L, Mueller H, Gérard P, Lepage P, Clavel T, Rohn S. Rapid analysis of bile acids in different biological matrices using LC-ESI-MS/MS for the investigation of bile acid transformation by mammalian gut bacteria. Anal Bioanal Chem. 2017;409(5):1231–45. doi:10.1007/s00216-016-0048-1. PMID:27822648.
- Haiser HJ, Gootenberg DB, Chatman K, Sirasani G, Balskus EP, Turnbaugh PJ. Predicting and manipulating cardiac drug inactivation by the human gut bacterium Eggerthella lenta. Science. 2013;341(6143):295–98. doi:10.1126/science.1235872. PMID:23869020.
- Ottesen EA, Leadbetter JR. Diversity of formyltetrahydrofolate synthetases in the guts of the wood-feeding cockroach Cryptocercus punctulatus and the omnivorous cockroach Periplaneta americana. Appl Environ Microbiol. 2010;76(14):4909–13. doi:10.1128/AEM.00299-10. PMID:20495046.
- Hädrich A, Heuer VB, Herrmann M, Hinrichs KU, Küsel K. Origin and fate of acetate in an acidic fen. FEMS Microbiol Ecol. 2012;81(2):339–54. doi:10.1111/j.1574-6941.2012.01352.x. PMID:22404042.
- Müller V, Frerichs J. Acetogenic Bacteria. In: eLS. Chichester: John Wiley & Sons, Ltd, 2013.
- Drake HL, Gössner AS, Daniel SL. Old acetogens, new light. Ann N Y Acad Sci. 2008;1125:100–28. doi:10.1196/annals.1419.016. PMID:18378590.
- Ragsdale SW. Enzymology of the Wood-Ljungdahl pathway of acetogenesis. Ann NY Acad Sci. 2009;1125:129–36. doi:10.1196/annals.1419.015.
- Tremblay PL, Zhang T, Dar SA, Leang C, Lovley DR. The Rnf complex of Clostridium ljungdahlii is a proton-translocating ferredoxin:NAD+ oxidoreductase essential for autotrophic growth. mBio. 2012;4(1):e00406–12. doi:10.1128/mBio.00406-12. PMID:23269825.
- Buckel W, Thauer RK. Energy conservation via electron bifurcating ferredoxin reduction and proton/Na(+) translocating ferredoxin oxidation. Biochim Biophys Acta. 2013;1827(2):94–113. doi:10.1016/j.bbabio.2012.07.002. PMID:22800682.
- Biegal E, Schmidt S, Gonzalez JM, Muller V. Biochemistry, evolution and physiological function of the Rnf complex, a novel ion-motive electron transport complex in prokaryotes. Cell Mol Life Sci. 2011;68:613–34. doi:10.1007/s00018-010-0555-8. PMID:21072677.
- Greening C, Biswas A, Carere CR, Jackson CJ, Taylor MC, Stott MB, Cook GM, Morales SE. Genomic and metagenomic surveys of hydrogenase distribution indicate H2 is a widely utilised energy source for microbial growth and survival. ISME J. 2016;10(3):761–77. doi:10.1038/ismej.2015.153. PMID:26405831.
- Wolf PG, Biswas A, Morales SE, Greening C, Gaskins HR. H2 metabolism is widespread and diverse among human colonic microbes. Gut Microbes. 2016;7(3):235–45. doi:10.1080/19490976.2016.1182288. PMID:27123663.
- Macdonald IA, Jellett JF, Mahony DE, Holdeman LV. Bile salt 3α- and 12α-hydroxysteroid dehydrogenases from Eubacterium lentum and related organisms. Appl Environ Microbiol. 1979;37:992–1000. PMID:39496.
- Francis MB, Allen CA, Shrestha R, Sorg JA. Bile acid recognition by the Clostridium difficile germinant receptor, CspC, is important for establishing infection. PLoS Pathog. 2013;9(5):e1003356. doi:10.1371/journal.ppat.1003356. PMID:23675301.
- Theriot CM, Young VB. Interactions between the gastrointestinal microbiome and Clostridium difficile. Annu Rev Microbiol. 2015;69:445–61. doi:10.1146/annurev-micro-091014-104115. PMID:26488281.
- Masuda N, Oda H. 1983. 7α-Dehydroxylation of bile acids by resting cells of an unidentified, gram-positive, nonsporeforming anaerobic bacterium. Appl Environ Microbiol. 45(2):456–62. PMID:6572491.
- Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296(5571):1313–16. doi:10.1126/science.1070477. PMID:12016314.
- Lee JY, Arai H, Nakamura Y, Fukiya S, Wada M, Yokota A. Contribution of the 7beta-hydroxysteroid dehydrogenase from Ruminococcus gnavus N53 to ursodeoxycholic acid formation in the human colon. J Lipid Res. 2013;54(11):3062–69. doi:10.1194/jlr.M039834. PMID:23729502.
- Odermatt A, Da Cunha T, Penno CA, Chandsawangbhuwana C, Reichert C, Wolf A, Dong M, Baker ME. Hepatic reduction of the secondary bile acid 7-oxolithocholic acid is mediated by 11beta-hydroxysteroid dehydrogenase 1. Biochem J. 2011;436(3):621–29. doi:10.1042/BJ20110022. PMID:21453287.
- Odermatt A, Klusonova P. 11beta-Hydroxysteroid dehydrogenase 1: Regeneration of active glucocorticoids is only part of the story. J Steroid Biochem Mol Biol. 2015;151:85–92. doi:10.1016/j.jsbmb.2014.08.011. PMID:25151952.
- Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3(5):543–53. doi:10.1016/S1097-2765(00)80348-2. PMID:10360171.
- Duboc H, Tachè Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis. 2014;46(4):302–12. doi:10.1016/j.dld.2013.10.021. PMID:24411485.
- Holdeman LV, Chen J. Anaerobe Laboratory Manual, 4th ed. Blacksburg, VA: Virginia Polytechnic and State Institute. 1977.
- Carbonero F, Benefiel AC, Gaskins HR. Contributions of the microbial hydrogen economy to colonic homeostasis. Nat Rev Gastroenterol Hepatol. 2012;9(9):504–18. doi:10.1038/nrgastro.2012.85. PMID:22585131.
- Loman NJ, Quinlan AR. Poretools: a toolkit for analyzing nanopore sequence data. Bioinformatics. 2014;30(23):3399–401. doi:10.1093/bioinformatics/btu555. PMID:25143291.
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19(5):455–77. doi:10.1089/cmb.2012.0021. PMID:22506599.
- Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL. Versatile and open software for comparing large genomes. Genome Biol. 2004;5(2):R12. doi:10.1186/gb-2004-5-2-r12. PMID:14759262.
- Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30(14):2068–69. doi:10.1093/bioinformatics/btu153. PMID:24642063.
- Kanehisa M, Sato Y, Morishima K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J Mol Biol. 2016;428(4):726–31. doi:10.1016/j.jmb.2015.11.006. PMID:26585406.
- Devendran S, Méndez-García C, Ridlon JM. Identification and characterization of a 20β-HSDH from the anaerobic gut bacterium Butyricicoccus desmolans ATCC 43058. J Lipid Res. 2017;58(5):916–25. doi:10.1194/jlr.M074914. PMID:28314858.
