ABSTRACT
Iron withholding, an essential component of nutritional immunity, plays a fundamental role in host resistance to Salmonella infection. Our previous study showed that SpvB, an important pSLT-encoded cytotoxic effector, facilitated Salmonella pathogenesis within macrophages via perturbing cellular iron metabolism. However, the underlying mechanisms of SpvB in Salmonella-relevant disorders of systemic iron metabolism have not yet been identified. Here, we demonstrated that SpvB facilitated Salmonella to scavenge iron from the host by modulating the hepcidin–ferroportin axis, a key regulator of systemic iron metabolism. We observed that SpvB enhanced hepatic hepcidin synthesis in a STAT3-dependent manner, but not the BMP/SMAD pathway. This subsequently resulted in a reduction of the unique cellular iron exporter ferroportin, which facilitated hypoferremia and hepatic iron accumulation and ultimately countered the limitation of iron availability, thereby improving the chances of Salmonella survival and replication. Moreover, SpvB promoted the production of proinflammatory molecules associated with the infiltration of inflammatory cells via highly upregulating TREM-1 signaling. Our data supported a role of TREM-1 in SpvB-related dysregulation of host iron metabolism and suggested that targeting TREM-1 might provide a potential therapeutic strategy to prevent or alleviate Salmonella pathogenesis.
Introduction
Salmonella enterica serovar Typhimurium (S. typhimurium) is a facultative intracellular gram-negative bacterial pathogen that causes severe gastroenteritis and systemic inflammation in both humans and animals. Approximately 180 million cases, or 9% of the diarrheal cases involving this bacterium, are reported annually around the world.Citation1 As one of most prevalent food- and water-borne pathogens, S. typhimurium gains access to the gastrointestinal barrier, which is associated with diarrhea and acute inflammation, then disseminates to the mesenteric lymph nodes, and eventually spreads to and colonizes other extra-intestinal tissues, such as the liver, during the late stage of infection.Citation2
S. typhimurium has evolved several mechanisms to counteract the host antimicrobial defense. The Salmonella plasmid virulence (spv) locus is a highly conserved region on the pSLT virulence plasmid from Salmonella strains of clinically significant serovars, e.g., S. typhimurium, S. enteritidis, S. choleraesuis, S. dublin, and S. arizona, et al.Citation3 The SpvB effector, which is one of the spv locus-encoded cytotoxic proteins, has been reported to participate in several facets of Salmonella pathogenesis. For instance, after translocating from the Salmonella-containing vacuole (SCV) into the cytoplasm of the host cell via Salmonella pathogenicity island-2 (SPI-2) type III secretion system (T3SS), SpvB induces apoptotic cell death in eukaryotic cells and contributes to severe inflammatory injury in miceCitation4,Citation5 In addition to its role in promoting Salmonella virulence, SpvB downregulates autophagic flux via the cytotoxic effects of actin depolymerization and contributes to the intracellular replication and survival of Salmonella.Citation6
Iron is essential for humans, as it functions as a redox catalyst for cellular enzymatic processes, and disorders of host iron homeostasis strongly influence the course and outcome of Salmonella infection. Iron is a necessary nutrient for nearly all bacteria, including Salmonella in their intracellular proliferation and survivalCitation7,Citation8 In the competition for host iron, Salmonella obtains iron resources to meet their own nutrient requirements, which results in hypoferremia and iron accumulation in the liver and spleen.Citation9 Therefore, it is significant to maintain physiological iron homeostasis during Salmonella infection.
There are two regulatory systems involved in iron metabolism: one that modulates cellular iron metabolism via iron-regulatory proteins and another that regulates systemic iron metabolism predominantly via the hepcidin–ferroportin (FPN) axis.Citation10 Hepcidin is a defensin-like, cysteine-rich antimicrobial peptide that prevents iron release from intracellular sources by directly binding to the sole mammalian iron exporter, FPN, thereby facilitating the internalization and lysosomal degradation of FPN and ultimately sequestering the iron in cellsCitation11,Citation12 Hepcidin is transcriptionally regulated by iron and inflammatory cytokines through the BMP/SMAD and JAK/STAT3 signaling pathways respectivelyCitation13,Citation14 In recent years, a growing number of experiments have indicated that enhanced hepcidin production during Salmonella infection contributes to bacterial growth and virulence. Despite this, however, the potential mechanism of how Salmonella modulates the hepcidin–FPN axis and subsequently responds to host iron withdrawal remains poorly understood. Previous work from our laboratory reported a novel contribution of SpvB to Salmonella pathogenesis by disturbing cellular iron metabolism. SpvB has been shown to inhibit FPN expression at the transcriptional level via a proteasome pathway dependent upon NRF2 reduction, thereby decreasing iron efflux and increasing intracellular iron concentration.Citation15 In this work, we aim to expand our understanding on the effect of SpvB in vivo by investigating the hypothesis that SpvB influences systemic iron metabolism in a hepcidin-dependent pathway.
Material and methods
Animal and ethics statement
C57BL/6 mice were obtained from the experimental animal center of Soochow University. Hepcidin (Hamp) heterozygous mice (on a C57BL/6 J genetic background) were a gift from Professor Youjia Xu (The Second Affiliated Hospital of Soochow University, China).Citation16 The generation and genotype identification of mice deficient in Hamp has been described previously. Mice were bred locally under specific-pathogen-free conditions in individually ventilated cages and received sterile water and food ad libitum. Eight- to ten- weeks old mice were used in this study. All animal experiments were approved by the Animal Experimental Committee of Soochow University (Grant 2111270) and complied with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH Guidelines).
Bacterial strains and growth condition
Wild-type (WT) S. typhimurium strain SL1344 was a gift from Professor Qian Yang (Nanjing Agricultural University, Nanjing, China). The spvB deletion mutant strain SL1344-ΔspvB and the spvB complemented strain SL1344-c-spvB were constructed in our previous work.Citation15 WT and ΔspvB strains were grown on Luria-Bertani (LB) agar (Hangwei, Hangzhou, China) plates or in LB medium (Hangwei), and c-spvB strain was grown in LB broth containing 100 μg/ml ampicillin (Sangon Biotech, Shanghai, China).
S. typhimurium infection in vivo
Salmonella strains were inoculated in LB medium overnight. On the day of experiments, bacterial cultures were diluted in a ratio of 1:100 and then agitated for a further 3 h. Upon washing 3 times with PBS, fresh bacterial suspension was ready for the infection experiment. Following orally administrated with 20 mg/mouse streptomycin (Sangon Biotech) for 24 h, mice were orally infected with 1 × 107 colony-forming units (CFUs) of SL1344 or ΔspvB strain. Samples were collected at indicated time points. Hepatic bacterial burden was quantified by plating homogenized hepatic tissues in appropriate dilutions on Salmonella-Shigella agar plates (Hangwei). The plates were incubated overnight at 37°C and colonies were counted the next day. To investigate the role of SpvB in Salmonella-associated dysregulation of systemic iron metabolism, S. typhimurium infected mice were administered the iron chelator deferasirox (DFX) ad libitum orally with a solution of 500 mg DFX (Meilunbio, Beijing, China) dissolved in 1000 ml drinking water.Citation17 The number of S. typhimurium in the liver was determined as described above. To analyze the relationship between SpvB and STAT3 pathway, mice were intraperitoneally treated with 20 mg/kg the STAT3 inhibitor Stattic as previously described,Citation18 and then infected with different S. typhimurium strains as mentioned above. To assess the role of TREM-1 in SpvB-mediated pathogenesis, mice were intraperitoneally injected with 100 μg/mouse LP17 peptide (LQVTDSGLYRCVIYHPP), a synthetic polypeptide inhibitor of TREM-1 dimerization and activation, at 1 day post-infection.Citation19
Quantitative PCR
GGTotal RNA was isolated from mouse liver using TRIzol reagent (Beyotime Biotechnology, Shanghai, China) and reverse-transcribed using the All-in-one RT MasterMix kit (Applied Biological Materials, Richmond, BC, Canada) according to the manufacturer’s instructions. cDNAs were analyzed by the ViiA7 real-time PCR instrument (Applied Biosystems, Carlsbad, CA, USA) using EvaGreen MasterMix-Low ROX (Applied Biological Materials). Specific primer sequences were listed in . All expression levels were normalized to Gapdh or β-ACTIN expression. Values were expressed as fold induction in comparison to untreated control mice.
Table 1. Primer sequences for quantitative PCR
m, mouse; h, human
Western blot analysis
Liver tissues were lysed in RIPA Lysis Buffer (Beyotime Biotechnology) containing a protease and phosphatase inhibitor, and protein concentrations were measured using the BCA Protein Assay (Beyotime Biotechnology). Protein extracts were separated on 12% gels and then electroblotted onto PVDF membranes (MilliporeSigma, Burlington, MA, USA). After blocking nonspecific binding with 5% nonfat dry milk powder in TBST, membranes were probed with primary antibodies overnight at 4°C and incubated with the appropriate HRP-labeled secondary antibodies at room temperature for 1 h. Membranes were then visualized with an enhanced chemiluminescence luminescence reagent (Meilunbio). The intensities of the bands were calculated by ImageJ Launcher broken symmetry software program (NIH, Bethesda, MD, USA). Antibodies to the following proteins were used: FPN (sc-49668; Santa Cruz Biotechnology, Dallas, TX, USA) for in vivo experiments; FPN (NBP1-21502; Novus Biologicals, Littleton, CO, USA) for in vitro experiments; Phospho-STAT3 (Tyr705) (YP0251; ImmunoWay, Plano, TX, USA); Phospho-Smad1 (Ser463/465)/Smad5 (Ser463/465)/Smad9 (Ser465/467) (D5B10) (13820; Cell Signaling Technology, Danvers, MA, USA); TREM-1 (MAB1187; R&D Systems, Minneapolis, MN, USA); IL6 (PB0060; Boster Biological Technology, Pleasanton, CA, USA); α-Tubulin (AF0001; Beyotime Biotechnology); β-Actin (bs-0061 R; Bioss, Woburn, MA, USA); and GAPDH (bs-10900 R; Bioss).
Isolation of liver non-parenchymal cells and flow cytometry analysis
Liver non-parenchymal cells were isolated as previously described.Citation20 In brief, livers were harvested, perfused with calcium and magnesium-free Hank’s Balanced Salt Solution (HBSS; Beyotime Biotechnology), and digested with collagenase type Ι (Worthington, Lakewood, NJ, USA). The cell suspension was filtered through a 70 μm cell strainer (Sorfa, Zhejiang, China), centrifuged to eliminate hepatocytes, and then eliminated erythrocyte the Red Blood Cell Lysis Buffer (Beyotime Biotechnology) according to the manufacturer’s instructions. The non-parenchymal cells were incubated with different antibodies at 4°C for 30 min. Antibodies were used as follows: F4/80 (123108; BioLegend, San Diego, CA, USA); CD11b (101212; BioLegend); FITC Rat IgG2a κ (400505; BioLegend) and APC Rat IgG2b κ (400611; BioLegend).
Iron measurement in the serum and liver
Blood samples collected from anesthetized mice through intracardiac puncture. Livers were harvested, weighed, mechanically homogenized in RIPA Lysis Buffer and mixed with HCl (0.01 M final concentration). Iron content was detected using an iron assay kit (BioAssay Systems, Hayward, CA, USA) according to the manufacturer’s instructions.
Histopathologic analysis and Perls’ Prussian Blue staining
Liver samples were fixed in 4% paraformaldehyde, embedded in paraffin and cut into 5-μm thick sections. To show inflammatory lesions, sections were subjected to hematoxylin-eosin (H&E) staining (Servicebio, Wuhan, China). To assess tissue iron concentration, sections were incubated in the Perls’ Prussian Blue working solution (Servicebio) at 60°C for 30 min and subsequently stained with nuclear fast red at room temperature for 5 min. All photomicrographs were taken with a Nikon Eclipse Ni-U fluorescence microscope (Nikon Corporation, Tokyo, Japan) with NIS-Elements F (Nikon Corporation).
Cell culture
HepG2 cells (human hepatoma cell line) were kindly provided by Professor Chengliang Gong (Soochow University, Suzhou, China). These cells were maintained in DMEM (HyClone Laboratories, Logan, UT, USA) containing 10% (v/v) heat-inactivated fetal bovine serum (FBS; Biological Industries, Kibbutz Beit-Haemek, Israel). THP-1 cells (human monocytic cell line), a gift from Professor Yanyun Zhang (Shanghai Institute of Nutrition and Health, Shanghai, China), were grown in RPMI 1640 (HyClone Laboratories) supplemented with 10% (v/v) heat-inactivated FBS.
Salmonella infection in vitro
The Salmonella suspension was prepared as described above. Following 1 h of incubation with bacteria, cells were washed 3 times with PBS and then placed in fresh medium containing 10% (v/v) heat-inactivated FBS and 100 μg/ml amikacin (MilliporeSigma). After 2 h, the cell culture medium was replaced with fresh medium containing 10% (v/v) heat-inactivated FBS and 10 μg/ml amikacin (MilliporeSigma).
Co-culture and treatment
THP-1 cells were differentiated into adherent macrophage-like cells by 100 nM phorbol 12-myristate 13-acetate (PMA; MilliporeSigma) for 48 h. A non-contact culture system was set up following a published protocolCitation21,Citation22 In brief, THP-1 macrophages were seeded at 5 × 105 cells per well on the top surface of the Transwell inserts (Corning-Costar, Corning, NY, USA). HepG2 cells were grown in 6-well plates. On the day of infection experiments, HepG2 cells were overlaid with Transwell inserts containing THP-1 macrophages, and the bacterial suspension was added to the apical side of the Transwell system. To assess iron content of macrophages in this co-cultured system, THP-1 macrophages were incubated with 100 μM ferrous chloride (Adamas, Shanghai, China) for 16 h prior to be placed above HepG2 cells.Citation22 The relative iron concentration in co-cultured macrophages lysates was determined using an iron assay kit (BioAssay Systems).
Transfection experiment
The HAMP small interfering RNA (siRNA), TREM-1 siRNA and negative control siRNA (NC siRNA) were purchased from GenePharma (Shanghai, China). Cells were transfected with siRNA using Lipofectamine RNAiMAX reagent (Thermo Fisher Scientific, Waltham, MA, USA) for 24 h according to the manufacturer’s instructions. Knockdown efficiency was assessed by quantitative PCR or Western blot with TREM-1 antibody (YT5133; ImmunoWay). The spvB overexpression plasmid pEGFP-spvB was constructed in our previous work.Citation6 THP-1 macrophages were transiently transfected with pEGFP-spvB or the control vector pEGFP-N1 by Lipofectamine 3000 (Thermo Fisher Scientific) according to the manufacturer’s instructions.
Statistics
Data are presented as mean ± SEM. Statistical analysis was performed using IBM SPSS statistics 22 (Chicago, IL, USA). Comparisons of 2 groups were analyzed using an independent Student’s t-test. Comparisons among multiple groups were performed with one-way ANOVA with Student-Newman-Keuls (S-N-K) correction. Values of P < .05 were considered statistically significant.
Results
SpvB effector protein is significant to S. typhimurium dysregulation of host iron metabolism
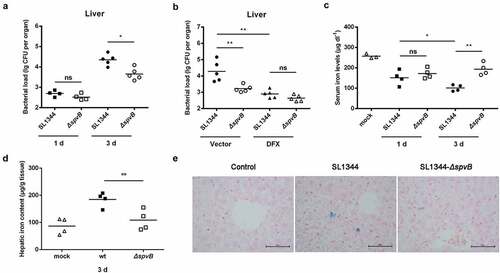
First, we assessed whether the Salmonella effector SpvB contributes to the hepatic Salmonella pathogenesis via weakening the host iron-withholding defenses. Streptomycin-pretreated mice were orally infected with either the WT S. typhimurium or the ΔspvB mutant S. typhimurium strain to study host-pathogen interactions. We counted the number of Salmonella in the liver at 1 and 3 days post-infection and found that even though there were no apparent differences between these S. typhimurium-infected mice at 1 day post-infection, mice infected with the WT strain contained a significantly higher hepatic bacterial burden than mice infected with the ΔspvB strain at 3 days post-infection (). We subsequently investigated whether limitation of iron availability for S. typhimurium might reverse this difference in vivo. S. typhimurium-infected mice were further orally treated with the iron chelator DFX. Our data showed that DFX not only decreased the hepatic bacterial load, but also eliminated the significant difference in S. typhimurium survival between mice infected with the WT and ΔspvB strains (). We further examined the changes in the host iron homeostasis in response to S. typhimurium infection. In agreement with our previous study, there was a significant decrease in serum iron levels in mice infected with the WT strain when compared to mice infected with the ΔspvB strain at 3 days post-infection (). Interestingly, the hepatic iron concentration in mice infected with the WT strain was significantly higher than that detected in mice infected with the ΔspvB strain (, e). These data demonstrated that SpvB is associated with S. typhimurium pathogenesis, at least partly through perturbing host iron metabolism.
Figure 1. SpvB effector protein is significant to S. typhimurium dysregulation of host iron metabolism. Streptomycin-pretreated mice were orally infected with 1 × 107 CFUs of either the WT or the ΔspvB mutant S. typhimurium strain. a) Hepatic bacterial load at 1 and 3 days post-infection was determined by plating. b) S. typhimurium-infected mice were administered either DFX dissolved in drinking water or the same volume of drinking water. Hepatic bacterial load at 3 days post-infection was determined by plating. c) Serum iron levels at 1 and 3 days post-infection were measured with a colorimetric assay. d) Hepatic iron content at 3 days post-infection was determined on the basis of a multiscan spectrum. e) Perls’ Prussian Blue staining of the liver at 3 days post-infection. Original magnification, ×40; scale bars: 50 μm. One of 3 representative histology experiments is shown. Statistical analysis was performed with IBM SPSS statistics 22. Data were compared with independent Student’s t-test. Values are expressed as the mean ± SEM, and statistically significant differences are indicated. *P< .05; **P< .01; ns, not significant
SpvB mediates alternation of serum and hepatic iron levels through activation of hepcidin expression
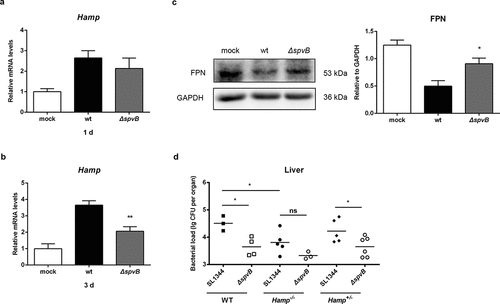
The hepcidin–FPN axis is considered to be a crucial regulator of host iron homeostasis. Therefore, we hypothesized that SpvB leads to changes in hepatic hepcidin expression and eventually contributes to Salmonella pathogenesis. At 1 day post-infection, the mRNA transcript level of hepatic hepcidin in WT-infected mice was similar to that in ΔspvB-infected mice (). However, at 3 days post-infection, mice infected with the WT strain showed significantly higher hepcidin expression than mice infected with the ΔspvB strain (). In line with this observation, mice infected with the WT strain displayed significantly lower FPN protein levels in the liver as compared with mice infected with the ΔspvB strain (). To better understand the role of hepcidin in SpvB-mediated Salmonella pathogenesis and iron metabolic disorders, WT (C57BL/6 J), Hamp gene knockout (Hamp−/−) and heterozygous (Hamp+/−) mice were infected orally with either the WT or ΔspvB S. typhimurium strain. Our data showed that the liver of Hamp−/− mice contained less bacteria than those of WT and Hamp+/− mice at 3 days post-infection, suggesting that hepcidin played a role in host defense against Salmonella infection. Importantly, there was no apparent difference in the number of Salmonella in the liver of Hamp−/− mice infected with the WT strain as compared to the ΔspvB strain (). These data suggested that SpvB contributes to dysregulation of host iron metabolism via acting on the hepcidin–FPN axis.
Figure 2. SpvB contributes to Salmonella-induced disorders of systemic iron metabolism by controlling the hepcidin-FPN axis. a-c) Streptomycin-pretreated mice were orally infected with 1 × 107 CFUs of either the WT or the ΔspvB mutant S. typhimurium strain. Hepatic Hamp levels at 1 day (a) or 3 days (b) post-infection were determined by quantitative PCR (n = 3–4 mice, respectively). c) Western blot analysis of whole liver lysates at 3 days post-infection with specific antibodies to FPN and the control GAPDH (n = 4 mice, respectively). Densitometric analysis of FPN relative to GAPDH protein and one of 4 representative western blot experiments are shown. d) Streptomycin-pretreated WT, Hamp+/− and Hamp−/− mice were orally infected with 1 × 107 CFUs of either the WT or the ΔspvB mutant S. typhimurium strain. Hepatic bacterial load at 3 days post-infection was determined by plating. Statistical analysis was performed with IBM SPSS statistics 22. Data were compared with independent Student’s t-test. Values are expressed as the mean ± SEM, and statistically significant differences are indicated. *P< .05; **P< .01; ns, not significant
SpvB induces hepatic hepcidin expression in a STAT3-dependent manner
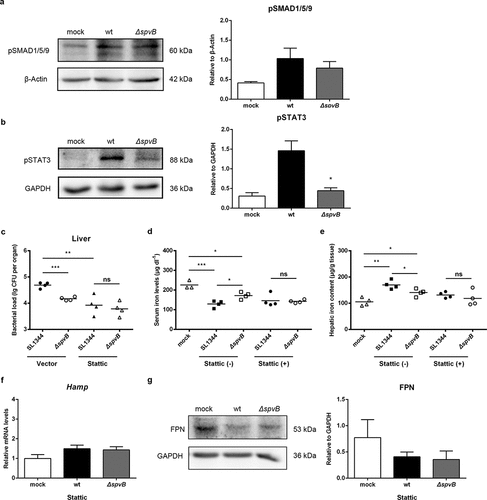
To gain a better understanding of the signaling mechanism by which SpvB regulates hepcidin expression, we examined both the BMP/SMAD and JAK/STAT3 pathways in the liver of WT-infected and ΔspvB-infected mice at 3 days post-infection. The protein level of SMAD1/5/9 phosphorylation was increased in S. typhimurium-infected mice compared with the control group, while there were no apparent differences in pSMAD1/5/9 expression between mice infected with the WT and ΔspvB strains. However, mice infected with the WT strain showed a significantly higher level of STAT3 phosphorylation as compared to mice infected with the ΔspvB strain (, b). To further examine the observation that SpvB might interfere with systemic iron metabolism via facilitating STAT3 activation, S. typhimurium-infected mice were intraperitoneally injected with the STAT3 inhibitor Stattic. Our data showed that inhibiting STAT3 with Stattic eliminated the significant difference in the hepatic bacterial load between mice infected with the WT and ΔspvB strains ( and Supplemental Fig. S1A). Importantly, it was found that the significant difference in serum iron concentration and hepatic iron content associated with the Salmonella effector SpvB were abrogated after Stattic treatment (, e). In line with these observations, the WT-infected mice showed a similar expression level of both hepcidin and FPN as compared with the ΔspvB-infected mice following Stattic treatment (, g). These data indicated that SpvB induced hepatic hepcidin expression through a STAT3-dependent manner, resulting in alternation of host iron metabolism and severe Salmonella pathogenesis.
Figure 3. SpvB increases hepatic hepcidin expression through the STAT3-dependent pathway. a, b) Streptomycin-pretreated mice were orally infected with 1 × 107 CFUs of either the WT or the ΔspvB mutant S. typhimurium strain and analyzed at 3 days post-infection. a) Western blot analysis of whole liver lysates with specific antibodies to pSMAD1/5/9 and the control β-Actin (n = 3 mice, respectively). Densitometric analysis of pSMAD1/5/9 relative to β-Actin protein and one of 3 representative western blot experiments are shown. b) Western blot analysis of whole liver lysates with specific antibodies to pSTAT3 and the control GAPDH (n = 3 mice, respectively). Densitometric analysis of pSTAT3 relative to GAPDH protein and one of 3 representative western blot experiments are shown. c-g) S. typhimurium-infected mice were administered i.p with either Stattic or the same volume of vector and analyzed at 3 days post-infection. c) Hepatic bacterial load was determined by plating. d) Serum iron levels were measured with a colorimetric assay. e) Hepatic iron content was determined on the basis of a multiscan spectrum. f) Hepatic Hamp levels were determined by quantitative PCR (n = 4 mice, respectively). g) Western blot analysis of whole liver lysates with specific antibodies to FPN and the control GAPDH (n = 3 mice, respectively). Densitometric analysis of FPN relative to GAPDH protein and one of 3 representative western blot experiments are shown. Statistical analysis was performed with IBM SPSS statistics 22. Data were compared with independent Student’s t-test. Values are expressed as the mean ± SEM, and statistically significant differences are indicated. *P< .05; **P< .01; ***P< .001; ns, not significant
SpvB causes an increase in hepatic inflammation following S. typhimurium infection
Since the efficacy of antimicrobial immune responses is intimately linked to iron metabolism, we subsequently examined the influence of SpvB on the levels of cytokines that are known to be important for host resistance to intracellular pathogens. The mRNA levels of interleukin 1β (IL1β) and tumor necrosis factor alpha (TNFα) in the liver of WT-infected mice were significantly higher than those in the liver of ΔspvB-infected mice at 3 days post-infection (, b). Importantly, interleukin 6 (IL6), the primary inducer of STAT3-mediated hepcidin synthesisCitation23,Citation24 was significantly increased in the liver of WT-infected mice as compared to ΔspvB-infected mice at 3 days post-infection (, d). These findings further confirmed our observation that SpvB regulates STAT3 activation.
Figure 4. SpvB promotes hepatic proinflammatory cytokine and chemokine expression in S. typhimurium infection. Streptomycin-pretreated mice were orally infected with 1 × 107 CFUs of either the WT or the ΔspvB mutant S. typhimurium strain and analyzed at 3 days post-infection. a-c) Hepatic Il1β (a), Tnfα (b) and Il6 (c) levels were determined by quantitative PCR (n = 4 mice, respectively). d) Western blot analysis of whole liver lysates with specific antibodies to IL6 and the control β-Actin (n = 3 mice, respectively). Densitometric analysis of IL6 relative to β-Actin protein and one of 3 representative western blot experiments are shown. e-g) Hepatic Ccl2 (e), Ccl3 (f) and Cxcl10 (g) levels were determined by quantitative PCR (n = 4 mice, respectively). Statistical analysis was performed with IBM SPSS statistics 22. Data were compared with independent Student’s t-test. Values are expressed as the mean ± SEM, and statistically significant differences are indicated. *P< .05; **P< .01; ***P< .001
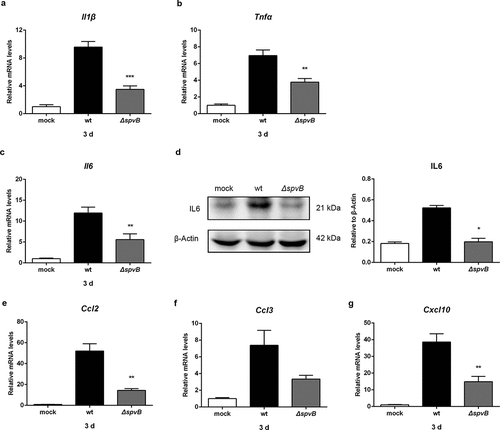
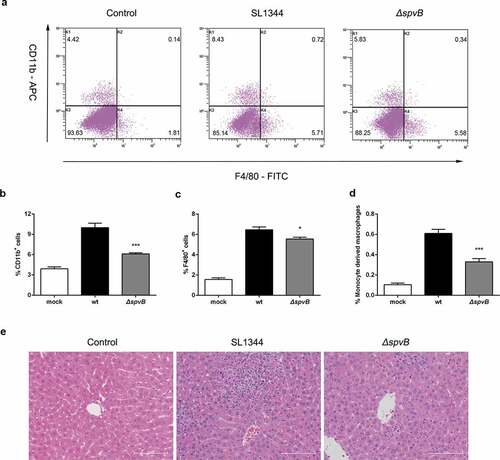
We further presumed that the Salmonella effector SpvB may be associated with inflammatory cells infiltration. At 3 day post-infection, higher mRNA levels of C-C motif chemokine ligand 2 (CCL2), C-C motif chemokine ligand 3 (CCL3) and C-X-C motif chemokine ligand 10 (CXCL10) were found in the liver of mice infected with the WT strain as compared to mice infected with the ΔspvB strain (). As a complementary approach, non-parenchymal cells isolated from the livers of WT-infected mice and ΔspvB-infected mice were analyzed by flow cytometry to confirm the observed effects of SpvB on inflammatory cell recruitment (). At 3 days post-infection, mice infected with the WT strain displayed a higher number of hepatic non-parenchymal cells expressing the pan-myeloid marker CD11b than mice infected with the ΔspvB strain (). Similar to this observation, more F4/80-positive non-parenchymal cells were found in the liver of WT-infected mice as compared with ΔspvB-infected mice (). Liver-associated F4/80+CD11b+ non-parenchymal cells are inflammatory monocyte-derived macrophages. Our data showed that mice infected with the WT strain contained a significantly higher number of hepatic F4/80+CD11b+ cells than mice infected with the ΔspvB strain at 3 days post-infection (). Additionally, the histopathological appearance of the liver showed severe inflammatory responses, such as more inflammatory cells around the hepatocytes, in mice infected with the WT strain as compared to mice infected with the ΔspvB strain (). These data suggested that SpvB contributes to an increased inflammatory response, a potential cause of iron metabolic disorder, in the liver during S. typhimurium infection.
Figure 5. SpvB increases inflammatory cell infiltration following S. typhimurium infection. Streptomycin-pretreated mice were orally infected with 1 × 107 CFUs of either the WT or the ΔspvB mutant S. typhimurium strain and analyzed at 3 days post-infection. a) Flow cytometric dot plots of hepatic non-parenchymal cells (n = 5 mice, respectively). b-d) Percentage of liver-infiltrated cell populations in S. typhimurium-infected mice. e) Histopathological analysis of the liver. Original magnification, ×20; scale bars: 100 μm. One of 3 representative histology experiments is shown. Statistical analysis was performed with IBM SPSS statistics 22. Data were compared with independent Student’s t-test. Values are expressed as the mean ± SEM, and statistically significant differences are indicated. *P< .05; ***P< .001
SpvB-mediated iron metabolic disorder is ameliorated by the TREM-1 inhibitor LP17
It has been demonstrated that triggering receptor expressed on myeloid cells 1 (TREM-1), an amplifier of inflammation that has limited expression in healthy livers, but upregulated expression during bacterial infections and liver injury, plays a fundamental role in promoting proinflammatory cytokine and chemokine secretion, as well as inflammatory cell infiltration.Citation20 Thus, we presumed that TREM-1 may be involved in SpvB-mediated increased hepatic inflammation and Salmonella virulence. In line with our hypothesis, TREM-1 expression at both the mRNA and protein levels were significantly higher in the livers of WT-infected mice than ΔspvB-infected mice at 3 days post-infection (, b). To investigate this possibility further, WT-infected mice and ΔspvB-infected mice were intraperitoneally injected with the TREM-1 inhibitor LP17. At 3 days post-infection, LP17 treatment reversed the significant difference in the hepatic bacterial burden in mice infected with the WT strain as compared to mice infected with the ΔspvB strain ( and Supplemental Fig. S1B).
Figure 6. SpvB-mediated iron metabolic disorder is ameliorated by the TREM-1 inhibitor LP17. a, b) Streptomycin-pretreated mice were orally infected with 1 × 107 CFUs of either the WT or the ΔspvB mutant S. typhimurium strain and analyzed at 3 days post-infection. a) Hepatic Trem1 levels were determined by quantitative PCR (n = 4 mice, respectively). b) Western blot analysis of whole liver lysates with specific antibodies to TREM-1 and the control α-Tubulin (n = 3 mice, respectively). Densitometric analysis of TREM-1 relative to α-Tubulin protein and one of 3 representative western blot experiments are shown. c-i) S. typhimurium-infected mice were administered i.p with either LP17 or the same volume of vector and analyzed at 3 days post-infection. c) Hepatic bacterial load was determined by plating. d) Serum iron levels were measured with a colorimetric assay. e) Hepatic iron content was determined on the basis of a multiscan spectrum. f) Hepatic Hamp levels were determined by quantitative PCR (n = 4 mice, respectively). g) Western blot analysis of whole liver lysates with specific antibodies to FPN and the control GAPDH (n = 3 mice, respectively). Densitometric analysis of FPN relative to GAPDH protein and one of 3 representative western blot experiments are shown. h) Hepatic Il6 levels were determined by quantitative PCR (n = 4 mice, respectively). i) Western blot analysis of whole liver lysates with specific antibodies to pSTAT3 and the control GAPDH (n = 3 mice, respectively). Densitometric analysis of pSTAT3 relative to GAPDH protein and one of 3 representative western blot experiments are shown. Statistical analysis was performed with IBM SPSS statistics 22. Data were compared with independent Student’s t-test. Values are expressed as the mean ± SEM, and statistically significant differences are indicated. *P< .05; **P< .01; ***P< .001; ns, not significant
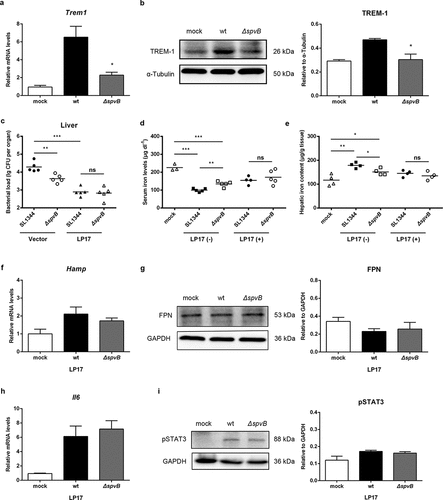
We subsequently investigated the potential role of TREM-1 in SpvB perturbing host iron homeostasis. At 3 days post-infection, S. typhimurium-infected mice receiving LP17 presented a less severe dysregulation of iron metabolism as evidenced by an increased serum iron level and reduction in hepatic iron content. LP17 administration also abrogated the effects of SpvB on host iron homeostasis (, e). In agreement, mice intraperitoneally injected with LP17 reversed the significant difference in hepatic hepcidin expression and FPN protein level between these two groups of S. typhimurium-infected mice (, g). Moreover, data showed that LP17 not only abrogated the significant difference in IL1β and TNFα expression, but also eliminated the apparent difference in CCL2 and CXCL10 expression between both groups of S. typhimurium-infected mice at 3 days post-infection (Supplemental Fig. S1C-G). These results indicated that TREM-1 contributes to SpvB-mediated inflammatory response. We further investigated whether LP17 influences the role of SpvB in activating IL6/JAK/STAT3 signaling. Indeed, both IL6 expression and STAT3 phosphorylation were similar in the livers of WT-infected mice as compared to ΔspvB-infected mice following LP17 treatment (, and Supplemental Fig. S1H). Taken together, these data demonstrated that inhibiting TREM-1 expression was beneficial to alleviating Salmonella pathogenesis and dysregulation of host iron homeostasis caused by the Salmonella effector SpvB.
SpvB interferes iron metabolism when macrophages co-cultured with hepatocytes
To further explore the role of SpvB in regulating hepcidin-FPN axis, we investigated whether SpvB could directly influence the transcription of hepcidin. As shown in , there were no apparent differences in HAMP expression among HepG2 cells infected with the WT, ΔspvB, or c-spvB strain. Subsequently, a HepG2 cells/THP-1 macrophages co-culture model was established, and we found that cells infected with the WT or the c-spvB strain displayed a higher mRNA transcript level of hepcidin as compared to those infected with the ΔspvB strain (). Meanwhile, FPN protein level was decreased more significantly in the WT or the c-spvB-infected cells than in the ΔspvB-infected cells (). Importantly, siRNA-mediated downregulation of HAMP in co-cultured HepG2 cells abrogated the significant difference in FPN expression among these Salmonella-infected THP-1 macrophages ( and Supplemental Fig. S1I). These observations indicated that cytokines such as IL6 produced by THP-1 cells were required for up-regulation of hepcidin transcription in HepG2 cells. To further investigate whether the dysregulation of systemic iron metabolism is directly caused by the effect of SpvB, THP-1 macrophages transfected with pEGFP-spvB or the control vector pEGFP-N1 were co-cultured with HepG2 cells with or without silencing of HAMP. As shown in Supplemental Fig. S1J, THP-1 macrophages transfected with pEGFP-spvB contained a significantly higher cellular iron concentration than those transfected with the control pEGFP-N1 vector. Importantly, siRNA-mediated downregulation of HAMP in co-cultured HepG2 cells resulted in a reduction of cellular iron content in THP-1 macrophages transfected with pEGFP-spvB, indicating that SpvB is per se to modulate hepcidin–FPN axis and regulate host systemic iron metabolism. In addition, when co-cultured with HepG2 cells with silencing of HAMP, we also found that iron load of THP-1 macrophages transfected with pEGFP-spvB was still higher than those transfected with the control pEGFP-N1 vector. This observation was in consistent with our previous observation that SpvB is per se to modulate cellular iron metabolism.Citation15
Figure 7. SpvB interferes macrophage iron metabolism when interacted with hepatocytes. a) HepG2 cells were infected with the WT, ΔspvB or c-spvB S. typhimurium strain at an MOI of 5, 10 or 20 for 8 h. HAMP levels were determined by quantitative PCR. b, c) Co-culture cells were infected with the WT, ΔspvB or c-spvB S. typhimurium strain at an MOI of 10 for 8 h. b) HAMP levels in co-cultured HepG2 cells were determined by quantitative PCR. c) Western blot analysis of co-cultured THP-1 macrophages lysates with specific antibodies to FPN and the control GAPDH. Densitometric analysis of FPN relative to GAPDH protein and one of 3 representative western blot experiments are shown. d) HepG2 cells with or without silencing of HAMP were co-cultured with THP-1 macrophages and then infected with the WT, ΔspvB or c-spvB S. typhimurium strain at an MOI of 10 for 8 h. Western blot analysis of co-cultured THP-1 macrophages lysates with specific antibodies to FPN and the control GAPDH. Densitometric analysis of FPN relative to GAPDH protein and one of 3 representative western blot experiments are shown. e, f) THP-1 cells with or without silencing of TREM-1 were co-cultured with HepG2 cells and then infected with the WT, ΔspvB or c-spvB S. typhimurium strain at an MOI of 10 for 8 h. e) HAMP levels in co-cultured HepG2 cells were determined by quantitative PCR. f) Western blot analysis of co-cultured THP-1 macrophages lysates with specific antibodies to FPN and the control GAPDH. Densitometric analysis of FPN relative to GAPDH protein and one of 3 representative western blot experiments are shown. Statistical analysis was performed with IBM SPSS statistics 22. The data were compared by ANOVA with Student-Newman-Keuls (S-N-K) correction. Values are expressed as the mean ± SEM of three independent experiments, and statistically significant differences are indicated. *P< .05; ns not significant
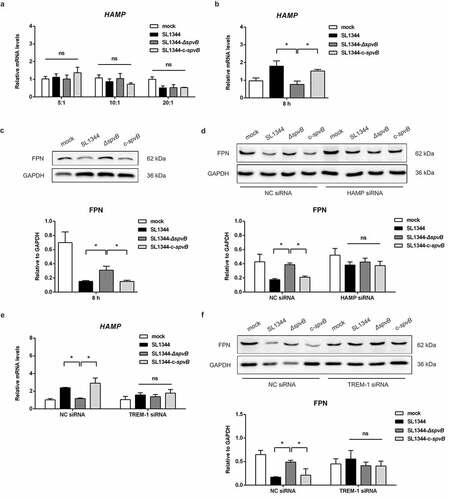
TREM-1 has been characterized as a receptor expressed on macrophages rather than hepatocytes.Citation20 TREM-1 knockdown in co-cultured THP-1 macrophages could reverse the effect of SpvB on hepcidin and FPN ( and Supplemental Fig. S1K). These data further verified our results obtained in vivo that SpvB regulates the hepcidin–FPN axis in a TREM-1-dependent manner, and targeting TREM-1 may provide potential therapeutics for treating salmonellosis.
Discussion
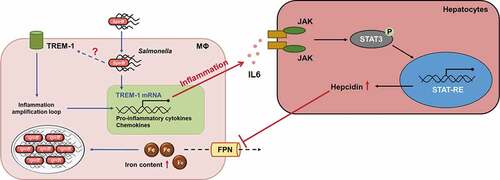
Host iron homeostasis is tightly controlled by cellular- and systemic-dependent regulatory systems.Citation10 Our previous study showed that SpvB plays an important role in promoting Salmonella virulence by facilitating Salmonella survival and proliferation within macrophages via interfering with cellular iron metabolism. However, the mechanisms of SpvB underlying Salmonella-relevant dysregulation of systemic iron metabolism have not been thoroughly studied. Here, we provided the first evidence, to our knowledge, that SpvB is one of the major participants of Salmonella-induced hepcidin expression leading to disorders of host iron homeostasis. We showed that the IL6/JAK/STAT3 pathway, but not the BMP/SMAD-dependent pathway, was involved in the SpvB-mediated upregulation of hepatic hepcidin and downregulation of FPN, which resulted in severe hypoferremia and strong iron accumulation in the liver. Moreover, SpvB promoted hepatic TREM-1 activation, thus facilitating secretion of proinflammatory cytokines and chemokines, as well as infiltration of inflammatory cells. Treatment of S. typhimurium-infected mice with the TREM-1 inhibitor LP17 was advantageous for ameliorating SpvB-mediated hepatic inflammation and, therefore, iron metabolic disorder ().
Figure 8. Model of SpvB in interfering with systemic iron metabolism. SpvB, an important pSLT-encoded cytotoxic effector, contributes to extensive and severe inflammation during S. typhimurium infection through activation of TREM-1 in macrophages. This subsequently induces hepatocyte hepcidin transcription via a IL6/JAK/STAT3-dependent manner. The induction of hepcidin expression in turn inhibits the sole iron exporter FPN, then results in an increased intramacrophage iron concentration and further facilitates hypoferremia and hepatic iron accumulation, which ultimately benefits for Salmonella survival and replication. MΦ, macrophage
Iron is an essential molecule in many biological processes, such as the host antimicrobial immune response.Citation8 During Salmonella infection, iron limitation is considered to be a central component of nutritional immunity.Citation25 Since the intracellular growth and replication of Salmonella is closely related to a sufficient iron supply, restriction in the availability of this essential nutrient metal can protect the host from severe symptoms. In response to changes in iron concentration within the microenvironment, we hypothesized that Salmonella may also employ strategies, especially the virulence factors located on the pSLT virulence plasmid, to circumvent the host iron limitation in vivo. To address this, we investigated the alternation of the host iron metabolism after oral infection of mice with the WT or ΔspvB S. typhimurium strain. Our results in this work, which demonstrated an increased hepatic bacterial burden associated with decreased serum iron content, were in line with our previous observation, which reported enhanced S. typhimurium pathogenesis and severe hypoferremia caused by SpvB. To our surprise, SpvB contributed to a significantly higher hepatic iron concentration and splenic iron overload in mice orally infected with S. typhimurium (data not shown), which was inconsistent with our previous research that demonstrated a lower splenic iron content attributed to SpvB in an intraperitoneal infection mouse model. It has been reported that mice infected with Salmonella through oral or intraperitoneal administration might cause divergent outcomes of host iron metabolism. For example, intraperitoneal infection of mice with S. typhimurium led to an increase in FPN expression, however, oral-infected mice showed a decrease in FPN levelCitation9,Citation17,Citation26 We speculated bacterial burden and their utilization of iron might be involved in differences of tissue iron concentration between oral and intraperitoneal administration.
Given that the hepcidin-FPN axis is believed to be a key regulator in the systemic regulation of iron metabolism, we presumed this essential iron regulatory axis was involved in the SpvB-mediated iron metabolic disorder within the liver. In support of this hypothesis, SpvB resulted in an increased expression of hepcidin, which was associated with a reduction in FPN protein levels. A previous study showed that hepcidin-deficient mice were more susceptible to S. typhimurium infection following intravenous administration.Citation27 Recently, a study from another laboratory reported that hepcidin deficiency had minimal effects on the growth of S. typhimurium in an intravenous infection mouse model.Citation28 However, our current data showed that there was a significant decrease of hepatic bacterial loads in Hamp−/− mice when compared to WT mice following oral infection, suggesting that hepcidin is one of the harmful factors involved in host resistance to S. typhimurium. In addition to this observation, we found that oral infection of Hamp−/− mice with the WT strain did not elicit apparent differences in the hepatic bacterial burden as compared to mice infected with the ΔspvB strain. These data suggested a role of SpvB in perturbing systemic iron metabolism by modulating the hepcidin–FPN axis. Taken together with our previous findings on SpvB interfering with cellular iron metabolism, we revealed that SpvB is an effective strategy utilized by Salmonella to scavenge iron from the host in order to achieve their own nutrient requirements.
To identify SpvB might act directly on hepatocytes to induce hepcidin transcription, either HepG2 cells only or HepG2 cells/THP-1 macrophages co-cultured model was exposed to the WT, ΔspvB or c-spvB strain. Interestingly, the hepcidin up-regulating activity caused by SpvB occurred in the presence of macrophages. During bacterial infections and inflammatory conditions, hepcidin transcription responds to a variety of inflammatory signals and mediatorsCitation23,Citation29 Our in vitro results supported these previous observations showing that cytokines secreted by macrophage play an integral role in altering hepcidin expression. Using ectopic expressed SpvB in co-cultured THP-1 macrophages, we confirmed that SpvB is per se to regulate systemic iron metabolism via modulating the hepcidin–FPN axis. Taken together, our previous publication showed that SpvB-mediated dysregulation of macrophage iron metabolism was present in early infection stage.Citation15 In current study, we further observed that SpvB could perturb macrophage iron homeostasis in late infection stage via regulating the hepcidin–FPN axis.
There are two major signaling pathways that are known to regulate hepcidin transcription in the liver and hence systemic iron metabolism: one is the BMP/SMAD pathway and the other is the JAK/STAT3 pathway.Citation30 In this study, our data showed that SpvB caused a significantly higher level of STAT3 phosphorylation, while no apparent difference was found in SMAD1/5/9 phosphorylation. Inhibition of STAT3 not only ameliorated symptoms in disorders of iron homeostasis but also reduced the hepatic bacterial burden that was mediated by SpvB. These observations demonstrated that the STAT3-dependent pathway, but not the BMP/SMAD pathway, was involved in the process of SpvB-mediated hepcidin synthesis. It has been suggested that the stimulus for the JAK/STAT3 pathway is primarily inflammatory cytokines. Among them, IL6, rather than cytokines associated with the type I response, such as TNFα, induces a signaling cascade that leads to the phosphorylation of STAT3.Citation31 Phosphorylated STAT3 subsequently translocates to the nucleus and promotes the transcription of hepcidinCitation32,Citation33 In line with our results regarding SpvB-induced STAT3 phosphorylation, a significant reduction in the expression of IL6 was found in mice infected with the ΔspvB strain as compared to those infected with the WT strain.
TREM-1, a cell-surface-activating receptor expressed on macrophages and monocytes in the liver, is elevated during bacterial challenge.Citation34 TREM-1 is considered to be an important therapeutic target in several inflammatory diseases, such as severe sepsis and pneumoniaCitation35,Citation36 A recent publication reported a new function of TREM-1 in intensifying hepatic inflammation and fibrogenesis.Citation20 However, to the best of our knowledge, it is not completely understood if TREM-1 signaling participates in Salmonella-relevant disorders of systemic iron metabolism. In this study, we showed that SpvB resulted in a significantly higher expression of TREM-1 and increased proinflammatory cytokine production such as CCL2, which was paralleled by an enhanced infiltration of inflammatory cells and hepatocellular damage in the liver. Our inhibitor assays also indicated that hepatic iron dysregulation was downstream of TREM-1 activation. When TREM-1 expression was inhibited by LP17, hypoferremia and hepatic iron accumulation caused by SpvB were abrogated. Importantly, similar S. typhimurium loads were observed in the liver of WT- and ΔspvB-infected mice after LP17 treatment, which suggested that targeting TREM-1 might provide potential therapeutic strategies to prevent or alleviate Salmonella pathogenesis. Furthermore, consistent with our results obtained in vivo, TREM-1 knockdown in macrophages could abrogate the effects of SpvB on hepcidin-FPN axis in hepatocytes/macrophages co-cultured system. However, even though the relationship between SpvB and TREM-1 has been demonstrated in this work, further investigation is required to identify the underlying mechanism. It has been reported that inhibition of the proteasome suppresses TREM-1 expressionCitation37,Citation38 Interestingly, our previous study demonstrated that proteasome pathways were involved in SpvB-relevant salmonellosis. However, whether SpvB activated TREM-1 expression via the proteasome pathway remains to be further discussed.
In summary, our findings further extended the knowledge regarding the SpvB-associated pathogenesis of Salmonella. As an extension of our previous study where we showed that SpvB disturbed cellular iron metabolism, in this study we revealed a novel contribution of SpvB to Salmonella-induced disorders of systemic iron metabolism by controlling the hepcidin-FPN axis. By activating TREM-1 signaling, SpvB contributes to extensive and severe hepatic inflammation. This subsequently induces hepcidin expression in an IL6/JAK/STAT3-dependent manner, and eventually interfering with the host nutritional immune strategy of iron withhold.
Author contributions
Q. Deng, S. Yang, and R. Huang designed and conducted the experiments; Q. Deng, S. Yang, Y. Li, and S. Wu contributed to development of methodology; Q. Deng, S. Yang, L. Sun, and K. Dong contributed to analysis and interpretation of data; Q. Deng, S. Yang, and L. Sun prepared figures and wrote the manuscript; Y. Li, S. Wu, and R. Huang supervised the project and edited the manuscript.
Abbreviation
Supplemental Material
Download Zip (841.3 KB)Acknowledgments
The authors thank Ying Xu, Ying Ye and Kai Huang (Cambridge Suda Genome Resource Center, Soochow University) for excellent technical support and for fruitful discussions. The authors also thank Hui Zhang (The Second Affiliated Hospital of Soochow University) for helping with animal experiments.
Supplementary material
Supplemental data for this article can be accessed on thepublisher’s website.
Additional information
Funding
References
- Besser JM. Salmonella epidemiology: A whirlwind of change. Food Microbiol. 2018;71:55–18. doi:10.1016/j.fm.2017.08.018.
- Fabrega A, Vila J. Salmonella enterica serovar Typhimurium skills to succeed in the host: virulence and regulation. Clin Microbiol Rev. 2013;26:308–341.
- Guiney DG, Fierer J. The role of the spv genes in Salmonella pathogenesis. Front Microbiol. 2011;2:129. doi:10.3389/fmicb.2011.00129.
- Kurita A, Gotoh H, Eguchi M, Okada N, Matsuura S, Matsui H, Danbara H, Kikuchi Y. Intracellular expression of the Salmonella plasmid virulence protein, SpvB, causes apoptotic cell death in eukaryotic cells. Microb Pathog. 2003;35(1):43–48. doi:10.1016/S0882-4010(03)00066-4.
- Browne SH, Hasegawa P, Okamoto S, Fierer J, Guiney DG. Identification of Salmonella SPI-2 secretion system components required for SpvB-mediated cytotoxicity in macrophages and virulence in mice. FEMS Immunol Med Microbiol. 2008;52(2):194–201. doi:10.1111/j.1574-695X.2007.00364.x.
- Chu Y, Gao S, Wang T, Yan J, Xu G, Li Y, Niu H, Huang R, Wu S. A novel contribution of spvB to pathogenesis of Salmonella Typhimurium by inhibiting autophagy in host cells. Oncotarget. 2016;7(7):8295–8309. doi:10.18632/oncotarget.6989.
- Bogdan AR, Miyazawa M, Hashimoto K, Tsuji Y. Regulators of iron homeostasis: new players in metabolism, cell death, and disease. Trends Biochem Sci. 2016;41(3):274–286. doi:10.1016/j.tibs.2015.11.012.
- Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe. 2013;13(5):509–519. doi:10.1016/j.chom.2013.04.010.
- Kim DK, Jeong JH, Lee JM, Kim KS, Park SH, Kim YD, Koh M, Shin M, Jung YS, Kim H-S. Inverse agonist of estrogen-related receptor gamma controls Salmonella typhimurium infection by modulating host iron homeostasis. Nat Med. 2014;20(4):419–424. doi:10.1038/nm.3483.
- Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142(1):24–38. doi:10.1016/j.cell.2010.06.028.
- Wang CY, Babitt JL. Liver iron sensing and body iron homeostasis. Blood. 2019;133:18–29.
- Ganz T. The discovery of the iron-regulatory hormone hepcidin. Clin Chem. 2019;65(10):1330–1331. doi:10.1373/clinchem.2019.306407.
- Ginzburg YZ, Feola M, Zimran E, Varkonyi J, Ganz T, Hoffman R. Dysregulated iron metabolism in polycythemia vera: etiology and consequences. Leukemia. 2018;32(10):2105–2116. doi:10.1038/s41375-018-0207-9.
- Houamel D, Ducrot N, Lefebvre T, Daher R, Moulouel B, Sari MA, Letteron P, Lyoumi S, Millot S, Tourret J. Hepcidin as a major component of renal antibacterial defenses against uropathogenic Escherichia coli. J Am Soc Nephrol. 2016;27(3):835–846. doi:10.1681/ASN.2014101035.
- Yang S, Deng Q, Sun L, Dong K, Li Y, Wu S, Huang R. Salmonella effector SpvB interferes with intracellular iron homeostasis via regulation of transcription factor NRF2. Faseb J. 2019;33(12):13450–13464. doi:10.1096/fj.201900883RR.
- Shen GS, Yang Q, Jian JL, Zhao GY, Liu LL, Wang X, Zhang W, Huang X, Xu YJ. Hepcidin1 knockout mice display defects in bone microarchitecture and changes of bone formation markers. Calcif Tissue Int. 2014;94(6):632–639. doi:10.1007/s00223-014-9845-8.
- Nairz M, Schleicher U, Schroll A, Sonnweber T, Theurl I, Ludwiczek S, Talasz H, Brandacher G, Moser PL, Muckenthaler MU. Nitric oxide-mediated regulation of ferroportin-1 controls macrophage iron homeostasis and immune function in Salmonella infection. J Exp Med. 2013;210(5):855–873. doi:10.1084/jem.20121946.
- Zhang FL, Hou HM, Yin ZN, Chang L, Li FM, Chen YJ, Ke Y, Qian ZM. Impairment of hepcidin upregulation by lipopolysaccharide in the interleukin-6 knockout mouse brain. Front Mol Neurosci. 2017;10:367. doi:10.3389/fnmol.2017.00367.
- Gibot S, Kolopp-Sarda MN, Bene MC, Bollaert PE, Lozniewski A, Mory F, Levy B, Faure GC. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J Exp Med. 2004;200(11):1419–1426. doi:10.1084/jem.20040708.
- Nguyen-Lefebvre AT, Ajith A, Portik-Dobos V, Horuzsko DD, Arbab AS, Dzutsev A, Sadek R, Trinchieri G, Horuzsko A. The innate immune receptor TREM-1 promotes liver injury and fibrosis. J Clin Invest. 2018;128(11):4870–4883. doi:10.1172/JCI98156.
- Chaston T, Chung B, Mascarenhas M, Marks J, Patel B, Srai SK, Sharp P. Evidence for differential effects of hepcidin in macrophages and intestinal epithelial cells. Gut. 2008;57(3):374–382. doi:10.1136/gut.2007.131722.
- Foka P, Dimitriadis A, Karamichali E, Kyratzopoulou E, Giannimaras D, Koskinas J, Varaklioti A, Mamalaki A, Georgopoulou U. Alterations in the iron homeostasis network: a driving force for macrophage-mediated hepatitis C virus persistency. Virulence. 2016;7(6):679–690. doi:10.1080/21505594.2016.1175700.
- Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276.
- Pietrangelo A, Dierssen U, Valli L, Garuti C, Rump A, Corradini E, Ernst M, Klein C, Trautwein C. STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology. 2007;132(1):294–300. doi:10.1053/j.gastro.2006.10.018.
- Nunez G, Sakamoto K, Soares MP. Innate nutritional immunity. J Immunol. 2018;201:11–18.
- Fang FC, Weiss G. Iron ERRs with Salmonella. Cell Host Microbe. 2014;15(5):515–516. doi:10.1016/j.chom.2014.04.012.
- Yuki KE, Eva MM, Richer E, Chung D, Paquet M, Cellier M, Canonne-Hergaux F, Vaulont S, Vidal SM, Malo D. Suppression of hepcidin expression and iron overload mediate Salmonella susceptibility in ankyrin 1 ENU-induced mutant. PLoS One. 2013;8:e55331.
- Willemetz A, Beatty S, Richer E, Rubio A, Auriac A, Milkereit RJ, Thibaudeau O, Vaulont S, Malo D, Canonne-Hergaux F. Iron- and hepcidin-independent downregulation of the iron exporter ferroportin in macrophages during Salmonella infection. Front Immunol. 2017;8:498. doi:10.3389/fimmu.2017.00498.
- Shanmugam NK, Chen K, Cherayil BJ. Commensal bacteria-induced interleukin 1beta (IL-1beta) secreted by macrophages up-regulates hepcidin expression in hepatocytes by activating the bone morphogenetic protein signaling pathway. J Biol Chem. 2015;290(51):30637–30647. doi:10.1074/jbc.M115.689190.
- Rochette L, Gudjoncik A, Guenancia C, Zeller M, Cottin Y, Vergely C. The iron-regulatory hormone hepcidin: a possible therapeutic target? Pharmacol Ther. 2015;146:35–52.
- Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101(7):2461–2463. doi:10.1182/blood-2002-10-3235.
- Rishi G, Wallace DF, Subramaniam VN. Hepcidin: regulation of the master iron regulator. Biosci Rep. 2015;35(3):3. doi:10.1042/BSR20150014.
- Verga Falzacappa MV, Vujic Spasic M, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109(1):353–358. doi:10.1182/blood-2006-07-033969.
- Han L, Fu L, Peng Y, Zhang A. Triggering receptor expressed on myeloid cells-1 signaling: protective and pathogenic roles on streptococcal toxic-shock-like syndrome caused by streptococcus suis. Front Immunol. 2018;9:577.
- Bouchon A, Facchetti F, Weigand MA, Colonna M. TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature. 2001;410(6832):1103–1107. doi:10.1038/35074114.
- Lin YT, Tseng KY, Yeh YC, Yang FC, Fung CP, Chen NJ. TREM-1 promotes survival during Klebsiella pneumoniae liver abscess in mice. Infect Immun. 2014;82(3):1335–1342. doi:10.1128/IAI.01347-13.
- Yuan Z, Syed MA, Panchal D, Rogers D, Joo M, Sadikot RT. Curcumin mediated epigenetic modulation inhibits TREM-1 expression in response to lipopolysaccharide. Int J Biochem Cell Biol. 2012;44(11):2032–2043. doi:10.1016/j.biocel.2012.08.001.
- Che X, Park KC, Park SJ, Kang YH, Jin HA, Kim JW, Seo DH, Kim DK, Kim TI, Kim WH. Protective effects of guggulsterone against colitis are associated with the suppression of TREM-1 and modulation of macrophages. Am J Physiol Gastrointest Liver Physiol. 2018;315(1):G128–G139. doi:10.1152/ajpgi.00027.2018.
