Abstract
Campylobacter concisus has been isolated from patients with gastroenteritis and inflammatory bowel disease (IBD), as well as healthy subjects. While strain differences may plausibly explain virulence differentials, an alternative hypothesis posits that the pathogenic potential of this species may depend on altered ecosystem conditions in the inflamed gut. One potential difference is oxygen availability, which is frequently increased under conditions of inflammation and is known to regulate bacterial virulence. Hence, we hypothesized that oxygen influences C. concisus physiology. We therefore characterized the effect of microaerophilic or anaerobic environments on C. concisus motility and biofilm formation, two important determinants of host colonization and dissemination. C. concisus isolates (n = 46) sourced from saliva, gut mucosal biopsies and feces of patients with IBD (n = 23), gastroenteritis (n = 8) and healthy subjects (n = 13), were used for this study. Capacity to form biofilms was determined using crystal violet assay, while assessment of dispersion through soft agar permitted motility to be assessed. No association existed between GI disease and either motility or biofilm forming capacity. Oral isolates exhibited significantly greater capacity for biofilm formation compared to fecal isolates (p<0.03), and showed a strong negative correlation between motility and biofilm formation (r = -0.7; p = 0.01). Motility significantly increased when strains were cultured under microaerophilic compared to anaerobic conditions (p<0.001). Increased biofilm formation under microaerophillic conditions was also observed for a subset of isolates. Hence, differences in oxygen availability appear to influence key physiological aspects of the opportunistic gastrointestinal pathogen C. concisus.
Introduction
While the human pathogen Campylobacter jejuni is recognized as a leading cause of gastroenteritis worldwideCitation1, other less characterized members of the same genus, such as Campylobacter concisus, are emerging as potential opportunistic instigators of acute and chronic gastrointestinal disordersCitation2–5. Although asymptomatic colonization with this Gram-negative organism has been observed throughout the gastrointestinal (GI) tractCitation3,Citation6,Citation7 in healthy adultsCitation3,Citation8, cases of C. concisus associated gastroenteritis with persistent symptomsCitation5 have been reported, particularly in infants and the elderlyCitation5,Citation9. Additionally, C. concisus is prevalent in IBD patientsCitation2–4 and increased risk of developing IBD has been described following cases of C. jejuni gastroenteritisCitation10,Citation11.
C. concisus has demonstrated pathogenic potential in vitro. It has been shown to induce pro-inflammatory responses, epithelial barrier dysfunction and apoptosis in gastrointestinal cell linesCitation12–14. The species is genetically heterogeneousCitation15–18 and strains isolated from different GI locations may encode distinct virulence factors, either as result of strain variation, or due to selection of strains with virulence attributes as a response to conditions in the host microenvironmentCitation8. Two distinct genomospecies (GS I and GS II) have previously been describedCitation15,Citation16. GS II was found to exhibit greater pathogenic potential in vitro and has been correlated with gastrointestinal symptomsCitation15,Citation16. Previously Nielsen et alCitation17 detected both genomospecies in patients with diarrhea, though no association between genomospecies and clinical disease severity was observed.
Environmental cues in the GI tract have previously been found to trigger virulence gene expression in pathogenic bacterial species such as Vibrio choleraeCitation19, and may facilitate physiological reprogramming from commensalism to pathogenic states in opportunistic microbial species. One such environmental cue is oxygen availability, which has previously been reported to influence virulence in Gram negative bacteriaCitation19, such as Salmonella enterica serovar TyphimuriumCitation20. C. concisus can be cultured under microaerophilic and anaerobic conditions in laboratory settings and requires hydrogen for growthCitation21. It has been suggested that C. concisus primarily grows under anaerobic conditions in the oral cavityCitation21. This is supported by the observation that this species frequently co-associates with strictly anaerobic bacteria in this nicheCitation21 including known hydrogen producersCitation22. H2 levels are higher in the lower gastrointestinal tract, and vary depending on the abundance of hydrogenogenic members of the gut microbiotaCitation21. The healthy gut is considered to be largely anaerobic, but oxygen concentrations can rise under inflammatory conditions, such as IBDCitation23. Based on these observations, we hypothesized that in the hydrogen-rich gut, increased oxygen availability alters the physiology of C. concisus, promoting opportunistic pathogenic traits such as increased motility (promoting dissemination) and biofilm formation (increased mucosal adherence). Flagellar motility and biofilm formation are dynamic features highly influenced by environmental cues including oxygen availabilityCitation24,Citation25, and are important virulence attributes of other pathogenic Campylobacter species such as C. jejuniCitation26,Citation27. However, knowledge of these properties remains sparse for C. concisus. Previous studies have indicated that C. concisus has a single polar flagellum, is motile under microaerophilic conditionsCitation28, and forms biofilms under both microaerophilic and anaerobic conditionsCitation27,Citation28. Nevertheless, the number of isolates assessed in previous studies was small and did not allow for comparisons between isolates from healthy subjects and patients with GI disease, between genomospecies or in response to oxygen availability. Hence, the aim of this study was to compare motility and biofilm formation under anaerobic and microaerophilic environments across a large set of C. concisus strains encompassing distinct genomospecies, isolated from distinct anatomical sites (oral cavity, gut mucosa and feces), across three clinical groups (IBD, gastroenteritis (GE) and healthy subjects (HS)).
Results
Variation in C. concisus motility and biofilm forming capacity is not related to health status or genomospecies
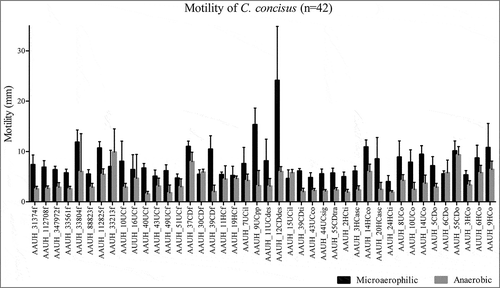
All 46 C. concisus clinical isolates exhibited the capacity to form biofilms, though this ability varied significantly across isolates and under microaerophilic or anaerobic conditions (Kruskal Wallis; p<0.01) (, Supplemental Fig 1). Biofilm formation did not differ significantly based on subject health status (IBD, GE or HS), irrespective of microaerophilic or anaerobic growth conditions. Thus, in vitro biofilm forming capacity did not relate to clinical assessments of gastrointestinal health (Kruskal-Wallis; p>0.05). Motility of 42 C. concisus isolates was assessed on soft agar plates under anaerobic conditions and compared to previous findings under microaerophilic conditions for the same cohort of isolatesCitation29. Motility varied significantly amongst isolates (Kruskal Wallis; p<0.01; ). However, irrespective of microaerophilic or anaerobic growth conditions there was no statistical significant difference in motility of isolates collected from patients with IBD, GE or HS (Kruskal-Wallis; p>0.05). Additionally, neither biofilm forming capacity nor motility significantly differed when isolates were stratified by genomospecies (Mann Whitney; p>0.05)
Figure 1. Biofilm forming capacity varies significantly amongst isolates (Kruskal Wallis; p < 0.05). Biofilms of 46 C. concisus isolates as measured by crystal violet staining (CV) absorbance at 550nm (A550 nm) under microaerophilic (black bars) or anaerobic (gray bars) conditions. Uninoculated BHI broth served as negative control. Asterix indicates strains that exhibit a significant difference (p<0.05) in biofilm formation between growth conditions.
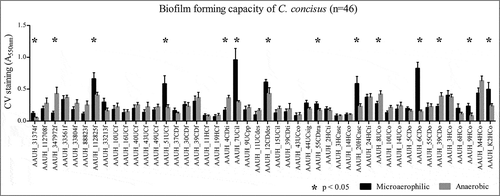
C. concisus oral isolates exhibit strongest biofilm forming capacity
A comparison of isolates originating from different anatomical sites along the GI-tract (oral cavity, gut mucosa and feces) revealed that regardless of subject health status, oral isolates exhibited significantly greater biofilm forming capacity compared with fecal isolates (Mann Whitney; p = 0.03 (MA), p = 0.07 (AN) (), but not in comparison to gut mucosal isolates. This phenomenon was genomospecies-independent (Chi-square; p>0.05). Whereas, motility was not related to anatomical site of isolation (Kruskal-Wallis; p>0.05).
Figure 3. Oral isolates exhibit greater biofilm forming potential than fecal isolates. Biofilms formation measured by crystal violet staining (CV) absorbance at 550nm (A550 nm) for 46 isolates stratified by anatomical sites of isolation; Oral (n = 11), Gut mucosa (n = 14), feces (n = 10) on the x-axis. Red dots represent isolates from IBD patients, blue dots represent isolates from healthy subjects. MA = microaerophilic growth, AN = Anaerobic growth.
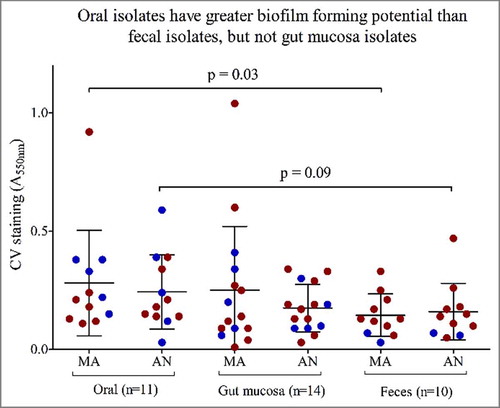
Motility and biofilm forming capacity are inversely correlated in oral isolates
When all isolates were considered, motility and biofilm formation were not significantly correlated, irrespective of whether microaerophilic or anaerobic growth conditions were used (Spearman correlation; r = 0.18(AN), r = 0.02(MA), p > 0.05) (a and e). However, under microaerophilic conditions, a strong inverse correlation was observed between the biofilm formation and motility of oral isolates (Spearman correlation; r = -0.7; p = 0.01) (h). A similar trend was also observed under anaerobic conditions (Spearman correlation; r = -0.5, p = 0.12) (d) for oral isolates. Under anaerobic conditions fecal isolates also exhibited a negative correlation between biofilm formation and motility (Spearman correlation; r = -0.41, p = 0.04) (b), whereas this was not observed under microaerophilic growth conditions for these isolates. No correlation between motility and biofilm formation was observed for gut mucosal isolates (c and g), nor amongst isolates when stratified by subject health status.
Figure 4. Correlation of biofilm and motility for 42 isolates. Anaerobic growth; A: All isolates, B: Fecal isolates, C: Gut mucosal isolates, D: Oral isolates. Microaerophilic growth: E: All isolates, F: Fecal isolates, G: Gut mucosal isolates, H: Oral isolates. r = Spearman correlation coefficient. Significant correlations are marked in blue boxes.
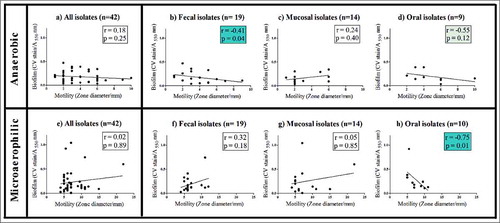
Microaerophilic environment increases motility and biofilm forming capacity
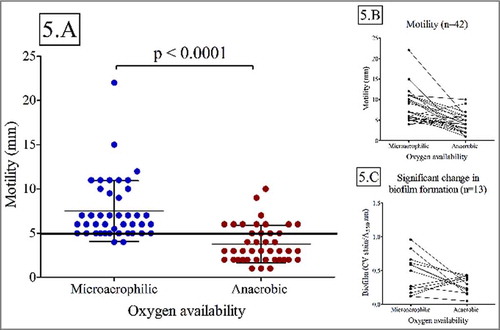
Microaerophilic environment was associated with a significant increase in motility (Wilcoxon paired rank test; p<0.01) (). A greater proportion of isolates exhibited a motile phenotype under microaerophilic conditions (95%; median: 8mm±3mm) compared to anaerobic growth conditions (33%, median: 4mm±2mm). Isolates that exhibited motility under both oxygen availability conditions (n = 14) exhibited a significant decrease in motility under anaerobic conditions (Wilcoxon paired rank test; p = 0.03) (). Increased motility under microaerophilic conditions was not related to disease outcome, anatomical site or genomospecies. (Chi-square; p>0.05).
Figure 5. Motility is significantly influenced by oxygen availability (Wilcoxon paired rank test; p < 0.01). Biofilm forming capacity was only influenced by oxygen availability in a subset of isolates A: Motility for all isolates (n = 42) under microaerophilic and anaerobic conditions. B: Motility for all isolates (n = 42) under microaerophilic and anaerobic conditions C: Subset of isolates with significant change in biofilm forming capacity.
We next hypothesized that exposure to microaerophilic or anaerobic growth conditions may influence microbial metabolism, leading to differences in production of diffusible or actively exported products that influence motility. To address this hypothesis we performed an experiment on three C. consuis isolates that had exhibited a significant increase in motility under microaerophilic growth conditions (AAUH_14HCco, AAUH_347972f, AAUH_12CDco). Each of these strains were cultured under anaerobic or microaerophilic conditions and cell free supernatant (CFS) was harvested and inoculated with each strain in soft agar plates to assess motility under anaerobic or microaerophilic growth conditions. For all isolates, under anaerobic culture conditions, CFS from microaerophilic or anaerobic growth did not significantly influence motility when compared to PBS (, panel A,B,C; p > 0.05, paired t-test or Wilcoxon paired rank test). However, under microaerophilic conditions two isolates exhibited a significant increase in motility following exposure to anaerobic (p < 0.01, t-test), but not to microaerophilic CFS (p > 0.05, paired t-test or Wilcoxon paired rank test; , panel A,B,C).
Figure 6. Effects of microaerophilic and anaerobic bacterial CFS on C. concisus motility: Motility measured under microaerophilic (red), or anaerobic (blue) growth conditions following exposure to PBS, microaerophilic CFS (MAs) or anaerobic CFS (ANs). A: Motility of isolate AAUH_14HCco, B: Motility of isolate AAUH_347972f, C: Motility of isolate AAUH_12CDco.
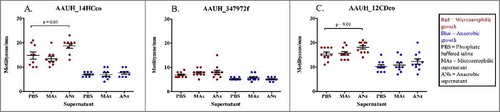
Figure 7. Images of soft BHI agar motility plates. Each plate has four zones of motility from isolate AAUH_14HCco. Plate 1: Microaerophilic growth; Plate 2 anaerobic growth. Circles indicate motility zones with a diameter line.

Increased oxygen availability was also associated with a significant difference in biofilm forming capacity (Wilcoxon paired rank test; p<0.05) for a subset of strains (n = 14), the majority of which produced larger biofilms under microaerophilic conditions. Again, no association was found between this observation and disease outcome, anatomical site or genomospecies (Chi-square; p>0.05).
Discussion
This is the first study to compare biofilm forming capacity and motility in a large set of clinical C. concisus isolates from inflammatory GI disease patients and healthy subjects, as well as from distinct anatomical sites of the GI tract. Although variation in motility and biofilm formation was observed across the isolates assayed, this variance was not related to disease status. This is in contrast to findings from a previous study of eight C. concisus strains, which differentiated into two sub-groups based on motility under microaerophilic conditionsCitation28. Strains originated from patients with different GI diseases (Crohn's Disease, acute or chronic gastroenteritis) and one strain from a healthy control. A tendency of greater motility was observed amongst strains from patients with chronic inflammatory diseases compared to acute or healthy statusCitation28. Differences between our study and that of Lavrencic et alCitation28, may be attibutable to strain variation, or to strain selection in distinct host niches in the GI tract. This is further supported by the observed difference in biofilm formation present across isolates from distinct anatomical sites – with greatest biofilm forming capacity observed in oral isolates, suggesting that niche-specialization may co-associate with physiological features of C. concisus strains.
Oral C. concisus isolates exhibited an inverse correlation between motility and biofilm forming capacity. Inhibition of motility is thought to be necessary to initiate biofilm formation, but in order to disperse from a biofilm, reactivation of motility is requiredCitation30,Citation31. Selective pressure of biofilm-proficient bacteria can result in permanent loss of motility due to loss-of-function mutationsCitation30. The observed negative relationship between biofilm formation and motility amongst oral C. concisus, was driven by isolates that exhibited either high motility and low biofilm formation, or conversely, high biofilm formation and low motility. While these strains may represent populations of C. concisus that consistently exhibit these physiological attributes, it is also possible that they represent distinct physiological states that co-exist within a C. concisus population in a given anatomical niche. Indeed, heterogeneity in physiological features of cells within biofilms, is well describedCitation30,Citation31. Compromised motility in favor of biofilm formation was not observed for gut isolates, suggesting that these isolates may originate from the planktonic subgroup of oral isolates, and that this sub-population has a greater capacity to both disseminate to, and colonize the lower GI-tract. This is further supported with whole genome sequencing data from this cohort showing that identical strains could be found in both the oral cavity and the lower GI-tractCitation32.
This is also the first study to compare motility and biofilm formation of C. concisus in microaerophilic and anaerobic environments. Interestingly, motility differed between growth environments with a significant decrease under anaerobic conditions. The same was observed for biofilm forming capacity, but only in a subset of isolates. Additionally, cell-free supernatants (CFS) generated under either microaerophilic or anaerobic conditions did not promote motility under subsequent anaerobic culture conditions. This may be due to inactivity of these product under anaerobic conditions, or that changes in bacterial physiology or metabolism induced under anaerobic conditions mute their signalling effectiveness. Thus it appears that C. concisus undergoes physiological changes in response to the presence of oxygen, which has also been observed for C. jejuni.Citation24,Citation25. Motility was, however, significantly increased in two mucosa isolates when anaerobic CFS were added to motility assays cultured under subsequent microaerophilic conditions, though it is plausible that increased metabolic activity and available substrate may promote this phenotype, factors which could be assessed in more detail in follow-up studies. A rise in oxygen levels may serve as an evolutionary environmental cue signaling proximity to the gut epithelial lining, as a micro-oxygenated zone of 70 μm exists around the mucosal surfaceCitation20. The ability to utilize oxygen as a signaling molecule that promotes chemotaxis is termed aerotaxis or energy taxis, and is typically used by motile microbes to navigate towards more favorable niches to optimize metabolic activityCitation33. C. jejuni has oxygen sensing genes (Aer, CetA and CetB) and exhibits aerotaxic behaviourCitation34,Citation35. To our knowledge, no data exists on oxygen sensing genes and aerotaxis for C. concisus, however it seems evident that this species can also sense and respond to changing oxygen availability.
C. concisus is able to respire and multiply under both anaerobic and microaerophilic conditions, indicative of active metabolism under both growth conditions. However, we and othersCitation21 observed smaller colonies and fewer colony forming units (CFU) under anaerobic conditions indicating slower metabolism and growth rates under conditions of anaerobiosis. Motility requires a high energy expenditureCitation30, and its down-regulation may represent an energy conservation mechanism under anaerobic conditions. Decreasing flagellar motility is also beneficial for phagocytic evasion, observed for other Gram negative bacteria such as P. aeruginosaCitation36. Decreased motility may occur through regulation of DNA supercoiling, which has been found to affect motility in strains of C. jejuniCitation37. A decrease in negative supercoiling was correlated with decreased motility via down-regulation of the FlgR and upregulation of FlgS, an important two-component regulatory system in this speciesCitation37. DNA supercoiling levels are influenced by the intracellular ATP/ADP ratio, with low ratios resulting in decreased DNA supercoiling regulated by DNA gyraseCitation38,Citation39. Interestingly, reduced ATP/ADP levels have been observed upon switch to anaerobic growth in E. coliCitation39. We speculate that a similar mechanism of decreased DNA supercoiling may arise as a result of anaerobic respiration, and explain the decreased motility observed for C. concisus isolates in our study. However, further research is needed to elucidate mechanisms of motility up- and downregulation in C. concisus. The altered response to oxygen observed for motility across the majority of our isolates did not extend to biofilm forming capacity. Only a subset of isolates exhibited upregulated biofilm formation under increased oxygen availability; some isolates exhibited the greatest biofilm forming capacity under anaerobic conditions. C. jejuni isolates have been shown to exhibit increased biofilm formation under microaerophilic conditions compared to aerobic conditionsCitation24,Citation40, however their response to anaerobic conditions remains unknown. In our study, the response to oxygen was not related to disease status, anatomical site of isolation or genomospecies. A number of C. concisus isolates exhibited stable biofilm forming capacity irrespective of oxygen availability, but increased motility under microaerophilic conditions. This suggests that the biofilm forming capacity of these strains occurs independent of oxygen availability, but that oxygen may serve as a signal to promote motility and dispersal from biofilms.
Our findings suggest that C. concisus exhibits enhanced motility in microaerophilic environments compared to conditions of anaerobiosis. While the healthy gut is considered to be largely anaerobic, the inflamed gut has increased levels of oxygen due to inflammatory processes such as neutrophil oxidative burst, and increased capillary permeabilityCitation41. C. concisus has the ability to cause epithelial damageCitation42, however this may require direct contact with the epithelial lining, and has only been investigated under microaerophilic conditions. Increased oxygen availability in the inflamed IBD gut may serve as a signal to C. concisus, enhancing motility and perhaps the capacity to access mucosal surfaces and may explain the higher prevalence of this species in the gut of IBD patients, compared to healthy subjects. Hence, IBD patients may be at greater risk for C. concisus gastroenteritis than the healthy population, and C. concisus infection may present as IBD flares. Therefore, it may be useful to include C. concisus in microbiological stool tests of IBD patients.
Gut oxygen availability is highly regulated by the gut microbiota and the abundance of facultative anaerobes have been correlated to increased oxygen concentrations in the distal gastrointestinal tractCitation43. Asymptomatic gastrointestinal colonization with C. concisus occurs in healthy subjects, and may be related to the relatively low abundance of facultative anaerobes in these subjects. Initially, the infant gut is not anaerobic, and is colonized by facultative anaerobes that subsequently create an anaerobic environment which promotes colonization by obligate anaerobes, such as ClostridiaCitation44. Additionally, increased abundance of facultative anaerobes is also observed in the elderly population, who exhibit evidence of gut microbiome diversity lossCitation45. Interestingly, C. concisus gastroenteritis is more prevalent in infants and the elderlyCitation5, which may be related to the gut oxygen availability signatures of these populations. Thus, we speculate that increased oxygen concentrations serve as an important regulatory switch for C. concisus that influences its physiology, which may enhance both survival and pathogenesis in the gastrointestinal tract. We note that both motility and biofilm forming capacity are regulated by various environmental inputs present in the gut, which are difficult to fully recapitulate in vitro. Especially important is the influence of other microorganisms on pathogenic physiologyCitation46, which should be the focus of future research.
In summary, our study demonstrates varying motility and biofilm forming capacity across Campylobacter concisus isolates with no relation to disease status or genomospecies. Biofilm forming capacity is related to anatomical site of isolation, with greater biofilm forming potential observed for oral isolates compared to that of fecal isolates. Oral isolates appear to segregate into two groups, as evidenced by the strong inverse correlation between motility and biofilm formation, suggestive of planktonic and biofilm sub-populations, and lower GI isolates may be sourced from the more motile population in oral cavity. In a microaerophilic environment C. concisus isolates exhibited increased motility, and for a subset of isolates increased biofilm forming capacity. Our findings suggest that a microaerophilic environment may serve as a physiological switch for C. concisus, leading to up-regulated motility and dissemination, thus providing a plausible explanation for the increased prevalence of this species in the inflamed gut. Thus, IBD patients as well as people with increased gut oxygen availability (such as infants and elderly population) may be at greater risk of C. concisus gastroenteritis and tests for C. concisus in these populations should be considered if clinically symptoms require microbiological examinations. The composition and metabolic activity of the gut microbiota may dictate C. concisus physiology and pathogenesis, and should form the focus of future studies.
Materials and methods
Clinical isolates and study population
C. concisus isolates were sourced from saliva (n = 12), gut mucosal biopsies (n = 15) and feces (n = 19) of patients with either Inflammatory Bowel Disease (IBD) (n = 23), acute gastroenteritis (GE) (n = 8) or from healthy subjects (HS) (n = 13), Citation3,Citation5. The IBD group consisted of patients with either Crohn's disease (CD), Ulcerative colitis (UC) or UC patients who had previous ileal-pouch-anal-anastomosis (IPAA) surgery. The healthy subjects group consisted of subjects who had undergone colonoscopy for screening purposes, including control of diverticulosis, genetic disposition for colorectal cancer or hereditary non-polyposis colorectal cancer. The acute gastroenteritis group consisted of patients with high levels of fecal calprotection (250–1820 mg/kg) and C. concisus as the only pathogen detectedCitation13. All strains were isolated at the Department of Clinical Microbiology, Aalborg University Hospital, by use of the passive filter technique and the Aalborg two-step culture method, as described elsewhereCitation3. For 26 isolates, whole-genome-sequencing data was available for genomospecies classification (GS I n = 10; GS II n = 16) and comparisonsCitation32.
Table 1. Participant demographics and isolate sources.
Bacterial culture
A total of 46 clinical isolates of C. concisus were cultured on 5% Horse Blood Agar plates with 1% added yeast extract for 48 hours under microaerophilic conditions (80% N2, 10% CO2, 5% H2, 5% O2). Subsequently, these isolates were cultured in Brain Heart Infusion broth (BHI) under either anaerobic (85% N2, 5% CO2, 10% H2, 0.05%< O2) or microaerophilic conditions. Anaerobic (AN) atmosphere was maintained in an anaerobic chamber (Coy Laboratory Products). Microaerophilic (MA) atmosphere was generated using CampyGen and CO2Gen gas generating kits (BD GasPaK™ EZ) and 0.2g sodium borohydride powder (Aldrich) dissolved in 10ml of sterile milli-Q water.
Biofilm Assay
The biofilm forming capacity of 46 C. concisus isolates was quantified following 72 hours of growth in BHI broth under microaerophilic or anaerobic conditions. Biofilm forming capacity was assayed using crystal violet staining in 96-well microtitre platesCitation47. Crystal violet staining was quantified by plate reader absorbance measured at 550 nm with four technical replicates per isolate. Median values from three independent experiments were calculated for each isolate and used in statistical analyses. Crystal violet absorbance values within one standard deviation of the negative control [0.1-0.5] were considered as non-biofilm forming isolates.
Motility Assay
A total of 42 clinical C. concisus isolates were assessed for motility under anaerobic conditions. Four isolates could not be resuscitated and were excluded from the motility assay. In brief, 5mL of 72-hr BHI culture for each strain was pelleted by centrifugation (200g for 20 minutes) and resuspended in phosphate-buffered-saline (PBS) to OD600 of 0.669. One microliter of this inoculum was applied to semi-solid 0.25% agar (37g BHI, 10g Blood Base) and incubated for 72 hours at 37°C in anaerobic chamber, after which motility zone diameters was measured. Motility zones below 5 mm were classified on non-motile in accordance to previous studiesCitation28,Citation29. Motility assay was repeated three times, each time with three technical replicates per isolate yielding a minimum of nine measurements per isolate. Motility evaluation under microaerophilic conditions was conducted using the same protocol and previously reported by Ovesen et al.Citation29.
Bacterial Supernatant supplemented Motility Assay
The effect of cell free supernatants (CFS) from microaerophilic or anaerobic growth on motility was assessed for three highly motile (under microaerophilic conditions) isolates (AAUH_12CDco, AAUH_347972 and AAUH_14HCco). In brief, 2 ml of 72-hr BHI cultures for each strain cultured under microaerophilic or anaerobic conditions were pelleted by centrifugation (200g for 20 minutes). Supernatants were filter sterilized with 0.22μm syringe filters (Millex). A small 10μl core was created in the semi-solid agar plates, and four microliters of sterile CFS were added. phosphate-buffered saline (PBS) filled cores were used as controls. Bacterial cultures were resuspended in PBS to McFarland Standard of 4 (OD600 = 0.669) and one microliter of this inoculum was added into each core. Plates were incubated under microaerophilic or anaerobic conditions using Anoxomat Mark II system (Advanced Instruments) for 72 hours, after which motility zone diameters were measured. The experiment was repeated three times for each of the three isolates, each time with minimum of three technical replicates.
Statistical analyses
Data were analyzed in the R statistical environment version 3.3.2 (R:A Language and Environment for Statistical Computing, R Core Team, R Foundation for Statistical Computing, Vienna, Austria, 2016, https://www.R-project.org/) and GraphPad Prism version 7.02 (GraphPad Software. La Jolla California, USA, www.graphpad.com) software. Data analyses were conducted with parametric test for normally distributed data (T-test, paired T-test) or non-parametric tests for non-normally distributed data (Mann Whitney U-test, Kruskal Wallis test and Wilcoxon paired rank test), where applicable. Correlations were analyzed by Spearman rank correlation test. Statistical significance was considered at p-values ≤0.05. Isolates used in this study were collected in part of two clinical studies, previously approved by the Regional Ethics Committee of Northern Jutland, Denmark (N-20080056, N-20130070).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgements
Supported in part by grants from the Lundbeck Foundation to the Innovation Centre, Denmark and UCSF to fund the Lundbeck Foundation Clinical Research Fellowship for Sandra Ovesen
References
- Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. Global epidemiology of campylobacter infection. Clin Microbiol Rev. 2015;28:687–720.
- Kaakoush NO, Mitchell HM, Man SM. Role of emerging Campylobacter species in inflammatory bowel diseases. Inflamm Bowel Dis. 2014;20:2189–97.
- Kirk KF, Nielsen HL, Thorlacius-Ussing O, Nielsen H. Optimized cultivation of Campylobacter concisus from gut mucosal biopsies in inflammatory bowel disease. Gut Pathog. 2016;8:27.
- Mahendran V, Riordan SM, Grimm MC, Tran TAT, Major J, Kaakoush NO, Mitchell H, Zhang L. Prevalence of Campylobacter species in adult Crohn's disease and the preferential colonization sites of Campylobacter species in the human intestine. PLoS One. 2011;6:e25417.
- Nielsen HL, Ejlertsen T, Engberg J, Nielsen H. High incidence of Campylobacter concisus in gastroenteritis in North Jutland, Denmark: A population-based study. Clin Microbiol Infect. 2013;19:445–50.
- Man SM, Castaño-Rodríguez N, Man SM, Mitchell HM. Is Campylobacter to esophageal adenocarcinoma as Helicobacter is to gastric adenocarcinoma? Trends Microbiol. 2015;23:455–62.
- von Rosenvinge EC, Song Y, White JR, Maddox C, Blanchard T, Fricke WF. Immune status, antibiotic medication and pH are associated with changes in the stomach fluid microbiota. ISME J. 2013;7:1354–66.
- Zhang L. Oral Campylobacter species: Initiators of a subgroup of inflammatory bowel disease? World J Gastroenterol. 2015;21:9239–44.
- Aabenhus R, Permin H, On SLW, Andersen LP. Prevalence of Campylobacter concisus in diarrhoea of immunocompromised patients. Scand J Infect Dis. 2002;34:248–52.
- Riddle MS, Gutierrez RL, Verdu EF, Porter CK. The chronic gastrointestinal consequences associated with Campylobacter. Curr Gastroenterol Rep. 2012;14:395–405.
- Gradel KO, Nielsen HL, Schønheyder HC, Ejlertsen T, Kristensen B, Nielsen H, et al. Increased short- and long-term risk of inflammatory Bowel disease. Gastroenterology. 2009;137:495–501.
- Kaakoush NO, Deshpande NP, Wilkins MR, Tan CG, Burgos-Portugal JA, Raftery MJ, Day AS, Lemberg DA, Mitchell H. The pathogenic potential of Campylobacter concisus strains associated with chronic intestinal diseases. PLoS One. 2011;6:e29045.
- Nielsen HL, Engberg J, Ejlertsen T, Nielsen H. Evaluation of fecal calprotectin in Campylobacter concisus and Campylobacter jejuni/coli gastroenteritis. Scand J Gastroenterol. 2013;48:633–5.
- Man SMS, Kaakoush NO, Leach ST, Nahidi L, Lu HK, Normann J, Day AS, Zhang L, Mitchell HM. Host Attachment, invasion, and stimulation of proinflammatory cytokines by Campylobacter concisus and other non-Campylobacter species. J Infect Dis. 2010;202:1855–65.
- Kalischuk LD, Inglis GD. Comparative genotypic and pathogenic examination of Campylobacter concisus isolates from diarrheic and non-diarrheic humans. BMC Microbiol. 2011;11:1–13.
- Aabenhus R, On SLW, Siemer BL, Permin H, Andersen LP. Delineation of Campylobacter concisus Genomospecies by amplified fragment length polymorphism analysis and correlation of results with clinical data. J Clin Microbiol. 2005;43:5091–6.
- Nielsen HL, Nielsen H, Torpdahl M. Multilocus sequence typing of Campylobacter concisus from Danish diarrheic patients. Gut Pathog. 2016;8:44.
- Kaakoush NO, Deshpande NP, Wilkins MR, Raftery MJ, Janitz K, Mitchell H. Comparative analyses of Campylobacter concisusstrains reveal the genome of the reference strain BAA-1457 is not representative of the species. Gut Pathog. 2011;3:15.
- Sengupta C, Ray S, Chowdhury R. Fine tuning of virulence regulatory pathways in enteric bacteria in response to varying bile and oxygen concentrations in the gastrointestinal tract. Gut Pathog. 2014;6:1–6.
- Marteyn B, Scorza FB, Sansonetti PJ, Tang C. Breathing life into pathogens: the influence of oxygen on bacterial virulence and host responses in the gastrointestinal tract. Cell Microbiol. 2011;13:171–6.
- Lee H, Ma R, Grimm MC, Riordan SM, Lan R, Zhong L, Raftery M, Zhang L. Examination of the Anaerobic Growth of Campylobacter concisus Strains. Int J Microbiol. 2014;1:ID 476047, 1–7.
- Washio J, Sato T, Koseki T, Takahashi N. Hydrogen sulfide-producing bacteria in tongue biofilm and their relationship with oral malodour. J Med Microbiol. 2017;54:889–95.
- Rigottier-Gois L. Dysbiosis in inflammatory bowel diseases: the oxygen hypothesis. ISME J. 2013;7:1256–61.
- Reeser RJ, Medler RT, Billington SJ, Jost BH, Joens LA. Characterization of Campylobacter jejuni biofilms under defined growth conditions. Appl Environ Microbiol. 2007;73:1908–13.
- Alm RA, Guerry P, Trust1 TJ. The Campylobacter u54 flaB flagellin promoter is subject to environmental regulation. J Bacteriol. 1993;175:4448–55.
- Baldvinsson SB, Holst Sørensen MC, Vegge CS, Clokie MRJ, Brøndsted L. Campylobacter jejuni motility is required for infection of the flagellotropic bacteriophage F341. Appl Environ Microbiol. 2014;80:7096–106.
- Gunther NW, Chen CY. The biofilm forming potential of bacterial species in the genus Campylobacter. Food Microbiol. 2009;26:44–51.
- Lavrencic P, Kaakoush NO, Huinao KD, Kain N, Mitchell HM. Investigation of motility and biofilm formation by intestinal Campylobacter concisus strains. Gut Pathog. 2012;4:22.
- Ovesen S, Kirk KF, Nielsen HL, Nielsen H. Motility of Campylobacter concisus isolated from saliva, feces, and gut mucosal biopsies. APMIS. 2017;125:230–5.
- Guttenplan SB, Kearns DB. Regulation of flagellar motility during biofilm formation. FEMS Microbiol Rev. 2013;37:849–71.
- Lynch AS, Robertson GT. Bacterial and fungal biofilm infections. Annu Rev Med. 2008;59:314–28.
- Kirk KF, Méric G, Nielsen HL, Pascoe B, Samuel K. Molecular epidemiology and comparative genomics of Campylobacter concisus strains from saliva, faeces and gut mucosal biopsies in inflammatory bowel disease. Sci Rep. 2018;8:1902.
- Schweinitzer T, Josenhans C. Bacterial energy taxis: a global strategy? Arch Microbiol. 2010;192:507–20.
- Hazeleger WC, Wouters JA, Rombouts FM. Physiological activity of campylobacter jejuni far below the minimal growth temperature. Appl Environ Microbiol. 1998;64:3917–22.
- Marchant J, Wren B, Ketley J. Exploiting genome sequence: predictions for mechanisms of Campylobacter chemotaxis. Trends Microbiol. 2002;10:155–9.
- Lovewell RR, Collins RM, Acker JL, Toole GAO, Wargo MJ. Step-wise loss of bacterial flagellar torsion confers progressive phagocytic evasion. PloS Pathog. 2011;7:e1002253.
- Shortt C, Scanlan E, Hilliard A, Cotroneo CE, Bourke B, Cróinín Ó. DNA supercoiling regulates the motility of campylobacter jejuni and is altered by growth in the presence of chicken mucus. MBio. 2016;7:e01227–16.
- Van Workum M, Oldenburg N, Hans V. DNA supercoiling depends on the phosphorylation potential in Escherichia coli. Mol Microbiol. 1996;20:351–60.
- Hsieh L, Burger RM, Drlicat K, Wang I. Bacterial DNA supercoiling and [ATP]/[ADP] changes associated with a transition to anaerobic growth. J Mol Biol. 1991;219:443–50.
- Oh E, Kim J, Jeon B. Stimulation of biofilm formation by oxidative stress in Campylobacter jejuni under aerobic conditions. Virulence. 2016;7:846–51.
- Zechner EL. Inflammatory disease caused by intestinal pathobionts. Curr Opin Microbiol. 2017;35:64–9.
- Nielsen HL, Nielsen H, Ejlertsen T, Engberg J, Günzel D, Zeitz M, Hering NA, Fromm M, Schulzke JD, Bücker R. Oral and fecal Campylobacter concisus strains perturb barrier function by apoptosis induction in HT-29/B6 intestinal epithelial cells. PLoS One. 2011;6:e23858.
- Rivera-Chávez F, Lopez CA, Bäumler AJ. Oxygen as a driver of gut dysbiosis. Free Radic Biol Med. 2017;105:93–101.
- Rodrı JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, Avershina E, Rudi K, Narbad A, Jenmalm MC, et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol. 2015;1:1–17.
- Biagi E, Candela M, Fairweather-tait S, Franceschi C, Brigidi P. Ageing of the human metaorganism: the microbial counterpart. Age (Omaha). 2012;34:247–67.
- Hoong K, Flint S, French N. Biofilm formation by Campylobacter jejuni in controlled mixed-microbial populations. Int J Food Microbiol. 2010;143:118–24.
- O'Toole GA. Microtiter dish biofilm formation assay. J Vis Exp. 2011;47:3–5.