ABSTRACT
Prebiotics are compounds in food that benefit health via affecting the gut microbiome. Omega-3 fatty acids have been associated with differences in gut microbiome composition and are widely accepted to have health benefits, although recent large trials have been inconclusive. We carried out a 6-week dietary intervention comparing the effects of daily supplementation with 500 mg of omega-3 versus 20 g of a well-characterized prebiotic, inulin. Inulin supplementation resulted in large increases in Bifidobacterium and Lachnospiraceae. In contrast, omega-3 supplementation resulted in significant increases in Coprococcus spp. and Bacteroides spp, and significant decreases in the fatty-liver associated Collinsella spp. On the other hand, similar to the results with inulin supplementation which resulted in significant increases in butyrate, iso-valerate, and iso-butyrate (p < .004), omega-3 supplementation resulted in significant increases in iso-butyrate and isovalerate (p < .002) and nearly significant increases in butyrate (p < .053). Coprococcus, which was significantly increased post-supplementation with omega-3, was found to be positively associated with iso-butyric acid (Beta (SE) = 0.69 (0.02), P = 1.4 x 10−3) and negatively associated with triglyceride-rich lipoproteins such as VLDL (Beta (SE) = −0.381 (0.01), P = .001) and VLDL-TG (Beta (SE) = −0.372 (0.04), P = .001) after adjusting for confounders. Dietary omega-3 alters gut microbiome composition and some of its cardiovascular effects appear to be potentially mediated by its effect on gut microbial fermentation products indicating that it may be a prebiotic nutrient.
Introduction
The human gut is home to trillions of bacteria forming a complex ecosystem that mediates host metabolic homeostasis.Citation1Diet can shape the composition of the gut microbiota. It is therefore possible to also alter the metabolic signatures of the gut microbial populationsCitation2–4 by influencing the level and variety of substrates available for gut bacteria to metabolize.Citation5 Although the majority of dietary components are directly absorbed in the upper digestive tract, some nutrients such as fiber remain undigested and are fermented in the large intestine and are commonly referred to as prebiotics. The breakdown of carbon sources, such a dietary fiber, by the gut microbiota leads to the production of short-chain fatty acids (SCFAs) and branched-chain fatty acids (BCFAs), Citation6 which in turn have been implicated in a variety of immunological, metabolic, and hormonal effects, such as promoting satiety, reducing inflammation, and improving glucose and lipid metabolism.Citation7,Citation8 Dietary fibers can modulate gut microbiota composition in through the enrichment of bacterial taxa that utilize the substrate and tolerate or benefit from the environmental changes caused by fiber fermentation.Citation9,Citation10 Therefore, the term ‘prebiotic’ has been recently revised to include ingredients that allow specific changes to not only the composition but also the activity of the gastrointestinal microflora that confers benefits upon host well-being and health.Citation11
Many recent publications have documented the effects of prebiotic dietary fiber on health-related traits via their effects on the gut microbiome.Citation9,Citation10,Citation12 On the other hand, the impact of dietary fats, such as omega-3 polyunsaturated fatty acids (PUFAs), on the gut microbiota is less well defined. Although studies have shown that the supplementation of omega-3 provides multiple health benefits against different chronic degenerative diseases, Citation12–16 recent large trials have been inconclusive for normal subjects.Citation17 The role of the gut microbiome and individual variation in modulating these effects are yet to be explored. The influence of omega-3 on the composition of the gut microbiome has been previously explored via observational studies.Citation18 Small randomized controlled trials that have found relatively small changes in the composition of the intestinal microbiomeCitation19–21 and the functional consequences, including the levels of SCFAs is a question that have been explored, but so far the latter has been done predominantly in animal models.Citation22–24 Some recent human interventional studies have investigated the effects of high, pharmacological doses of EPA and DHA supplementationCitation21 on gut microbiome composition the effect of omega-3 intake in doses compatible with dietary intake from food (as could correspond to eating oily fish two times per week) on both gut microbiome composition and SCFA/BCFA production are lacking. In this study, we have investigated the prebiotic potential of omega 3 compared to a well-characterized prebiotic, inulin fiber. The prebiotic potential of omega 3 was evaluated based on its effect on the gut microbiome composition and function (measured by associations of the composition of the gut microbiome with changes in short-chain fatty acids and lipid metabolites) at the end of a 6-week intervention.
Methods
Study population
Study subjects were enrolled from the TwinsUK registry, a national register of adult twins recruited as volunteers without selecting for any particular disease or trait traits. A total of 69 subjects were enrolled into the study and randomized into either the omega 3 or fiber arm.
Study design and intervention
Participant eligibility included those aged >18 y who had a body mass index (BMI) between 20 and 39.9 kg/m2 and had a low habitual fiber consumption of less than 15 g/d. The following exclusion criteria were considered: ongoing or planned regular use of other omega-3 PUFA or cod liver oil supplements; seafood allergy; concomitant use of non-steroidal anti-inflammatory medications, including aspirin; current treatment for any chronic inflammatory condition or malignancy; previous colonic or small bowel resection; current smoker (minimum 6 months smoking cessation) and pregnancy. If an individual was eligible, he/she was consented and booked in for their baseline clinical visit at the clinical research facility at St Thomas’ Hospital, London, UK. Participants were randomized to take either 20 g of inulin fiber or 500 mg of omega-3 supplements daily (165 mg of EPA, 110 mg DHA, in gelatin capsules) for a period of 6 weeks. Neither participants nor researchers were blinded to the interventions and hence allocation order. The participants were booked in for a follow-up visit at the end of the 6-week intervention period. Randomization was performed using an online software (www.sealedenvelope.co.uk). All participants provided written informed consent. The trial was approved by the West Midlands Black Country Research Ethics Committee (18/WM/0066) and is registered under the clinicaltrials.gov database (NCT03442348).
Sample collection
Blood, stool, and anthropometric measures (height, weight, blood pressure, body composition) were collected at both the baseline and follow-up visits. Blood samples were collected from participants between 8:30am and 10am during each visit. Participants were instructed to come in fasted state at least since 9 pm the night before (i.e. minimum fasting time was 11.5 hours). Blood samples were collected using Serum Separator Tubes (SST) and were processed within 2–3 hours of collection for separating serum and aliquoted for storage at −20 C until the end of the intervention period.
Diet and lifestyle patterns were measured at baseline, mid-intervention (i.e. 3 weeks into the intervention), and at follow-up using a set of validated questionnaires including the EPIC-Norfolk: Food Frequency Questionnaire;Citation25 the Bristol stool form scaleCitation26, and the SF-12 quality of life questionnaire.Citation27 Fecal samples were provided at study visits and immediately frozen at −80°C until DNA extraction, which occurred as soon as the study was completed.
Microbiota analysis
The stool DNA extraction is detailed in Goodrich et al.Citation28 of 100 mg were taken from the sample and used for extraction. There was no homogenization prior to this step. Fecal samples were collected and the composition of the gut microbiome was determined by 16 S rRNA gene sequencing carried out as previously described.Citation29,Citation30
Briefly, the V4 region of the 16S rRNA gene was amplified using universal primers 355 F (CCAGACTCCTACGGGAGGCAGC) and 806 R (GGACTACHVGGGTWTCTAAT). Amplified DNA was sequenced on the MiSeq platform (Illumina). Read filtering and clustering were carried out using the MYcrobiota pipeline.Citation31 Chimeric sequences were filtered using the VSEARCH algorithm within Mothur, and reads were clustered into OTUs using closed-reference clustering against the SILVA database v132 based on a 97% similarity. Diversity metrics (Shannon index, observed OTUs, and Unweighted UniFrac) were calculated by rarefying the OTU table down to 7000 sequences per sample 50 times and taking the average. These analyses were carried out in QIIME 2 (v2018.11).
Metabolite analysis
Serum short and branched chain fatty acids:
The method employed for the serum SCFA and BCFA was based on the in-situ pentafluorbenzylation of the free acid species, followed by GC-NCI-MS determination of the resulting derivatives. Measures were only obtained from serum and not feces given recent reports indicating that circulating levels but not fecal levels, in much larger sample sizes, correlate with clinical traits.Citation32 All reagents and primary standards for acetic, propionic, iso-butyric, butyric, isovaleric, valeric acids including the internal standard (d4 acetic acid) were purchased from Sigma Aldrich.100 µl of serum were dispensed into an Eppendorf tube, followed by the addition of 100 µl of acetonitrile containing internal standard (d4 acetic acid) at 6 µmol/l. After vortexing for 30s, the mixture was treated with 5 µl of neat pentafluorobenzyl bromide, followed by 3 µl of neat diisopropylethylamine and then incubated for 30 min at 60°C to effect derivatization. On cooling, 100 µl of heptane were added and the mixture vortexed briefly to facilitate analyte transfer. Upon a brief centrifugation, the supernatant (containing the pentafluorbezyl derivatives) was transferred to a glass insert vial for GC-MS analysis. The analysis was performed on an Agilent Technologies 6890 gas chromatograph interfaced with a 5973 mass spectral detector operated in negative chemical ionization mode. The system inlet was operated in splitless mode with the injection point temperature set at 220°C. The short-chain fatty acid derivatives were separated on a DB-5 MS capillary column of dimensions 30 m × 250 µm ×0.25 µm using temperature programming and a constant carrier flow rate. Mass spectral data were acquired in selected ion monitoring mode with masses corresponding to the carboxylate anion chosen for both qualification and quantitation. Five level calibration curves were generated by preparing standards spanning the concentration range of interest and running under identical conditions. Briefly, a binary stock solution containing acetic acid and propionic was prepared at concentrations of 1.5 mg/ml and 0.15 mg/ml, respectively. Similarly, a stock solution containing iso-Butyric, Butyric, iso-Valeric, and Valeric was prepared with concentrations of 0.15 mg/ml, 0.27 mg/ml, 0.04 mg.ml and 0.15 mg/ml, respectively. It was necessary to have acetic/propionic calibrators independent of the other species to prevent traces of the other species present contributing to the lower levels of butyric onwards. These two independent stock mixtures were then diluted to form working calibrators which in turn were serially diluted to form the basis of the calibration. Hundred-microliter aliquots of the resulting calibrants were prepared as per serum.
Lipids and gut-derived metabolites:
Circulating levels of cholesterol and triglyceride fractions from fasting serum samples were measured using the high-throughput 1 H-NMR metabolomics platform (Nightingale Health Ltd., Helsinki, Finland; nightingalehealth.com/).Citation33 In addition, circulating levels of Docosahexaenoic acid (DHA) and Total omega 3 were also measured using the same platform.
Certain gut-derived metabolites such as TMAO and IPA were measured using tandem mass spectrometry with the Biocrates MxP Qaunt 500 kit (Biocrates Life Science AG, Innsbruck, Austria).Citation34
Statistical analysis
OTUs with a relative abundance of <0.1% in every sample were removed, and zero inflated relative OTU abundances were inverse normal transformed before further analysis. We investigated the different effects of fiber and omega-3 interventions on changes in OTU abundances (genus level) by running general linear models, with change in OTU abundance as the outcome and fiber/omega-3 intervention as the exposure. We adjusted for age, gender, and BMI and multiple testing using false discovery rate (FDR<0.05). Linear regressions were employed to investigate the association between OTUs and serum metabolites adjusting for covariates such as age, sex, BMI, and multiple testing (FDR < 0.05). All statistical analyses were carried out in R v3.5.2.
Results
Sixty-nine participants were randomized into either the omega-3 or inulin fiber intervention arms. The descriptive characteristics of study participants are shown in .
Table 1. Descriptive characteristics of participants in the fiber and omega 3 intervention arms at baseline and follow-up, respectively, both interventions were well tolerated with no major adverse events (AEs) reported. No differences in measures of habitual diet as assessed by FFQ were observed in either arm between baseline and follow-up (not shown)
Diversity indices
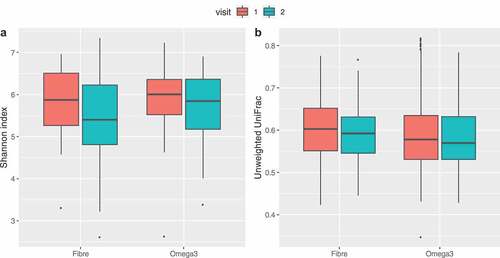
No significant change between baseline and follow-up was observed in average alpha (both Shannon and observed OTUs) and beta diversities for omega-3 and inulin intervention arms as shown in .
OTUs and serum metabolites associated with both treatment arms
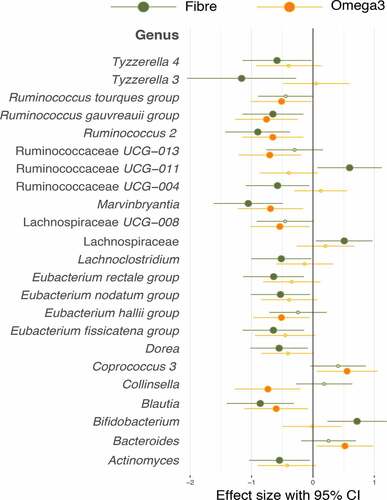
Most significantly associated with omega-3 supplementation were Coprococcus and Bacteroides, whereas Bifidobacterium, Ruminococcaceae UCG-011 and an unidentified taxon belonging to a genus from the Lachnospiraceae family was found to be significantly increased in the fiber group. There were certain species of the Lachnospiraceae related genus which were also increased in the omega-3 group, however this did not reach statistical significance as shown in .
Figure 2. Forest plot of effect sizes with 95% confidence intervals showing association of all significant (FDR adjusted p value <.05) OTUs in the Fiber and omega-3 intervention arms. Smaller dots on the fiber and omega-3 arm indicate a loss of statistical significance. Association was tested by paired t-tests between baseline and follow-up
In order to assess if the relatively small changes in the intestinal microbiome that we observed at the end of the 6-week intervention period had functional consequences, we measured gut microbiome-derived metabolites. There were significant increases in the levels of certain SCFAs and BCFAs in both the fiber and omega-3 arms with fiber eliciting a greater effect on SCFAs and BCFAs increase (). However, no significant differences were seen in the levels of TMAO and IPA in either of the arms. The levels of circulating docosahexaenoic acid (DHA) and FAW3 total omega-3 as a proportion of total fatty acids (FA) shows a significant increase in the omega-3 arm.
Table 2. Changes in the levels of serum metabolites in both arms (p-values from paired t-tests)
Association of the gut microbiome composition with short-chain fatty acids and cardiovascular markers
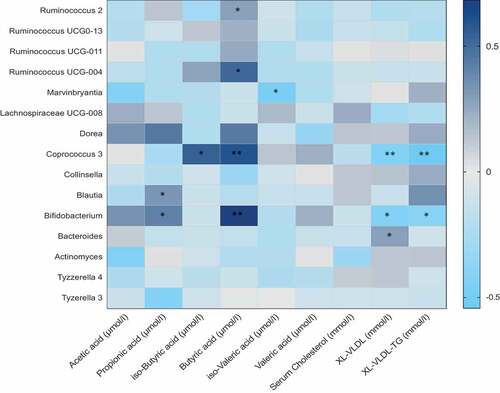
We then looked at the association between all genera that were significantly altered in one of the two interventions and SCFA, BCFA, and serum metabolites. We observed that in most cases, genera positively associated with SCFAs or BCFAs were also negatively associated with serum lipids as shown in . For instance, the relative abundance of Coprococcus, that was significantly increased in the omega-3 arm at follow-up compared to baseline, was found to be positively associated with iso-butyric acid (Beta (SE) = 0.69 (0.02), P = 1.4 x 10−3). In addition to these, the other genera that were increased in the omega-3 arm such as Ruminococcaceae UCG-004, showed positive significant associations with SCFAs such as butyrate (Beta (SE) = 0.67 (0.04), P = 1.1 x 10−3) and valerate (Beta (SE) = 0.62 (0.02), P = 1.3x 10−3) respectively. The genera Bifidobacterium, one of the genera which was significantly increased in the inulin fiber arm only was positively associated with butyrate (Beta (SE) = 0.780 (0.02), P = 1.5 x 10−5). In addition to the associations with short-chain fatty acids, we associated the different genera with markers of cardiovascular disease. We found that Coprococcus was negatively associated with VLDL (Beta (SE) = −0.381 (0.01), P = .001), VLDL-TG (Beta (SE) = −0.372 (0.04), P = .001). The genera Bifidobacterium was also negatively associated with XL-VLDL and VLDL-TG (Beta (SE) = −0.472 (0.05), P = .002; Beta (SE) = −0.463 (0.03), P = .001) respectively.
Figure 3. Heat map showing OTUs clustered at genus level and their association with serum metabolites. Values are beta coefficients from linear models adjusted for BMI, age and gender. The heat map is color coded by correlation according to the table legend (dark blue for positive and light blue for negative correlations). p values are adjusted for FDR and are indicated as FDR p < .05 (*) p < .01 (**)
Effect of BMI on gut microbiome composition and serum metabolite levels
Since the study included obese participants, we carried out sub-analyses stratifying obese (BMI>30) and non-obese subjects (BMI<30) (Supplementary Table 1). We found no significant differences in the composition of the gut microbiome between the two groups except for specific bacterial species such as Coprococcus 3 which was marginally significant in the high BMI/obese group (>30) in the omega 3 arm (p = .04). Similarly, we saw a significant association of Bifidobacterium in low BMI/lean group in the fiber arm only (p = .02). However, we found no significant associations of BMI with SCFAs, BCFAs, and cardiovascular markers (Supplementary Table 1)
Discussion
In this study, we report small, consistent changes in the human intestinal microbiome associated with 6-weeks of supplementation with 500 mg of omega-3 FA and we compare them to changes seen with inulin fiber supplementation for the same length of time. There were significant changes in the levels of bacterial fermentation products following a 6-week intervention with omega-3 supplementation and the overall effects were comparable to inulin fiber supplementation supporting the role of omega-3 as a potential prebiotic.
As expected for inulin supplementation, we observed large and significant increases in Bifidobacterium and Lachnospiraceae and in butyrate production. In the case of Omega-3, the largest increases were in Coprococcus and Bacteroides. An increase in Coprococcus abundance due to omega-3 supplementation was also reported by Waston and coworkersCitation21 using a much larger dose of omega-3 (4 g). In that study, significant increases in the abundance of Bifidobacterium were found, which we failed to observe (see ) suggesting that this effect may be dose dependent. However, in both cases, we see significant drops in the relative abundance of some SCFA producing bacteria, such as Eubacterium and some types of Ruminococaccae. This is consistent with what is known about how prebiotics affect bacterial communities.Citation35 Different substrates increase the relative abundance of different species and this in turn, results in decreases of other species, some of which may also be SCFA producers or be involved in some of the health benefits linked to the gut microbiome. This suggests that optimal prebiotic supplementation strategies should focus on feeding bacterial communities and requires an understanding of the interdependencies between bacterial strains, rather than the increase of a single carbon source.
The lack of significant change in microbial diversity associated with omega-3 PUFA intervention is consistent with previous studies, in which there was either no change, or a small change in α diversity.Citation21 However, the current study highlights significant shifts in the composition of the gut microbiome with specific short-chain fatty acid-producing bacteria that increased in not only the fiber group but also in the omega-3 group.
Emerging data have demonstrated that an aberrant gut microbiota composition is associated with several diseases, including metabolic disorders. One of the mechanisms by which the microbiota affects human health and disease is its capacity to produce metabolites which are either associated with the development of disease or those that protect against disease. One such versatile class of microbial metabolites are short and branched chain fatty acids that are commonly produced from the microbial fermentation of dietary fibers and are likely to have broad impacts on various aspects of host physiology.Citation8 In this study, we observed significant differences in the levels of short and branched chain fatty acids which were found to be positively associated with specific SCFA-producing bacteria. Coprococcus was significantly increased post-supplementation with Omega-3. The increase in Coprococcus was positively associated with iso-butyric and butyric acid levels which are fatty acids that are produced by the breakdown of amino acids rather than the breakdown of carbohydrates and are also referred to as branched-chain fatty acids.Citation36 The association of Coprococcus with butyrate has been well established in previous studies, Citation37,Citation38 however, its association with iso-butyrate has not been previously reported. The results of the correlation analysis suggested significant positive associations of the genus Coprococcus with iso-butyric and butyric acid production. We note however, that although the increase of Coprococcus in the omega-3 arm was more evident among obese individuals than among non-obese individuals, we found no corresponding difference in levels of SCFAs or other markers. Several studies support the role of both the microbiota and n-3 PUFAs in regulating inflammatory, cardiovascular, and immune markers.Citation8,Citation10,Citation12,Citation39,Citation40 In the current study, we report negative associations of Coprococcus with triglyceride-rich lipoproteins such as VLDL and VLDL-TG. The current findings suggest that the cardiovascular benefits of omega 3 supplementation may be mediated by the gut microbiome, however this requires further investigations along with the potential differences of Coprococcus effects on health among obese and non-obese individuals.
Previous larger scale trials such as ASCEND and REDUCE-IT; which tested the role of omega-3 on reducing cardiovascular events have generated conflicting results.Citation41,Citation42 Although the results from these studies highlighted that the cardio-metabolic effects could be dependent on dosage of omega-3 supplementation and cardiovascular risk score of the subjects, Citation43 the role of the gut microbiome interacting with omega-3 could also be a crucial factor that could result in the variability observed. Interestingly, we also find that omega-3 supplementation results in a strong significant decrease in the relative abundance of the genus Collinsella which we have recently reported to be increased by 3-fold in individuals with non-alcohol fatty liver disease.Citation44 Given that NAFLD is known to be a risk factor for both insulin resistance and cardiometabolic disease this suggests an important potential microbiome pathway by which omega-3 has a positive effect on health.
We acknowledge several limitations in our study. Firstly, the trial lacked direct comparisons to a placebo arm; however, the prebiotic effect of omega-3 was compared to inulin fiber, a well-characterized prebiotic. Secondly, the participants were predominantly female and therefore our results may not generalize to diverse populations. This may have also had some effects on our results in relation to the gut microbiome; however, the effect of gender has been adjusted for in all statistical analyses. Thirdly, although it has been shown that over 90% of microbes are associated with a vast proportion of the measured gut metabolites (>80%), the effects of these associations on host health are mainly derived from microbial metabolic pathways that are shared amongst microbial communities interacting with their surrounding environment.Citation45 Therefore a combined approach of metagenomics and metabolomics may provide a better understanding of the functional role of microbial species and communities in mediating immune and cardiovascular benefits. Lastly, although circulating plasma SCFA levels are more directly linked to metabolic health, they may not truly reflect the levels of SCFAs produced and absorbed by the gut.Citation46 In addition, the complexity and challenges faced by the handling of these volatile molecules adds the uncertainty of replicating levels from both sources.Citation47 Therefore, our results apply solely to the effect of gut microbes on circulating levels of SCFAs and BCFAs and we cannot extrapolate our conclusions to fecal levels of these compounds. Based on the current findings, we suggest that we can consider omega-3 fatty acids to possess the functional properties of a prebiotic due to the fact that they not only have the potential to induce small changes in the composition of the gut microbiome but also increase the levels of certain gut-derived metabolites such as BCFAs and SCFAs that have shown to positively impact metabolic health.Citation48 Although previous studies have shown increased butyrogenic capacity and therapeutic effects on trialing relatively large doses of omega 3 (greater than 4 g/d), Citation21,Citation49,Citation50 these doses could be challenging to achieve through a normal diet.Citation51 However, the current study has highlighted that prebiotic effects of omega 3 can be achieved by taking as little as 500 mg of EPA + DHA daily for 6 weeks. Furthermore, observational and clinical trials have widely demonstrated the potential benefits of prebiotics on human healthCitation52 and therefore the next steps to improve public health in the context of non-communicable diseases would be to test combinations of prebiotics to target specific diseases.
Supplemental Material
Download Zip (334 KB)Acknowledgments
We thank the Mass Spectrometry team at King’s College London for generating the Short-Chain Fatty Acid data. We wish to express our appreciation to all study participants of the TwinsUK cohort.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012 June 08;336(6086):1262–11. doi:10.1126/science.1223813.
- Holmes E, Kinross J, Gibson GR, Burcelin R, Jia W, Pettersson S, Nicholson JK. Therapeutic modulation of microbiota-host metabolic interactions. Sci Transl Med. 2012 June 06;4(137):137rv6. doi:10.1126/scitranslmed.3004244.
- Donovan SM. Introduction to the special focus issue on the impact of diet on gut microbiota composition and function and future opportunities for nutritional modulation of the gut microbiome to improve human health. Gut Microbes. 2017 Mar 4;8(2):75–81. doi:10.1080/19490976.2017.1299309.
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014 Jan 23;505(7484):559–563. doi:10.1038/nature12820.
- Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HMB, Coakley M, Lakshminarayanan B, O’Sullivan O et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012 August 09;488(7410):178–184. doi:10.1038/nature11319.
- Rentas MF, Pedreira RS, Perini MP, Risolia LW, Zafalon RVA, Alvarenga IC, Vendramini THA, Balieiro JCC, Pontieri CFF, Brunetto MA, et al. Galactooligosaccharides and a prebiotic blend improve colonic health and immunity of adult dogs. PLoS One. 2020;15(8):e0238006. doi:10.1371/journal.pone.0238006.
- Makki K, Deehan EC, Walter J, Backhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018 June 13;23(6):705–715. doi:10.1016/j.chom.2018.05.012.
- Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016 June 02;165(6):1332–1345. doi:10.1016/j.cell.2016.05.041.
- Nguyen NK, Deehan EC, Zhang Z, Jin M, Baskota N, Perez-Muñoz ME, Cole J, Tuncil YE, Seethaler B, Wang T, et al. Gut microbiota modulation with long-chain corn bran arabinoxylan in adults with overweight and obesity is linked to an individualized temporal increase in fecal propionate. Microbiome. 2020;8(1):118. doi:10.1186/s40168-020-00887-w.
- Deehan EC, Duar RM, Armet AM, Perez-Muñoz ME, Jin M, Walter J. Modulation of the gastrointestinal microbiome with nondigestible fermentable carbohydrates to improve human health. Microbiol Spectr. 2017;5(5). doi:10.1128/microbiolspec.BAD-0019-2017.
- Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD et al. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017 August 01;14(8):491–502. doi:10.1038/nrgastro.2017.75.
- Armet AM, Deehan EC, Thone JV, Hewko SJ, Walter J. The effect of isolated and synthetic dietary fibers on markers of metabolic diseases in human intervention studies: a systematic review. Adv Nutr. 2019 July 25. doi:10.1093/advances/nmz074.
- Watanabe Y, Tatsuno I. Omega-3 polyunsaturated fatty acids for cardiovascular diseases: present, past and future. Expert Rev Clin Pharmacol. 2017 August 01;10(8):865–873. doi:10.1080/17512433.2017.1333902.
- Miles EA, Calder PC. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br J Nutr. 2012 June 01;107(Suppl 2):171. doi:10.1017/S0007114512001560.
- Calder PC. Fatty acids and immune function: relevance to inflammatory bowel diseases. Int Rev Immunol. 2009;28(6):506–534. doi:10.3109/08830180903197480.
- Mori TA, Beilin LJ. Omega-3 fatty acids and inflammation. Curr Atheroscler Rep. 2004 November 01;6(6):461–467. doi:10.1007/s11883-004-0087-5.
- Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019 January 03;380(1):23–32. doi:10.1056/NEJMoa1811403.
- Menni C, Zierer J, Pallister T, Jackson MA, Long T, Mohney RP, Steves CJ, Spector TD, Valdes AM. Omega-3 fatty acids correlate with gut microbiome diversity and production of N-carbamylglutamate in middle aged and elderly women. Sci Rep. 2017 September 11;7(1):11079. doi:10.1038/s41598-017-10382-2.
- Balfego M, Canivell S, Hanzu FA, Sala-Vila A, Martinez-Medina M, Murillo S, Mur T, Ruano EG, Linares F, Porras N, et al. Effects of sardine-enriched diet on metabolic control, inflammation and gut microbiota in drug-naive patients with type 2 diabetes: a pilot randomized trial. Lipids Health Dis. 2016 April;18(15):78. doi:10.1186/s12944-016-0245-0.
- Rajkumar H, Mahmood N, Kumar M, Varikuti SR, Challa HR, Myakala SP. Effect of probiotic (VSL#3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: a randomized, controlled trial. Mediators Inflamm. 2014;2014:348959. doi:10.1155/2014/348959.
- Watson H, Mitra S, Croden FC, Taylor M, Wood HM, Perry SL, Spencer JA, Quirke P, Toogood GJ, Lawton CL et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut. 2018 November 01;67(11):1974–1983. doi:10.1136/gutjnl-2017-314968.
- Zhu L, Sha L, Li K, Wang Z, Wang T, Li Y, Liu P, Dong X, Dong Y, Zhang X, et al. Dietary flaxseed oil rich in omega-3 suppresses severity of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in rats. Lipids Health Dis. 2020;19(1):20. doi:10.1186/s12944-019-1167-4.
- Hereu M, Ramos-Romero S, Busquets C, Atienza L, Amézqueta S, Miralles-Pérez B, Nogués MR, Méndez L, Medina I, Torres JL, et al. Effects of combined D-fagomine and omega-3 PUFAs on gut microbiota subpopulations and diabetes risk factors in rats fed a high-fat diet. Sci Rep. 2019;9(1):16628. doi:10.1038/s41598-019-52678-5.
- El-Ansary A, Al-Ayadhi L. Relative abundance of short chain and polyunsaturated fatty acids in propionic acid-induced autistic features in rat pups as potential markers in autism. Lipids Health Dis. 2014;13:140. doi:10.1186/1476-511X-13-140.
- Welch AA, Luben R, Khaw KT, Bingham SA. The CAFE computer program for nutritional analysis of the EPIC-Norfolk food frequency questionnaire and identification of extreme nutrient values. J Hum Nutr Diet. 2005;18(2):99–116. doi:10.1111/j.1365-277X.2005.00593.x.
- Blake MR, Raker JM, Whelan K. Validity and reliability of the bristol stool form scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2016;44(7):693–703. doi:10.1111/apt.13746.
- Melville MR, Lari MA, Brown N, Young T, Gray D.. Quality of life assessment using the short form 12 questionnaire is as reliable and sensitive as the short form 36 in distinguishing symptom severity in myocardial infarction survivors. Heart. 2003;89(12):1445–1446. doi:10.1136/heart.89.12.1445.
- Goodrich JK, Waters J, Poole A, Sutter J, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell J, et al. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–799. doi:10.1016/j.cell.2014.09.053.
- Goodrich JK, Davenport E, Beaumont M, Jackson M, Knight R, Ober C, Spector T, Bell J, Clark A, Ley R, et al. Genetic determinants of the gut microbiome in UK twins. Cell Host Microbe. 2016;19(5):731–743. doi:10.1016/j.chom.2016.04.017.
- Jackson MA, Goodrich JK, Maxan M-E, Freedberg DE, Abrams JA, Poole AC, Sutter JL, Welter D, Ley RE, Bell JT, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65(5):749–756. doi:10.1136/gutjnl-2015-310861.
- Boers SA, Hiltemann SD, Stubbs AP, Jansen R, Hays JP. Development and evaluation of a culture-free microbiota profiling platform (MYcrobiota) for clinical diagnostics. Eur J Clin Microbiol Infect Dis. 2018 June 01;37(6):1081–1089. doi:10.1007/s10096-018-3220-z.
- Kurilshikov A, van den Munckhof ICL, Chen L, Bonder JM, Schraa K, Rutten WHJ, Ricksen PN, Graaf de J, Oosting M, Sanna S et al. Gut microbial associations to plasma metabolites linked to cardiovascular phenotypes and risk. Circ Res. 2019;124(12):1808–1820. doi:10.1161/CIRCRESAHA.118.314642.
- Soininen P, Kangas AJ, Würtz P, Suna T, Ala-Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ Cardiovasc Genet. 2015;8:192–206. doi:10.1161/CIRCGENETICS.114.000216.
- Roberts JA, Varma VR, Huang C-W, An Y, Oommen A, Tanaka T, Ferucci L, Elango P, Takebayashi T, Harada S et al. Blood Metabolite Signature of Metabolic Syndrome Implicates Alterations in Amino Acid metabolism: findings from the baltimore longitudinal study of aging (BLSA) and the tsuruoka metabolomics cohort study (TMCS). Int J Mol Sci. 2020;21:1249. doi:10.3390/ijms21041249.
- Reichardt N, Vollmer M, Holtrop G, Farquharson FM, Wefers D, Bunzel M, Duncan SH, Drew JE, Williams LM, Milligan G et al. Specific substrate-driven changes in human fecal microbiota composition contrast with functional redundancy in short-chain fatty acid production. Isme J. 2018 February 01;12(2):610–622. doi:10.1038/ismej.2017.196.
- Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003 February 01;62(1):67–72. doi:10.1079/PNS2002207.
- Duncan SH, Barcenilla A, Stewart CS, Pryde SE, Flint HJ. Acetate utilization and butyryl coenzyme A (CoA): acetate-coatransferase in butyrate-producing bacteria from the human large intestine. Appl Environ Microbiol. 2002 October 01;68(10):5186–5190.
- Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014 October 01;12(10):661–672. doi:10.1038/nrmicro3344.
- Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012 September 12;308(10):1024–1033. doi:10.1001/2012.jama.11374.
- Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002 December 01;21(6):495–505. doi:10.1080/07315724.2002.10719248.
- ASCEND Study Collaborative Group, Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Bartin J, Murphy K, Aung T, Haynes R et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med;2018 October 18:379(16):1540–1550
- Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Juliano RA, Jiao L, Granowitz C et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019 January 03;380(1):11–22. doi:10.1056/NEJMoa1812792.
- Back M, Hansson GK. Omega-3 fatty acids, cardiovascular risk, and the resolution of inflammation. Faseb J. 2019 February 01;33(2):1536–1539. doi:10.1096/fj.201802445R.
- Astbury S, Atallah E, Vijay A, Aithal PG, Grove J, Valdes MA. Lower gut microbiome diversity and higher abundance of proinflammatory genus Collinsella are associated with biopsy-proven nonalcoholic steatohepatitis. Gut Microbes. 2019 November;07:1–12.
- Visconti A, Le Roy CI, Rosa F, Rossi N, Martin TC, Mohney RP, Li W, de Rinaldis E, Bell JT, Venter JC et al. Interplay between the human gut microbiome and host metabolism. Nat Commun. 2019 October 03;10(1):4505–z. doi:10.1038/s41467-019-12476-z.
- Nishitsuji K, Xiao J, Nagatomo R, Umemoto H, Morimoto Y, Akatsu H, Inoue K, Tsuneyama K. Analysis of the gut microbiome and plasma short-chain fatty acid profiles in a spontaneous mouse model of metabolic syndrome. Sci Rep. 2017 Nov 20;7(1):15876. doi:10.1038/s41598-017-16189-5.
- Liebisch G, Ecker J, Roth S, Schweizer S, Öttl V, Schött HF, Yoon H, Haller D, Holler E, Burkhardt R, et al. Quantification of fecal short chain fatty acids by liquid chromatography tandem mass spectrometry-investigation of pre-analytic stability. Biomol. 2019 Mar 28;9(4):121. doi:10.3390/biom9040121.
- Muller M, Hernandez MAG, Goossens GH, Reijnders D, Holst JJ, Jocken JWE, van Eijk H, Canfora EE, Blaak EE. Circulating but not fecal short-chain fatty acids are related to insulin sensitivity, lipolysis and GLP-1 concentrations in humans. Sci Rep. 2019 August 29;9(1):12515. doi:10.1038/s41598-019-48775-0.
- Ueshima H, Stamler J, Elliott P, Chan Q, Brown IJ, Carnethon MR, Daviglus ML, He K, Moag-Stahlberg A, Rodriguez BL, et al. Food omega-3 fatty acid intake of individuals (total, linolenic, long-c hain) and their blood pressure: INTERMAP study. Hypertension. 2007;50:313–319. doi:10.1161/HYPERTENSIONAHA.107.090720.
- Mori TA. Omega-3 fatty acids and blood pressure. Cell Mol Biol. 2010;56:83–92.
- Carboni S, Kaur G, Pryce A, McKee K, Desbois AP, Dick JR, Galloway SDR, Hamilton DL. Mussel consumption as a “food first” approach to improve omega-3 status. Nutrients. 2019 Jun 19;11(6):1381. doi:10.3390/nu11061381.
- Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019 February 02;393(10170):434–445. doi:10.1016/S0140-6736(18)31809-9.