ABSTRACT
Alcohol is well known for promoting systemic inflammation and aggravating multiple chronic health conditions. Thus, alcohol may also be expected to serve as a risk factor in autoimmune diseases. However, emerging data from human and animal studies suggest that alcohol may in fact be protective in autoimmune diseases. These studies point toward alcohol’s complex dose-dependent relationship in autoimmune diseases as well as potential modulation by duration and type of alcohol consumption, cultural background and sex. In this review, we will explore alcohol’s pro- and anti-inflammatory properties in human and animal autoimmune diseases, including autoimmune diabetes, thyroid disease, systemic lupus erythematosus, rheumatoid arthritis, experimental autoimmune encephalomyelitis and multiple sclerosis. We will also discuss potential mechanisms of alcohol’s anti-inflammatory effects mediated by the gut microbiome.
Introduction
Autoimmune diseases arise from aberrant immune system activation against self-antigens and affect approximately 24 million people in the United States, with rising incidence in recent years.Citation1 Women tend to be disproportionately affected, with female-to-male odds ratios of up to 9:1 in some autoimmune diseases.Citation2 While there are known genetic risk alleles,Citation3 environmental factors are increasingly seen as major contributors to triggering autoimmunity.Citation4 Among environmental factors, diet and the composition of the gut microbiome are being closely studied for their role in the initiation and progression of autoimmune disorders.Citation5,Citation6
Alcohol is a widely available dietary factor in our society and its pro-inflammatory effects and end-organ damage are well documented at high doses.Citation7 However, alcohol’s role in inflammation and autoimmunity at moderate doses has been relatively less well understood. While it may be hypothesized that alcohol could serve as an environmental inflammatory risk factor, recent evidence actually points toward alcohol’s protective effects in several autoimmune diseases, including autoimmune thyroid disease, autoimmune diabetes, systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) and multiple sclerosis (MS), both in human and animal studies.Citation8–15 Although it is perplexing to explain alcohol’s apparent protective role in autoimmune diseases given its pro-inflammatory properties, current evidence suggests that alcohol has pleiotropic tissue-specific and sex-specific anti-inflammatory actions in the body at different doses.
In this review, we will examine alcohol’s dose-dependent effects and potential mechanisms in autoimmune diseases in human and animal studies with a focus on the role of alcohol in modulating the gut microbiome in autoimmunity.
Alcohol metabolism and dosing in human and animal studies
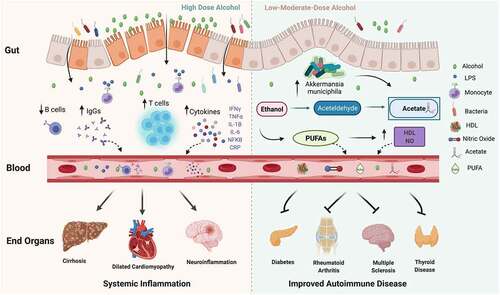
Shortly after consumption, alcohol is absorbed into the bloodstream from the stomach and small intestine, and subsequently diffuses to different body organs. Alcohol is metabolized to acetaldehyde and acetate primarily within the gastrointestinal (GI) tract, but also in other organs, such as the brain.Citation16 Both alcohol and acetaldehyde can induce systemic inflammation via 1) activation of toll-like receptors (TLRs) 2, 3, 4 and NOD-like receptor family pyrin containing 3 (NLRP3) inflammasome complex on immune cells, 2) bacterial overgrowth in the GI tract and production of a bacterial breakdown product, lipopolysaccharide (LPS) and 3) generation of free oxygen radical species and inducible nitric oxide synthase (iNOS), both of which directly affect the permeability of gut tight junctions leading to LPS leakage into the bloodstreamCitation17–20. LPS is a strong TLR agonist and leads to the activation and maturation of macrophages and other innate immune cells.Citation21 Thus, the combination of increased gut permeability, LPS translocation and alcohol-mediated immune activation can predispose to a pro-inflammatory state ().
Figure 1. Alcohol has pleiotropic effects in the body. At high doses, alcohol destabilizes the gut barrier and can lead to dysbiosis, increase in bacterial wall product, liposaccharide (LPS), which can stimulate toll-like receptors (TLR) on immune cells and lead to an increase in monocytes, T cells, cytokines and immunoglobulin (IgG) levels as well as a decrease in B cells. In turn, circulating inflammatory cytokines, IgGs and immune cells contribute to end-organ damage. At low-moderate doses, alcohol has been demonstrated to improve autoimmune disease risk and progression. Although the precise mechanism is not well understood, low-moderate alcohol may have a positive impact on inflammation via increase in Akkermansia muciniphila and other protective gut microbes, as well as contribute to increases in acetate, polyunsaturated fatty acids (PUFAs), high-density lipoprotein (HDL) and nitric oxide (NO)
In interpreting human and animal alcohol studies, it is important to closely consider the administered quantity of alcohol. Patterns of human drinking are typically divided into light, moderate and heavy consumption. For humans, a standard alcoholic drink is defined as approximately 14 g of alcohol.Citation22 According to the CDC, light drinking is considered to be three or fewer alcoholic drinks per week. Moderate drinking is defined as one alcoholic drink per day for women and two drinks per day for men, though variations across studies exist for this definition. Heavy alcohol consumption is defined as having four or more drinks/day for females and five or more drinks/day for males.Citation22 Accurate human consumption can be challenging to quantify due to participant subjective memory and accurate reporting.
In animal studies, it is also important to account for variable physiological effects due to administered dose, route of administration (e.g. oral vs. gavage vs. intravenous), consumption pattern (e.g. voluntary vs. non-voluntary), as well as differences in body weight and metabolism between animal species. Rodents, for example, metabolize alcohol approximately five times faster than humans, which results in relatively lower voluntary consumption as rodents rapidly experience the deleterious effects of acetaldehyde accumulation.Citation23 In animal studies, alcohol consumption is usually measured in grams of pure ethanol per kilogram of body weight (g/kg), though some studies may report values as blood ethanol concentration (BEC) or as alcohol by volume (ABV).Citation24 For murine alcohol studies, light, moderate and heavy alcohol consumption are generally within 0–1.5 g/kg, 2.5 g/kg and 3–6 g/kg, respectively.Citation25–27
Protective role of light-moderate dose of alcohol in autoimmune diseases
Alcohol consumption may be expected to contribute toward an increased risk of or exacerbation of autoimmune diseases given its pro-inflammatory properties. Indeed, in some inflammatory diseases, such as irritable bowel syndrome (IBS) and perennial allergies, there is a direct correlation between consumption of high alcohol doses and disease onset.Citation28,Citation29 However, impressively, in multiple studies across autoimmune diseases, light-moderate alcohol consumption appears to reduce the disease risk, severity and progression (). In the following section, we will delineate the known alcohol dose-dependent effects on autoimmune diseases.
Table 1. Alcohol’s therapeutic effects in autoimmune diseases
Autoimmune diabetes
The beneficial effects of moderate alcohol have been documented in both non-autoimmune type 2 diabetes and in autoimmune type 1 diabetes in adults (LADA)Citation30–34 (). In a study of LADA, there was a 60% risk reduction in patients who consumed 2–7 g/day compared with patients consuming 0.01–2 g/day. This study also noted higher anti‐glutamic acid decarboxylase antibody (GAD Ab) levels and lower C‐peptide in abstainers compared with alcohol consumers, with a more pronounced effect in men. In another LADA study, a 46% risk reduction was noted in men and women consuming greater than 25 g/day.Citation34 The effect appeared to be strongest in patients with low anti-GAD Ab levels and was restricted to wine drinkers compared to beer or liquor consumers.Citation34 The authors surmised that patients with low anti-GAD Ab levels may be most similar to patients with type 2 diabetes, and it may be polyphenols and hydroxystilbenes in wine that promote anti-oxidative or anti-inflammatory effects of alcohol in autoimmunity.Citation34
Autoimmune thyroid diseases
Similarly to diabetes, moderate alcohol has been demonstrated to be protective in both autoimmune hypothyroidism and hyperthyroidism (). For example, moderate alcohol was correlated with decreased risk of hypothyroidism and Grave’s disease in a dose-dependent manner compared to controls, regardless of gender or type of alcohol consumed.Citation10,Citation35 Several studies also found that moderate alcohol consumption of >10 units/weekCitation36 or at least 35 g of alcohol per dayCitation37 was associated with a lower probability of autoimmune thyroid disease and development of positive thyroid peroxidase antibodies.
Systemic lupus erythematosus
A significant dose-dependent association between moderate alcohol and SLE risk has been identified in multiple case-control, cohort and cross-sectional studiesCitation9,Citation38–41 (). In a meta-analysis, protective effects of moderate alcohol were tied to the duration of SLE, with significance seen in patients treated for less than 10 years compared to patients treated for less than 5 years.Citation40 Another study concluded that moderate alcohol may lower the chance of ANA-positive patients to progress to SLE.Citation42 Smaller case–control SLE studies, which tend to be more prone to recall bias and reverse causation bias, have either not identified an association with alcohol consumption and SLE risk or have detected a slightly higher risk.Citation43,Citation44
Rheumatoid arthritis
Similar to thyroid disease, diabetes and SLE, multiple epidemiological studies and several mechanistic studies support the protective role of light to moderate alcohol in RA in a J- or U-shaped dose-dependent mannerCitation8,Citation15,Citation45–54 (). In a meta-analysis study, both men and women had a reduction in RA risk over 10 years, with women experiencing the highest risk reduction.Citation49 In other studies, women consuming moderate alcohol reported lower disease activity and higher quality of life compared to men.Citation48 However, it has also been documented that alcohol may prevent radiological progression in men and increase radiological progression in women.Citation53 A significantly lower Modified Health Assessment Questionnaire scores (suggestive of improved functional status) have also been found in RA patients consuming moderate alcohol compared to nondrinkers.Citation55 This effect was stronger in patients who were positive for HLA-DRB1 shared epitope.Citation55 Thus, there is likely to be a beneficial but complex relationship between alcohol, gender and genetic make-up in RA.
Multiple sclerosis
Likewise, in MS there is also evidence for protective effects of moderate alcohol in decreasing disease risk and/or disease progression (). Several large population studies have demonstrated a dose-dependent inverse association between alcohol and MS risk in both sexes.Citation12,Citation56 Moderate consumption of red wine appears to correlate with a lower Expanded Disability Status Scale score, suggesting improved function, though patients drinking moderate alcohol exhibited an increase in T2 lesion volume on brain MRI.Citation57 Conversely, high doses of alcohol may contribute to increased risk of MS, particularly in men.Citation58,Citation59
Some studies have noted no association between different doses of alcohol and the risk of developing MS. In these studies, gender may be a variable that may explain alcohol’s effects in MS. For example, in a female Nurses’ Health Study (NHS) I and II, there was no association between different types of alcohol and the risk of MS.Citation60 Although this was a large study of >90,000 women between the two NHS studies, the cohort of MS patients of 258 cases was relatively smaller and it is also possible that females may not experience the degree of protective effects of alcohol compared to males. For example, in an animal model of MS, experimental autoimmune encephalomyelitis (EAE), it was recently shown that primarily male mice improved in disease scores on a moderate alcohol diet.Citation11
Alcohol’s pro- and anti-inflammatory effects on the immune system
It is well known that chronic high-dose alcohol consumption can lead to a higher infectious disease burden in alcoholics and more difficulty in clearing pathogens such as Listeria monocytogenes, Mycobacterium tuberculosis and influenza.Citation61–63 However, while chronic high-level alcohol consumption is known to induce systemic inflammation, there is an increasing understanding that alcohol’s effects on the innate and adaptive immune system are dose-dependent.
For example, alcohol has prominent dose-dependent effects on microglia, the innate immune cells of the central nervous system (CNS). In mouse models of acute alcohol abuse, cerebellar microglia display no inflammatory cytokine production following a single moderate-dose alcohol exposure of 3 g/kg and only a transient IL-1β/TNF-α increase following high-dose administration 5 g/kg.Citation64 At much higher alcohol doses of up to 10 g/kg/day, microglia display increased activation in association with the production of different inflammatory cytokines, including IL-1β, IL-18, IL-10, interferon-gamma (IFN-γ), transformative growth factor beta (TGF-β) and chemokines, CXCL2, CX3CL1. In turn, these cytokines and chemokines can lead to peripheral lymphocyte translocation across the blood–brain barrier (BBB) and further CNS inflammation.Citation65–68
Alcohol also modulates the adaptive immune system in a dose-dependent manner. Chronic moderate alcohol consumption leads to T and B cell activation and proliferation,Citation69 while chronic heavy consumption is associated with T and B cell depletion and apoptosis as well as an increase in immunoglobulins.Citation70 Additionally, chronic binge alcohol consumption changes T cell phenotypes leading to a decreased percentage of naive T lymphocytes and higher percentages of memory T-cells.Citation71,Citation72 Conversely, moderate alcohol consumption has been linked to modulation of T follicular helper (TFH) cells.Citation8
Cytokines and inflammatory markers are also affected by alcohol in a dose-dependent manner. For instance, C-reactive protein (CRP) and interleukin 6 (IL-6) are elevated in human heavy drinkers but relatively reduced in moderate drinkers compared to nondrinkers.Citation73 CRP effects may also be sexually dimorphic, with some studies indicating that alcohol-induced CRP reduction is specific to women,Citation74 though other studies suggest that moderate consumption reduces CRP in a U-shaped pattern regardless of sex.Citation75
Pro- and anti-inflammatory dose-dependent alcohol effects on the immune system in rheumatoid arthritis
In RA, a J-shaped association has been noted with CRP levels, with patients consuming 1–7 drinks/week had the lowest CRP levels.Citation51 RA patients consuming moderate alcohol display a U-shaped association with IL-6 levels prior to symptom development and an inverse relationship between alcohol consumption and soluble tumor necrosis factor receptor 2 (TNFR2) levels.Citation76 As alcohol can contribute to liver damage, a study evaluating the relationship between alcohol consumption and liver inflammation reported that >21 units per week correlated with transaminitis, while <14 units per week did not.Citation48 Moderate alcohol consumption has also been associated with 50% reduction in RA risk in patients positive for anticitrullinated protein antibodies (ACPA), and a 30% disease risk reduction in ACPA-negative RA in an inverse dose–response relationship.Citation47
Dose-dependent effects of alcohol on the immune system are also noted in RA mouse models. In a model of collagen-induced arthritis (CIA), mice on a moderate alcohol diet experienced a 40% lower incidence of CIA and >50% decrease in radiological disease severity compared to non-alcohol controls.Citation8 Alcohol-consuming mice also had lower levels of IL-21 and IL-17A, neutrophils, monocytes, plasma B cells and IgG levels.Citation8 The authors also found that both alcohol and acetate affected the functional state of T follicular helper (TFH) cells in vitro and in vivo, leading to suppression of IL-21 secretion.Citation8 These findings are intriguing as TFH cells are often found in synovial joints in RA patients and are also important mediators of gut immunity, suggesting a possible link between immune processes in the gut and RA. In another CIA study, moderate alcohol (10% ethanol in water) delayed the onset and ameliorated the progression of CIA via an increase in endogenous testosterone, inhibition of nuclear factor B activation and down-regulation of leukocyte migration.Citation77
Alcohol’s pro- and anti-inflammatory effects on the gut
The gut microbiome, composed of trillions of microorganisms, is increasingly considered to be one of the critical environmental factors in modulating the immune system and risk of autoimmune diseases. For example, fecal transplantation of gut bacteria from patients with SLE and MS to animal models can reproduce disease symptoms in animals.Citation78,Citation79 Conversely, supplementation of a dysbiotic microbiome with diverse commensal species through fecal transplant has therapeutic benefits in IBS and MS.Citation80–82 Similarly, in animal studies, antibiotic ablation of the gut flora is sufficient to prevent EAE onset entirely, while the introduction of strains of Erysipelotrichaceae and Lactobacillus reuteri exacerbates EAE symptoms and increases autoreactive T-cells.Citation83 In an RA model in germ-free mice, the introduction of segmented filamentous bacteria (SFB) was sufficient for the development of arthritis via Th17 response.Citation84 Likewise, in non-germ-free RA mice, oral gavage of SFB drove the differentiation and migration of TFH cells systemically, leading to autoantibody generation and arthritis exacerbation.Citation85
As alcohol is largely metabolized within the GI tract, it is a prime factor to impact gut microbiome composition, gut immune system and downstream systemic immune communications with other organs. In the following section, we will focus on alcohol’s effects on the gut, gut immune system and gut metabolism of fatty acids and how these effects may translate into pro-inflammatory vs protective effects in autoimmune diseases.
Pro-inflammatory effects of high-dose alcohol on the gastrointestinal tract, gut microbiome, gut metabolites and nutrients
Microbiome transfer from high-dose alcohol-fed mice to alcohol-naïve germ-free mice has been shown to induce intestinal inflammation in the recipient mice.Citation86 Potential explanations for how high-dose alcohol may induce gut inflammation include alteration of the gut microbiome (dysbiosis) in alcohol-metabolizing species, bacterial cytotoxicity, changes to enteric peristalsis and hepatotoxicity (liver steatosis and cirrhosis).Citation87–90 Microbiome dysbiosis can lead to impaired intestinal permeability and promote inflammation via systemic translocation of gut bacterial endotoxin, LPS, activation of TLR and nuclear factor-κB (NF-kB) on immune cells and induction of inflammatory iNOSCitation17,Citation19,Citation20. In turn, hepatotoxicity interferes with the liver’s ability to detoxify substances, which results in systemic accumulation of alcohol’s toxic metabolite, acetaldehyde.Citation91 In addition, LPS-mediated activation of liver-resident macrophages, Kupffer cells, further contributes to pro-inflammatory cytokine release and propagation of systemic inflammation.Citation92
There are also specific microbiota changes that have been described in animal and human high-dose alcohol studies (). In human alcohol use disorder (AUD) studies, dysbiosis has been characterized by lower Bacteroidetes,Citation89,Citation93 lower Akkermansia muciniphila,Citation94Citation121Citation95 and higher Proteobacteria.Citation89 In animal models of high-dose alcohol consumption, alcohol-consuming animals have reduced bacterial diversity along with lower Bacteroidetes, elevated Proteobacter, elevated Actinobacter,Citation94 reduction in Firmicutes and elevation in Bacteroidetes.Citation96 However, not all studies have noted a reduction in Firmicutes in response to high-dose alcohol. For example, voluntary self-administration of chronic high-dose alcohol in macaques resulted in reduced Bacteroidetes, elevated Firmicutes and a complete absence of Akkermansia muciniphila during the drinking period, while abstinence from drinking restored baseline bacterial species.Citation90
Table 2. Alcohol induced gut microbiota alterations by dose and alcohol diet duration
In addition to changes in microbiota, high-dose alcohol can also decrease immunomodulatory gut metabolites such as aryl hydrocarbon receptor (AhR).Citation97 AhR is a transcriptional factor expressed in immune cells and is known to impact T cell differentiation, effector and regulatory T cell functions. AhR aberrant expression has also been linked to autoimmune dysregulation.Citation98,Citation99 As AhR ligand administration is beneficial in autoimmune diseases, lower levels of AhR due to chronic high-dose alcohol may potentially contribute to autoimmune disease exacerbation.
Lastly, chronic high-dose alcohol intake can cause malabsorption and nutrient deficiencies.Citation100 In turn, nutrient deficiencies for vitamins such as thiamine, cyanocobalamin and vitamin D can exacerbate different autoimmune conditions.Citation101
Anti-inflammatory effects of low-to-moderate alcohol on the gut microbiome, gut metabolites and fatty acids
An important way in which alcohol may beneficially impact autoimmune inflammation is via its effects on fatty acid metabolism in the gut. While at high doses alcohol is known to lead to fatty acid dysregulation and development of fatty liver disease,Citation102–104 at lower doses, alcohol may contribute to the generation of gut-derived anti-inflammatory fatty acids, such as short-chain fatty acids (SCFAs) and polyunsaturated fatty acids (PUFAs)Citation103,Citation104 ().
SCFAs, including formate, acetate, propionate and butyrate, are a class of carboxylic acids produced primarily through microbial fermentation of dietary fiber.Citation106 Importantly, SCFAs are known to have important anti-microbial and anti-inflammatory properties and to reduce inflammation in autoimmune diseases.Citation107,Citation108 Oral supplementation of SCFA cocktail has been shown to be sufficient to reduce autoreactive Th1 and Th17 cell activity and to dampen disease severity in animal models of autoimmune colitis and EAE.Citation109,Citation110
There are potentially two ways in which low-to-moderate alcohol consumption can modulate SCFA production. First, low-to-moderate alcohol can alter SCFA-producing microbial communities in the gut, such as Akkermansia muciniphila.Citation111 Caslin, Maguire et al. showed that moderate alcohol consumption (2.6% ABV Lieber-DeCarli diet) increased levels of Akkermansia muciniphila in the gut in association with reducing disease severity in EAE.Citation11 Similarly, Lee et al. found that short-term alcohol consumption (5 days of 0.8 g/kg intragastric) elevated Akkermansia muciniphila levels in mice, an elevation not observed in groups consuming fermented rice liquor (FRL) of equivalent alcoholic strength.Citation112 In addition, alcohol itself is metabolized into the SCFA, acetate,Citation113 and animals fed a Lieber-DeCarli diet for 8 weeks show elevated levels of acetic acid compared to controls.Citation114 Thus, low-to-moderate alcohol could impact SCFA balance both by influencing SCFA producing bacteria and via acetate production ().
Another potential mechanism of low-to-moderate alcohol’s protection in autoimmune diseases may rely on alcohol’s important role in the metabolism of essential PUFAs, such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA).Citation103,Citation105 These PUFAs can reduce reactive oxygen species formation and act as anti-inflammatory molecules. Low-to-moderate dose alcohol has been shown to increase PUFA production,Citation115 while at high alcohol doses, PUFA concentration decreases due to increased fatty acid catabolism.Citation103 Of note, PUFAs and PUFA derivatives, such as resolvins, lipoxins and protection, have been linked to the mitigation of autoimmune diseases.Citation116,Citation117 In addition, an increase in PUFAs has also been shown to be cardioprotective in multiple studies.Citation118–120 As cardiovascular health is becoming an important factor in autoimmune disease outcomes, it is possible that this may be another protective mechanism mediated by low-to-moderate alcohol ().
Protective effects of low-moderate alcohol on the gut microbiome in models of multiple sclerosis
In an animal model of MS, EAE, Caslin, Maguire et al. administered a 2.6% moderate alcohol or isocaloric diet to both male and female C57BL/6 J mice and observed that males on the diet exhibited a greater long-term disease remission while females initially experienced remission and a subsequent disease exacerbation.Citation11 In this study, a moderate alcohol diet resulted in sex-specific gut microbiota alterations in individual immunoregulatory taxa, such as Turicibacter, Akkermansia and Prevotella, and also led to significant enrichment in beneficial Clostridial and Firmicute networks of bacteria.Citation11 Another study evaluating a moderate alcohol model of 10% (vol/vol) in EAE also documented EAE amelioration in alcohol-consuming C57BL/6 J mice and implicated gut-related TFH cells mechanistically.Citation8
Conclusions and future directions
Overall, current evidence points to a dose-dependent association between alcohol and disease severity in multiple autoimmune diseases, including autoimmune thyroid disease, diabetes, SLE, RA and MS. At low-to-moderate doses, alcohol appears to have protective effects, while at higher consumption patterns, alcohol can be addictive and can contribute to detrimental symptomatic effects on the host and worse autoimmune disease outcomes.
Though the exact mechanism by which low-to-moderate alcohol mediates autoimmune disease amelioration remains to be fully understood, emerging mechanistic studies suggest that low-to-moderate alcohol likely has both a systemic immunomodulatory role, such as shifting the balance of anti-inflammatory innate and adaptive immune cells and cytokines/chemokines, as well as a role in sculpting the composition of the gut microbiome and their fatty acid metabolites, such as SCFAs and PUFAs.
Future prospective patient studies that account for sex, age, cultural and socioeconomic background, alcohol type, timing of administration and mechanistic animal model studies on the gut microbiome and the immune system will be critical to better understand alcohol’s role in autoimmune diseases. In turn, this knowledge will help guide the creation of specific clinical recommendations on alcohol consumption in patients with autoimmune diseases as well as help identify protective immune and gut-derived biomarkers that could be used in the treatment of autoimmune diseases independently of alcohol.
Contributions
BC, KM, ST and EM wrote the first draft of the paper. ST and KM made the first draft of the tables. EM edited the manuscript, created the figure and final tables.
Acknowledgments
We are grateful to Dr R. Adron Harris, Dr William Schwartz and Cole Maguire for their advice and support in preparing this review. was created with Biorender.com.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Roberts MH, Erdei E. Comparative United States autoimmune disease rates for 2010-2016 by sex, geographic region, and race. Autoimmun Rev. 2020;19(1):102423. doi:10.1016/j.autrev.2019.102423.
- Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35:347–15.
- Ceccarelli F, Agmon-Levin N, Perricone C. Genetic factors of autoimmune diseases 2017. J Immunol Res. 2017;2017:2789242. doi:10.1155/2017/2789242.
- Costenbader KH, Gay S, Alarcon-Riquelme ME, Iaccarino L, Doria A. Genes, epigenetic regulation and environmental factors: which is the most relevant in developing autoimmune diseases? Autoimmun Rev. 2012;11:604–609. doi:10.1016/j.autrev.2011.10.022.
- Yamamoto EA, Jørgensen TN. Relationships between vitamin D, gut microbiome, and systemic autoimmunity. Front Immunol. 2020;10:3141. doi:10.3389/fimmu.2019.03141.
- Hills RD, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut microbiome: profound implications for diet and disease. Nutrients. 2019;11:1613. doi:10.3390/nu11071613.
- Bishehsari F, Magno E, Swanson G, Desai V, Voigt RM, Forsyth CB, Keshavarzian A. Alcohol and gut-derived inflammation. Alcohol Res. 2017;38:163–171.
- Azizov V, Dietel K, Steffen F, Dürholz K, Meidenbauer J, Lucas S, Frech M, Omata Y, Tajik N, Knipfer L, et al. Ethanol consumption inhibits TFH cell responses and the development of autoimmune arthritis. Nat Commun. 2020;11:1998. doi:10.1038/s41467-020-15855-z.
- Barbhaiya M, Lu B, Sparks JA, Malspeis S, Chang S-C, Karlson EW, Costenbader KH. Influence of alcohol consumption on the risk of systemic lupus erythematosus among women in the nurses’ health study cohorts. Arthritis Care Res (Hoboken). 2017;69:384–392. doi:10.1002/acr.22945.
- Carle A, Pedersen IB, Knudsen N, Perrild H, Ovesen L, Rasmussen LB, Jørgensen T, Laurberg P. Moderate alcohol consumption may protect against overt autoimmune hypothyroidism: a population-based case–control study. Eur J Endocrinol. 2012;167(4):483–490. doi:10.1530/EJE-12-0356.
- Caslin B, Maguire C, Karmakar A, Mohler K, Wylie D, Melamed E. Alcohol shifts gut microbial networks and ameliorates a murine model of neuroinflammation in a sex-specific pattern. Proc Natl Acad Sci U S A. 2019;116:25808–25815. doi:10.1073/pnas.1912359116.
- HedströmAK,HillertJ,OlssonT,AlfredssonL.Alcoholasamodifiablelifestylefactoraffectingmultiplesclerosisrisk.JAMANeurol.2014;71:300–305. doi:10.1001/jamaneurol.2013.5858.
- Kiyohara C, Washio M, Horiuchi T, Asami T, Ide S, Atsumi T, Kobashi G, Tada Y, Takahashi H. Cigarette smoking, alcohol consumption, and risk of systemic lupus erythematosus: a case-control study in a Japanese population. J Rheumatol. 2012;39:1363–1370. doi:10.3899/jrheum.111609.
- Lu B, Solomon DH, Costenbader KH, Karlson EW. Alcohol consumption and risk of incident rheumatoid arthritis in women: a prospective study. Arthritis Rheumatol. 2014;66:1998–2005. doi:10.1002/art.38634.
- Rasouli B, Ahlbom A, Andersson T, Grill V, Midthjell K, Olsson L, Carlsson S. Alcohol consumption is associated with reduced risk of type 2 diabetes and autoimmune diabetes in adults: results from the Nord-Trondelag health study. Diabet Med. 2013;30:56–64. doi:10.1111/j.1464-5491.2012.03713.x.
- Cederbaum AI. Alcohol metabolism. Clin Liver Dis. 2012;16:667–685. doi:10.1016/j.cld.2012.08.002.
- Lawrimore CJ, Crews FT. Ethanol, TLR3, and TLR4 agonists have unique innate immune responses in neuron-like SH-SY5Y and microglia-like BV2. Alcohol Clin Exp Res. 2017;41:939–954. doi:10.1111/acer.13368.
- Iracheta-Vellve A, Petrasek J, Gyogyosi B, Bala S, Csak T, Kodys K, Szabo G. Interleukin-1 inhibition facilitates recovery from liver injury and promotes regeneration of hepatocytes in alcoholic hepatitis in mice. Liver Int. 2017;37:968–973. doi:10.1111/liv.13430.
- Wang H, Khor TO, Saw CL, Lin W, Wu T, Huang Y, Kong ANT. Role of Nrf2 in suppressing LPS-induced inflammation in mouse peritoneal macrophages by polyunsaturated fatty acids docosahexaenoic acid and eicosapentaenoic acid. Mol Pharm. 2010;7:2185–2193. doi:10.1021/mp100199m.
- Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50:538–547. doi:10.1016/j.jhep.2008.10.028.
- Lien E, Means TK, Heine H, Yoshimura A, Kusumoto S, Fukase K, Fenton MJ, Oikawa M, Qureshi N, Monks B, et al. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest. 2000;105:497–504. doi:10.1172/JCI8541.
- Rolland B, Chazeron I, Carpentier F, Moustafa F, Viallon A, Jacob X, Lesage P, Ragonnet D, Genty A, Geneste J, et al. Comparison between the WHO and NIAAA criteria for binge drinking on drinking features and alcohol-related aftermaths: results from a cross-sectional study among eight emergency wards in France. Drug Alcohol Depend. 2017;175:92–98. doi:10.1016/j.drugalcdep.2017.01.034.
- Holmes RS, Duley JA, Algar EM, Mather PB, Rout UK. Biochemical and genetic studies on enzymes of alcohol metabolism: the mouse as a model organism for human studies. Alcohol Alcohol. 1986;21:41–56.
- Leeman RF, Heilig M, Cunningham CL, Stephens DN, Duka T, O’Malley SS. Ethanol consumption: how should we measure it? Achieving consilience between human and animal phenotypes. Addict Biol. 2010;15:109–124. doi:10.1111/j.1369-1600.2009.00192.x.
- Bjork JM, Gilman JM. The effects of acute alcohol administration on the human brain: insights from neuroimaging. Neuropharmacology. 2014;84:101–110. doi:10.1016/j.neuropharm.2013.07.039.
- Kanuri G, Wagnerberger S, Landmann M, Prigl E, Hellerbrand C, Bischoff SC, Bergheim I. Effect of acute beer ingestion on the liver: studies in female mice. Eur J Nutr. 2015;54:465–474. doi:10.1007/s00394-014-0730-z.
- Pruett S, Tan W, Howell GE 3rd, Nanduri B. Dosage scaling of alcohol in binge exposure models in mice: an empirical assessment of the relationship between dose, alcohol exposure, and peak blood concentrations in humans and mice. Alcohol. 2020;89:9–17. doi:10.1016/j.alcohol.2020.03.011.
- Bendtsen P, Gronbaek M, Kjaer SK, Munk C, Linneberg A, Tolstrup JS. Alcohol consumption and the risk of self-reported perennial and seasonal allergic rhinitis in young adult women in a population-based cohort study. Clin Exp Allergy. 2008;38:1179–1185. doi:10.1111/j.1365-2222.2008.02945.x.
- Hsu TY, He GY, Wang YC, Chen C-Y, Wang S-H, Chen W-K, Kao C-H. Alcohol use disorder increases the risk of irritable bowel disease: a nationwide retrospective cohort study. Medicine (Baltimore). 2015;94:e2334. doi:10.1097/MD.0000000000002334.
- Carlsson S, Hammar N, Grill V. Alcohol consumption and type 2 diabetes meta-analysis of epidemiological studies indicates a U-shaped relationship. Diabetologia. 2005;48:1051–1054. doi:10.1007/s00125-005-1768-5.
- Joosten MM, Chiuve SE, Mukamal KJ, Hu FB, Hendriks HF, Rimm EB. Changes in alcohol consumption and subsequent risk of type 2 diabetes in men. Diabetes. 2011;60:74–79. doi:10.2337/db10-1052.
- Kerr WC, Williams E, Li L, Lui CK, Ye Y, Greenfield TK, Lown EA. Alcohol use patterns and risk of diabetes onset in the 1979 National Longitudinal Survey of Youth Cohort. Prev Med. 2018;109:22–27. doi:10.1016/j.ypmed.2018.01.010.
- Li XH, Yu FF, Zhou YH, He J. Association between alcohol consumption and the risk of incident type 2 diabetes: a systematic review and dose-response meta-analysis. Am J Clin Nutr. 2016;103:818–829. doi:10.3945/ajcn.115.114389.
- Rasouli B, Andersson T, Carlsson PO, Dorkhan M, Grill V, Groop L, Martinell M, Tuomi T, Carlsson S. Alcohol and the risk for latent autoimmune diabetes in adults: results based on Swedish ESTRID study. Eur J Endocrinol. 2014;171:535–543. doi:10.1530/EJE-14-0403.
- Carle A, Bulow Pedersen I, Knudsen N, Perrild H, Ovesen L, Rasmussen LB, Jørgensen T, Laurberg P. Graves’ hyperthyroidism and moderate alcohol consumption: evidence for disease prevention. Clin Endocrinol (Oxf). 2013;79:111–119. doi:10.1111/cen.12106.
- Effraimidis G, Tijssen JG, Wiersinga WM. Alcohol consumption as a risk factor for autoimmune thyroid disease: a prospective study. Eur Thyroid J. 2012;1:99–104. doi:10.1159/000338920.
- Huang Y, Cai L, Zheng Y, Pan J, Li L, Zong L, Lin W, Liang J, Huang H, Wen J, et al. Association between lifestyle and thyroid dysfunction: a cross-sectional epidemiologic study in the She ethnic minority group of Fujian Province in China. BMC Endocr Disord. 2019;19:83. doi:10.1186/s12902-019-0414-z.
- Hardy CJ, Palmer BP, Muir KR, Sutton AJ, Powell RJ. Smoking history, alcohol consumption, and systemic lupus erythematosus: a case-control study. Ann Rheum Dis. 1998;57:451–455. doi:10.1136/ard.57.8.451.
- Bengtsson AA, Rylander L, Hagmar L, Nived O, Sturfelt G. Risk factors for developing systemic lupus erythematosus: a case-control study in southern Sweden. Rheumatology (Oxford). 2002;41:563–571. doi:10.1093/rheumatology/41.5.563.
- Wang J, Pan HF, Ye DQ, Su H, Li XP. Moderate alcohol drinking might be protective for systemic lupus erythematosus: a systematic review and meta-analysis. Clin Rheumatol. 2008;27:1557–1563. doi:10.1007/s10067-008-1004-z.
- Cozier YC, Barbhaiya M, Castro-Webb N, Conte C, Tedeschi S, Leatherwood C, Costenbader KH, Rosenberg L. A prospective study of obesity and risk of systemic lupus erythematosus (SLE) among Black women. Semin Arthritis Rheum. 2019;48(6):1030–1034. doi:10.1016/j.semarthrit.2018.10.004.
- Hahn J, Leatherwood C, Malspeis S, Liu X, Lu B, Roberts AL, Sparks JA, Karlson EW, Feldman CH, Munroe ME, et al. Associations between daily alcohol consumption and systemic lupus erythematosus-related cytokines and chemokines among US female nurses without SLE. Lupus. 2020;29:976–982. doi:10.1177/0961203320929427.
- Formica MK, Palmer JR, Rosenberg L, McAlindon TE. Smoking, alcohol consumption, and risk of systemic lupus erythematosus in the Black women’s health study. J Rheumatol. 2003;30:1222–1226.
- Kim SK, Lee SS, Choe JY, Park SH, Lee H. Effect of alcohol consumption and smoking on disease damage in systemic lupus erythematosus: data from the Korean Lupus Network (KORNET) registry. Lupus. 2017;26:1540–1549. doi:10.1177/0961203317709346.
- Baker JF, England BR, Mikuls TR, Hsu JY, George MD, Pedro S, Sayles H, Michaud K. Changes in alcohol use and associations with disease activity, health status, and mortality in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2020;72:301–308. doi:10.1002/acr.23847.
- Bergman S, Symeonidou S, Andersson ML, Soderlin MK, Group B. Alcohol consumption is associated with lower self-reported disease activity and better health-related quality of life in female rheumatoid arthritis patients in Sweden: data from BARFOT, a multicenter study on early RA. BMC Musculoskelet Disord. 2013;14:218. doi:10.1186/1471-2474-14-218.
- Hedstrom AK, Hossjer O, Klareskog L, Alfredsson L. Interplay between alcohol, smoking and HLA genes in RA aetiology. RMD Open. 2019;5:e000893. doi:10.1136/rmdopen-2019-000893.
- Humphreys JH, Warner A, Costello R, Lunt M, Verstappen SMM, Dixon WG. Quantifying the hepatotoxic risk of alcohol consumption in patients with rheumatoid arthritis taking methotrexate. Ann Rheum Dis. 2017;76:1509–1514. doi:10.1136/annrheumdis-2016-210629.
- Jin Z, Xiang C, Cai Q, Wei X, He J. Alcohol consumption as a preventive factor for developing rheumatoid arthritis: a dose-response meta-analysis of prospective studies. Ann Rheum Dis. 2014;73:1962–1967. doi:10.1136/annrheumdis-2013-203323.
- Kallberg H, Jacobsen S, Bengtsson C, Pedersen M, Padyukov L, Garred P, Frisch M, Karlson EW, Klareskog L, Alfredsson L, et al. Alcohol consumption is associated with decreased risk of rheumatoid arthritis: results from two Scandinavian case-control studies. Ann Rheum Dis. 2009;68:222–227. doi:10.1136/ard.2007.086314.
- Mangnus L, Van Steenbergen HW, Nieuwenhuis WP, Reijnierse M, Van Der Helm-van Mil AHM. Moderate use of alcohol is associated with lower levels of C reactive protein but not with less severe joint inflammation: a cross-sectional study in early RA and healthy volunteers. RMD Open. 2018;4:e000577. doi:10.1136/rmdopen-2017-000577.
- Maxwell JR, Gowers IR, Moore DJ, Wilson AG. Alcohol consumption is inversely associated with risk and severity of rheumatoid arthritis. Rheumatology (Oxford). 2010;49:2140–2146. doi:10.1093/rheumatology/keq202.
- Sageloli F, Quesada JL, Fautrel B, Salliot C, Gaudin P, Baillet A. Moderate alcohol consumption is associated with increased radiological progression in women, but not in men, with early rheumatoid arthritis: results from the ESPOIR cohort (Etude et Suivi des Polyarthrites Indifferenciees Recentes). Scand J Rheumatol. 2018;47:440–446. doi:10.1080/03009742.2018.1437216.
- Scott IC, Tan R, Stahl D, Steer S, Lewis CM, Cope AP. The protective effect of alcohol on developing rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology (Oxford). 2013;52:856–867. doi:10.1093/rheumatology/kes376.
- Lu B, Rho YH, Cui J, Iannaccone CK, Frits ML, Karlson EW, Shadick NA. Associations of smoking and alcohol consumption with disease activity and functional status in rheumatoid arthritis. J Rheumatol. 2014;41:24–30. doi:10.3899/jrheum.130074.
- Andersen C, Sondergaard HB, Bang Oturai D, Laursen JH, Gustavsen S, Larsen NK, Magyari M, Just-Østergaard E, Thørner LW, Sellebjerg F, et al. Alcohol consumption in adolescence is associated with a lower risk of multiple sclerosis in a Danish cohort. Mult Scler. 2019;25:1572–1579. doi:10.1177/1352458518795418.
- Diaz-Cruz C, Chua AS, Malik MT, Kaplan T, Glanz BI, Egorova S, Guttmann CRG, Bakshi R, Weiner HL, Healy BC, et al. The effect of alcohol and red wine consumption on clinical and MRI outcomes in multiple sclerosis. Mult Scler Relat Disord. 2017;17:47–53. doi:10.1016/j.msard.2017.06.011.
- Gili-Miner M, Lopez-Mendez J, Vilches-Arenas A, Ramírez-Ramírez G, Franco-Fernández D, Sala-Turrens J, Béjar-Prado L. Multiple sclerosis and alcohol use disorders: in-hospital mortality, extended hospital stays, and overexpenditures. Neurologia. 2016. doi:10.1016/j.nrl.2016.07.008.
- Pakpoor J, Goldacre R, Disanto G, Giovannoni G, Goldacre MJ. Alcohol misuse disorders and multiple sclerosis risk. JAMA Neurol. 2014;71:1188–1189. doi:10.1001/jamaneurol.2014.1795.
- Massa J, O’Reilly EJ, Munger KL, Ascherio A. Caffeine and alcohol intakes have no association with risk of multiple sclerosis. Multiple Sclerosis. 2013;19:53–58. doi:10.1177/1352458512448108.
- Gurung P, Young BM, Coleman RA, Wiechert S, Turner LE, Ray NB, Waldschmidt TJ, Legge KL, Cook RT. Chronic ethanol induces inhibition of antigen specific CD8+ but not CD4+ immunodominant T cell responses following Listeria monocytogenes inoculation. J Leukoc Biol. 2009;85:34–43. doi:10.1189/jlb.0208101.
- Meyerholz DK, Edsen-Moore M, McGill J, Coleman RA, Cook RT, Legge KL. Chronic alcohol consumption increases the severity of murine influenza virus infections. J Immunol. 2008;181:641–648. doi:10.4049/jimmunol.181.1.641.
- Mason CM, Dobard E, Zhang P, Nelson S. Alcohol exacerbates murine pulmonary tuberculosis. Infect Immun. 2004;72:2556–2563. doi:10.1128/IAI.72.5.2556-2563.2004.
- Ahlers KE, Karacay B, Fuller L, Bonthius DJ, Dailey ME. Transient activation of microglia following acute alcohol exposure in developing mouse neocortex is primarily driven by BAX-dependent neurodegeneration. Glia. 2015;63:1694–1713. doi:10.1002/glia.22835.
- Alfonso-Loeches S, Urena-Peralta J, Morillo-Bargues MJ, Gomez-Pinedo U, Guerri C. Ethanol-induced TLR4/NLRP3 neuroinflammatory response in microglial cells promotes leukocyte infiltration across the BBB. Neurochem Res. 2016;41:193–209. doi:10.1007/s11064-015-1760-5.
- Warden AS, Azzam M, DaCosta A, Mason S, Blednov YA, Messing RO, Mayfield RD, Harris RA. Toll-like receptor 3 activation increases voluntary alcohol intake in C57BL/6J male mice. Brain Behav Immun. 2019;77:55–65. doi:10.1016/j.bbi.2018.12.004.
- Warden AS, Wolfe SA, Khom S, Varodayan FP, Patel RR, Steinman MQ, Bajo M, Montgomery SE, Vlkolinsky R, Nadav T, et al. Microglia control escalation of drinking in alcohol-dependent mice: genomic and synaptic drivers. Biol Psychiatry. 2020;88:910–921. doi:10.1016/j.biopsych.2020.05.011.
- Marshall SA, Geil CR, Nixon K. Prior binge ethanol exposure potentiates the microglial response in a model of alcohol-induced neurodegeneration. Brain Sci. 2016;6:6. doi:10.3390/brainsci6020016.
- Romeo J, Warnberg J, Nova E, Diaz LE, Gomez-Martinez S, Marcos A. Moderate alcohol consumption and the immune system: a review. Br J Nutr. 2007;98(Suppl 1):S111–115. doi:10.1017/S0007114507838049.
- Slukvin II, Jerrells TR. Different pathways of in vitro ethanol-induced apoptosis in thymocytes and splenic T and B lymphocytes. Immunopharmacology. 1995;31:43–57. doi:10.1016/0162-3109(95)00032-4.
- Appay V, Sauce D. Naive T cells: the crux of cellular immune aging?Exp Gerontol. 2014;54:90–93. doi:10.1016/j.exger.2014.01.003.
- Zhang H, Meadows GG. Chronic alcohol consumption in mice increases the proportion of peripheral memory T cells by homeostatic proliferation. J Leukoc Biol. 2005;78:1070–1080. doi:10.1189/jlb.0605317.
- Volpato S, Pahor M, Ferrucci L, Simonsick EM, Guralnik JM, Kritchevsky SB, Fellin R, Harris TB. Relationship of alcohol intake with inflammatory markers and plasminogen activator inhibitor-1 in well-functioning older adults: the health, aging, and body composition study. Circulation. 2004;109:607–612. doi:10.1161/01.CIR.0000109503.13955.00.
- Oliveira A, Rodriguez-Artalejo F, Lopes C. Alcohol intake and systemic markers of inflammation–shape of the association according to sex and body mass index. Alcohol Alcohol. 2010;45:119–125. doi:10.1093/alcalc/agp092.
- Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W. Effect of alcohol consumption on systemic markers of inflammation. Lancet. 2001;357:763–767. doi:10.1016/S0140-6736(00)04170-2.
- Lu B, Solomon DH, Costenbader KH, Keenan BT, Chibnik LB, Karlson EW. Alcohol consumption and markers of inflammation in women with preclinical rheumatoid arthritis. Arthritis Rheum. 2010;62:3554–3559. doi:10.1002/art.27739.
- Jonsson IM, Verdrengh M, Brisslert M, Lindblad S, Bokarewa M, Islander U, Carlsten H, Ohlsson C, Nandakumar KS, Holmdahl R, et al. Ethanol prevents development of destructive arthritis. Proc Natl Acad Sci U S A. 2007;104:258–263. doi:10.1073/pnas.0608620104.
- Ma Y, Xu X, Li M, Cai J, Wei Q, Niu H. Gut microbiota promote the inflammatory response in the pathogenesis of systemic lupus erythematosus. Mol Med. 2019;25:35. doi:10.1186/s10020-019-0102-5.
- Berer K, Gerdes LA, Cekanaviciute E, Jia X, Xiao L, Xia Z, Liu C, Klotz L, Stauffer U, Baranzini SE, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A. 2017;114:10719–10724. doi:10.1073/pnas.1711233114.
- Meighani A, Hart BR, Bourgi K, Miller N, John A, Ramesh M. Outcomes of fecal microbiota transplantation for Clostridium difficile infection in patients with inflammatory bowel disease. Dig Dis Sci. 2017;62:2870–2875. doi:10.1007/s10620-017-4580-4.
- Li K, Wei S, Hu L, Yin X, Mai Y, Jiang C, Peng X, Cao X, Huang Z, Zhou H, et al. Protection of fecal microbiota transplantation in a mouse model of multiple sclerosis. Mediators Inflamm. 2020;2020:2058272. doi:10.1155/2020/2058272.
- Makkawi S, Camara-Lemarroy C, Metz L. Fecal microbiota transplantation associated with 10 years of stability in a patient with SPMS. Neurol Neuroimmunol Neuroinflamm. 2018;5:e459. doi:10.1212/NXI.0000000000000459.
- Miyauchi E, Kim SW, Suda W, Kawasumi M, Onawa S, Taguchi-Atarashi N, Morita H, Taylor TD, Hattori M, Ohno H, et al. Gut microorganisms act together to exacerbate inflammation in spinal cords. Nature. 2020;585:102–106. doi:10.1038/s41586-020-2634-9.
- Wu YY, Hsu JL, Wang HC, Wu SJ, Hong CJ, Cheng IH. Alterations of the neuroinflammatory markers IL-6 and TRAIL in Alzheimer’s disease. Dement Geriatr Cogn Dis Extra. 2015;5:424–434. doi:10.1159/000439214.
- Teng F, Klinger CN, Felix KM, Bradley C, Wu E, Tran N, Umesaki Y, Wu H-J. Gut microbiota drive autoimmune arthritis by promoting differentiation and migration of Peyer’s patch T follicular helper cells. Immunity. 2016;44:875–888. doi:10.1016/j.immuni.2016.03.013.
- Canesso MCC, Lacerda NL, Ferreira CM, Jl G, D A, C G, G C, Sh P, C M, Fs M, et al. Comparing the effects of acute alcohol consumption in germ-free and conventional mice: the role of the gut microbiota. BMC Microbiol. 2014;14:240. doi:10.1186/s12866-014-0240-4.
- Bode JC, Bode C, Heidelbach R, Durr HK, Martini GA. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology. 1984;31:30–34.
- Chang CS, Chen GH, Lien HC, Yeh HZ. Small intestine dysmotility and bacterial overgrowth in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 1998;28:1187–1190. doi:10.1002/hep.510280504.
- Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302:G966–978. doi:10.1152/ajpgi.00380.2011.
- Zhang X, Yasuda K, Gilmore RA, Westmoreland SV, Platt DM, Miller GM, Vallender EJ. Alcohol-induced changes in the gut microbiome and metabolome of rhesus macaques. Psychopharmacology (Berl). 2019;236:1531–1544. doi:10.1007/s00213-019-05217-z.
- Tuma DJ. Role of malondialdehyde-acetaldehyde adducts in liver injury. Free Radic Biol Med. 2002;32:303–308. doi:10.1016/S0891-5849(01)00742-0.
- Zeng T, Zhang CL, Xiao M, Yang R, Xie KQ. Critical roles of Kupffer cells in the pathogenesis of alcoholic liver disease: from basic science to clinical trials. Front Immunol. 2016;7:538. doi:10.3389/fimmu.2016.00538.
- Puri P, Liangpunsakul S, Christensen JE, Shah VH, Kamath PS, Gores GJ, Walker S, Comerford M, Katz B, Borst A, et al. The circulating microbiome signature and inferred functional metagenomics in alcoholic hepatitis. Hepatology. 2018;67:1284–1302. doi:10.1002/hep.29623.
- Dubinkina VB, Tyakht AV, Odintsova VY, Yarygin KS, Kovarsky BA, Pavlenko AV, Ischenko DS, Popenko AS, Alexeev DG, Taraskina AY, et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome. 2017;5:141. doi:10.1186/s40168-017-0359-2.
- Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, Gobejishvili L, Joshi-Barve S, Ayvaz T, Petrosino J, et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One. 2013;8:e53028. doi:10.1371/journal.pone.0053028.
- Yan AW, Schnabl B. Bacterial translocation and changes in the intestinal microbiome associated with alcoholic liver disease. World J Hepatol. 2012;4:110–118. doi:10.4254/wjh.v4.i4.110.
- Zhang HF, Lin XH, Yang H, Zhou LC, Guo YL, Barnett JV, Guo ZM. Regulation of the activity and expression of aryl hydrocarbon receptor by ethanol in mouse hepatic stellate cells. Alcohol Clin Exp Res. 2012;36:1873–1881. doi:10.1111/j.1530-0277.2012.01787.x.
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T reg and TH 17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi:10.1038/nature06880.
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld J-C, Stockinger B. The aryl hydrocarbon receptor links TH 17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi:10.1038/nature06881.
- Barr TM. Impact of alcohol on intestinal homeostasis: UC Riverside, 2018.
- Ogunsakin O, Hottor T, Mehta A, Lichtveld M, McCaskill M. Chronic ethanol exposure effects on vitamin D levels among subjects with alcohol use disorder. Environ Health Insights. 2016;10(EHI):S40335. doi:10.4137/EHI.S40335.
- Mann RE, Smart RG, Govoni R. The epidemiology of alcoholic liver disease. Alcohol Res Health. 2003;27:209.
- Pawlosky RJ, Salem N Jr. Perspectives on alcohol consumption: liver polyunsaturated fatty acids and essential fatty acid metabolism. Alcohol. 2004;34:27–33. doi:10.1016/j.alcohol.2004.07.009.
- Bjørkhaug ST, Aanes H, Neupane SP, Bramness JG, Malvik S, Henriksen C, Skar V, Medhus AW, Valeur J. Characterization of gut microbiota composition and functions in patients with chronic alcohol overconsumption. Gut Microbes. 2019;10:663–675. doi:10.1080/19490976.2019.1580097.
- De Lorgeril M, Salen P, Martin J-L, Boucher F, De Leiris J. Interactions of wine drinking with omega-3 fatty acids in patients with coronary heart disease: a fish-like effect of moderate wine drinking. Am Heart J. 2008;155:175–181. doi:10.1016/j.ahj.2007.08.009.
- Flint HJ, Duncan SH, Scott KP, Louis P. Links between diet, gut microbiota composition and gut metabolism. Proc Nutr Soc. 2015;74:13–22. doi:10.1017/S0029665114001463.
- Correa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MA. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunol. 2016;5:e73. doi:10.1038/cti.2016.17.
- Parada Venegas D, De La Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277. doi:10.3389/fimmu.2019.00277.
- Luu M, Visekruna A. Short-chain fatty acids: bacterial messengers modulating the immunometabolism of T cells. Eur J Immunol. 2019;49:842–848. doi:10.1002/eji.201848009.
- Mizuno M, Noto D, Kaga N, Chiba A, Miyake S, Ashour HM. The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS One. 2017;12:e0173032. doi:10.1371/journal.pone.0173032.
- Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi:10.1080/19490976.2015.1134082.
- Lee JE, Ha JS, Park HY, Lee E. Alteration of gut microbiota composition by short-term low-dose alcohol intake is restored by fermented rice liquor in mice. Food Res Int. 2020;128:108800. doi:10.1016/j.foodres.2019.108800.
- Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30:5–13.
- Xie G, Zhong W, Li H, Li Q, Qiu Y, Zheng X, Chen H, Zhao X, Zhang S, Zhou Z, et al. Alteration of bile acid metabolism in the rat induced by chronic ethanol consumption. FASEB J. 2013;27:3583–3593. doi:10.1096/fj.13-231860.
- Denkins Y, Woods J, Whitty J, Hannigan JH, Martier SS, Sokol RJ, Salem N. Highly unsaturated fatty acids in nutrition and disease prevention-maternal nutrition-effects of gestational alcohol exposure on the fatty acid composition of umbilical cord serum in humans. Am J Clin Nutr. 2000;71:300S. doi:10.1093/ajcn/71.1.300s.
- Cas MD, Roda G, Li F, Secundo F. Functional lipids in autoimmune inflammatory diseases. Int J Mol Sci. 2020;21:3074. doi:10.3390/ijms21093074.
- Abdolmaleki F, Kovanen PT, Mardani R, Gheibi-hayat SM, Bo S, Sahebkar A. Resolvins: emerging players in autoimmune and inflammatory diseases. Clin Rev Allergy Immunol. 2020;58:82–91. doi:10.1007/s12016-019-08754-9.
- Massaro M, Scoditti E, Carluccio MA, De Caterina R. Nutraceuticals and prevention of atherosclerosis: focus on ω3 polyunsaturated fatty acids and Mediterranean diet polyphenols. Cardiovasc Ther. 2010;28:e13–e19. doi:10.1111/j.1755-5922.2010.00211.x.
- Skulas-Ray AC, Wilson PW, Harris WS, Brinton EA, Kris-Etherton PM, Richter CK, Jacobson TA, Engler MB, Miller M, Robinson JG, et al. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. 2019;140:e673–e691. doi:10.1161/CIR.0000000000000709.
- Chaddha A, Eagle KA. Omega-3 fatty acids and heart health. Circulation. 2015;132:e350–e352. doi:10.1161/CIRCULATIONAHA.114.015176.
- Yagi S, Fukuda D, Aihara K-I, Akaike M, Shimabukuro M, Sata M. N-3 polyunsaturated fatty acids: promising nutrients for preventing cardiovascular disease. J Atheroscler Thromb. 2017;24(10):999–1010. doi:10.5551/jat.RV17013.
