ABSTRACT
Clostridium difficile has been documented as a major cause of uncontrolled outbreaks of enteritis in neonatal pigs and antibiotic-associated infections in clinical settings. It belongs to the natural cohort of early colonisers of the gastrointestinal tract of pigs and can be detected in faeces up to two weeks post-partum. In older pigs, it often remains under the detection limit. Most neonatal pigs show no clinical signs of disease although C. difficile and its toxins can be detected at high levels in faeces. Increased mortality rates associated with C. difficile on pig farms are, so far, considered “spontaneous” and the predisposing factors are mostly not defined. The infection caused by C. difficile is multifactorial and it is likely that the repertoire of maternal factors, host physiology, the individually developing gut microbiota, co-infections and environmental stress define the conditions for disease development. In this addendum to our recently published work on CDI in neonatal piglets, we discuss the “early-life events” that influence C. difficile spread and infection in neonatal piglets.
Introduction
For more than a decade Clostridium difficile has been documented as a major cause of uncontrolled enteritis outbreaks in neonatal pigs.Citation1,Citation2 Nowadays, it is known that different farm animal species can be affected, making them also a potential reservoir for C. difficile infection (CDI) in humans.Citation3 Besides the historic ribotype 027, a new type of C. difficile, ribotype 078, originating from pigs, has been found to transmit to farmworkers and cause CDI.Citation4 In the western industrialised countries, in hospitalised patients, C. difficile is a leading cause of nosocomial infection, morbidity, and mortality where the latter is typically evident in the elderly (> 80 years). Besides nosocomial infections, community acquired CDI is increasingly important and the newly-reported ribotype such as 078 has linked disease in humans and pigs.Citation5 Current treatment of CDI in pigs and humans includes the use of antibiotics, however treatment failure and infection relapse can occur.Citation6 A promising solution in current clinical practice is faecal microbiome transplantation (FMT) which supports the concept of “colonisation resistance”. The outcomes of clinical trials with the use of FMT are characterised by high cure rates (up to 95%) and the method is gaining interest among both health practitioners and patients.Citation7–Citation9
Spores of C. difficile facilitate a rapid spread of the bacterium between animals and in the environment.Citation10,Citation11 The diagnosis of CDI in pigs and humans usually includes diarrhoea and colitis as well as the identification of virulent C. difficile and detection of toxins. However, clinical symptoms often do not correlate with C. difficile and their toxins, making the diagnosis of CDI extremely difficult.Citation12,Citation13 Interestingly, C. difficile belongs to the natural early colonisers of the gastrointestinal tract of pigs and up to 100% of piglets test positive (and so increasing the probability of being colonised by toxigenic ribotypes) within two days after birth followed by a rapid decline with age.Citation10,Citation11 Increased pre-weaning mortality rates associated with C. difficile on pig farms are so far termed “spontaneous” and the predisposing factors are largely not known.Citation14 It is very likely that maternal factors, host physiology, the individually developing gut microbiota, co-infections and environmental stress are important determinants.
In this Addendum to our recently published work on CDI in neonatal piglets,Citation13 we aim at discussing the “early-life events” that influence the spread of C. difficile infection in neonatal piglets.
C. difficile infection in pigs
C. difficile colonises the piglet gut at birth and can be detected in faeces up to two weeks post-partum. Even more, most neonatal pigs show no clinical signs of disease although C. difficile and its toxins are present at high levels in the faeces. C. difficile is also found in adult pigs though often at a very low level.Citation15 Although neonatal piglets are normally asymptomatic carriers of C. difficile (including toxigenic ribotypes), more severely infected animals may be underweight by 10–15% and exhibit decreased growth. The mortality rate among diarrhoeic neonatal piglets can be up to 14%.Citation16 Pathogenic C. difficile have the ability to produce several toxins which may be associated with CDI symptoms in neonatal piglets. The action of the two major exotoxins secreted by the bacterium, toxin A (TcdA) and toxin B (TcdB) is related to the modulation of the intestinal epithelial cell physiology and disruption of barrier function. The toxins can inactivate Rho proteins involved in the formation of the cell cytoskeleton, leading to disruption of tight junctions (TJ) and finally epithelial integrity.Citation17 Thus, in the last phase of C. difficile infection, intestinal pathology develops due to a fluent inflammatory response induced by the toxins, virulence factors and additionally by translocation of the gut microbiota. The loss of epithelial integrity and accompanying reduction of transepithelial resistance can also be demonstrated in an IPEC-J2 cell culture model and it seems to be toxin dose-dependent (). The induction of pro-inflammatory cytokines by the toxins leads to the migration of neutrophils and macrophages into the site of infection and formation of mesocolonic oedema.Citation18,Citation19 Typical clinical symptoms in infected piglets include pasty-to-watery diarrhoea, anorexia, growth retardation and dehydration. These manifestations may finally lead to animal death.Citation16 The inflammation leads to harmful epithelial damage that is responsible for the clinical course of the infection and it triggers an adaptive immune reaction that is essential as a long-lasting specific defence. Besides the influence of the inflammatory response and microbiome shift on the course of the disease, CDI is a risk factor for resistome expansion in pigs and humans; in humans treated in intensive care units or suffering from CDI, shifts in the intestinal microbiome were recently linked with resistome changes.Citation20
Figure 1. Response of the IPEC-J2 to different concentrations of toxin A (TcdA) and toxin B (TcdB) as measured by transepithelial electrical resistance (TEER) in an in vitro assay up to 20 h of incubation. Control: growth media (Dulbecco’s Modified Eagle’s Medium – DME, DMEM | Sigma-Aldrich). Spent supernatant containing TcdA (1 291 ng/ml) and TcdB (829 ng/ml) diluted 1:2, 1:10, 1:100 and 1:1 000. Methods in Supplementary file S1.
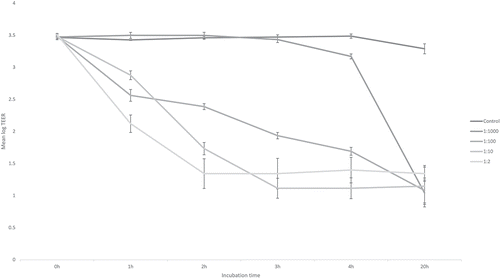
The susceptibility to CDI in pigs decreases with ageCitation21 but the reasons for this are not clear. Differences in the susceptibility to CDI in neonatal piglets could result from the presence of toxin receptors or intestinal concentration of bile salts which are critical for the germination of C. difficile spores in the intestine.Citation22,Citation23 In addition, the synthesis of C. difficile toxins (major virulence factors) have been found to depend on the presence of certain amino acids (e.g., cysteine or proline as inhibitors) and short chain fatty acids (e.g. butyric acid as inducer)Citation24,Citation25 and are thus related to the intestinal micro-environment.
Microbial dysbiosis in C. difficile infection
Still little is known about the phenomenon of resistance and susceptibility to CDI in piglets. It is unclear why and under which conditions piglets become sick or are asymptomatic carriers of toxigenic ribotypes. Interestingly, the natural colonisation of porcine and human neonates with C. difficile is more prominent when they are formula fed.Citation13,Citation26 The pathogenesis of CDI is most likely multifactorial and thus accompanied by co-infections with other pathogens or a predisposing intestinal dysbiosis.Citation27,Citation28 There is increasing evidence that certain diseases are not only associated and due to a single causative pathogenCitation29 but rather, are associated (and may be due to) a collection of different microbes (i.e., the “pathobiome hypothesis”).Citation30 Thus, any disruption of the natural colonisation process or perturbances of the intestinal ecosystem could enhance the susceptibility to CDI. In line with this hypothesis, our recent data on the CDI model show a high abundance of Proteobacteria including putative pathogens such as Escherichia spp. or Shigella spp..Citation13
In humans, C. difficile is probably the best-known pathogen that follows antibiotic-mediated changes in the gut microbiome.Citation28 Whether this is also true in the pig is yet not clear. However, we showed in a previous study that antibiotic treatment in a sow was associated with an increased concentrations of C. difficile and toxins in her piglets as compared to non-treated sows.Citation15 Antibiotics are known to alter the structure and metabolism of the gut microbiota allowing the expansion of opportunistic pathogens including C. difficile. However, non-antibiotic treated piglets may also develop CDI.Citation31 A deeper knowledge regarding the influence of feeding on microbiome signatures, resistome, and C. difficile pathology could assist the development of protective strategies to combat CDI in piglets.
Neonatal microbial programming through mother-offspring association
The association between mother and offspring gut microbiota during early life is a critical factor for the subsequent succession of intestinal commensal bacteria and immune development later on.Citation32,Citation33 The newborn piglet is continuously exposed to microbes from its mother and the environment, which enter the gut together with the mother’s milk. The early gut colonisers in neonatal piglets between 1 and 3 days of age include clostridia, enterobacteria, enterococci, streptococci and peptostreptococci, whereas lactobacilli and other species become predominant afterwards.Citation34 This early neonatal phase also seems to determine the microbial profile and intestinal health later in life.Citation35 In contrast to early-life colonisation patterns, changes in the intestinal microbial ecosystem during the abrupt weaning process in pigs have been studied intensively during the past decades and are accompanied with functional adaptations related to diet complexity.Citation36,Citation37 Compared to humans,Citation26,Citation32,Citation38 the aspect of mother-offspring association and its effect on early microbial programming in pigs has not been studied in detail. Still, little is known about the impact of diet on the microbial association between the mother sows and their offspring as well as the establishment of the infant gut microbiota early in life. Few studies suggest a positive impact of sow milk on the intestinal microbiota and immune system of the pigletsCitation39,Citation40 and that certain probiotics given to sows may alter the microbiota composition and immune status of their offspring.Citation41,Citation42
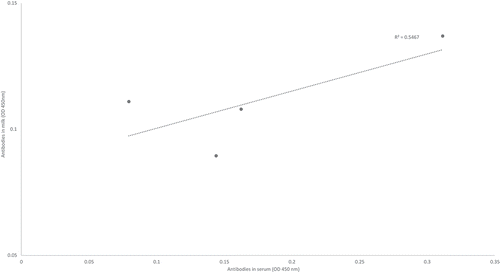
The only source of nutrients for the new-born piglet is milk, which contains numerous growth factors, microbial antigens and host antibodies e.g., directed against certain pathogens and contributing to passive immunisation in the offspring.Citation43,Citation44 Interestingly, antibodies against TcdA have been identified in human blood serumCitation45 which may protect against CDI.Citation46 We could demonstrate that the IgG antibodies against TcdA can be found in sows’ blood serum and milk (). Recent studies have shown that the administration of TcdB-specific bovine colostrum could prevent and treat CDI in mice and reduced disease recurrence by 67%.Citation47 Thus, immunisation offers promising tools to actively protect the individuals against CDI. The above data suggest that manipulating the mother’s antibody repertoire in milk could protect neonatal piglets from CDI. In several studies it has been demonstrated that the amount of immunoglobulins targeting toxin A and B are decisive for asymptomatic carriage or recurrent courses.Citation45,Citation46 In addition to the humoral reaction of the host itself, also intravenously administered human monoclonal antibodies that bind to TcdB show evidence of protection.Citation48 Although one must suppose that serum titres of antibodies only reflect the amount of mucosal antibody production that is a prerequisite for a direct defence of the mucosal surface, nothing is known about the mucosal secretion of immunoglobulins specific for toxins. In addition, the mechanism of how intravenous administered antibody can protect mucosal surfaces from toxin-mediated damage has to be elucidated. Interestingly, human milk oligosaccharides were found to adhere to TcdA and TcdB and slightly inactivate their toxicity in in vitro cell cultureCitation49 and such an effect might also be demonstrated using porcine milk oligosaccharides but this has yet to be studied . In addition, porcine milk oligosaccharides could provide an important selective advantage to some bacteria, thereby modulating C. difficile colonisation.
Figure 2. Estimation of the strength of the relationship between the antibody titres (anti-IgG-anti-toxinA) in serum and milk from four lactating sows, as assessed by enzymatic immunoassay method and measured by spectrophotometry. Methods in Supplementary file S2. Animals and study approval were described previously.Citation13.
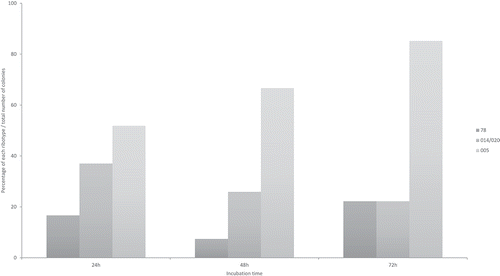
Mechanisms by which other bacteria could prevent colonisation by virulent C. difficile have not been clarified in pigs. The diversity of the gut microbiome may influence on the complete recovery from CDI or recurrent disease: patients with severe disease harbour a significantly less diverse microbiome as compared to patients with non-recurrent infections.Citation50 Therefore, a phenomenon termed “colonisation resistance”, where C. difficile is replaced by other bacteria in the developing ecosystem could thereby contribute to protection of the host from CDI.Citation51 For example, it has been shown in humans that Clostridium scindens can successfully outcompete C. difficile and prevent or ameliorate CDI.Citation52 Even more, non-pathogenic or less virulent C. difficile ribotypes (natural colonisers of neonatal piglets) may successfully outcompete the toxigenic ribotypes, which we have recently observed in co-culture, in vitro (). Similar effects have been previously demonstrated in neonatal pigsCitation53 and hamsters.Citation54 These observations indicate that colonisation with the commensal microbiota including non-toxigenic C. difficile could provide protection to CDI. However, the horizontal transfer of toxin genes between toxigenic and non-toxigenic C. difficile cannot be overruled,Citation55,Citation56 especially when using this bacterium as a probiotic.
Figure 3. Percentage distribution of three C. difficile ribotypes (078, 014/020, 005) co-incubated all together at equal concentrations in BHIS media, plated and identified from a mixed culture by PCR-ribotyping coupled with Agilent 2100 Bioanalyzer (Agilent; Santa Clara, CA-USA). Methods in Supplementary file S3.
Among the abundant commensal microorganisms, lactic acid bacteria are generally considered as beneficial due to their antagonistic properties against putative pathogens.Citation57 Despite (lactic) acid production, they can modulate the intestinal environment and host metabolism through bile salt deconjugation and dehydroxylation,Citation58 which in turn may affect the growth and activity of C. difficile. In fact, primary bile salts (e.g. taurocholate) act as germinants for C. difficile, while secondary bile salts (e.g. deoxycholate) inhibit its growth in vitro.Citation22,Citation23 Changing the activity of lactic acid bacteria could therefore change the susceptibility of the host to CDI.
Finally, the normal proteolytic activity of the developing gut microbiota could also have some advantage in suppressing CDI by contributing to the biological inactivation of the clostridial toxins TcdA and TcdB (N-terminal glucosyltransferase domain, responsible for the initiation of infection). Such mechanisms could in part explain the lack of clinical manifestation of infection in piglets, although the toxins are still detectable using immunochemical or cell culture tools.Citation15
Taken together, the above-mentioned factors point towards the importance of mother-offspring interaction early in life and that maternal nutrition may play a role in CDI in neonatal pigs.
C. difficile and diet
To date, only a few animal studies have focused directly on the influence of diet on C. difficile colonisation and susceptibility to CDI in animals. It has been reported that hamsters and mice fed either atherogenic, axenic or elemental diets demonstrate higher C. difficile and toxin concentrations in their gut and their survival rates are lower as compared to animals fed normal diets.Citation59–Citation62 The results highlight the potential to directly manipulate the susceptibility to CDI by dietary means, at least in these animal models. However, direct or indirect dietary effects on C. difficile colonisation and infection in pigs still need to be clarified.
A negative impact of high levels of SCFA and low pH on C. difficile growth and toxin production has been shown in a previous in vitro study.Citation63 A higher concentration of SCFA and low pH could inhibit or stimulate toxin production by C. difficile, in vitro.Citation25,Citation63 Therefore, this approach could offer an attractive way to control the colonisation patterns in the offspring via modulation of the mother sow diet and thereby protect against C. difficile expansion in suckling piglets.
Conclusions
C. difficile is still one of the most important emerging pathogens in clinical settings and has been shown to be relevant for pig farming. The disease is multifactorial and the repertoire of different maternal and environmental factors seems to set the conditions for C. difficile expansion and development of infection. There are fundamental knowledge gaps that define an urgent need to substantially expand research into the conditions and factors that contribute to the transmission and development of CDI. One relevant gap is a better insight into a very short “window of opportunity” for C. difficile to outgrow in the colon of piglets which is up to two weeks of age only, while beyond this the bacterium remains under current detection limits. Such early colonisation of C. difficile in neonatal piglets only up to two weeks post-partum could raise questions about when the piglet starts to contact the environmental microbiota. Next is an explanation of how healthy carriers of toxigenic C. difficile can remain free of symptoms since asymptomatic carriage of this bacterium in piglets and humans is well recognised. In addition, the zoonotic potential of C. difficile and resistant pathogens as a consequence of antibiotic treatment is of major importance for human health. Thus, the long-term goal would be to develop strategies to modulate the resistance patterns to CDI under normal conditions. Finally, the emerging presence of C. difficile in animals and foods and the need for science-based prevention strategies of serious CDI necessitates a deeper knowledge about the protective potential of diet, microbiota, immunoglobulins, mucosal immune reactions following distinct host factors that determine the course of infection in piglets and humans.
Disclosure of interest
The authors report no conflict of interest.
Supplemental Material
Download Zip (56.5 KB)Acknowledgments
The authors would like to thank Ms B. Martínez-Vallespín, PhD, Ms P. Huck, Ms M. Eitinger, Ms S. Bordessoule and Ms H. de Thomasson for their excellent assistance in the in vitro assays.
Supplementary Materials
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Songer JG. The emergence of clostridium difficile as a pathogen of food animals. Anim Heal Res Rev. [Internet] 2004;5:321–326. https://www.cambridge.org/core/article/emergence-of-clostridium-difficile-as-a-pathogen-of-food-animals/2DDA4F2A72086FB977DD9C68B361E755. doi:10.1079/AHR200492.
- Songer JG, Anderson MA. Clostridium difficile: an important pathogen of food animals. Anaerobe. [Internet] 2006;12:1–4. [accessed 2015 Feb 4]. http://www.ncbi.nlm.nih.gov/pubmed/16701605. doi:10.1016/j.anaerobe.2005.09.001.
- Indra A, Lassnig H, Baliko N, Much P, Fiedler A, Huhulescu S, Allerberger F. Clostridium difficile: a new zoonotic agent? Wien Klin Wochenschr. [Internet] 2009;121:91–95. [accessed 2014 Nov 4]. http://www.ncbi.nlm.nih.gov/pubmed/19280132. doi:10.1007/s00508-008-1127-x.
- Goorhuis A, Debast SB, van Leengoed LAMG, Harmanus C, Notermans DW, Bergwerff AA, Kuijper EJ. Clostridium difficile PCR ribotype 078: an emerging strain in humans and in pigs? J Clin Microbiol. [Internet] 2008;46:1157. author reply 1158. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2268365&tool=pmcentrez&rendertype=abstract. doi:10.1128/JCM.01536-07.
- Fawley WN, Davies KA, Morris T, Parnell P, Howe R, Wilcox MH. Enhanced surveillance of Clostridium difficile infection occurring outside hospital, England, 2011 to 2013. Eurosurveillance. [Internet] 2016;21:30295. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=22535. doi:10.2807/1560-7917.ES.2016.21.29.30295.
- McFarland LV, Surawicz CM, Rubin M, Fekety R, Elmer GW, Greenberg RN. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol. 1999. doi:10.1086/501553.
- Mullish BH, Marchesi JR, Thursz MR, Williams HRT. Microbiome manipulation with faecal microbiome transplantation as a therapeutic strategy in Clostridium difficile infection. QJM. [Internet] 2015;108:355–359. http://qjmed.oxfordjournals.org.abc.cardiff.ac.uk/content/108/5/355.full. doi:10.1093/qjmed/hcu182.
- Fareed S, Sarode N, Stewart F, Malik A, Peer JEL. 2018 undefined. Applying fecal microbiota transplantation (FMT) to treat recurrent Clostridium difficile infections (rCDI) in children. SearchProquestCom. [Internet] 2018;1–19. http://search.proquest.com/openview/e59c7c1b9bb6ea770be7faf0930fb137/1?pq-origsite=gscholar&cbl=2045935.
- Meyers S, Shih J, Neher JO, Safranek S. Clinical inquiries: how effective and safe is fecal microbial transplant in preventing C difficile recurrence? J Fam Pract. [Internet] 2018;67:386–388. http://www.ncbi.nlm.nih.gov/pubmed/29879241.
- Grześkowiak Ł, Zentek J, Vahjen W. Physical pre-treatment improves efficient DNA extraction and qPCR sensitivity from clostridium difficile spores in faecal swine specimens. Curr Microbiol. 2016;73:727–731. doi:10.1007/s00284-016-1123-8.
- Hopman NEM, Keessen EC, Harmanus C, Sanders IMJG, van Leengoed LAMG, Kuijper EJ, Lipman LJA. Acquisition of Clostridium difficile by piglets. Vet Microbiol. [Internet] 2011;149:186–192. [accessed 2014 Nov 19]. http://www.ncbi.nlm.nih.gov/pubmed/21111541. doi:10.1016/j.vetmic.2010.09.036.
- Burnham C-AD, Carroll KC. Diagnosis of Clostridium difficile infection: an ongoing conundrum for clinicians and for clinical laboratories. Clin Microbiol Rev. [Internet] 2013;26:604–630. doi:10.1128/CMR.00016-13.
- Grześkowiak Ł, Martínez-Vallespín B, Dadi TH, Radloff J, Amasheh S, Heinsen F-A, Franke A, Reinert K, Vahjen W, Zentek J, et al. Formula feeding predisposes neonatal piglets to Clostridium difficile gut infection. J Infect Dis. [Internet] 2018;217:1442–1452. http://academic.oup.com/jid/advance-article/doi/10.1093/infdis/jix567/4584511.
- Moono P, Foster NF, Hampson DJ, Knight DR, Bloomfield LE, Riley TV. Clostridium difficile infection in production animals and avian species: A review. Foodborne Pathog Dis. [Internet] 2016;XX:fpd.2016.2181. doi:10.1089/fpd.2016.2181.
- Grześkowiak Ł, Zentek J, Vahjen W. Determination of the extent of Clostridium difficile colonisation and toxin accumulation in sows and neonatal piglets. Anaerobe. [Internet] 2016;40:5–9. http://linkinghub.elsevier.com/retrieve/pii/S1075996416300397. doi:10.1016/j.anaerobe.2016.04.012.
- Squire MM, Carter GP, Mackin KE, Chakravorty A, Norén T, Elliott B, Lyras D, Riley TV. Novel molecular type of clostridium difficile in neonatal pigs, Western Australia. Emerg Infect Dis. 2013;19:790–792. doi:10.3201/eid1909.130682.
- Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev. [Internet] 2005;18:247–263. http://cmr.asm.org.ezproxy.sussex.ac.uk/cgi/content/abstract/18/2/247. doi:10.1128/CMR.18.2.247-263.2005.
- Peretz A, Tkhawkho L, Pastukh N, Brodsky D, Halevi CN, Nitzan O. Correlation between fecal calprotectin levels, disease severity and the hypervirulent ribotype 027 strain in patients with Clostridium difficile infection. BMC Infect Dis. [Internet] 2016;1–5. doi:10.1186/s12879-016-1618-8.
- Keel MK, Songer JG. The comparative pathology of Clostridium difficile-associated disease. Vet Pathol. [Internet] 2006;43:225–240. doi:10.1354/vp.43-3-225.
- Millan B, Park H, Hotte N, Mathieu O, Burguiere P, Tompkins TA, Kao D, Madsen KL. Fecal microbial transplants reduce antibiotic-resistant genes in patients with recurrent Clostridium difficile infection. Clin Infect Dis. 2016;62:1479–1486. doi:10.1093/cid/ciw185.
- Alvarez-Perez S, Blanco JL, Bouza E, Alba P, Gibert X, Maldonado J, Garcia ME. Prevalence of Clostridium difficile in diarrhoeic and non-diarrhoeic piglets. Vet Microbiol. 2009;137:302–305. doi:10.1016/j.vetmic.2009.01.015.
- Sorg JA, Sonenshein AL. Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol. [Internet] 2008;190:2505–2512. [accessed 2014 Nov 14]. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2293200&tool=pmcentrez&rendertype=abstract. doi:10.1128/JB.01765-07.
- Wheeldon LJ, Worthington T, Hilton AC, Elliott TSJ, Lambert PA. Physical and chemical factors influencing the germination of Clostridium difficile spores. J Appl Microbiol. [Internet] 2008;105:2223–2230. [accessed 2014 Nov 9]. http://www.ncbi.nlm.nih.gov/pubmed/19120667. doi:10.1111/j.1365-2672.2008.03965.x.
- Karlsson S, Burman LG, Åkerlund T. Induction of toxins in Clostridium difficile is associated with dramatic changes of its metabolism. Microbiology. 2008;154:3430–3436. doi:10.1099/mic.0.2008/019778-0.
- Karlsson S, Lindberg A, Norin E, Burman LG, Akerlund T. Toxins, butyric acid, and other short-chain fatty acids are coordinately expressed and down-regulated by cysteine in Clostridium difficile. Infect Immun. 2000;68:5881–5888. doi:10.1128/IAI.68.10.5881-5888.2000.
- Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–521. doi:10.1542/peds.2005-2824.
- Antharam VC, Li EC, Ishmael A, Sharma A, Mai V, Rand KH, Wang GP. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J Clin Microbiol. 2013;51:2884–2892. doi:10.1128/JCM.00749-13.
- Schubert AM, Rogers MAM, Ring C, Mogle J, Petrosino JP, Young VB, Aronoff DM, Schloss PD. Microbiome data distinguish patients with clostridium difficile infection and non-c. Difficile-associated diarrhea from healthy controls. MBio. 2014;5. doi:10.1128/mBio.01021-14.
- Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. [Internet] 2016;8. doi:10.1186/s13073-016-0294-z.
- Vayssier-Taussat M, Albina E, Citti C, Cosson J-F, Jacques M-A, Lebrun M-H, Le Loir Y, Ogliastro M, Petit M-A, Roumagnac P, et al. Shifting the paradigm from pathogens to pathobiome: new concepts in the light of meta-omics. Front Cell Infect Microbiol. [Internet] 2014;4:1–7. doi:10.3389/fcimb.2014.00029/abstract.
- Rodriguez-Palacios A, Borgmann S, Kline TR, LeJeune JT. Clostridium difficile in foods and animals: history and measures to reduce exposure. Anim Health Res Rev. [Internet] 2013;14:11–29. http://www.ncbi.nlm.nih.gov/pubmed/23324529. doi:10.1017/S1466252312000229.
- Grönlund M, Grzeskowiak Ł, Isolauri E, Salminen S. Influence of mother’s intestinal microbiota on gut colonization in the infant. Gut Microbes. [Internet] 2011;2:1–7. [accessed 2014 Oct 12]. http://www.landesbioscience.com/journals/30/article/16799/. doi:10.4161/gmic.2.4.16799.
- Schierack P, Filter M, Scharek L, Toelke C, Taras D, Tedin K, Haverson K, Lübke-Becker A, Wieler LH. Effects of Bacillus cereus var. toyoi on immune parameters of pregnant sows. Vet Immunol Immunopathol. [Internet] 2009;127:26–37. [accessed 2015 Jan 30]. http://www.ncbi.nlm.nih.gov/pubmed/18986709. doi:10.1016/j.vetimm.2008.09.006.
- Bian G, Ma S, Zhu Z, Su Y, Zoetendal EG, Mackie R, Liu J, Mu C, Huang R, Smidt H, et al. Age, introduction of solid feed and weaning are more important determinants of gut bacterial succession in piglets than breed and nursing mother as revealed by a reciprocal cross-fostering model. Environ Microbiol. 2016;18:1566–1577. doi:10.1111/1462-2920.13272.
- Thompson CL, Wang B, Holmes AJ. The immediate environment during postnatal development has long-term impact on gut community structure in pigs. ISME J. [Internet] 2008;2:739–748. http://www.ncbi.nlm.nih.gov/pubmed/18356821. doi:10.1038/ismej.2008.29.
- Pieper R, Janczyk P, Zeyner A, Smidt H, Guiard V, Souffrant WB. Ecophysiology of the developing total bacterial and Lactobacillus communities in the terminal small intestine of weaning piglets. Microb Ecol. 2008;56:474–483. doi:10.1007/s00248-008-9366-y.
- Frese SA, Parker K, Calvert CC, Mills DA. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome. [Internet] 2015;3:28. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4499176/. doi:10.1186/s40168-015-0091-8.
- Grześkowiak Ł, Grönlund -M-M, Beckmann C, Salminen S, von Berg A, Isolauri E. The impact of perinatal probiotic intervention on gut microbiota: double-blind placebo-controlled trials in Finland and Germany. Anaerobe. [Internet] 2012;18:7–13. [accessed 2014 Sep 4]. http://www.ncbi.nlm.nih.gov/pubmed/21979491. doi:10.1016/j.anaerobe.2012.08.007.
- Inman CF, Haverson K, Konstantinov SR, Jones PH, Harris C, Smidt H, Miller B, Bailey M, Stokes C. Rearing environment affects development of the immune system in neonates. Clin Exp Immunol. 2010;160:431–439. doi:10.1111/j.1365-2249.2010.04090.x.
- Balzola F, Bernstein C, Ho GT, Lees C. Environmentally-acquired bacteria influence microbial diversity and natural innate immune responses at gut surfaces: commentary. Inflamm Bowel Dis Monit. 2010;10:134.
- Starke IC, Pieper R, Neumann K, Zentek J, Vahjen W. Individual responses of mother sows to a probiotic Enterococcus faecium strain lead to different microbiota composition in their offspring. Benef Microbes. [Internet] 2013;4:345–356. [accessed 2014 Sep 10]. http://www.ncbi.nlm.nih.gov/pubmed/24311318. doi:10.3920/BM2013.0021.
- Scharek-Tedin L, Kreuzer-Redmer S, Twardziok SO, Siepert B, Klopfleisch R, Tedin K, Zentek J, Pieper R. Probiotic treatment decreases the number of CD14-expressing cells in porcine milk which correlates with several intestinal immune parameters in the piglets. Front Immunol. [Internet] 2015;6:1–10. doi:10.3389/fimmu.2015.00108/abstract.
- Klobasa F, Werhahn E, Butler JE. Composition of sow milk during lactation. J Anim Sci. 1987;64:1458–1466. doi:10.2527/jas1987.6451458x.
- Klobasa F, Butler JE. Absolute and relative concentrations of immunoglobulins G, M, and A, and albumin in the lacteal secretion of sows of different lactation numbers. Am J Vet Res. 1987;48:176–182.
- Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000;342:390–397. doi:10.1056/NEJM200002103420604.
- Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357:189–193. doi:10.1016/S0140-6736(00)03592-3.
- Hutton ML, Cunningham BA, Mackin KE, Lyon SA, James ML, Rood JI, Lyras D. Bovine antibodies targeting primary and recurrent Clostridium difficile disease are a potent antibiotic alternative. Sci Rep. 2017;7:5–9. doi:10.1038/s41598-017-03982-5.
- Wilcox MH, Gerding DN, Poxton IR, Kelly C, Nathan R, Birch T, Cornely OA, Rahav G, Bouza E, Lee C, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile Infection. N Engl J Med. [Internet] 2017;376:305–317. doi:10.1056/NEJMoa1602615.
- El-Hawiet A, Kitova EN, Kitov PI, Eugenio L, Ng KKS, Mulvey GL, Dingle TC, Szpacenko A, Armstrong GD, Klassen JS. Binding of Clostridium difficile toxins to human milk oligosaccharides. Glycobiology. 2011;21:1217–1227. doi:10.1093/glycob/cwr055.
- Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, Schmidt TM, Young VB. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile –associated diarrhea. J Infect Dis. [Internet] 2008;197:435–438. doi:10.1086/525047.
- Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol. [Internet] 2013;13:790–801. [accessed 2014 Sep 6]. http://www.ncbi.nlm.nih.gov/pubmed/24096337. doi:10.1038/nri3535.
- Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A, et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. [Internet] 2015;517:205–208. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4354891&tool=pmcentrez&rendertype=abstract. doi:10.1038/nature13828.
- Songer JG, Jones R, Anderson MA, Barbara AJ, Post KW, Trinh HT. Prevention of porcine Clostridium difficile-associated disease by competitive exclusion with nontoxigenic organisms. Vet Microbiol. [Internet] 2007;124:358–361. [accessed 2015 Jan 29]. http://www.ncbi.nlm.nih.gov/pubmed/17493774. doi:10.1016/j.vetmic.2007.04.019.
- Sambol SP, Merrigan MM, Tang JK, Johnson S, Gerding DN. Colonization for the prevention of Clostridium difficile disease in Hamsters. J Infect Dis. 2002;186:14–16. doi:10.1086/345676.
- Brouwer MSM, Roberts AP, Hussain H, Williams RJ, Allan E, Mullany P. Horizontal gene transfer converts non-toxigenic Clostridium difficile strains into toxin producers. Nat Commun. [Internet] 2013;4:2601. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3826655&tool=pmcentrez&rendertype=abstract. doi:10.1038/ncomms3601.
- Brouwer MSM, Mullany P, Allan E, Roberts AP. Investigating transfer of large chromosomal regions containing the pathogenicity locus between clostridium difficile strains. Methods Mol Biol. 2016;1476:215–222. doi:10.1007/978-1-4939-6361-4_16.
- Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev. 2004;28:405–440. doi:10.1016/j.femsre.2004.01.003.
- Corzo G, Gilliland SE. Bile salt hydrolase activity of three strains of Lactobacillus acidophilus. J Dairy Sci. [Internet] 1999;82:472–480. doi:10.3168/jds.S0022-0302(99)75256-2.
- Mahe S, Corthier G, Dubos F. Effect of various diets on toxin production by two strains of Clostridium difficile in gnotobiotic mice. Infect Immun. 1987;55:1801–1805.
- Frankel WL, Choi DM, Zhang W, Roth JA, Don SH, Afonso JJ, Lee FH, Klurfeld DM, Rombeau JL. Soy fiber delays disease onset and prolongs survival in experimental Clostridium difficile ileocecitis. JPEN J Parenter Enteral Nutr. 1994;18:55–61. doi:10.1177/014860719401800155.
- Blankenship-Paris TL, Walton BJ, Hayes YO, Chang J. Clostridium difficile infection in hamsters fed an atherogenic diet. Vet Pathol. 1995;32:269–273. doi:10.1177/030098589503200308.
- Iizuka M, Itou H, Konno S, Chihara J, Tobita M, Oyamada H, Toyoshima I, Sasaki K, Sato A, Horie Y, et al. Elemental diet modulates the growth of Clostridium difficile in the gut flora. Aliment Pharmacol Ther. 2004;20(Suppl 1):151–157. doi:10.1111/j.1365-2036.2004.01969.x.
- May T, Mackie RI, Fahey GC, Cremin JC, Garleb KA. Effect of fiber source on short-chain fatty acid production and on the growth and toxin production by Clostridium difficile. Scand J Gastroenterol. [Internet] 1994;29:916–922. http://www.ncbi.nlm.nih.gov/pubmed/7839098. doi:10.3109/00365529409094863.
