ABSTRACT
It has long been acknowledged that dietary fibres are important to maintain a healthy gut. Over the past decade, several studies have shown that loss of complex polysaccharides from the Western diet has resulted in alterations to our colonic microbiota. The concurrent increase in the incidence of inflammatory bowel disease in the Western world has driven us to explore the potential mechanistic link between diet, the microbiota and the host defence systems that normally prevent inflammation. Using mice fed a low fibre Western-style diet and robust live tissue analytical methods we have now provided evidence that this diet impairs the colonic inner mucus layer that normally separates bacteria from host cells. Western societies urgently need to develop their understanding of the molecular mechanisms of the diet-microbiota-mucus axis and its implications for inflammatory diseases.
A major lifestyle difference between Western(ised) and non-Western regions is diet. The traditional omnivorous human diet is low in fat and rich in complex plant-derived carbohydrates (dietary fibre), and this has remained constant for most of our evolutionary history. However, the diet of some modern societies has radically altered over the past century with the advent of high calorie, low-fibre processed food resulting in an up to seven-fold decrease in dietary fibre intake in Western nations compared to populations in the developing world. This shift has correlated with the emergence of a number of mucosal inflammatory diseases, raising the possibility that diet might be a contributing environmental factor.
Under healthy conditions, colonic inflammation is prevented by a mucus layer that physically separates the microbiota from the epithelial cell surface.Citation1 Mucus is produced by epithelial goblet cells that secrete a core set of proteins including the gel-forming mucin MUC2, a large glycoprotein (2.5 MDa) with long central proline/threonine/serine rich sequences (PTS/mucin domains) that are densely O-glycosylated to form stiff linear rods.Citation2 MUC2 monomers are assembled into polymers via covalent C-terminal dimerization and N-terminal trimerization and once secreted form enormous net-like sheets.Citation3 These polymers form the structural skeleton of the mucus layer, as evidenced by the observation that Muc2−/- mice lack detectable intestinal mucus.Citation1 Stratified MUC2 polymeric sheets form a mucus layer that is anchored to the epithelium. This inner mucus layer is impenetrable to most bacteria and is proteolytically converted to a detached and expanded outer layer that serves as a habitat for the microbiota. Crucially, genetic ablation of Muc2 results in spontaneous colitis and colorectal cancer development.Citation1 This is similar to the pathology of UC, where a penetrable inner mucus layer is observed in patients with active inflammation.Citation4
The impact of a Western-style diet on the colonic mucus barrier
We recently sought to test the hypothesis that diet affects the function on the colonic mucus using mice fed a Western-style diet high in fat and simple sugars and low in complex fibre ().Citation5 Mice were assessed for metabolic parameters, stool microbiota configuration and colonic mucus layer properties. Importantly, we employed live tissue analytical methods that allow simultaneous quantification of inner mucus layer thickness, mucus penetrability (‘barrier function’) and mucus growth rate (‘inner mucus layer-to-outer mucus layer conversion’) and collection of mucus for proteomic analyses.Citation6 This ex vivo approach allows robust quantification of multiple mucus parameters and avoids the artefacts that occur upon fixation and sectioning which limit the quality of histochemical mucus layer quantification.
Figure 1. Switching from a traditional/high fibre diet to a Western-style low fibre diet drives alterations in microbiota composition and metabolite production and results in inner mucus layer barrier dysfunction.
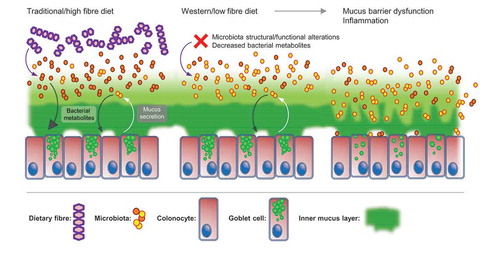
Our results demonstrated that exposure to a Western diet results in rapid alterations in microbiota composition and reduced inner mucus layer thickness, increased mucus penetrability and growth rate after only three days of Western diet feeding. While the overall mucus proteome was not significantly altered, the absolute concentration of MUC2 molecules was decreased, implying that a decrease in the density of the MUC2 polymeric network might contribute to the increased mucus penetrability. The mucus barrier defects were not replicated in genetically obese hyperphagic Ob/Ob mice suggesting that dietary composition, as opposed to overnutrition, was a key factor. Crucially, microbiota transplantation from control diet (low fat, high fibre)-fed mice prevented the inner mucus layer dysfunction in Western diet -fed mice, providing evidence of a causal role for the microbiota in the driving these effects.
A notable alteration in microbiota composition after Western diet-feeding was a decline in the relative abundance of the genus Bifidobacterium. The levels of these bacteria were found to positively correlate with mucus barrier function across all of our experimental interventions, which was of particular interest as Bifidobacteria are commonly used as probiotics. Supplementation of Western diet-fed mice with Bifidobacterium longum resulted in restoration of the mucus growth rate, but did not rescue the penetrability defect. In contrast, administration of a single type of dietary fibre (inulin) restored and normalized mucus barrier function, but did not affect growth rate.
Combined, our results highlight the existence of a deterministic diet-microbiota-mucus barrier axis, whereby alterations in dietary composition drive sequential changes in the microbiota composition and metabolism, which in turn results in mucus dysfunction. Consequently, the observations support the hypothesis that the Western diet can cause increased mucus penetrability, allowing bacteria to come closer to the host epithelium, which may ultimately trigger inflammation.
The microbiota-mucus-host balance
The fact that only two bacterial phyla, the Bacteroidetes and Firmicutes, dominate the adult gut is a testament to the fact that our microbiota has co-evolved with us. This specialization can broadly be characterized as the ability to persistently colonize the host combined with the capacity to replicate using the metabolic substrates that are available in the intestine. In humans, these substrates consist of the complex polysaccharide fibres that we lack the enzymatic capacity to digest as well as the glycans of the host mucus. The microbiome encodes several thousand carbohydrate-active enzymes which allows bacteria to degrade and feed on these complex glycans. Microbiota carbohydrate fermentation generates metabolites, such as short chain fatty acids (SCFA), which can be absorbed and used as an energy source by the host, a process that is considered a cornerstone of host-microbiota mutualism.
Notably, mutualistic bacteria that target dietary polysaccharides may also encode enzymes that allow them to degrade mucin O-glycans.Citation7 Mucin glycan catabolism by the microbiota can be seen as an example of host-microbiota mutualism, as MUC2 polymer biosynthesis is a complex process that requires a high level of resource commitment by the host. Thus, cleavage and fermentation of MUC2 O-glycans allows a proportion of these resources to be recycled back to the colonic epithelium as SCFA. However, the balance between MUC2 secretion by the host and degradation by the microbiota must be finely balanced in order to prevent mucus layer disruption. Most important for maintaining this balance is that that the inner mucus layer remains impenetrable to bacteria and thus bacteria will normally not degrade the inner mucus layer mucin polymer network. Dietary changes, as observed after exposure to a Western diet, allow bacteria to penetrate the inner mucus layer, thus shifting this delicate balance.
The relationship between the host and the microbiota has evolved in the context of a high-fibre environment. Therefore, it is not hard to imagine that alterations in this environment might destabilise this relationship via its effects on the mucus barrier, as we have observed in our Western diet-feeding experiments. Indeed, previous investigations using gnotobiotic mouse models and dietary interventions suggest that the lack of fibre is key to driving microbiota-dependent mucus barrier dysfunction.Citation8,Citation9 Importantly, Desai et al. found that dietary fibre depletion resulted in the upregulation of bacterial gene clusters related to mucin degradation, which correlated with a decrease in inner mucus layer thickness. Thus, current research supports the concept that dietary fibre depletion causes inner mucus layer dysfunction via its effect on the microbiota (). However, the mechanistic basis for this remains unclear.
Microbiota-dependent mucus barrier dysfunction
It has been suggested that a switch to mucin O-glycan degradation might cause microbiota-dependent mucus barrier disruption, as bacteria can strip the glycans that normally shield the MUC2 protein core from proteolysis. This idea has yet to been proven, although it can be argued that it is supported by previous observations that genetic deletion of the Core 1 and 3 glycosyltransferases that extend the mucin O-glycan chains results in a dysfunctional mucus barrier.Citation10,Citation11 Nevertheless, one must take into account that glycosylation not only protects the MUC2 protein core, but also has great influence on the water binding capacity of the mucin and its expansion upon secretion.Citation3 Furthermore, our results showing that supplementation with a simple polysaccharide (1% inulin) can rescue diet-induced inner mucus layer disruption do not fully support the idea that a diet rich in complex fibres is necessary to prevent inner mucus layer dysfunction.Citation5 Moreover, germ free mice have been shown to have a penetrable inner colonic mucus layer,Citation12 which, due to the nature of these mice, cannot be caused by bacterial degradation. Therefore, while bacterial carbohydrate hydrolase-mediated MUC2 degradation may play a role in diet-induced inner mucus layer barrier dysfunction, additional mechanisms must exist.
More important is probably the metabolic alterations of the microbiota, supported by the observation that it was only three days between diet change and the initiation of the mucus defect. Some alterations in the microbial composition were observed, but this was not dramatic. Alterations in diet can quickly alter the production of small molecule metabolites by the microbiota. Western diet causes a decrease in SCFA production. Importantly, loss of SCFA is known to result in epithelial cells switching from beta-oxidation to the less efficient fermentation of glucose to lactate.Citation13 Biosynthesis of MUC2, and other mucus components, has a high energy cost and therefore a general decrease in available ATP may negatively influence the production of a functional inner mucus layer. Bacteria also carry numerous polygenes that provide the capacity to metabolize small molecules that have been suggested to affect the host.Citation14,Citation15 A number of such small microbial metabolites will probably have different effects on the epithelium and might be the missing link connecting the impact of diet on the microbiota to mucus properties.
No matter the underlying mechanistic cause, diet-induced colonic mucus barrier dysfunction has clear implications for human health. The emergent chronic inflammatory bowel disease ulcerative colitis specifically affects the colon.Citation16 It is thought to be caused by excessive mucosal immune responses to the microbiota, and is strongly associated with an increase in colonic mucus barrier penetrability.Citation4 Strikingly, the global epidemiology of ulcerative colitis demonstrates that the disease is primarily restricted to Western nations and increasing in regions that have adopted a Western lifestyle. As consumption of a Western diet is considered to be a risk-factor for inflammatory bowels diseases,Citation17 this has led to the concept that dietary composition might affect mucus barrier function as possible mechanistic link between lifestyle and ulcerative colitis. The results of our current investigation clearly support this hypothesis.
In conclusion, the field of diet-microbiota-host mucus protection axis has made significant progress in understanding how modern shifts in the human diet may have disturbed the ancient evolutionary balance between our symbiotic gut microbiota and the mucus layer that separates the bacteria from the host. However, a clear mechanistic understanding of this phenomenon is still lacking on many levels. Only by developing this understanding will we be able to therapeutically target the complex interactions between diet, microbiota, mucus and epithelium. In the future, this will allow us to handle modern emergent diseases like ulcerative colitis as well as provide scientifically based information that will assist the public to pursue a healthy dietary lifestyle.
Additional information
Funding
References
- Johansson MEV, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064–15069. doi:10.1073/pnas.0803124105.
- Johansson MEV, Hansson GC. Immunological aspects of intestinal mucus and mucins. Nature Rev Immunol. 2016;16:639–649. doi:10.1038/nri.2016.88.
- Ambort D, Johansson MEV, Gustafsson JK, Nilsson HE, Ermund A, Johansson BR, Koeck PJB, Hebert H, Hansson GC. Calcium and pH-dependent packing and release of the Gel-forming MUC2 mucin. Proc Natl Acad Sci USA. 2012;109:5645–5650. doi:10.1073/pnas.1120269109.
- Johansson MEV, Gustafsson JK, Holmen-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjövall H, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and in patients with ulcerative colitis. Gut. 2014;213:281–291. doi:10.1136/gutjnl-2012-303207.
- Schroeder BO, Birchenough GMH, Stahlman M, Arike L, Johansson MEV, Hansson GC, Bäckhed F. Bifidobacterium or fiber protect against diet-induced deterioration of the inner colonic mucus layer. Cell Host Microbe. 2018;23:1–14. doi:10.1016/j.chom.2017.11.004.
- Gustafsson JK, Ermund A, Johansson MEV, Schutte A, Hansson GC, Sjovall, H. An ex vivo method for studying mucus formation, properties and thickness in human colonic biopsies and mouse small and large intestinal explants. Am J Physiol. 2012;302:G430–G438.
- Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Micro. 2012;10:323–335. doi:10.1038/nrmicro2746.
- Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167:1339–1353. doi:10.1016/j.cell.2016.10.043.
- Zou J, Chassaing B, Singh V, Pellizzon M, Ricci M, Fythe MD, Kumar MV, Gewirtz AT. Fiber-mediated nourishment of gut microbiota 1 protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host Microbe. 2018;23:1–13. doi:10.1016/j.chom.2017.11.003.
- Bergstrom K, Fu J, Johansson ME, Liu X, Gao N, Wu Q, Song J, McDaniel JM, McGee S, Chen W, et al. Core 1- and 3-derived O-glycans collectively maintain the colonic mucus barrier and protect against spontaneous colitis in mice. Mucosal Immunol. 2016;10:91–103. doi:10.1038/mi.2016.45.
- Sommer F, Adam N, Johansson MEV, Xia L, Hansson GC, Bäckhed F, Sanz Y. Altered mucus glycosylation in core 1 o-glycan-deficient mice affects microbiota composition and intestinal architecture. PLoS One. 2014;9:e85254. doi:10.1371/journal.pone.0085254.
- Johansson MEV, Jacobsson HE, Holmén-Larsson J, Schütte A, Ermund A, Rodríguez-Piñeiro AM, Arike L, Wising C, Svensson F, Bäckhed F, et al. Normalization of the host intestinal mucus systems requires long-term colonization. Cell Host Microbe. 2015;18:582–592. doi:10.1016/j.chom.2015.10.007.
- Donohoe DR, Wali A, Brylawski BP, Bultman SJ, Bereswill S. Microbial regulation of glucose metabolism and cell-cycle progression in Mammalian colonocytes. PLoS ONE. 2012;7:e46589. doi:10.1371/journal.pone.0046589.
- Donia M, Cimermancic P, Schulze C, Wieland Brown LC, Martin J, Mitreva M, Clardy J, Linington RG, Fischbach MA. A systematic analysis of biosynthetic gene clusters in the human microbiome reveals a common family of antibiotics. Cell. 2014;158:1402–1414. doi:10.1016/j.cell.2014.08.032.
- Wlodarska M, Luo C, Kolde R, d’Hennezel E, Annand JW, Heim CE, Krastel P, Schmitt EK, Omar AS, Creasey EA, et al. Indoleacrylic acid produced by commensal peptostreptococcus species suppresses inflammation. Cell Host Microbe. 2017;22:25–37. doi:10.1016/j.chom.2017.06.007.
- Danese S, Fiocchi C. Ulcerative colitis. New Engl J Med. 2011;365:1713–1725. doi:10.1056/NEJMra1102942.
- Zuo T, Kamm MA, Colombel JF, Ng SC. Urbanization and the gut microbiota in health and inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2018;15:440–452.
