ABSTRACT
Alcohol-induced liver disease is closely related to translocation of bacterial products and bacteria from the intestine to the liver. However, it is not known whether bacterial translocation to the liver depends on certain intestinal microbiota changes that would predispose bacteria to translocate to the liver. In this study, we investigated the microbiota in the jejunum, ileum, cecum, feces and liver of mice subjected to chronic ethanol feeding using a Lieber DeCarli diet model of chronic ethanol feeding for 8 weeks. We demonstrate that chronic ethanol administration changes alpha diversity in the ileum and the liver and leads to compositional changes especially in the ileum. This is largely driven by an increase in gram-negative phyla – the source of endotoxins. Moreover, gram-negative Prevotella not only increased in the mucus layer of the ileum but also in liver samples. These results suggest that bacterial translocation to the liver might be associated with microbiota changes in the distal gastrointestinal tract.
Introduction
Alcohol consumption can lead to hepatic steatosis, which can progress to liver cirrhosis. Nearly 50% of cirrhosis-associated deaths are related to increased alcohol intake.Citation1 Patients with alcohol abuse and alcohol-associated liver disease have a different intestinal microbial composition compared to healthy controls.Citation2,Citation3 This alcohol-associated dysbiosis is characterized not only by bacterial overgrowth in the small intestine, that is, an increase in numbers of bacteria,Citation4,Citation5 but also by compositional changes of the bacteria in the intestinal lumen.Citation6 Microbial changes are thought to be crucial for the progression of alcohol-induced liver disease, as mice treated with nonabsorbable antibiotics had less features of liver disease.Citation7–Citation9
Translocation of bacterial products such as lipopolysaccharide (LPS) in alcohol-induced liver disease is thought to be mediated by dysfunction of the intestinal barrier: antimicrobial peptides in the mucus layer are reduced,Citation5,Citation10 the epithelial cell function is disturbed,Citation11 and intestinal inflammation is increased.Citation12 The associated liver damage is mainly caused by toll-like receptor 4-induced activation of Kupffer cells (resident liver macrophages) with subsequent release of inflammatory cytokines in the liver.Citation13,Citation14
We have recently shown that an ethanol-induced increase of bacteria in the intestinal mucus and epithelial cell layer in alcoholic liver disease is linked to increased bacterial translocation to mesenteric lymph nodes and the liver – as shown by bacterial cultures and increased 16S gene copies.Citation15 If gastric acid production in mice is impaired, liver damage has even been associated with translocation of a certain bacterial species, that is, Enterococcus faecalis.Citation16 The microbial composition in the liver was investigated in patients with cholangiocarcinomaCitation17 and hepatocellular carcinomaCitation18 but not in patients with alcoholic liver disease.
The above demonstrates that alcoholic liver disease is closely related to translocation of bacteria and bacterial products to the liver, while nothing is known about the microbial composition in the liver, and whether bacterial translocation to the liver depends on compositional microbiome changes at certain intestinal sites.
Here, we investigated changes in the microbial composition of ethanol-fed mice at different intestinal sites as well as in the liver to clarify which microbial compositions might facilitate bacterial translocation and which sites might be the source of translocating bacteria.
Results
Bacterial diversity in control- versus ethanol-fed mice
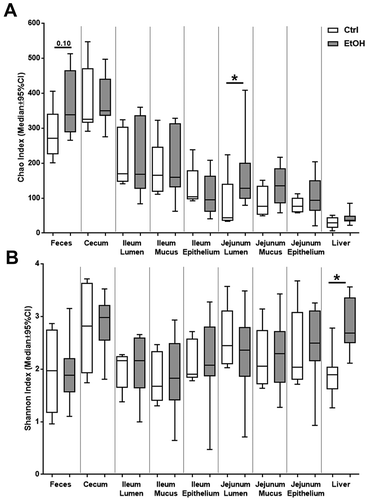
Wild-type C57BL/6 mice were fed a control- or ethanol-containing diet for 8 weeks, and microbiota was analyzed after 16S rRNA sequencing of feces and cecal contents, as well as lumen, mucus layer and epithelial cell layer of both jejunum and ileum, and from liver samples. Bacterial richness, that is, the numbers of different bacterial species within each sample, is represented by the Chao indices in . As expected, richness was highest in the feces and cecum and declined towards the oral gastrointestinal tract. Lowest values were found in the liver. A difference between ethanol feeding and controls was only present in the lumen of the jejunum, with ethanol leading to an increase in bacterial richness in the jejunal lumen. However, this difference was not present in the mucus/epithelial cell layer nor the liver.
Bacterial evenness, that is, how equal different bacterial species are distributed within each sample, is represented by the Shannon indices in . Low values for evenness correspond to bacterial communities that are dominated by only a few species. The distribution of bacterial species at most investigated sites was not significantly different between control and ethanol feeding. Evenness was increased only in the liver following chronic ethanol feeding, suggesting that chronic ethanol administration facilitates translocation of only certain bacterial species to the liver. In conjunction with the data from the Chao indices (), this indicates that ethanol leads to a more equal distribution of the few differing bacterial species that are present in the liver.
Bacterial community changes with chronic ethanol feeding
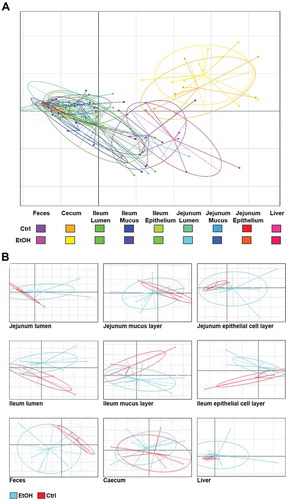
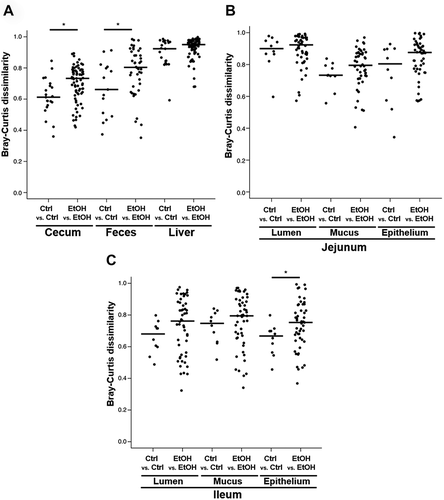
Combined principal component analysis (PCA) plots of bacterial communities are displayed in . PCA plots separated by site are displayed in . To test for the ethanol-related variability in species composition, we applied Bray–Curtis distance analyses ().Citation19,Citation20 Ethanol-related differences in bacterial community composition were detected in feces, cecum () and the epithelial cell layer of the ileum (). Using unweighted PCA with Bray–Curtis dissimilarity matrices compared by permutational multivariate analysis of variance (PERMANOVA) as a secondary approach, chronic ethanol feeding induced significant differences in the microbiota of both cecum and feces and, in addition, in microbiota from livers and luminal contents of the jejunum.
Chronic ethanol feeding leads to changes in bacterial colonization patterns
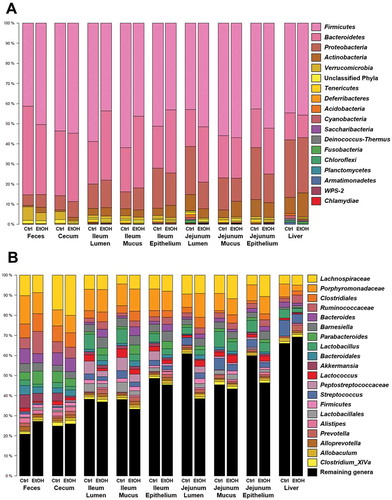
To elucidate, if above-mentioned bacterial community changes might be driven by distinct bacterial phyla, we further analyzed bacterial colonization pattern and community compositions. depicts the colonization pattern of the 20 most prevalent bacteria at the investigated sites. Ethanol-associated changes in bacterial colonization pattern were distinct in the intestine and liver at the phylum () and the genus level ().
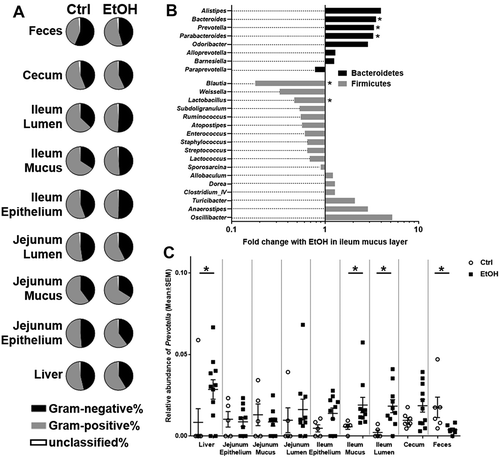
Gram-negative bacteria are the source of LPS, which is considered an endotoxin. LPS is an important mediator during experimental alcoholic liver disease, it is increased in binge-ethanol consumption and it correlates with mortality in cirrhosis.Citation7,Citation11,Citation21–Citation23 Grouping of bacteria into gram-negative and gram-positive phyla revealed that the proportion of gram-negative bacteria was reduced in feces, cecal contents, jejunal sites and the liver of ethanol-fed mice. In the ileum, ethanol rather seemed to increase the proportion of gram-negative bacteria, and this ethanol-associated change was more pronounced as the above-mentioned changes at other sites ().
Looking further into the most abundant gram-negative (Bacteroidetes) and gram-positive (Firmicutes) phyla in the mucus layer of the ileum, we found changes in several genera following chronic ethanol feeding supporting the above findings (). Relative abundance of most genera belonging to Bacteroidetes increased, while there were several genera belonging to Firmicutes that decreased following chronic ethanol feeding. Significant changes with ethanol feeding were seen for Bacteroides, Prevotella and Parabacteroides (increase), as well as for Blautia and Lactobacillus (decrease). Next, we were interested if any of those genera can be found in liver samples as well and if ethanol-induced changes would be similar. And indeed, we saw an increase in Prevotella in the livers of ethanol-fed mice (), suggesting that the relative increase of Prevotella in the mucus layer of the ileum is associated with bacterial translocation to the liver.
Discussion
We have previously shown that ethanol impairs the expression of intestinal antimicrobial proteins, which induces a quantitative increase of bacteria in the mucus and epithelial cell layer.Citation15 This was linked to increased bacterial translocation to mesenteric lymph nodes and the liver, and to increased ethanol-induced liver disease in mice.Citation15 Conversely, transgenic overexpression of regenerating islet derived (REG) 3g in intestinal epithelial cells reduced ethanol-associated bacterial overgrowth in the mucus and epithelial cell layer, and decreased bacterial translocation and ethanol-induced liver disease.Citation15 This study investigated ethanol-associated compositional microbiome changes along the intestinal tract and in the liver of mice. Ethanol increased bacterial richness in the jejunum and changed bacterial communities in the ileum by inducing a shift towards gram-negative bacteria. The relative abundance of gram-negative Prevotella was increased in the mucus layer of the ileum, and Prevotella was also detected in the liver, suggesting that bacterial translocation possibly occurs from the distal gastrointestinal tract in ethanol-induced liver disease.
Several studies have investigated ethanol-induced microbiome changes in rodent cecal, fecal, ileal and colon samples.Citation3,Citation22 A recent publication assessed ethanol-induced changes of the fecal microbiome and metabolome in mice.Citation24 In line with our findings, alpha diversity measures were similar and community clustering differed in control- versus ethanol-fed mouse feces. In a study with rats, ethanol reduced abundance of Lactobacillus and increased Bacteroides and Parabacteroides, which was also observed in our study.Citation25 In human stool samples, patients with high intestinal permeability and ethanol consumption had increased Blautia and Dorea and less Ruminococcus and Clostridia.Citation2 In contrast, we saw less Blautia in the ileum, while the other genera were unchanged not only in the ileum but also in feces (data not shown). However, neither the animal nor the human studies have investigated the ethanol-associated microbiota changes in the lumen, mucus layer and epithelial cell layer at different intestinal sites.
As one major finding we saw an increase of Prevotella in the mucus layer of the ileum and in the liver. The available literature on Prevotella in liver disease shows inconsistent results: In duodenal aspirates of patients with ethanol-related cirrhosis, Prevotella was decreased compared to patients with hepatitis C virus-related cirrhosis.Citation26 With respect to chronic ethanol consumption, Prevotella was also decreased in stool samples of cirrhotics but not in alcohol-dependent subjects without cirrhosis.Citation27 In contrast, ethanol consumption increased Prevotella in the oral cavity.Citation28 In addition, Prevotella was increased in patients with cirrhosis (for various etiologies) versus controls in saliva and stool samples.Citation29 Prevotella was also increased in duodenal mucosa samples of patients with hepatitis B-cirrhosis.Citation30 The varying results may be explained by high genetic diversity of Prevotella species.Citation31
Prevotella are gram-negative, obligate anaerobes.Citation32 LPS released from Prevotella could enhance ethanol-induced liver disease as LPS is an important mediator of alcoholic liver disease.Citation23,Citation33 Increased levels of Prevotella copri (detected in human stool samples) correlated with endotoxin levels and insulin resistance in type 2 diabetes.Citation34,Citation35 Because Prevotella are abundant in the human gingival grove, their role in the development of periodontitis has been extensively studied, and some of the disease-promoting effects might also play a role for liver disease.Citation32 Prevotella intermedia was found to be capable to invade oral epithelial cells, as it secretes exopolysaccharides to form biofilms and is a producer of several proteases.Citation36–Citation39 In addition, P. intermedia induced the secretion of tumor necrosis factor-alpha (TNF) from human monocyte-derived THP-1 macrophages.Citation40 Other species, P. gingivalis and P. nigrescens, induced arthritic bone erosions by toll-like receptor-2- and interleukin (IL)-1-dependent Th17-cell activation in mice.Citation41 IL-1β mediates ethanol-induced liver disease in mice.Citation42
In the gut, mucosal invasion of Prevotella was detected in Crohn’s disease.Citation43 Moreover, a proinflammatory microbiome containing Prevotella induced spontaneous colitis in mice by intestinal induction of CC-chemokine ligand 5 (CCL5).Citation44 CCL5 expression in the liver is increased in ethanol-induced liver disease.Citation16,Citation45,Citation46 It induces T-cell mediated inflammation,Citation47 and just recently inhibition of CCL5 signaling by a CCR2/5 inhibitor prevented and reversed ethanol-induced liver injury in mice.Citation45
The above shows that besides the release of LPS, which is known to enhance ethanol-induced liver disease, Prevotella-induced release of either TNF, IL-1β or CCL5 might trigger ethanol-induced liver injury. However, it is a limitation of our study that we can only speculate about a functional link between Prevotella and ethanol-induced liver disease, as well as bacterial translocation from the ileum to the liver. Further studies are required to determine whether Prevotella promotes experimental ethanol-induced liver disease, and if so, which virulence factors mediate a liver disease-promoting effect. In addition, functional studies with labeled bacteria to enable tracking of bacterial translocation might elucidate, if viable Prevotella actually translocate from the ileum to the liver.
To the best of our knowledge, we present the first comprehensive microbiome analysis from samples of the entire intestinal tract and the liver of ethanol-fed mice. Microbial differences between the investigated intestinal sites are important to consider for future studies. In particular, human studies might have to include mucosal biopsies from the ileum.
Materials and methods
Mice
The analysis was done with tissues obtained during a recently published mouse study by Wang et al.Citation15 Female wild-type littermate mice (C57BL/6 background) were used. Mice were housed in pairs and received a liquid ethanol-containing Lieber DeCarli diet to mimic chronic alcohol feeding for 8 weeks.Citation15 Pair-fed control mice received a similar diet with ethanol being replaced by isocaloric amounts of dextrose. All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of the University of California, San Diego.
DNA extraction
16S rRNA sequencing was done from DNA isolated from fecal pellets, cecal content, lumen (i.e., content), mucus layer and epithelial cell layer of the jejunum and ileum, as well as from the liver. Numbers of biological replicates are shown in . The different layers from jejunum and ileum were obtained as described.Citation15 In brief, luminal contents from 2-cm pieces of intestine were obtained from flushing the intestinal pieces with 1 ml of phosphate-buffered saline (PBS); mucus layer was obtained by subsequent cutting of the intestine and vigorous washing with 1 ml of PBS; the epithelial cell layer was analyzed from the remaining tissue. DNA extraction was done as described previouslyCitation:48 the materials were homogenized in 1 ml sterile PBS using lysing matrix C tubes (MP116912, MP Biomedicals, Santa Ana, CA) and a Mini-BeadBeater-96 (GlenMills, Clifton, NJ). This was followed by digestion with proteinase K (Am2546, Thermo Fisher Scientific, Waltham, MA), RNAse A (19101, Qiagen, Valencia, CA) and 10% sodium dodecyl sulfate (SDS; L3771, Sigma-Aldrich, St. Louis, MO) at 55°C for 1 h. Suspensions were transferred to lysing matrix B tubes (MP116911, MP Biomedicals, Santa Ana, CA) and homogenized after addition of phenol (15513, Thermo Fisher Scientific, Waltham, MA). Using phenol/chloroform/isoamyl alcohol (15593, Thermo Fisher Scientific, Waltham, MA), the lysate was extracted three times followed by one extraction with chloroform and sodium acetate buffer solution (S7899, Sigma-Aldrich, St. Louis, MO). DNA was finally precipitated and washed using ethanol and resuspended in sterile water (BP561, Thermo Fisher Scientific, Waltham, MA).
Table 1. Numbers of biological replicates.
16S rRNA profiling
The extracted DNA was amplified using primers that target the V4 region of the 16S rRNA gene.Citation49 These primers included the i5 and i7 adaptor sequences for dual index Illumina MiSeq pyrosequencing as well as unique 8 bp indices incorporated onto both primers such that each sample receives its own unique barcode pair. Using approximately 100 ng of extracted DNA, the amplicons were generated with Platinum Taq polymerase (Life Technologies, Carlsbad, CA). Amplicons were purified using the QIAquick PCR purification kit (Qiagen, Valencia, CA), quantified using Tecan fluorometric methods (Tecan Group, Maennedorf, Switzerland), normalized and finally pooled in preparation for Illumina MiSeq sequencing using the dual index V2 chemistry 2 × 250 bp format (Illumina, San Diego, CA) following the manufacturer’s protocol.
Statistical analyses
Analyses and data plots from OTU counts were done with R, version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism 7.04 (GraphPad Software, Inc., La Jolla, CA), respectively. Differences between groups were assessed by Mann-Whitney or Wilcoxon rank-sum test. The permutational multivariate analysis of variance (PERMANOVA) calculations were performed using the VEGAN package for R. Significant differences are marked with asterisk if p < 0.05.
Abbreviations
| CCL5 | = | CC-chemokine ligand 5 |
| Ctrl | = | Control |
| EtOH | = | Ethanol feeding |
| IL | = | Interleukin |
| LPS | = | Lipopolysaccharide |
| PERMANOVA | = | Permutational multivariate analysis of variance |
| TNF | = | Tumor necrosis factor alpha |
Accession Numbers
Sequence data were registered at NCBI under BioProject PRJNA512118. Sequence reads are available at NCBI under the following consecutive BioSample IDs (SAMN10661899–SAMN10662037).
Disclosure of interest
The authors report no conflict of interest.
Author Contributions
S.B. wrote this manuscript, analyzed data and generated figures and tables. L.W. provided mice and specialized knowledge. M.T., C.K., K.M., H.S. and D.E.F. performed microbiota sequencing and analyses and generated figures. H.S. performed PERMANOVA analyses. D.E.F. also supervised the microbiome sequencing, performed microbiome data submission, wrote text and edited the manuscript. B.S. supervised the study and edited the manuscript. All authors approved the final version of this manuscript.
Additional information
Funding
References
- Rehm J, Shield KD. Global alcohol-attributable deaths from cancer, liver cirrhosis, and injury in 2010. Alcohol Res. 2013;35:174–183.
- Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Starkel P, Windey K, Tremaroli V, Backhed F, Verbeke K, et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014;111:E4485–93. doi:10.1073/pnas.1415174111.
- Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302(9):G966–78. doi:10.1152/ajpgi.00380.2011.
- Bode JC, Bode C, Heidelbach R, Durr HK, Martini GA. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology. 1984;31(1):30–34.
- Yan AW, Fouts DE, Brandl J, Starkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53(1):96–105. doi:10.1002/hep.24018.
- Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, Gobejishvili L, Joshi-Barve S, Ayvaz T, Petrosino J, et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One. 2013;8(1):e53028. doi:10.1371/journal.pone.0053028.
- Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108(1):218–224.
- Chen P, Starkel P, Turner JR, Ho SB, Schnabl B. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology. 2015;61:883–894. doi:10.1002/hep.27489.
- Llopis M, Cassard AM, Wrzosek L, Boschat L, Bruneau A, Ferrere G, Puchois V, Martin JC, Lepage P, Le Roy T, et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut. 2016;65:830–839. doi:10.1136/gutjnl-2015-310585.
- Wang Y, Kirpich I, Liu Y, Ma Z, Barve S, McClain CJ, Feng W. Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. Am J Pathol. 2011;179:2866–2875. doi:10.1016/j.ajpath.2011.08.039.
- Bluemel S, Williams B, Knight R, Schnabl B. Precision medicine in alcoholic and nonalcoholic fatty liver disease via modulating the gut microbiota. Am J Physiol Gastrointest Liver Physiol. 2016;311:G1018–G36. doi:10.1152/ajpgi.00245.2016.
- Hendrikx T, Duan Y, Wang Y, Oh JH, Alexander LM, Huang W, Starkel P, Ho SB, Gao B, Fiehn O, et al. Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut. 2018. doi:10.1136/gutjnl-2018-317232.
- Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. 1994;20:453–460.
- Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi:10.1038/nm1663.
- Wang L, Fouts DE, Starkel P, Hartmann P, Chen P, Llorente C, DePew J, Moncera K, Ho SB, Brenner DA, et al. Intestinal REG3 lectins protect against alcoholic steatohepatitis by reducing mucosa-associated microbiota and preventing bacterial translocation. Cell Host Microbe. 2016;19:227–239. doi:10.1016/j.chom.2016.01.003.
- Llorente C, Jepsen P, Inamine T, Wang L, Bluemel S, Wang HJ, Loomba R, Bajaj JS, Schubert ML, Sikaroodi M, et al. Gastric acid suppression promotes alcoholic liver disease by inducing overgrowth of intestinal enterococcus. Nat Commun. 2017;8:837. doi:10.1038/s41467-017-00796-x.
- Chng KR, Chan SH, Ng AHQ, Li C, Jusakul A, Bertrand D, Wilm A, Choo SP, Tan DMY, Lim KH, et al. Tissue microbiome profiling identifies an enrichment of specific enteric bacteria in opisthorchis viverrini associated cholangiocarcinoma. EBioMedicine. 2016;8:195–202. doi:10.1016/j.ebiom.2016.04.034.
- Asmaa Ezzat MNMI, Irni Suhayu S, Zeinat K. Abd ElHady AE, Ekram H, Mahmoud EH. Metagenomic study of the liver microbiota in liver cancer - Metagenomic and metatranscriptomic analyses of the hepatocellular carcinoma-associated microbial communities and the potential role of microbial communities in liver cancer. J Gastroint Dig Syst. 2014;4:228. doi:10.4172/2161-069X.1000228.
- Anderson MJ, Ellingsen KE, McArdle BH. Multivariate dispersion as a measure of beta diversity. Ecol Lett. 2006;9:683–693. doi:10.1111/j.1461-0248.2006.00926.x.
- Bray JR, Curtis JT. An ordination of the upland forest communities of Southern Wisconsin. Ecol Monogr. 1957;27:326–349. doi:10.2307/1942268.
- Hartmann P, Chen P, Wang HJ, Wang L, McCole DF, Brandl K, Starkel P, Belzer C, Hellerbrand C, Tsukamoto H, et al. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology. 2013;58:108–119. doi:10.1002/hep.26321.
- Hartmann P, Seebauer CT, Schnabl B. Alcoholic liver disease: the gut microbiome and liver cross talk. Alcohol Clin Exp Res. 2015;39:763–775. doi:10.1111/acer.12704.
- Inokuchi S, Tsukamoto H, Park E, Liu ZX, Brenner DA, Seki E. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin Exp Res. 2011;35:1509–1518. doi:10.1111/j.1530-0277.2011.01487.x.
- Wang G, Liu Q, Guo L, Zeng H, Ding C, Zhang W, Xu D, Wang X, Qiu J, Dong Q, et al. Gut microbiota and relevant metabolites analysis in alcohol dependent mice. Front Microbiol. 2018;9:1874. doi:10.3389/fmicb.2018.01874.
- Kosnicki KL, Penprase JC, Cintora P, Torres PJ, Harris GL, Brasser SM, Kelley ST. Effects of moderate, voluntary ethanol consumption on the rat and human gut microbiome. Addict Biol. 2018. doi:10.1111/adb.12626.
- Jacobs JP, Dong TS, Agopian V, Lagishetty V, Sundaram V, Noureddin M, Ayoub WS, Durazo F, Benhammou J, Enayati P, et al. Microbiome and bile acid profiles in duodenal aspirates from patients with liver cirrhosis: the microbiome, microbial markers and liver disease study. Hepatol Res. 2018. doi:10.1111/hepr.13207.
- Dubinkina VB, Tyakht AV, Odintsova VY, Yarygin KS, Kovarsky BA, Pavlenko AV, Ischenko DS, Popenko AS, Alexeev DG, Taraskina AY, et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome. 2017;5:141. doi:10.1186/s40168-017-0359-2.
- Fan X, Peters BA, Jacobs EJ, Gapstur SM, Purdue MP, Freedman ND, Alekseyenko AV, Wu J, Yang L, Pei Z, et al. Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Microbiome. 2018;6:59. doi:10.1186/s40168-018-0432-5.
- Bajaj JS, Betrapally NS, Hylemon PB, Heuman DM, Daita K, White MB, Unser A, Thacker LR, Sanyal AJ, Kang DJ, et al. Salivary microbiota reflects changes in gut microbiota in cirrhosis with hepatic encephalopathy. Hepatology. 2015;62:1260–1271. doi:10.1002/hep.27819.
- Chen Y, Ji F, Guo J, Shi D, Fang D, Li L. Dysbiosis of small intestinal microbiota in liver cirrhosis and its association with etiology. Sci Rep. 2016;6:34055. doi:10.1038/srep34055.
- Larsen JM. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology. 2017;151:363–374. doi:10.1111/imm.2017.151.issue-4.
- Chapter 4 - Subgingival microbes. In: ZhouX, Li Y, editors. Atlas of oral microbiology. Academic Press; 2015. p. 67–93, ISBN 9780128022344. doi:10.1016/B978-0-12-802234-4.00004-5.
- Brandl K, Schnabl B. Is intestinal inflammation linking dysbiosis to gut barrier dysfunction during liver disease? Expert Rev Gastroenterol Hepatol. 2015;9:1069–1076. doi:10.1586/17474124.2015.1057122.
- Leite AZ, Rodrigues NC, Gonzaga MI, Paiolo JCC, de Souza CA, Stefanutto NAV, Omori WP, Pinheiro DG, Brisotti JL, Matheucci Junior E, et al. Detection of increased plasma interleukin-6 levels and prevalence of Prevotella copri and bacteroides vulgatus in the feces of type 2 diabetes patients. Front Immunol. 2017;8:1107. doi:10.3389/fimmu.2017.01107.
- Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E, Falony G, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376–381. doi:10.1038/nature18646.
- Dorn BR, Leung KL, Progulske-Fox A. Invasion of human oral epithelial cells by Prevotella intermedia. Infect Immun. 1998;66:6054–6057.
- Jansen HJ, Grenier D, Van der Hoeven JS. Characterization of immunoglobulin G-degrading proteases of Prevotella intermedia and Prevotella nigrescens. Oral Microbiol Immunol. 1995;10:138–145.
- Shibata Y, Fujimura S, Nakamura T. Purification and partial characterization of an elastolytic serine protease of Prevotella intermedia. Appl Environ Microbiol. 1993;59:2107–2111.
- Yamanaka T, Furukawa T, Matsumoto-Mashimo C, Yamane K, Sugimori C, Nambu T, Mori N, Nishikawa H, Walker CB, Leung KP, et al. Gene expression profile and pathogenicity of biofilm-forming Prevotella intermedia strain 17. BMC Microbiol. 2009;9:11. doi:10.1186/1471-2180-9-11.
- Kim SJ, Choi EY, Kim EG, Shin SH, Lee JY, Choi JI, Choi IS. Prevotella intermedia lipopolysaccharide stimulates release of tumor necrosis factor-alpha through mitogen-activated protein kinase signaling pathways in monocyte-derived macrophages. FEMS Immunol Med Microbiol. 2007;51:407–413. doi:10.1111/j.1574-695X.2007.00318.x.
- de Aquino SG, Abdollahi-Roodsaz S, Koenders MI, van de Loo FA, Pruijn GJ, Marijnissen RJ, Walgreen B, Helsen MM, van Den Bersselaar LA, de Molon RS, et al. Periodontal pathogens directly promote autoimmune experimental arthritis by inducing a TLR2- and IL-1-driven Th17 response. J Immunol. 2014;192:4103–4111. doi:10.4049/jimmunol.1301970.
- Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, Barrieau M, Min SY, Kurt-Jones EA, Szabo G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476–3489. doi:10.1172/JCI60777.
- Chiodini RJ, Dowd SE, Galandiuk S, Davis B, Glassing A. The predominant site of bacterial translocation across the intestinal mucosal barrier occurs at the advancing disease margin in Crohn’s disease. Microbiology. 2016;162:1608–1619. doi:10.1099/mic.0.000336.
- Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi:10.1016/j.cell.2011.04.022.
- Ambade A, Lowe P, Kodys K, Catalano D, Gyongyosi B, Cho Y, Iracheta Vellve A, Adejumo A, Saha B, Calenda C, et al. Pharmacological inhibition of CCR2/5 signaling prevents and reverses alcohol-induced liver damage, steatosis and inflammation in mice. Hepatology. 2019 Mar;69(3):1105–1121. doi: 10.1002/hep.30249.
- Maltby J, Wright S, Bird G, Sheron N. Chemokine levels in human liver homogenates: associations between GRO alpha and histopathological evidence of alcoholic hepatitis. Hepatology. 1996;24:1156–1160. doi:10.1053/jhep.1996.v24.pm0008903391.
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi:10.1016/j.it.2004.09.015.
- Bluemel S, Wang L, Martino C, Lee S, Wang Y, Williams B, Horvath A, Stadlbauer V, Zengler K, Schnabl B. The role of intestinal C-type regenerating islet derived-3 lectins for nonalcoholic steatohepatitis. Hepatol Commun. 2018;2:393–406. doi:10.1002/hep4.1165.
- Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. 2013;79:5112–5120. doi:10.1128/AEM.01043-13.