ABSTRACT
We recently reported an increased colon cancer risk associated with oral antibiotic use in a large United Kingdom population. This association between antibiotic exposure and cancer risk adds to a growing body of evidence that antibiotic use has unintended off-target long-term health consequences. This addendum highlights major studies linking antibiotic use and chronic disease in pediatric and adult populations. Microbiota dysbiosis is the key proposed mechanism underlying antibiotic:disease associations, resulting in alterations in gene expression, epigenetic modification, colonization by pathogenic bacteria, instigation of biofilms, and immune regulation and inflammation. These adverse outcomes of antibiotic exposure underscore the need for diagnostic and antibiotic stewardship, as well as the urgency for further development of non-antibiotic therapies for bacterial infections.
Antibiotic use and overuse
Antibiotic overuse is a pressing public health problem worldwide. An estimated 70 billion antibiotic doses are consumed annually.Citation1 Since the beginning of the 21st century, global antibiotic consumption has continued to rise, with an overall 65% increase in defined daily doses of antibiotics. This trend is largely driven by increased antibiotic use in low- and middle-income countries, which consume fewer antibiotics per person than high-income countries, but account for the majority of antibiotic doses given (24.5 billion defined daily doses (DDDs) out of a total 34.8 billion worldwide).Citation2 In the United States, 270 million antibiotic prescriptions are generated annually,Citation3 with more prescriptions given to older adults >65 years of age as compared to children and adolescents.Citation3 Though the overall number of prescriptions has not changed between 2000 and 2010, a significant increase in prescriptions of broad-spectrum antibiotics has been reported across all ages.Citation4 The most recent published data indicate that, remarkably, there are 836 antibiotic prescriptions per 1000 persons annually – nearly one per person each year–in the United States, and 606 per 1000 persons annually in the United Kingdom. Respiratory tract infections are the most common diagnosis associated with outpatient oral antibiotic prescriptions, accounting for >50% of prescriptions, followed by urinary tract infections.Citation5 Notably, studies estimate that 23-30% of antibiotic prescriptions are inappropriate.Citation6,Citation7 In both the US and UK and indeed globally, penicillins are the most commonly prescribed antibiotic class, followed by macrolides.Citation2,Citation3,Citation5
Oral antibiotic use is associated with increased colon cancer risk
Given this widespread use of antibiotics, investigating its association with health outcomes has key implications for public health. Several studies have shown that use of antibiotics, even narrow spectrum antibiotics, exert strong, persistent effects on the structure and composition of the gut microbiota. The gut microbiota is known to interact with the host immune system and increasingly associated with changes in human physiology that potentially drive, in part, disease development in multiple systems. Murine models and human studies from our laboratory have established the association of colon biofilms, assemblages of mucus-invasive bacteria, with pro-carcinogenesis, particularly in the proximal colon.Citation8–Citation11
Under the working hypothesis that antibiotics disrupt gut microbiota and result in mucosal inflammation that contributes to cancer formation, we conducted a matched case-control study on antibiotic-cancer association in the United Kingdom Clinical Practice Research Datalink (CPRD), the world’s largest electronic medical record database with population-based primary care data collected prospectively.Citation12 A total of 166,057 participants were analyzed, making it the largest cohort analysis to date of the relationship of oral antibiotic use with CRC. With a median follow-up of 8 years, 7 out of 10 participants received oral antibiotics, with most prescribed more than one type of antibiotic over time. After controlling for confounding factors, a positive association of antibiotic exposure and colon cancer risk was observed, with effects significantly increased for even minimal antibiotic use and in a dose-response pattern (1–15 days vs no use, AORs: 1.08, 95% CI [1.04–1.13]; 16–30 days vs no use, AORs: 1.14, 95% CI [1.08–1.20]; 31–60 days vs no use, AORs 1.15, 95% CI [1.09–1.22]; >60 days vs no use, AORs: 1.17, 95% CI [1.10–1.23], ptrend<0. 001). Notably, the effect of antibiotic use on colon cancer risk was only observed in the proximal colon, which is the site first exposed to antibiotics not absorbed in the small intestine and likely prior to possible drug modification or degradation in the colon. Antibiotics that kill anaerobic bacteria appeared to drive the risk, particularly penicillins (AOR 1.09, 95% CI [1.05–1.13]), but not cephalosporins, quinolones, macrolides, or sulfamethoxazole/trimethoprim. A particularly convincing aspect of the analysis was that the association of increased risk for colon cancer after antibiotic exposure was linked to antibiotic use more than 10 years before the cancer diagnosis (AOR, 1.17, 95% CI [1.06–1.31]). This result suggested a long term impact of antibiotics on the gut microbiota and aligned with the usual slow growth of colon tumors. Namely, it is estimated that the time from tumor initiation to the visualization of a mucosal polyp is, most often, at least 10 years.
In contrast and also of interest in the field of gastrointestinal tract neoplasia, we observed that antibiotic exposure, particularly to tetracyclines, was associated with decreased rectal cancer risk but only after 60 days of antibiotic exposure (AOR, 0.85, 95% CI [0.79–0.93], p = .003). The differences observed in the associations between antibiotic exposure and colon versus rectal cancer are at least consistent with the differing biology of these two types of cancer. Our work supports, but does not prove, the idea that there is a potential causal link between disrupted microbiota and CRC.
Four additional studies have also suggested a positive antibiotic-colon cancer association in North American, European, and Asian populations ().Citation13–Citation15 The Harvard Nurses’ Health Study additionally showed that increased risk of colon polyps (precancerous lesions) after age 60 is correlated with antibiotic use in early-to-middle adulthood (at ages 20–39 and 40–59).Citation16 This effect was not seen for antibiotic use within the last 4 years, suggesting a latent period between antibiotic exposure and the development of adenomas. Several epidemiologic studies have examined the possible impact of antibiotic exposure on other cancer types, such as lung, breast, prostate, liver, and skin cancers, yet these results are conflicting.Citation12,Citation14,Citation15,Citation17,Citation18 However, most studies failed to show a dose-response pattern by antibiotic exposure levels, temporal trends, or class-specific effects, likely suggesting a non-causal association for those cancer types or reflecting an insufficient sample size or database to address these critical points that would strengthen the analyses.
Table 1. Previous studies on antibiotic-cancer association.
Chronic diseases associated with antibiotic exposure
In addition to its link to colon cancer, antibiotic use has been associated with several chronic diseases, including allergic, autoimmune, metabolic, and psychiatric diseases (). Animal models suggest an even larger number of chronic disease associations than those observed clinically to date. Numerous studies have identified links between perinatal or early childhood antibiotic use and the development of allergic, autoimmune, and metabolic diseases during childhood. Other studies have found associations with antibiotic use and chronic disease in adults but lack historical data on earlier exposures in childhood. More longitudinal studies are needed to identify associations with significant latency between antibiotic exposure and disease expression and outcome. Such studies are most easily accomplished with large, longitudinal patient databases in countries with national healthcare infrastructures, as less comprehensive data (and often only cross-sectional data) are available in places like the United States. Common themes arising from these studies are that antibiotic:disease associations are often strongest with young age of antibiotic exposure and tend to be dose-dependent.
Table 2. Associations of Antibiotic Exposure and Chronic Disease: Selected Studies.
Disrupted microbiota: key mechanisms linking antibiotics and disease outcomes
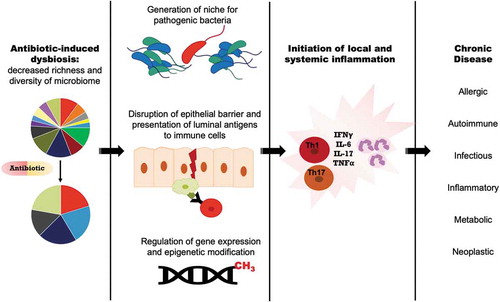
Microbiota dysbiosis or alteration is the central mechanism by which antibiotics are thought to contribute to the development of chronic disease. Antibiotic exposure disrupts the gut microbiome by eradicating taxa and reducing overall diversity.Citation31,Citation32 Healthcare providers often counsel patients that antibiotics will temporarily alter their gut microbiome; however, it is now becoming clear that even brief exposure to antibiotics can lead to long-lasting alterations in the microbiome of the human gut.Citation33,Citation34 Studies have shown that a single short course of antibiotics can drastically alter an individual’s gastrointestinal flora, leading to changes in the composition, diversity, metabolic function, and resistance gene expression of the microbiome.Citation35,Citation36 Dethelfsen et al. found that 5 days of ciprofloxacin, a narrow spectrum antibiotic, was sufficient to reduce the richness and diversity of the distal colon microbiome, but these perturbations resolved by 4 weeks post-treatment.Citation33 However, repeated exposure with a second course of ciprofloxacin led to persistent alterations in distal gut microbiome composition.Citation34 Additionally, the magnitude of microbiome perturbation differed across patients, indicating some degree of variability that may put individual patients at lesser or greater risk of subsequent long-term consequences of antibiotic exposure. In another study, patients received a single 7-day course of oral clindamycin, dramatically reducing the diversity of Bacteroides isolates (as assessed by repetitive sequence-based PCR), consistent with the anti-anaerobic spectrum of clindamycin. This Bacteroides population shift persisted during 2 years of follow-up, suggesting that even a single antibiotic course can induce profound long-lasting alterations in specific subpopulations of the microbiota.Citation37 Furthermore, the different patterns of long-term microbiota impact for ciprofloxacin vs clindamycin suggest that the effects from antibiotic use are dose and drug-dependent.Citation37 Consistently, in our CPRD cohort, we observed that colon cancer risk increased markedly with minimal antibiotic use (1–15 cumulative days) and was most significant after oral exposure to anti-anaerobic agents, particularly penicillins. These observations coincide with the fact that the gut microbiota is predominately composed of anaerobes and that anti-anaerobic agents markedly disrupt the microbiota organization and structure in the colon. There are, however, many gaps in our knowledge about the impact of specific antibiotics, duration of therapy and dosage on the gut microbiota as well as microbiota at other body sites. Similarly, we do not understand the impact of intravenous antibiotics on the human microbiota.
Gut dysbiosis triggered by antibiotics can likely lead to complex downstream effects on gut barrier function, gene expression and epigenetic modifications, and available niches for pathogenic bacteria and biofilms, ultimately altering local and systemic immune responses (). Exposure to antibiotics during critical developmental periods appears to have profound long-term effects. In humans, prenatal exposure to antibiotics is associated with aberrant DNA methylation, and these epigenetic changes correlate with alterations in metabolism and growth, specifically low birth weight.Citation38 Low birth weight, in turn, is associated with adulthood obesity, cardiovascular disease, Type II diabetes, and malignancy, suggesting that in utero antibiotic exposure confers long-term risk for chronic disease. Indeed, the gut microbiota is critical to postnatal epigenetic modification of the colonic epithelium through DNA methylation, with long term consequences for barrier and immune function.Citation39 Prenatal antibiotic exposure has also been shown to alter gene expression in the developing gut in an animal model, and to correlate with dysfunctional barrier function, leading to the exposure of the mucosal immune system to luminal antigens.Citation40 Gut barrier dysfunction and host exposure to new antigens is a common proposed mechanism for many antibiotic disease associations. In mice treated with antibiotics, commensal bacteria translocate across the colonic epithelium into mesenteric lymph nodes via goblet cell-associated antigen passage.Citation41,Citation42 In a colitis model, this phenomenon was associated with enhanced inflammatory responses. Enhanced inflammation was only observed in mice that received certain types of antibiotics (e.g. ampicillin, but not tetracycline) that altered barrier function and allowed live commensal organisms to translocate.Citation41 In addition to disrupting barrier function, another potential consequence of dysbiosis is development of a gut mucosal or luminal niche for pathogenic bacteria or microbial communities. This has been well-described after antibiotic treatment where colonization with enteric pathogens such as Clostridioides difficile and Salmonella typhimurium may occur.Citation32
Though gut microbial dysbiosis has been reported in oral, lung, breast and liver cancers, as yet, no functional experiments have causally linked the state of dysbiosis to carcinogenesis in these organs.Citation43 In contrast, microbial dysbiosis has been extensively studied in CRC, and preclinical models have demonstrated clear functional consequences.Citation44 Along the digestive tract from mouth to anus, the colon harbors the highest density of microorganisms.Citation45 Thus, the colon is likely the most affected site from oral antibiotic exposure. Antibiotic use can disrupt the commensal microbiota and facilitate invasion of tumorigenic microbes. Several studies show that CRC tissues are enriched for polymicrobial biofilms, particularly on proximal colon tumors.Citation8,Citation10,Citation11 This observation correlates with our CPRD cohort findings in which antibiotic exposure was associated with increased risk of proximal/right colon cancer. While the exact mechanism of differential antibiotic-cancer association by anatomic location is unknown, we hypothesize that putative carcinogenic bacteria, such as Fusobacterium, Porphyromonas, Enterococcaceae, and Bacteroides–Prevotella, as well as toxin-producing species including some B. fragilis and E. coli, may be differentially distributed along the colorectal tract. We hypothesize that depletion of the protective commensal microbiota by antibiotics facilitates pathogenic bacteria invasion into the colon mucus and mucosa. Another possible hypothesis is that colon epithelial cells with differing function along the colonic axis display differing regional sensitivity to dysbiosis. Furthermore, we and others have linked the acquisition of carcinogenic microbes or development of colon mucosal biofilms with chronic mucosal inflammation and subsequent tumor formation.Citation8,Citation9,Citation46,Citation47
Strategies for reducing antibiotic use
As evidence mounts that antibiotic use can have varied off-target and long-lasting negative consequences, there is increasing urgency for physicians and other health care providers to be better antibiotic stewards. The studies reviewed herein have focused on outpatient oral antibiotic prescriptions, which comprise the majority of antibiotic use. Yet, outpatient antibiotic stewardship programs are in their infancy compared to more robust and effective inpatient programs. Studies show that inpatient antibiotic stewardship programs are effective at reducing the number and duration of antibiotic prescriptions; however, without paired outpatient stewardship programs in place, these gains can be undone. A large study of fluoroquinolone stewardship at 48 Michigan hospitals demonstrated that inpatient stewardship efforts resulted in an 11% decrease in the number of patients receiving fluoroquinolones, and 814 fewer treatment days per 1000 patients.Citation48 This was driven by decreased administration of fluoroquinolones during inpatient hospitalization. However, 2/3 of fluoroquinolone treatment days occurred in the outpatient setting after hospital discharge, and in hospitals with inpatient stewardship programs, there was no change in the number of discharge prescriptions for fluoroquinolones. When compared to hospitals without inpatient stewardship programs, those with such programs had twice as many post-discharge fluoroquinolone prescriptions. This suggests that without an accompanying outpatient stewardship program, inpatient efforts to curb antibiotic use are later reversed by outpatient prescribing practices.
Providers can and should decline requests for antibiotics from patients with viral syndromes, noninfectious conditions, or asymptomatic colonization with bacteria (e.g. asymptomatic bacteriuria). When clinical uncertainty exists, there is increasing effort being devoted to development and deployment of novel diagnostics aimed at distinguishing viral from bacterial infections. Coupling of ‘diagnostic stewardship’ with ‘antimicrobial stewardship’ is the goal going forward. For patients who do require antibiotic therapy, stewardship efforts should be aimed at promoting the narrowest and shortest effective course. There are numerous clinical scenarios in which shorter antibiotic courses have been shown to be non-inferior to longer durations of therapy. For example, many patients with community-acquired pneumonia can be treated with 3–5 days of antibiotics, rather than the previous standard of 7–10 days.Citation49–Citation51 Studies have similarly supported shortened courses of therapy for urinary tract infections, intra-abdominal infections, and bacteremia, among others.Citation52,Citation53
There is also increased research targeting development of novel approaches to treating infections without the use of antibiotics. One exciting area is the use of bacteriophages to target bacterial infections. Although rigorous studies are required to validate this therapy, published case reports indicate that phage therapy holds promise.Citation54–Citation56 Demonstrations of safety, efficacy, generalizability, and cost-effectiveness are required before phage therapy becomes mainstream. However, it is an appealing approach to infection because of its demonstrated potential for treating extremely drug resistant bacteria (albeit case reports), and because it is a uniquely targeted therapy that theoretically would not broadly alter the host microbiome. Bacteriocins and antimicrobial peptides have also been proposed as potential alternatives to antibiotic therapy.Citation57,Citation58 Antimicrobial peptides have been further refined with the development of specifically targeted antimicrobial peptides (STAMPs), which consist of an antimicrobial peptide fused to a targeting domain.Citation59 One such example is STAMP C16G2, which was shown to selectively kill Streptococcus mutans in a polymicrobial biofilm, suggesting a high-degree of specificity that would preserve the host microbiota.Citation59 Indeed, in a small clinical trial, this constructed peptide selectively removed S. mutans from dental plaque without disrupting the oral microbiota as a whole.Citation60 Further work is needed to validate, test safety and determine the cost-effectiveness of these novel therapeutics for broader clinical use.
Future directions for investigation
Our understanding of the microbiome and its complex interactions with the human host have grown exponentially in the last decade. Basic and clinical research have uncovered intriguing associations between antibiotic-induced microbial dysbiosis and the development of various chronic diseases, including colon cancer. Questions remain as to links between different antibiotic classes, disruption of specific members of the aerobic and anaerobic microbial community, and mechanisms of pro-carcinogenesis or other disease induction resulting from antibiotic exposure. What is clear is that most patients are prescribed antibiotics at least once in their lives, and the consequences of these exposures are neither innocuous nor short-lived. We have a responsibility to reduce unnecessary antibiotic use, and to explore non-antibiotic options for treating infections that preserve the integrity of our natural microbiome homeostasis. If we fail our patients in this regard, the consequences certainly include increased susceptibility to pathogens like Clostridioides difficile and increasingly drug-resistant bacteria as well as quite likely the development of chronic disease, enhanced risk for colon cancer and/or perhaps other malignancies. More studies are warranted to investigate how intestinal microbiota exerts its modulatory role on carcinogenesis, particularly differential mechanisms along the colorectal continuum. Future work is needed to identify high risk patients who may be more susceptible to microbiome disruption and to develop strategies to modulate or manipulate gut microorganisms for therapeutic purposes.
Competing Interests
CLS discloses research grants from Bristol Myers Squibb and Janssen as well as a personal fee from Merck (2/19). CLS is a reviewer and author for UpToDate. No other authors have disclosures.
Acknowledgments
The authors thank all members of the Sears laboratory as well as Charles Haines, MD, PhD; Alastair Watson, MD; Andrew Hart, MD; Mary Jane Platt, MD; Drew M. Pardoll, MD, PhD; Sara E. Cosgrove, MD, MS and Kelly A. Gebo, MD, MPH
Additional information
Funding
References
- Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, Laxminarayan R. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014;14(8):742–750. doi:10.1016/S1473-3099(14)70780-7.
- Klein EY, Van Boeckel TP, Martinez EM, Pant S, Gandra S, Levin SA, Goossens H, Laxminarayan R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A. 2018;115(15):E3463–E3470. doi:10.1073/pnas.1717295115.
- Prevention CfDCa. Outpatient antibiotic prescriptions — United States. Outpatient Antibiotic Prescriptions — United States; 2016.
- Lee GC, Reveles KR, Attridge RT, Lawson KA, Mansi IA, Lewis JS, Frei CR. Outpatient antibiotic prescribing in the united states: 2000 to 2010. BMC Med. 2014;12(96). doi:10.1186/1741-7015-12-96.
- Dolk FCK, Pouwels KB, Smith DRM, Robotham JV, Smieszek T. Antibiotics in primary care in england: which antibiotics are prescribed and for which conditions? J Antimicrob Chemother. 2018;73(suppl_2):ii2–ii10. doi:10.1093/jac/dkx504.
- Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM, Finkelstein JA, Gerber JS, Hyun DY, Linder JA, et al. Prevalence of inappropriate antibiotic prescriptions among us ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864–1873. doi:10.1001/jama.2016.4151.
- Smieszek T, Pouwels KB, Dolk FCK, Smith DRM, Hopkins S, Sharland M, Hay AD, Moore MV, Robotham JV. Potential for reducing inappropriate antibiotic prescribing in english primary care. J Antimicrob Chemother. 2018;73(suppl_2):ii36–ii43. doi:10.1093/jac/dkx500.
- Drewes JL, White JR, Dejea CM, Fathi P, Iyadorai T, Vadivelu J, Roslani AC, Wick EC, Mongodin EF, Loke MF, et al. High-resolution bacterial 16s rrna gene profile meta-analysis and biofilm status reveal common colorectal cancer consortia. NPJ Biofilms Microbiomes. 2017;3(34). doi:10.1038/s41522-017-0040-3.
- Dejea CM, Fathi P, Craig JM, Boleij A, Taddese R, Geis AL, Wu X, DeStefano Shields CE, Hechenbleikner EM, Huso DL, et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science. 2018;359(6375):592–597. doi:10.1126/science.aah3648.
- Dejea CM, Wick EC, Hechenbleikner EM, White JR, Mark Welch JL, Rossetti BJ, Peterson SN, Snesrud EC, Borisy GG, Lazarev M, et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci U S A. 2014;111(51):18321–18326. doi:10.1073/pnas.1406199111.
- Tomkovich S, Dejea CM, Winglee K, Drewes JL, Chung L, Housseau F, Pope JL, Gauthier J, Sun X, Mühlbauer M, et al. Human colon mucosal biofilms from healthy or colon cancer hosts are carcinogenic. J Clin Invest. 2019;130:1699–1712. doi:10.1172/JCI124196.
- Zhang H, García Rodríguez LA, Hernández-Díaz S. Antibiotic use and the risk of lung cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1308–1315. doi:10.1158/1055-9965.EPI-07-2817.
- Kilkkinen A, Rissanen H, Klaukka T, Pukkala E, Heliövaara M, Huovinen P, Männistö S, Aromaa A, Knekt P. Antibiotic use predicts an increased risk of cancer. Int J Cancer. 2008;123(9):2152–2155. doi:10.1002/ijc.v123:9.
- Yang B, Hagberg KW, Chen J, Sahasrabuddhe VV, Graubard BI, Jick S, McGlynn KA. Associations of antibiotic use with risk of primary liver cancer in the clinical practice research datalink. Br J Cancer. 2016;115(1):85–89. doi:10.1038/bjc.2016.148.
- Boursi B, Mamtani R, Haynes K, Yang YX. Recurrent antibiotic exposure may promote cancer formation–another step in understanding the role of the human microbiota?. Eur J Cancer. 2015;51(17):2655–2664. doi:10.1016/j.ejca.2015.08.015.
- Cao Y, Wu K, Mehta R, Drew DA, Song M, Lochhead P, Nguyen LH, Izard J, Fuchs CS, Garrett WS, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut. 2018;67(4):672–678. doi:10.1136/gutjnl-2016-313413.
- Tamim HM, Hajeer AH, Boivin JF, Collet JP. Association between antibiotic use and risk of prostate cancer. Int J Cancer. 2010;127(4):952–960. doi:10.1002/ijc.25139.
- Velicer CM, Heckbert SR, Lampe JW, Potter JD, Robertson CA, Taplin SH. Antibiotic use in relation to the risk of breast cancer. JAMA. 2004;291(7):827–835. doi:10.1001/jama.291.7.827.
- Hoskin-Parr L, Teyhan A, Blocker A, Henderson AJ. Antibiotic exposure in the first two years of life and development of asthma and other allergic diseases by 7.5 yr: A dose-dependent relationship. Pediatr Allergy Immunol. 2013;24(8):762–771. doi:10.1111/pai.12153.
- Risnes KR, Belanger K, Murk W, Bracken MB. Antibiotic exposure by 6 months and asthma and allergy at 6 years: findings in a cohort of 1,401 us children. Am J Epidemiol. 2011;173(3):310–318. doi:10.1093/aje/kwq400.
- Arvonen M, Virta LJ, Pokka T, Kröger L, Vähäsalo P. Repeated exposure to antibiotics in infancy: A predisposing factor for juvenile idiopathic arthritis or a sign of this group’s greater susceptibility to infections? J Rheumatol. 2015;42(3):521–526. doi:10.3899/jrheum.140348.
- Card T, Logan RF, Rodrigues LC, Wheeler JG. Antibiotic use and the development of crohn’s disease. Gut. 2004;53(2):246–250. doi:10.1136/gut.2003.025239.
- Hviid A, Svanström H, Frisch M. Antibiotic use and inflammatory bowel diseases in childhood. Gut. 2011;60(1):49–54. doi:10.1136/gut.2010.219683.
- Kronman MP, Zaoutis TE, Haynes K, Feng R, Coffin SE. Antibiotic exposure and ibd development among children: A population-based cohort study. Pediatrics. 2012;130(4):e794–803. doi:10.1542/peds.2011-3886.
- Boursi B, Mamtani R, Haynes K, Yang YX. The effect of past antibiotic exposure on diabetes risk. Eur J Endocrinol. 2015;172(6):639–648. doi:10.1530/EJE-14-1163.
- Mueller NT, Whyatt R, Hoepner L, Oberfield S, Dominguez-Bello MG, Widen EM, Hassoun A, Perera F, Rundle A. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes (Lond). 2015;39(4):665–670. doi:10.1038/ijo.2014.180.
- Scott FI, Horton DB, Mamtani R, Haynes K, Goldberg DS, Lee DY, Lewis JD. Administration of antibiotics to children before age 2 years increases risk for childhood obesity. Gastroenterology. 2016;151(1):120–129.e125. doi:10.1053/j.gastro.2016.03.006.
- Schwartz BS, Pollak J, Bailey-Davis L, Hirsch AG, Cosgrove SE, Nau C, Kress AM, Glass TA, Bandeen-Roche K. Antibiotic use and childhood body mass index trajectory. Int J Obes (Lond). 2016;40(4):615–621. doi:10.1038/ijo.2015.218.
- Tasian GE, Jemielita T, Goldfarb DS, Copelovitch L, Gerber JS, Wu Q, Denburg MR. Oral antibiotic exposure and kidney stone disease. J Am Soc Nephrol. 2018;29(6):1731–1740. doi:10.1681/ASN.2017111213.
- Lurie I, Yang YX, Haynes K, Mamtani R, Boursi B. Antibiotic exposure and the risk for depression, anxiety, or psychosis: A nested case-control study. J Clin Psychiatry. 2015;76(11):1522–1528. doi:10.4088/JCP.15m09961.
- Yassour M, Vatanen T, Siljander H, Hämäläinen AM, Härkönen T, Ryhänen SJ, Franzosa EA, Vlamakis H, Huttenhower C, Gevers D, et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. 2016;8(343):343ra381. doi:10.1126/scitranslmed.aad0917.
- Lichtman JS, Ferreyra JA, Ng KM, Smits SA, Sonnenburg JL, Elias JE. Host-microbiota interactions in the pathogenesis of antibiotic-associated diseases. Cell Rep. 2016;14(5):1049–1061. doi:10.1016/j.celrep.2016.01.009.
- Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16s rrna sequencing. PLoS Biol. 2008;6:e280.
- Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4554–4561. doi:10.1073/pnas.1000087107.
- Pérez-Cobas AE, Artacho A, Knecht H, Ferrús ML, Friedrichs A, Ott SJ, Moya A, Latorre A, Gosalbes MJ. Differential effects of antibiotic therapy on the structure and function of human gut microbiota. PLoS One. 2013;8(11):e80201. doi:10.1371/journal.pone.0080201.
- Pérez-Cobas AE, Gosalbes MJ, Friedrichs A, Knecht H, Artacho A, Eismann K, Otto W, Rojo D, Bargiela R, von Bergen M, et al. Gut microbiota disturbance during antibiotic therapy: A multi-omic approach. Gut. 2013;62(11):1591–1601. doi:10.1136/gutjnl-2012-303184.
- Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. Isme J. 2007;1(1):56–66. doi:10.1038/ismej.2007.3.
- Vidal AC, Murphy SK, Murtha AP, Schildkraut JM, Soubry A, Huang Z, Neelon SE, Fuemmeler B, Iversen E, Wang F, et al. Associations between antibiotic exposure during pregnancy, birth weight and aberrant methylation at imprinted genes among offspring. Int J Obes (Lond). 2013;37(7):907–913. doi:10.1038/ijo.2013.47.
- Yu DH, Gadkari M, Zhou Q, Yu S, Gao N, Guan Y, Schady D, Roshan TN, Chen MH, Laritsky E, et al. Postnatal epigenetic regulation of intestinal stem cells requires dna methylation and is guided by the microbiome. Genome Biol. 2015;16:211. doi:10.1186/s13059-015-0763-5.
- Schumann A, Nutten S, Donnicola D, Comelli EM, Mansourian R, Cherbut C, Corthesy-Theulaz I, Garcia-Rodenas C. Neonatal antibiotic treatment alters gastrointestinal tract developmental gene expression and intestinal barrier transcriptome. Physiol Genomics. 2005;23(2):235–245. doi:10.1152/physiolgenomics.00057.2005.
- Knoop KA, McDonald KG, Kulkarni DH, Newberry RD. Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut. 2016;65(7):1100–1109. doi:10.1136/gutjnl-2014-309059.
- Knoop KA, Gustafsson JK, McDonald KG, Kulkarni DH, Kassel R, Newberry RD. Antibiotics promote the sampling of luminal antigens and bacteria via colonic goblet cell associated antigen passages. Gut Microbes. 2017;8(4):400–411. doi:10.1080/19490976.2017.1299846.
- Pevsner-Fischer M, Tuganbaev T, Meijer M, Zhang SH, Zeng ZR, Chen MH, Elinav E. Role of the microbiome in non-gastrointestinal cancers. World J Clin Oncol. 2016;7(2):200–213. doi:10.5306/wjco.v7.i2.200.
- Tsilimigras MC, Fodor A, Jobin C. Carcinogenesis and therapeutics: the microbiota perspective. Nat Microbiol. 2017;2:17008. doi:10.1038/nmicrobiol.2017.8.
- Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 2016;14(1):20–32. doi:10.1038/nrmicro3552.
- Chen J, Domingue JC, Sears CL. Microbiota dysbiosis in select human cancers: evidence of association and causality. Semin Immunol. 2017;32:25–34. doi:10.1016/j.smim.2017.08.001.
- Mima K, Cao Y, Chan AT, Qian ZR, Nowak JA, Masugi Y, Shi Y, Song M, da Silva A, Gu M, et al. Fusobacterium nucleatum in colorectal carcinoma tissue according to tumor location. Clin Transl Gastroenterol. 2016;7(11):e200. doi:10.1038/ctg.2016.53.
- Vaughn VM, Gandhi T, Conlon A, Chopra V, Malani AN, Flanders SA. The association of antibiotic stewardship with fluoroquinolone prescribing in michigan hospitals: A multi-hospital cohort study. Clin Infect Dis. 2019;69(8):1269–1277. doi:10.1093/cid/ciy1102.
- Dunbar LM, Wunderink RG, Habib MP, Smith LG, Tennenberg AM, Khashab MM, Wiesinger BA, Xiang JX, Zadeikis N, Kahn JB. High-dose, short-course levofloxacin for community-acquired pneumonia: A new treatment paradigm. Clin Infect Dis. 2003;37(6):752–760. doi:10.1086/cid.2003.37.issue-6.
- El Moussaoui R, de Borgie CA, van den Broek P, Hustinx WN, Bresser P, van den Berk GE, Poley JW, van den Berg B, Krouwels FH, Bonten MJ, et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ. 2006;332(7554):1355. doi:10.1136/bmj.332.7554.1355.
- Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, Cooley LA, Dean NC, Fine MJ, Flanders SA, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the american thoracic society and infectious diseases society of america. Am J Respir Crit Care Med. 2019;200(7):e45–e67.
- Royer S, DeMerle KM, Dickson RP, Prescott HC. Shorter versus longer courses of antibiotics for infection in hospitalized patients: A systematic review and meta-analysis. J Hosp Med. 2018;13(5):336–342. doi:10.12788/jhm.2905.
- Yahav D, Franceschini E, Koppel F, Turjeman A, Babich T, Bitterman R, Neuberger A, Ghanem-Zoubi N, Santoro A, Eliakim-Raz N, et al. Seven versus 14 days of antibiotic therapy for uncomplicated gram-negative bacteremia: A noninferiority randomized controlled trial. Clin Infect Dis. 2019;69(7):1091–1098. doi:10.1093/cid/ciy1054.
- LaVergne S, Hamilton T, Biswas B, Kumaraswamy M, Schooley RT, Wooten D. Phage therapy for a multidrug-resistant. Open Forum Infect Dis. 2018;5(4):ofy064. doi:10.1093/ofid/ofy064.
- Chan BK, Turner PE, Kim S, Mojibian HR, Elefteriades JA, Narayan D. Phage treatment of an aortic graft infected with. Evol Med Public Health. 2018;2018(1):60–66. doi:10.1093/emph/eoy005.
- Schooley RT, Biswas B, Gill JJ, Hernandez-Morales A, Lancaster J, Lessor L, Barr JJ, Reed SL, Rohwer F, Benler S, et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant acinetobacter baumannii infection. Antimicrob Agents Chemother. 2017;61(10). doi:10.1128/AAC.00954-17.
- Ahmad V, Khan MS, Jamal QMS, Alzohairy MA, Al Karaawi MA, Siddiqui MU. Antimicrobial potential of bacteriocins: in therapy, agriculture and food preservation. Int J Antimicrob Agents. 2017;49(1):1–11. doi:10.1016/j.ijantimicag.2016.08.016.
- Mahlapuu M, Håkansson J, Ringstad L, Björn C. Antimicrobial peptides: an emerging category of therapeutic agents. Front Cell Infect Microbiol. 2016;6:194. doi:10.3389/fcimb.2016.00194.
- Eckert R, He J, Yarbrough DK, Qi F, Anderson MH, Shi W. Targeted killing of streptococcus mutans by a pheromone-guided “Smart” Antimicrobial peptide. Antimicrob Agents Chemother. 2006;50(11):3651–3657. doi:10.1128/AAC.00622-06.
- Sullivan R, Santarpia P, Lavender S, Gittins E, Liu Z, Anderson MH, He J, Shi W, Eckert R. Clinical efficacy of a specifically targeted antimicrobial peptide mouth rinse: targeted elimination of streptococcus mutans and prevention of demineralization. Caries Res. 2011;45(5):415–428. doi:10.1159/000330510.
- Wang JL, Chang CH, Lin JW, Wu LC, Chuang LM, Lai MS. Infection, antibiotic therapy and risk of colorectal cancer: A nationwide nested case-control study in patients with type 2 diabetes mellitus. Int J Cancer. 2014;135(4):956–967. doi:10.1002/ijc.28738.
- Dik VK, van Oijen MG, Smeets HM, Siersema PD. Frequent use of antibiotics is associated with colorectal cancer risk: results of a nested case-control study. Dig Dis Sci. 2016;61(1):255–264. doi:10.1007/s10620-015-3828-0.