ABSTRACT
As Helicobacter pylori management has become more challenging and less efficient over the last decade, the interest in innovative interventions is growing by the day. Probiotic co-supplementation to antibiotic therapies is reported in several studies, presenting a moderate reduction in drug-related side effects and a promotion in positive treatment outcomes. However, the significance of gut microbiota involvement in the competence of probiotic co-supplementation is emphasized by a few researchers, indicating the alteration in the host gastrointestinal microbiota following probiotic and drug uptake. Due to the lack of long-term follow-up studies to determine the efficiency of probiotic intervention in H. pylori eradication, and the delicate interaction of the gut microbiota with the host wellness, this review aims to discuss the gut microbiota alteration by probiotic co-supplementation in H. pylori management to predict the comprehensive effectiveness of probiotic oral administration.
Abbreviations: acyl-CoA- acyl-coenzyme A; AMP- antimicrobial peptide; AMPK- AMP-activated protein kinase; AP-1- activator protein 1; BA- bile acid; BAR- bile acid receptor; BCAA- branched-chain amino acid; C2- acetate; C3- propionate; C4- butyrate; C5- valeric acid; CagA- Cytotoxin-associated gene A; cAMP- cyclic adenosine monophosphate; CD- Crohn’s disease; CDI- C. difficile infection; COX-2- cyclooxygenase-2; DC- dendritic cell; EMT- epithelial-mesenchymal transition; FMO- flavin monooxygenases; FXR- farnesoid X receptor; GPBAR1- G-protein-coupled bile acid receptor 1; GPR4- G protein-coupled receptor 4; H2O2- hydrogen peroxide; HCC- hepatocellular carcinoma; HSC- hepatic stellate cell; IBD- inflammatory bowel disease; IBS- irritable bowel syndrome; IFN-γ- interferon-gamma; IgA immunoglobulin A; IL- interleukin; iNOS- induced nitric oxide synthase; JAK1- janus kinase 1; JAM-A- junctional adhesion molecule A; LAB- lactic acid bacteria; LPS- lipopolysaccharide; MALT- mucosa-associated lymphoid tissue; MAMP- microbe-associated molecular pattern; MCP-1- monocyte chemoattractant protein-1; MDR- multiple drug resistance; mTOR- mammalian target of rapamycin; MUC- mucin; NAFLD- nonalcoholic fatty liver disease; NF-κB- nuclear factor kappa B; NK- natural killer; NLRP3- NLR family pyrin domain containing 3; NOC- N-nitroso compounds; NOD- nucleotide-binding oligomerization domain; PICRUSt- phylogenetic investigation of communities by reconstruction of unobserved states; PRR- pattern recognition receptor; RA- retinoic acid; RNS- reactive nitrogen species; ROS- reactive oxygen species; rRNA- ribosomal RNA; SCFA- short-chain fatty acids; SDR- single drug resistance; SIgA- secretory immunoglobulin A; STAT3- signal transducer and activator of transcription 3; T1D- type 1 diabetes; T2D- type 2 diabetes; Th17- T helper 17; TLR- toll-like receptor; TMAO- trimethylamine N-oxide; TML- trimethyllysine; TNF-α- tumor necrosis factor-alpha; Tr1- type 1 regulatory T cell; Treg- regulatory T cell; UC- ulcerative colitis; VacA- Vacuolating toxin A.
Introduction
Gastric carcinoma, as one of the leading causes of cancer-associated deaths, is mainly developed as a result of Helicobacter pylori (H. pylori) infection. The prevalence of H. pylori infection exceeds half of the world’s population; however, the likelihood of affecting health or disease is not uniform and largely relies on host genetics, bacterial virulence, and environmental conditions.Citation1 By leveraging several virulence factors, H. pylori interferes with various cellular components of the host to induce proliferation, apoptosis, migration, and inflammatory responses.Citation2 H. pylori has a substantial association with chronic gastritis, gastric ulcer, mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric adenocarcinoma.Citation3 The combination of up to four drugs including two or three types of antibiotics as well as a proton-pump inhibitor for two weeks is suggested as the first-line of H. pylori treatment.Citation4–6 However, the ideal approach for H. pylori eradication remains elusive and current prescriptions are mostly empirical, heedless of the bacterial antibiotic susceptibility.Citation7
The increased prevalence of antibiotic resistance and antibiotic-associated adverse effects are the primary reasons explaining the requirement for alternative approaches to manage H. pylori infection.Citation8 The interaction of probiotics with the host and gastrointestinal microbiome through alteration in the gut microbiota composition, competition for accessible nutrients and attachment sites, and prevention of bacterial colonization to mediate health benefits indicates the advantage of probiotic co-supplementation in antibiotic treatments.Citation9 Intervention studies have demonstrated a reduction in gastrointestinal symptoms and drug-related side effects by probiotic oral administration.Citation10 However, long-term follow-up investigations are required to elucidate the efficiency of adjuvant interventions on H. pylori treatment.
Here, we aim to highlight the great significance of the host gut microbiota involvement in the competence of probiotic supplementation. We will further discuss the bidirectional interaction of probiotic strains and indigenous gastrointestinal microbiota to predict the effectiveness of this adjuvant therapy and provide an outlook for future investigations within the nascent and promising research field.
Gut microbiota
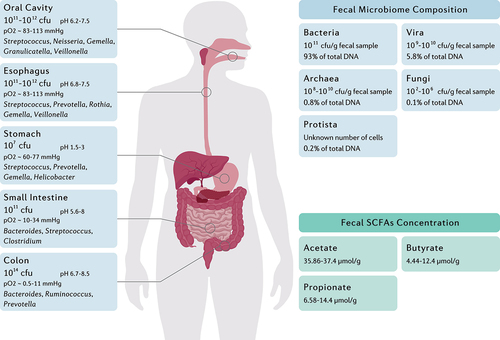
In addition to the tremendous community of microorganisms inside and on the human body, the gastrointestinal tract harbors a diverse and dynamic consortia of commensal or mutualistic microorganisms, mainly consisting of Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Verrucomicrobia phyla.Citation11 Based on the ecological characteristics of the gastrointestinal tract, the microbial load ranges from 1012 CFU/ml in the oral cavity and a narrow diversity of 107 CFU/ml in the stomach and duodenum to a vast diversity of 1014 CFU/ml in the colon ().Citation12 Due to the reduction in oxygen concentration along the longitudinal axis, the upper gastrointestinal tract is the residence of Gram-positive cocci, such as Gemella and Streptococcus, whereas the intestines and colon are enriched with anaerobes including the Clostridium and Faecalibacterium genera.Citation16 Furthermore, the luminal to mucosal axis organizes the bacteria based on their ability for mucus degradation. Bacteroides thetaiotaomicron, Akkermansia muciniphila, Ruminoccous gnavus, Bacteroides fragilis, and Bifidobacterium bifidium are predominant bacteria within the mucus layer that utilize glycans as their energy source by glycosidase, sulphatase, and sialidase enzymes.Citation17 Despite the dynamic colonization of indigenous commensals within the intestinal niches created by glycans, the lack of dietary fiber polysaccharides potentially emphasizes the significance of the host intestinal mucin as a reliable energy source for the gut microbiota.Citation18
Figure 1. The main genera and total abundance of bacteria vary along the gastrointestinal tract. The colon is characterized by low levels of oxygen as well as the presence of enormous numbers and species of bacteria. On the other hand, the microbial composition and metabolite concentration of stool samples are distinguished from gut biopsies, in which the bacteria and the fungi constitute the majority and minority of total fecal DNA, respectively.Citation12–15 Fecal concentration of SCFAs are also demonstrated as they might be considered key regulators of the intestinal homeostasis.
The role of immune system in shaping gut microbiota
A distinctive characteristic of the intestinal immune system is its capacity to distinguish mutualistic microorganisms from pathogens and further establish active tolerance toward commensal bacteria.Citation19 Identification of microbe-associated molecular patterns (MAMPs) by pattern recognition receptors (PRRs), such as toll-like receptors (TLRs) and nucleotide-binding oligomerization domain receptors (NODs), leads to the activation of various cellular signaling pathways. Consequently, modulation of gene expression by multiple ligands, transcription factors, and kinases can modify the production levels of inflammatory cytokines, chemokines, and immunoreceptors.Citation20 Although pathogens and commensals share common ligands that activate the TLRs, several mechanisms are considered for TLR-mediated discrimination of gut bacteria. Commensals can be simply distinguished from pathogens owing to the lack of virulence factors and differences in invasiveness. Furthermore, the cellular location of TLRs on the intestinal epithelium is inaccessible to commensal bacteria. Different PAMP affinity for TLRs and activation of ligand-specific signaling pathways are other possible mechanisms to identify commensals from pathogens.Citation21 On the other hand, NOD2 recognizes conserved motifs of bacterial peptidoglycan and maintains mucus layer activity; thereby, NOD2 deficiency or mutation might lead to pathogen overgrowth, inflammation, and colon cancer.Citation22 A recent study indicated that NOD2 knockout mice demonstrated an impaired recovery of gut microbiota composition following an antibiotic intervention, suggesting the remarkable contribution of this receptor in shaping the gut microbial community.Citation23 Furthermore, NOD1 activation as a consequence of peptidoglycan recognition can trigger both immune memory and tolerance.Citation24 Irving et al. demonstrated the development of peptidoglycan-specific immunity following H. pylori infection and the subsequent NOD1 activation and autophagy induction.Citation25
The mucus layer of the intestinal epithelium intervenes between the resident microbiome and epithelial layer to form a static shield and narrow the immunogenicity of antigens by provoking dendritic cells (DCs) to an anti-inflammatory response. Moreover, the complex architecture of the intestinal epithelium, as well as their secretions, such as antimicrobial peptides (AMPs) and immunoglobulins, preserve the functionality of the mucosal barrier.Citation26 The most abundant AMPs are defensins that develop small pores in bacterial membranes to disrupt cellular integrity. α- and β-defensins are the two subfamilies of defensins, predominantly released by Paneth cells and colonic epithelial cells, respectively.Citation27 In addition to pore formation, these AMPs can trap bacteria by degenerating the bacterial cytoplasm and developing extracellular net-like structures.Citation28 Furthermore, cathelicidin is the primary AMP expressed during infancy regardless of the bacterial presence and remarkably influences the early development of gut microbiota.Citation29 Perturbation of the gastrointestinal microbiota of preterm and term infants may lead to persistent immune and metabolic disorders.Citation30 Collectively, the intestinal epithelium can establish an efficacious physico-chemical barrier that prevents pathogen colonization on the mucosal surface while creating immune tolerance against commensal bacteria.
In addition to the innate immune system, recent studies exhibited a mutualistic interaction of the adaptive immune system in shaping gut microbial composition. B cells are critical modulators of intestinal homeostasis, mainly through expressing secretory immunoglobulin A (SIgA) in response to commensal recognition.Citation31 The pivotal and often oversimplified role of SIgA depends on the gut microbial community. Chaotic or excessive reaction to alteration in the richness or pro-inflammatory behavior of particular strains by SIgA influences not only the specific bacteria but probably the whole microbiota.Citation32 SIgA predominantly prevents the translocation of microorganisms from lamina propria to the bloodstream, interferes with conjugative plasmid transfer, and facilitates the colonization of commensal bacteria.Citation33 On the other hand, T follicular helper cells are specialized to cooperate with B cells and modify humoral immunity.Citation34 Although several studies began to elucidate the mechanistic interaction of cellular immunity with gut microbiota through inflammatory signaling pathways, we have yet to fully understand the aspects of the adaptive immune system in shaping the gut microbiota.
Gut microbial metabolites in preserving homeostasis
The gut microbiota plays a critical role in preserving the normal bioactivity of the host through gut microbiota-derived metabolites, especially bile acids (BAs), short-chain fatty acids (SCFAs), branched-chain amino acids (BCAAs), trimethylamine N-oxide (TMAO), tryptophan, and indole derivatives.Citation35 Nevertheless, the knowledge concerning the direct effect of the gut microbiota on the host metabolism remains scarce; however, the gastrointestinal microbiota has a particular interaction with mitochondria owing to their common origin.Citation36 It has been recently indicated that delta-valerobetaine production by the gut microbiome reduces cellular carnitine and mitochondrial long-chain acyl-coenzyme A (acyl-CoA); consequently, this obesogenic metabolite prevents mitochondrial fatty acid oxidation and leads to diet-dependent obesity.Citation37
SCFAs are saturated fatty acids acquired from microbiota-accessible carbohydrates and mainly include acetate (C2), propionate (C3), butyrate (C4), and valeric acid (C5) in the human body.Citation38,Citation39 Nevertheless, the abundance of each SCFA depends on substrate availability, gut microbiota composition, and gastrointestinal transit time. SCFAs exhibit several local effects, such as preserving the intestinal barrier integrity and pH reduction as their concentration increase from the distal ileum (6.5–7.5) to the proximal colon (5.5–7.5).Citation40,Citation41 Moreover, SCFAs promote the induction and expansion of intestinal regulatory T cells,Citation42 DCs, and macrophages,Citation43 exert an anti-carcinogenic and anti-oxidative effect in the intestine,Citation44 and suppress pathogen-induced inflammation ().Citation45
Figure 2. The interplay between the gut metabolome, H. pylori, and the host immune system. H. pylori induces chronic gastric inflammation through the activation of transcriptional factors such as NF-κB. By stimulating the production of BCAA from the gut microbiota, H. pylori activates the mTORC1 complex and ultimately inhibits autophagic response. H. pylori further disrupts the integrity of the gastric epithelial barrier by suppressing the expression of tight junction proteins. On the other hand, microbiota production of SCFAs and secondary bile acids modulate gastric inflammation and immune system activation by reducing NF-κB activation, promoting the secretion of anti-inflammatory cytokines, AMPs, and IgA, and preserving the integrity of the gut barrier.
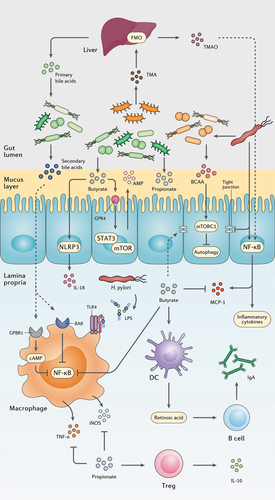
Hepatocytes synthesize primary bile acids from cholesterol, conjugate them to taurine or glycine, and then release them into the gall bladder to form bile in combination with cholesterol, phospholipids, minerals, electrolytes, bilirubin, biliverdin, and protein.Citation46 Intestinal bacteria will deconjugate primary BAs that fail reabsorption in the terminal ileum and thereby convert them to secondary BAs by microbial biotransformation, including dehydroxylation, epimerization, and oxidation of hydroxyl groups.Citation47 Secondary BAs are involved in the modulation of cell signaling, microbial composition, intestinal metabolism, and the host immune response. Reduced BA deconjugation is associated with inflammatory bowel diseases (IBD) including ulcerative colitis (UC) and Crohn’s disease (CD), as well as irritable bowel syndrome (IBS).Citation48 Free BAs, such as cholic acid, deoxycholic acid, and chenodeoxycholic acid, can stimulate apoptosis and reduce interleukin 6 (IL-6) production, while conjugated BAs such as glycolic acid, glycodeoxycholic acid, and glycochenodeoxycholic acid promote cell growth and induce IL-6 production.Citation49 However, excessive production of the secondary BA deoxycholic acid triggers the expression of inflammatory and tumorigenic factors in hepatic stellate cells (HSCs), contributing to hepatocellular carcinoma development.Citation50 Secondary BAs might also activate farnesoid X receptor (FXR) and elevate the risk of developing colorectal cancer and hepatocellular carcinoma.Citation51
As an essential amino acid in the human body, tryptophan must be obtained by diet and further metabolized through host or microbial pathways. The indole pathway for tryptophan metabolism is mediated by the gut microbiome leading to a variety of indole metabolites, some of which are involved in mucosal homeostasis, gastrointestinal motility, and the host immune response.Citation52 However, BCAAs valine, leucine, and isoleucine are possible biomarkers in human carcinogens owing to their requirement in cancer cell growth and tumor progression.Citation53 Although BCAAs are involved in carcinogenesis and metabolic disorders, such as obesity, insulin resistance, and type 2 diabetes (T2D), sports supplements with these amino acids might improve strenuous training.Citation54
Gut bacteria produces TMA, which is transferred to the liver through the bloodstream and further converted to TMAO by hepatic flavin monooxygenases (FMOs). Animal products such as meat, fish, and eggs are rich in TMA precursors.Citation55 TMAO is a major risk factor for cardiovascular disease, renal fibrosis and functional impairment, atherosclerosis, and colorectal cancer.Citation56,Citation57 It is further indicated that a precursor to TMAO, trimethyllysine (TML), alone and combined with TAMO, is involved in cardiovascular events for patients with the acute coronary syndrome.Citation58
Gut microbial dysbiosis
Defining the gut microbiota composition and function of metabolically healthy individuals is a prerequisite for claiming gut dysbiosis and identifying disease-related biomarkers. The efforts in this field are encountered with an intimidating complexity in the host–microbiota interaction, which needs comprehensive, multidisciplinary approaches for further elucidation.Citation59 Although a healthy microbiome composition is yet to be determined, the relative alteration of gastrointestinal microorganisms in disease conditions can be mainly classified as pathobiont enrichment, commensal depletion, or diversity reduction.Citation60 Pathobionts are among the host indigenous microbiome that can trigger or accelerate diseases in particular genetic or environmental conditions.Citation61 An increased proportion of Enterobacteriaceae, including Escherichia coli, Klebsiella spp., and Proteus spp., is a typical example of pathobionts enrichment. This family of Gram-negative symbionts is commonly overgrown in multiple inflammatory situations including intestinal bowel disease, obesity, celiac disease, colon cancer, and antibiotic therapies.Citation62 In contrast to the overgrowth of pathobionts, the gut microbial community frequently suffers a tremendous depletion or total loss of some commensal bacteria following microbial elimination or reduced bacterial proliferation.Citation60 Commensal bacteria are responsible for providing energy resources for the host enterocytes,Citation63 inhibiting pathogen colonization,Citation64 preserving lymphoid tissue architecture, and regulating the immune response.Citation26 Bio-engineered commensal supplementation is an innovative strategy, recently used for delivering tailored substances to target particular metabolic pathways.Citation65 On the other hand, a common and recurrent feature of disease-related dysbiosis is reduced microbial diversity. Although reduced alpha diversity might be the effect rather than the cause of disorders, this characteristic is correlated to gastrointestinal and extra-gastrointestinal diseases, such as CD, IBS, colorectal cancer, and autism.Citation66 Furthermore, the development of a mature microbiome through lifespan highly relies on alpha diversity. Interestingly, specific bacteria can be used as markers for the development and maturation of the microbiota such as R. gnavus, which is inversely correlated to microbial richness at all ages and reduces from childhood toward adulthood.Citation67 Accordingly, there is a delicate interaction between gut homeostasis and the host biological function. Disruption of the intricate equilibrium of metabolic interactions by pathogen colonization or microbiota modifying interventions can damage the integrity of the gut barrier, change the host indigenous bacteria, and further lead to metabolic disorders.
H. pylori and gut microbiota
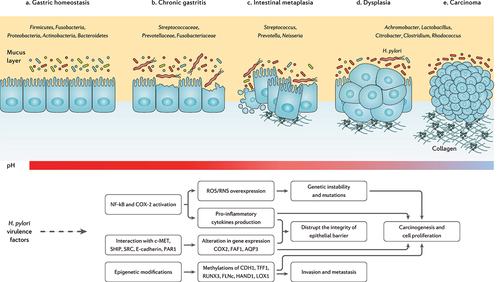
The clinical implications of H. pylori infection are not limited to gastrointestinal disorders but also include H. pylori association with obesity, diabetes, IBD, allergic disorders, as well as cardiovascular, hepatobiliary, skin, kidney, autoimmune, neurologic, and psychiatric diseases.Citation68 This might indicate the importance of H. pylori and gut microbiota crosstalk, as several mechanisms are reported for this pathogen influencing the host microbiome.Citation69 Modulation of the host immune response, manipulation of the cellular signaling, impairment of the epithelial cell polarity, and alteration of gastric acidity are the primary mechanisms contributing to gut microbiota alteration during H. pylori infection.Citation70 Below, we discuss several aspects of H. pylori infection interacting with gastric and intestinal microbiome, as well as gut microbial metabolites ().
Figure 3. The progression of chronic gastritis toward gastric carcinoma has been characterized by the reduction in the Helicobacter genus, overgrowth of opportunistic bacteria, increased apoptosis, necrosis, and collagen production, changes in the cytoskeleton and polarity of the gastric epithelium, and gradual suppression of gastric acidity. The main mechanisms of action through which H. pylori virulence factors promote the risk of developing gastric cancer are further depicted.Citation71–73
Gastric microbiota
In the last decade, several studies have compared the gut microbiota composition of H. pylori-infected and non-infected individuals, reporting controversial data even regarding the diversity and richness of the microbial community.Citation74 It is possibly due to the remarkable dependence of microbiota composition on individual and environmental factors, such as host genetics, ethnicity, geography, socioeconomic status, and diet.Citation75,Citation76 Furthermore, the microbial community is highly variable along the longitudinal axis of gastrointestinal tract. Hence, in a recent study, corpus and antrum bacteria were reported to significantly differ between individuals positive or negative for H. pylori, while the bacterial community from the lower gastrointestinal tract and stool samples were comparable.Citation77
Although H. pylori antigen load exhibited a reverse relationship with Fusicatenibacter, Alistipes, Bacteroides, and Barnesiella genera, gut microbiota composition is mainly dominated by the same phyla yet different richness in H. pylori-infected and non-infected individuals.Citation72 Streptococcus, Neisseriae, Prevotella, Rothia, Fusobacterium, Veillonella, and Haemophilus are considered the main gastric bacterial genera enriched in H. pylori-positive individuals, compared to H. pylori-negative subjects.Citation78,Citation79 Likewise, the overgrowth of Candida species in the stomach has been reported during H. pylori infection, which might result in synergistic effects on the H. pylori pathogenesis.Citation80 However, H. pylori-induced gastric microbiota alteration is strain-specific and independent of the host-microbial colonization burden. A recent in vivo study demonstrated the substantial reduction of Akkermansia, Bacteroides, and Lachnospiraceae genera in gerbils infected with a cytotoxin-associated gene A (CagA)-positive H. pylori strain compared to a CagA-negative strain. Yet, comparable alpha diversity for the gastric microbiota has been reported for the investigated groups.Citation81 Furthermore, allelic variation in the H. pylori vacuolating toxin A (VacA) is associated with distinct modification of the gastric microbiota.Citation82
The microbiome alteration further relies on the stages of gastric tumorigenesis along with substantial enrichment of oral microbial species including Peptostreptococcus stomatis, Streptococcus anginosus, Parvimonas micra, Slackia exigua, and Dialister pneumosintes toward carcinogenesis.Citation83 Some studies reported an increased colonization burden and microbial diversity, as well as the overgrowth of cancer promoting bacteria in the gastric mucosa of patients with gastric cancer compared to gastritis.Citation84,Citation85 However, a metagenomics study indicated that the microbiota tends to be gradually depleted in the gastric mucosa from non-atrophic gastritis toward intestinal metaplasia and gastric cancer. In this regard, a significant reduction in TM7, Porphyromonas sp, Neisseria sp, and Streptococcus sinensis, as well as a substantial enrichment in Lactobacillus coleohominis and Lachnospiraceae have been further reported.Citation86
Even though Helicobacter is the most abundant genus in chronic gastritis, gastric carcinoma is reported with a significant reduction in the proportion of this genus. Meanwhile, certain commensals but potentially opportunistic pathogenic taxa such as Citrobacter, Clostridium, Lactobacillus, Achromobacter, and Rhodococcus were found to be enriched among gastric microbiota in gastric cancer.Citation87 Another study further reported Streptococcus, Lactobacillus, Veillonella, Prevotella, Neisseria, and Haemophilus as the highly prevalent gastric microbial genera in patients with gastric carcinoma.Citation88 Consistent with the foregoing data, an enriched proportion of Fusobacterium, Neisseria, Prevotella, Veillonella, and Rothia genera have been characterized in patients with advanced gastric lesion compared to the healthy/superficial gastritis group.Citation89
Lactic acid bacteria are mainly reported as protective bacteria in gastric carcinoma, while their increased abundance during cancer progression might indicate otherwise. Reactive oxygen species (ROS), N-nitroso compounds (NOC), and lactate production, as well as induction of epithelial–mesenchymal transition (EMT) and immune tolerance, are among carcinogenic factors promoted by lactic acid bacteria.Citation90 On the other hand, the destruction of stomach hydrochloric acid-producing glands by H. pylori infection increases the stomach pH and eventually promotes the colonization of NOC-producing bacteria.Citation91,Citation92 Veillonella, Clostridium, Haemophilus, Staphylococcus, Neisseria, Lactobacillus, and Nitrospirae are involved in gastric carcinogenesis by NOC production and further induction of mutagenesis, angiogenesis, and proto-oncogenes expression as well as apoptosis prevention.Citation90
Intestinal microbiota
Compared to studies exploring the influence of H. pylori on the gastric microbiota, a limited number of studies investigated the effect of H. pylori on the intestinal microbiota. Considering the intestinal microbiota at the phylum level, Firmicutes, Proteobacteria, Actinobacteria, and Acidobacteria have been elevated, while Bacteroidetes has been reduced following H. pylori infection.Citation93,Citation94 At the genus level, Bacteroides, Barnesiella, Alistipes, and Fusicatenibacter have been negatively associated with H. pylori stool antigen load.Citation95 Lapidot et al. also demonstrated a strong association between H. pylori infection and Prevotella copri and Eubacterium biforme in school-age children.Citation96 Additionally, long-term H. pylori infection of Mongolian gerbils has been characterized by Akkermansia enrichment in the colon.Citation97 Moreover, Candida glabrata and other unclassified fungi have been reported to be increased in stool samples following H. pylori infection in adults.Citation98 However, regarding the alpha diversity of the intestinal microbiota, contradictory reports indicated microbial enrichment,Citation98–100 microbial depletionCitation101 or no significant alterationCitation102–104 in H. pylori-infected patients. Except for one study, no significant alteration has been indicated for microbial alpha diversity following H. pylori infection. This might suggest that H. pylori promotes the host’s resilience against microbial depletion, reflecting the co-evolution of H. pylori and humans over tens of thousands of years.Citation95,Citation105 Furthermore, the geological and cultural differences among the investigated population might be responsible for the inconsistency in the aforementioned studies.Citation106 Several aspects of H. pylori-induced alteration of intestinal microbiota remain to be further investigated. However, H. pylori-induced gastric immunopathogenesis including hypochlorhydria and hypergastrinemia is held responsible for H. pylori-associated intestinal dysbiosis.Citation107,Citation108
Gut metabolome
H. pylori interactions with epithelial cells results in disruption of tight junctions and activation of the host inflammatory responses ().Citation109 This recalcitrant pathogen provokes the activity of the nuclear factor kappa B (NF-κB) transcription factor, stimulates the expression of monocyte chemoattractant protein-1 (MCP-1) from epithelial cells to induce monocyte infiltration, and activates monocytes through LPS interaction with TLR4. Consequently, H. pylori infection leads to the overexpression of pro-inflammatory cytokines including induced nitric oxide synthase (iNOS), tumor necrosis factor-α (TNF-α), interferon-gamma (IFN-γ), IL-8, IL-6, IL-4, and IL-1β.Citation110
The interaction between H. pylori infection and SCFA is far from being fully elucidated, yet the reduction of SCFA has been reported in the feces of H. pylori-infected mice.Citation111 Specifically, butyrate promotes intestinal barrier function via activating AMP-activated protein kinase (AMPK) or inhibiting claudin-2 production to stimulate the expression of tight junction proteins.Citation40 Through the G protein-coupled receptor 4 (GPR4) and mammalian target of rapamycin (mTOR)/signal transducer and activator of transcription 3 (STAT3) signaling pathway, butyrate promotes AMPs expression in epithelial cells. SCFAs might lead to NLR family pyrin domain containing 3 (NLRP3) inflammasome activation by GPR4 receptor inducing IL-18 secretion from the epithelium. GPR109A is a surface receptor on DCs and macrophages that detects butyrate and further induces the development of regulatory T cells (Treg) and prevents the proliferation of T helper 17 (Th17) cells.Citation112 Moreover, butyrate can suppress the production of iNOS, TNF-α, IL-6, MCP-1, and IFN-γ by inhibiting NF-κB activation.Citation113 On the other hand, propionate downregulates the production of pro-inflammatory cytokines including IL-4, IL-5, and IL-17A, and stimulates Treg cells to release the anti-inflammatory cytokine IL-10. In LPS-activated monocytes, propionate is reported to inhibit TNF-α and iNOS expression.Citation45 It is also suggested that the interaction of SCFAs with DCs elevates retinoic acid (RA) production and consequently increases IgA secretion by B cells in lamina propria.Citation114
BAs interaction with bile acid receptor (BAR) in LPS-activated macrophages inhibits NF-κB transcription; therefore, downregulates the overexpression of pro-inflammatory cytokines. Furthermore, G-protein-coupled bile acid receptor 1 (GPBAR1) activation by BAs stimulates cyclic adenosine monophosphate (cAMP) production; therefore, BAs interfere with the NF-κB signaling pathway either directly or through competition of cAMP for the transcription region.Citation115
VacA, as a major virulence factor in H. pylori bacteria, induces cellular autophagy to promote the growth and colonization of this pathogen in the mucosal layer.Citation116 Thereafter, H. pylori may provoke the gut microbiome to produce BCAAs isoleucine, leucine, and valine, and thereby activates the mTORC1 complex to inhibit autophagy within the gut epithelium and further induces chronic inflammation.Citation117 Another inflammatory metabolite in the intestine is TAMO, which induces NF-κB activation and promotes the expression of pro-inflammatory cytokines; consequently, a positive correlation has been reported between TAMO circular concentration and serum levels of IL-8 and TNF-α.Citation118,Citation119
Microbiome modifying interventions
Antibiotic therapy
Clinical studies have used innovative approaches including targeted sequencing of 16S ribosomal RNA (rRNA), PICRUSt (phylogenetic investigation of communities by reconstruction of unobserved states), and high-throughput DNA sequencing to facilitate the identification of microbial gene or taxon as disease biomarkers. Nonetheless, intra-individual variability of the gut microbiota, as well as microbiologically heterogeneous subjects has forced the host–microbiota interaction to remain fraught and challenging.Citation120 Notwithstanding the individual distinctions in microbial composition and the enormous differences in pathologies of metabolic diseases, intervention in the fragile host-microbiota crosstalk can lead to joint and disease-specific alteration in the community and activity of gut microbiota. Obesity, T2D, cardio-metabolic disease, metabolic liver disease, and malnutrition are primary metabolic disorders resulting from microbiome dysbiosis.Citation121
Antibiotic treatment as a major disrupter of the gastrointestinal microbial community may lead to alpha diversity reduction, metabolome alteration, and antibiotic resistance.Citation122 Antibiotic administration not only influences the resistome of the subject to whom it is given, but also the whole population owing to selection for resistance to its function.Citation123 The propagation and spread of antibiotic resistance genes in the mucus layer is a defensive function for gut microbiota to minimize the effect of antibiotics, yet short-term antibiotic therapy can cause a long-term reduction in certain commensal bacteria.Citation124 In addition to the antibiotic-directed modification of the gut microbiota, researchers have reported that intervention therapies can remodel the gene expression and overall metabolic activity of the gastrointestinal microbiota.Citation125 Moreover, PPIs as essential drugs in H. pylori eradication can directly disrupt microbial composition, in addition to increasing the stomach pH and thereby influencing which bacteria reach the intestine.Citation126 It is also suggested that the gut microbiota response to antibiotic treatment is determined by particular bacteria in the pre-treatment microbiome; thereby, targeting these bacteria may reduce the risk of dysbiosis and antibiotic-related metabolic disorders.Citation127
Probiotic supplementation
Multiple microorganisms comply with the definition of probiotics as live microorganisms providing a health benefit when supplemented in sufficient amounts.Citation128 The empirical top-down strategy to study indigenous bacteria enriched in healthy subjects is still a major approach to identify probiotic strains with sufficient beneficial effects on human health.Citation129 Common probiotics classify as probiotic lactic acid bacteria (LAB) such as Lactobacillus spp., Bifidobacterium spp., and Streptococcus spp., non-LAB probiotics, such as Clostridium butyricum, Bacillus spp., and E. coli Nissle 1917, and next-generation probiotics, such as Akkermansia muciniohila, Faecalibacterium prausnitzii, and Bacteroides species.Citation130
The impact of probiotic supplementation on human health has been largely investigated and reported to interfere with acute diarrhea, improve IBD, reduce the risk for late-onset neonatal sepsis, cardiometabolic syndrome, and necrotizing enterocolitis, increase H. pylori eradication rate, decrease the prevalence and intensity of respiratory infection, ease depression and manage atopic dermatitis.Citation131 Although several studies have failed to investigate mucosal or fecal microbiota composition of individuals during therapeutic interventions, strong evidence points out that the effectiveness of probiotic strains might not rely on colonizing the gastrointestinal tract but rather reside in their capacity of sharing genes and metabolites, reinforcing disturbed bacteria, and directly affecting the gut barrier and immune cells.Citation132 The differences in responding to the same probiotic supplementation in healthy adults further suggest that an individual’s basal gut microbiota influences the body’s response to probiotic strains.Citation133 Considering the variabilities in the host genetics, diet, disease-associated dysbiosis, and indigenous gut microbiota composition, the responses to the same intervention therapy might differ within the study population.
Next-generation probiotic supplementation
Next-generation probiotics, also termed as live biotherapeutics, emphasize emerging microorganisms not being used as health-promoting factors to date, which will probably be taken under a drug regulatory framework. Regarding the importance of the gut microbiota, these probiotic strains mainly originate from the human microbiome symbionts including A. muciniphila, F. prausnitzii, and several Bacteroides species.Citation134 A. muciniphila as an abundant bacterium within the host intestine is involved in regulating metabolic pathways, modulating the immune response, and preserving the intestinal barrier.Citation135 The prevalence of this bacterium is negatively associated with obesity, T2D, IBD, and appendicitis.Citation136 Daily administration of 1010 A. muciniphila bacteria to obese volunteers for 90 days is reported to reduce insulin resistance, plasma cholesterol, and the risk for developing liver dysfunction and inflammation, whereas no significant alteration is demonstrated in the gut microbiota.Citation137 On the other hand, F. prausnitzii is reported to be reduced in patients with IBD,Citation138 IBS,Citation139 colorectal cancer,Citation140 obesity, and diabetes.Citation141 Owing to the oxygen sensitivity of this bacterium and several other candidate strains, little is known about their efficiency and safety as probiotic supplements.Citation142 It is suggested that prebiotic co-supplementation with next-generation probiotics may promote the survivability and activity of probiotic strains in the human gut.Citation143 Nevertheless, the development of gastrointestinal modeling through organoid technology can deepen our knowledge of the complexity of probiotic-host interaction and provide the opportunity of designing personalized therapeutics and develop next-generation probiotics.Citation144
H. pylori eradication
International guidelines highly recommend H. pylori eradication for individuals who test positive.Citation145,Citation146 According to the test-and-treat strategy, randomized clinical trials were conducted to demonstrate the long-term safety of H. pylori treatment and further report that despite the transient alteration in gastrointestinal microbiota and elevation in specific antibiotic resistance, this perturbation diminished 8 weeks or one year after treatment. Meanwhile, the reduction in insulin resistance and triglyceride serum concentrations were demonstrated as the advantages of H. pylori management.Citation147 Moreover, the incidence of developing gastric carcinogenesis can be decreased by 50% following therapeutic management of H. pylori infection.Citation148 However, H. pylori eradication not only stimulates gut dysbiosis but may also selects out drug-resistant species from the gut microbiota and further expands single-drug resistance (SDR) and multiple-drug resistance (MDR) mechanisms in other microbial species.Citation149 Furthermore, H. pylori eradication can lead to major drug-related side effects including T2D and gastric adenocarcinoma.Citation150,Citation151 The tight interaction of the gastrointestinal microbiota and host wellness, as well as microbiome alteration and alpha diversity reduction during intervention therapies suggest a substantial involvement of the host microbiota in the adverse effects of H. pylori treatment.Citation152
As the gut microbiota can potentially spread the resistance genes from commensals to pathogens and regulate the host bioactivity,Citation54 reducing antibiotic resistance genes and preserving the intrinsic gut microbiota composition might increase H. pylori eradication rate and reduce collateral damages. Probiotic supplementation during treatment can preserve the host indigenous microbiota, facilitate rebiosis, and restore the intrinsic balance of bacteria in the gastrointestinal tract.Citation153,Citation154 It has been recently indicated that probiotic administration reduces the resistome configuration in colonization-permissive individuals. However, post-treatment probiotic supplementation has been reported to inhibit the reduction of antibiotic resistance genes number and further spread the resistance mechanisms in the intestinal mucosa.Citation155 Cifuentes et al. reported a substantial reduction in resistant genes for lincosamides, tetracyclines, MLS-B (macrolide, lincosamide, and streptogramin B), and beta-lactam class following Saccharomyces boulardii CNCM I-745 supplementation during H. pylori eradication.Citation156 Moreover, a recent meta-analysis of 5792 cases indicated that probiotic supplementation significantly increases the H. pylori eradication rate. Zhang et al. further reported that long-term (>10 days) probiotic administration leads to a statistically higher eradication rate compared with short-term administration.Citation157 However, limited effectiveness has been obtained in H. pylori eradication through probiotic supplementation as the main treatment strategy without being co-supplemented with conventional antibiotic regiments.Citation158
Owing to the high prevalence of H. pylori infection in childhood, mostly adolescence, or young adulthood should be considered for screening studies.Citation148 Clinical symptoms, epidemiology, diagnostic approaches, antibiotic susceptibility, and treatment strategies for H. pylori infection significantly differ from the ones in adults and children.Citation159 Yet, significant improvement has been obtained in H. pylori management, decreasing clinical manifestations, and the incidence of antibiotic-related side effects through probiotic supplementation in children. Lactobacillus casei strains and multi-strain consortia of Lactobacillus acidophilus and Lactobacillus rhamnosus are reported as the foremost adjuvant supplement in promoting H. pylori eradication rate and reducing drug-associated adverse effects in children, respectively.Citation160 However, major limitations to meta-analysis studies include the different study designs, the wide spectrum of the co-supplemented antibiotic regimen, and the few studies conducted on the same probiotic strain.Citation160
Probiotics mechanism of action in modulating H. pylori infection
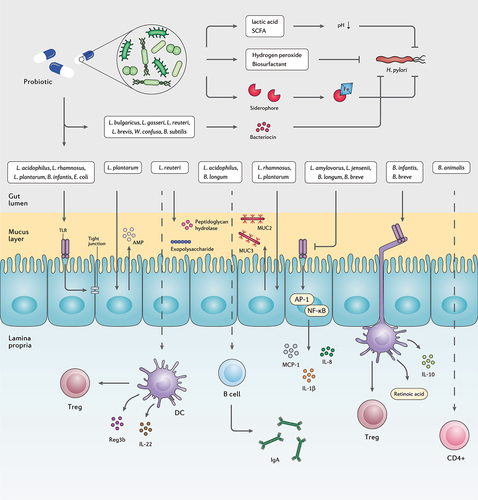
Studies have indicated that advantageous impacts of probiotics against H. pylori infection occur through a variety of mechanisms, such as reinforcement of gut mucosal barrier, elimination of pathogens, enhancement of the host immune system, and microbiome modification ().Citation161 Several probiotic species are antagonistic toward invasive pathogens, yet in H. pylori eradication, solid proof indicates that probiotics mainly reduce antibiotic-induced side effects.Citation162 However, there are considerable limitations in these mechanistic studies including high reliability on cell-culture systems not attributed to complex intestinal environment and low colonization capacity of human probiotic strains in the gastrointestinal tract of mice models.Citation131 Nonetheless, multiple key mechanisms are demonstrated for probiotic administration in clinical, in vitro, and in vivo studies, as detailed further below.
Figure 4. The interplay between probiotic strains, H. pylori, and the host immune system. Several probiotic strains can directly eliminate H. pylori cells by producing bacteriocins, siderophore, hydrogen peroxide, biosurfactant, lactic acid, and SCFAs. Probiotic bacteria can retain the activity of the gut barrier by stimulating the production of mucin and tight junction proteins. Certain probiotic species preserve the inherent structure of the gut microbiota by increasing the concentration of AMPs, peptidoglycan hydrolase, and exopolysaccharides. Furthermore, several probiotic bacteria regulate the host inflammatory response and prevent the development of chronic inflammation.
Promotion of mucosal barrier
The gastrointestinal epithelium as the front line of the host innate defense against pathogenic invaders is required to preserve the integrity of the gastrointestinal barrier. Despite the uncovered mechanisms concerning the exact relationship between the intestinal barrier and inflammatory disorders, a defective epithelial barrier rather than immune dysfunction may result in chronic inflammation.Citation163 Accordingly, H. pylori-associated carcinogenesis is either indirectly accelerated by chronic inflammation and tumorigenesis or directly through induction of epigenetic alteration in the gastric epithelial cells by bacterial factors.Citation164
The protective properties of the mucosal barrier largely rely on the gut microbiota community and their components and metabolites. Due to the presence of mucin glycan, the so-called mucus-associated microorganisms can colonize and attach to the intestinal mucus layer.Citation165 Recent advances in characterizing the beneficial mechanisms of commensal bacteria have led to novel strategies to maintain and promote intestinal barrier function. Lactobacillus plantarum ZS2058 as a probiotic can preserve the gut barrier function and permeability by modulating the expression of tight junctions and improving the intestinal epithelium.Citation166 L. plantarum 299 v and L. rhamnosus GG promote the expression of key mucin genes mucin 2 (MUC2) and MUC3 to maintain the integrity of the intestinal barrier.Citation167 Moreover, L. plantarum ZS2058 is reported enhancing the host defense peptides such as pBD2 and PG1-5; therefore, elevating the intestinal barrier function.Citation166 As a key bacterium in healthcare-related gastrointestinal infection, Clostridioides difficile colonization in the intestine contributes to nosocomial diarrhea with significant morbidity and mortality.Citation168 For which, Lactobacillus reuteri LMG P-27481 is demonstrated to provoke IL-10 production in immature DCs, repair the mucosal barrier function, and obtain a distinguish outcome in preventing C. difficile colonization and toxin load possibly by expressing bioactive molecules including exopolysaccharide and peptidoglycan hydrolases.Citation169
H. pylori can overcome the epithelial barrier by mislocalizing or reducing the expression of tight junction transmembrane protein components including junctional adhesion molecule A (JAM-A) and further disrupt the tight junctional defense barrier.Citation170 The aforementioned mechanism highlights the activity of probiotics, such as L. rhamnosus GG, L. acidophilus, L. plantarum MB452, Bifidobacterium infantis BB-02, and E. coli Nissle 1917 that stimulate TLR activation and further promote epithelial barrier by regulation of tight junction proteins production.Citation171 Nevertheless, some strains such as Lactobacillus amylovorus DSM 16698 T and Lactobacillus jensenii TL2937 negatively regulate TLR activation to inhibit the expression of pro-inflammatory cytokines IL-8 and IL-1β. Moreover, Bifidobacterium longum BB536 and Bifidobacterium breve M-16 V can significantly suppress IL-8, IL-6, and MCP-1 secretion by inhibiting activator protein 1 (AP-1) and NF-κB activation through interaction with TLR and increasing the expression of ubiquitin editing protein A20.Citation172
Secretion of antimicrobial substances
Lactic acid, SCFAs, hydrogen peroxide, and bacteriocin are the major antibacterial substances secreted from probiotics. The incomplete ionization of lactic acid and SCFAs act as proton carriers, lowering the cytoplasmic pH and accumulating toxic anions to prevent H. pylori colonization. Probiotics can further eliminate H. pylori by generating hydrogen peroxide (H2O2) and damaging pathogenic proteins, membrane lipids, and DNA of the bacterial cell.Citation173 However, due to their oxygen tolerance, lactic acid bacteria have anti-oxidative properties suppressing oxidative stress through radical scavenging, metal ion chelation, antioxidant enzyme expression, and host antioxidant and ROS-producing enzyme regulation.Citation174
Bacteriocin expression has been considered as a pivotal property of probiotics to inhibit pathogen colonization and obtain a competitive advantage. The antimicrobial mechanisms of action differ among bacteriocins, yet common mechanisms are the elevation of membrane permeability and prevention of nucleic acid and/or cell wall protein synthesis.Citation175 Bacillus subtilis 3, Weissella confuse PL9001, Lactobacillus gasseri Kx110A1, Lactobacillus brevis ATCC 14869, Lactobacillus bulgaricus, and L. reuteri ATCC 55730 demonstrated inhibitory activity against H. pylori through bacteriocin production.Citation167,Citation173 Less-studied antimicrobial compounds in probiotics are siderophores that prevent pathogen access to iron, biosurfactants that interrupt or lyse pathogen cell membrane, and adhesion inhibitors, which interfere with the pathogen adhesion to epithelial cells and consequently prevent its virulence function.Citation175
Immune promotion
Probiotic strains may indirectly suppress H. pylori infection through the host immune response promotion by stimulating the activity of phagocytoses and natural killer (NK) cells, modifying phenotype and cytokine pattern of DCs, as well as increasing antibody and anti-inflammatory cytokines secretion.Citation176 Interestingly, researchers reported that viable and non-viable bacteria had a different impact on the host cellular gene expression, suggesting the importance of both microbial cell surface and actively released substances on the gut transcriptome.Citation177
B. infantis 35624 and B. breve YIT10347 activate the intestinal DCs by interacting with TLRs and stimulating RA metabolism. As a result, DCs activation elevates the expression of IL-10 and the number of Foxp3+ Treg and type 1 regulatory T (Tr1) cells. Moreover, L. rhamnosus GG and L. acidophilus can reduce the number of Th17 cells and the expression of IL-23 and IL-17 cytokines through prevention of STAT3 and NF-κB signaling and further shift the balance between pro-inflammatory M1 and immunosuppressive M2 macrophage toward M2 phenotype.Citation178 In contrast, Bifidobacterium animalis spp. lactis Bl 5764 is able to promote IL-17A expression by CD4+ T lymphocytes in vitro. L. reuteri Lr 5454 co-culture with DCs can promote Tregs, and regenerating islet-derived protein 3-beta (Reg3b) expression in a NOD2-dependent manner and further induce IL-22 production.Citation179 IL-22 plays an imperative role in preserving gut homeostasis and tissue regeneration. Furthermore, this cytokine accelerates the colonization of Phascolarctobacterium bacterium and thereby prevents C. difficile infection (CDI).Citation180
Immunoglobulin A (IgA) as the main immunoglobulin isotype in the gut mucosa, regulates bacterial translocation and interferes with bacterial toxicity.Citation181 L. acidophilus and B. longum are the major probiotic species demonstrated to increase IgA production from B cells in the intestinal lamina propia.Citation182 Intestinal secretory IgA antibodies coat bacteria to prevent them from adhering the epithelium and barricading inflammation development. However, in vitro studies indicated that commensal microorganisms coated with IgA can grow without remarkable alteration. Moreover, high-affinity IgA coating elevates the risk of bacterial invasion and activation of inflammatory pathways. As H. pylori expresses receptors detecting IgA glycoprotein motifs, IgA attachment to these surface receptors improves H. pylori adhesion to the epithelial layer and facilitates its colonization.Citation183
Probiotic supplementation and gut microbiota alteration
Regarding H. pylori eradication, multiple studies investigated the impact of probiotic administration on the gut microbiota composition (). In the following sections, we aim to discuss the bioactivity of microbiota that noted significantly altered within the gastrointestinal tract of individuals who underwent H. pylori eradication by probiotic supplementation.
Table 1. Summary of studies examining the effects of probiotic co-supplementation to H. pylori eradication on the human gut microbiota.
Single-strain probiotic supplementation
C. butyricum is an anaerobic bacterium that consumes undigested dietary fibers and mainly produces butyrate and acetate. Although some C. butyricum strains are equipped with toxins, others are antibiotic-sensitive and free of pathogenic markers and clostridial toxin genes.Citation193 In particular, C. butyricum CBM 588 can inhibit gastrointestinal inflammation and side effects of antibiotic treatments, such as diarrhea. Consequently, oral administration of this probiotic might prevent inflammation-associated diseases such as UC.Citation194 Chen et al. reported that C. butyricum CBM 588 co-supplementation with H. pylori quadruple therapy exhibited a significant reduction in Fusobacteria and Tenericutes phyla as well as an increase in Actinobacteria phylum following H. pylori eradication. However, Lactococcus raffinolactis, Lactobacillus sakei, and Acinetobacter baumannii NIPH60 were significantly increased only in the antibiotic group.Citation99
Disregarding health conditions, over 100 uncultured Tenericutes have been recently discovered in the human gastrointestinal metagenome. Although the complex behavior of this phylum is not fully understood, Tenericutes bacteria in the host gastrointestinal tract demonstrated a substantial reduction in their genomes and metabolic capacities compared to environmental Tenericutes.Citation195 Furthermore, Tenericutes is suggested to play a key role in the host metabolic pathways, such as bile acid metabolism.Citation196 However, pathogenic species of this phylum are presented with virulence factors including hydrogen peroxide, toxins, surface polysaccharides, and sialic acid catabolism.Citation195 Therefore, the reduction in the population of this taxon may cause various metabolic changes in the host, which needs further in-depth investigations at the strain level. Moreover, Fusobacteria is not prevalent nor relatively enriched in non-colorectal cancer individuals.Citation197 This genus can stimulate cancer cell survival through modulation of STAT3, janus kinase 1 (JAK1), and MYC oncogenes and further induce tumor cell invasion by promoting IL-8 expression.Citation198 Consequently, Fusobacteria depletion in the probiotic supplemented group may indicate a potentially beneficial effect for C. butyricum CBM 588 consumption during H. pylori eradication. On the other hand, A. baumannii bacteria are opportunistic pathogens and mainly contribute to ventilator-associated pneumonia and bloodstream infections.Citation199 This pathogen has become a global health-care problem owing to the several mechanisms underlying its antibiotic resistance.Citation200 As a result, the enrichment of this pathogenic species in the antibiotic group is consistent with the foregoing favorable effectiveness of probiotic administration. However, L. sakei that enriched in the antibiotic group is beneficially involved in obesity, cardiovascular disease, and gastrointestinal inflammation.Citation201
Enterococcus faecium strains are particularly adaptive to their respective environment owing to their salt and acid tolerance. Although E. faecium are antibiotic-resistant infectious agents, they are hardly reported to induce infection in the human body. Moreover, certain E. faecium and E. faecalis strains are the only enterococci bacteria supplemented as probiotics.Citation202 Biofermin-R (multidrug-resistant preparation of E. faecium 129 BIO 3B-R) administration with H. pylori triple therapy demonstrated beneficial advantages.Citation188 This probiotic strain was reported to promote Blautia genus colonization, which is most commonly accompanied by probiotic activities.Citation203 The reduced proportion of Bifidobacterium genus in the antibiotic treated group further highlights the delicate impact of Biofermin-R supplementation on preserving the abundance of probiotic genera among gut bacteria.
Multi-strain probiotic supplementation
A randomized, controlled trial conducted in Germany demonstrated the advantage of probiotic co-supplementation in H. pylori eradication.Citation190 In this study, the intestine of probiotic supplemented individuals was the residence of a higher proportion of Slackia bacteria that are suggested beneficially involved in the host isoflavone, fat, and energy metabolism.Citation204,Citation205 On the other hand, Fusobacterium that was enriched in the antibiotic group was correlated to digestive disorders, gastrointestinal inflammation, and colorectal carcinoma.Citation206,Citation207 However, Desulfovibrio, as Gram-negative sulfate-reducing bacteria, produce hydrogen sulfide and lipopolysaccharide and might contribute to the pathogenesis of Parkinson’s disease;Citation208 consequently, the increased proportion of these bacteria in the gut bacterial community may cause post-therapy adverse effects following probiotic consumption. Moreover, Methanobrevibacter that enriched in the probiotic group are reported more abundant in Parkinson’s disease and gut dysbiosis.Citation209 On the other hand, Roseburia, as major butyrate-producing bacteria in the intestine, can reduce oxidative stress, repair intestinal mucosa, and suppress intestinal inflammation.Citation210 Therefore, the increased abundance of Roseburia bacteria in the antibiotic group may accelerate gut rebiosis after H. pylori treatment.
B. subtilis bacteria are consists of mesophilic, neutrophilic, and some pH tolerant strains with the capacity to produce a vast diversity of antimicrobial compounds.Citation211 Several studies used B. subtilis and E. faecium combination as oral supplemented probiotic and further evaluated their synergic effect on H. pylori eradication, such as the research conducted by Oh et al. exhibiting that resistant bacteria to clarithromycin and amoxicillin, including Citrobacter, Klebsiella, Pseudomonas, and Escherichia, were significantly enriched in the antibiotic group than probiotic-supplemented group.Citation184 Klebsiella pneumoniae, known as Gram-negative opportunistic pathogens, are responsible for the respiratory tract, urinary tract, and bloodstream infections. Due to the antibiotic resistance and hypervirulent characteristic of Klebsiella pneumoniae strains, clinical management of this pathogen has become progressively challenging.Citation212 Oh et al. further reported the increased abundance of Prevotella stercorea in the antibiotic group, whereas Lactobacillus ruminis were enriched in the probiotic group.Citation185 P. stercorea has been suggested to be positively correlated with the expression of mucosal pro-inflammatory cytokines especially TNF-α.Citation213 Despite the poorly understood interaction of L. ruminis with the host biofunction, these bacteria may stimulate immune response through TLR2-mediated NF-κB activation and inhibit the growth of pathogens by acid secretion and competition for binding sites.Citation214
As a result of probiotic administration for H. pylori treatment, Tang et al. reported the enrichment of beneficial bacteria including Oscillospira, Lactobacillales, and Phascolarctobacterium in the feces of probiotic-supplemented individuals.Citation189 Although some studies indicated a positive correlation between Oscillospira and intestinal inflammation, it has been demonstrated that the relative abundance of Oscillospira is negatively associated with the expression of pro-inflammatory MCP-1, as well as the development of UC, IBD, and pediatric nonalcoholic fatty liver disease (NAFLD); therefore, Oscillospira is a candidate for the next-generation probiotics.Citation215 Moreover, Lactobacillales of the Bacilli family can stimulate the innate and adaptive immune system and suppress inflammation by regulating IL-17 production.Citation216 Phascolarctobacterium are reduced in hepatitis B virus-infected patientsCitation217 as well as individuals with postpartum depression disorder.Citation218 As succinate consumers, Phascolarctobacterium bacteria can interfere with the colonization of succinate-consuming bacteria; therefore, preventing CDI.Citation219 On the other hand, pathogenic bacteria have been reported to be enriched in the antibiotic group as Dialister, Sutterella, and Collinsella are mainly responsible for gut inflammation, liver diseases, and digestive disorder.Citation220–222 Furthermore, Anaerotruncus that enriched in the antibiotic group are butyrate-producing bacteria with a positive correlation to saturated fatty acid and cholesterol intake; therefore, they are involved in obesity and NAFLD-associated hepatocellular carcinoma (HCC).Citation223,Citation224 Citrobacter genus presented a low virulence activity following their colonization in the gastrointestinal tract. However, increased abundance of Citrobacter species might lead to severe diseases in respiratory and urinary tract, central nervous system, bloodstream, and intestines in the probiotic supplemented patients.Citation225 Anaerofustis genus is associated with movement and psychiatric disorders as well as pro-inflammatory activities.Citation226 Furthermore, decreased starch degradation, possibly as a result of Collinsella reduction, leads to low levels of SCFAs production and weakens the gut epithelial barrier and host immune response in the probiotic group.Citation227 Moreover, certain commensal bacteria including Megasphaera, Ruminococcus, and Coprococcus were significantly increased in the antibiotic group. Although some studies indicated that Ruminococcus species, particularly Ruminococcus gnavus, are correlated with T2D, CD, and UC,Citation228,Citation229 certain species such as Ruminococcus bromii, are abundant in healthy individuals and may lower cardiovascular risk and provide anti-inflammatory compounds through carbohydrate degradation.Citation230,Citation231 Furthermore, the proportion of gut Ruminococcus species is possibly associated with the number of CD8+ Treg cells in the human body, and thereby Ruminococcus bacteria may lower the risk for developing type 1 diabetes (T1D).Citation232 Megasphaera are capable of SCFAs synthesis, osmotic diarrhea regulation, and host immune response promotion.Citation233 Moreover, Coprococcus are inversely correlated to depression, lung cancer, and Parkinson’s disease.Citation234–236
In consistent with the aforementioned studies, Wu et al. reported Dialister and Plesiomonas as the main genera in the patients undergoing H. pylori triple therapy regimen,Citation186,Citation187in which Plesiomonas shigelloides, as a single species in the Plesiomonas genus, is involved in gastrointestinal disorders including gastroenteritis and diarrhea.Citation237 Nevertheless, some pathogenic bacteria, such as Achromobacter, Actinomyces, and Cupriavidus, were enriched during the study follow-up of the probiotic-supplemented group.
Non-viable probiotic supplementation
The capacity of supplemented probiotics to temporarily or persistently colonize the gut mucosa and whether it is essential for their effects on the host biofunction are yet to be fully elucidated. A vast majority of researchers examined the successful probiotic colonization in the host mucosal layer by the proportion of probiotic bacteria in stool without direct assessment of mucosal samples.Citation238 In a recent study, the comparison of fecal and mucosal expansion of supplemented probiotic species demonstrated that fecal presence of probiotic strains cannot identify permissive and resistant individuals, suggesting the passage of probiotic bacteria through the gastrointestinal tract without substantial adhesion nor colonization.Citation239 Consequently, some studies investigated the effects of probiotic strains without the colonization capacity through the administration of dead and inactivated microorganisms, also termed as paraprobiotics.
Through leveraging a non-viable probiotic to reduce the cost and biological risk of treatment, Yang et al. demonstrated Fusicatenibacter, Bacteroides, Faecalibacterium, and Subdoligranulum as the main genera in the stool sample of individuals undergoing H. pylori triple therapy plus probiotic regimen.Citation191 Several Bacteroides species are commensal bacteria providing nutrition and vitamins and playing a key role in cancer immunotherapy and prevention.Citation240 Faecalibacterium genus mainly promote the host immune system by producing anti-inflammatory substances such as butyric acid and bioactive peptides; thereby, the reduced proportion of Faecalibacterium bacteria is correlated with the progression of IBD.Citation241 Although the exact bioactivity of Subdoligranulum are not fully understood, this genus is suggested to have probiotic properties, particularly in the host metabolic health.Citation242 Moreover, Fusicatenibacter are involved in butyric acid production and inversely correlated with IL-8 expression.Citation243 On the other hand, Escherichia-Shigella, as the abundant bacteria in the antibiotic group, are associated with macrophage cell death, gut inflammation, and diarrhea.Citation244,Citation245
A recent study conducted in China reported the advantage of multi-strain probiotic administration in which detrimental bacteria were enriched in the antibiotic group while commensal bacteria were more abundant in the probiotic group.Citation192 Lachnospiraceae UCG 006 and Eubacterium ventriosum, as commensal bacteria, can protect the human intestinal against colorectal cancer by producing SCFAs.Citation246,Citation247 Furthermore, Ruminococcaceae bacteria are one of the main butyrate producers in the human digestive tract; therefore, promoting the integrity of the gut barrier.Citation248 On the other hand, the increased proportion of Leptotrichia is a risk factor for colorectal cancer;Citation249 however, certain Leptotrichia species might be inversely correlated to pancreatic cancer.Citation250 Moreover, Leptotrichia is reported as an oral health-related genus, substantially enriched in healthy individuals without dental caries experience.Citation251
As one of the major causes of gastroenteritis, Campylobacter genus prevalence increased during the last decade globally. Well-studied species within the Campylobacter genus are C. jejuni and C. fetus, mainly responsible for the vast majority of reported Campylobacter infections and bloodstream infections, respectively.Citation252 Therefore, the enhanced colonization of Campylobacter bacteria in the antibiotic group may further emphasize the beneficial effect of paraprobiotic consumption. Although Erysipelatoclostridium are SCFAs producers, the relative abundance of this genus is demonstrated to be enriched in the intestine of patients with gout, metabolic syndrome, and IBS.Citation253,Citation254 Furthermore, Ralstonia is a genus of Gram-negative opportunistic bacteria causing infection in immunocompromised hosts.Citation255 However, these bacteria are more abundant in H. pylori-negative individuals than infected patients.Citation256 Consequently, Erysipelatoclostridium and Ralstonia enrichment may increase the risk of developing gastrointestinal inflammation and immune disorders in the antibiotic group.
The pros and cons of probiotic supplementation
While the safety of probiotic strains constitutes decades of ongoing conflict, researchers have generally reported beneficial advantages of probiotic supplementation in maintaining the host indigenous microbiome and reducing drug-related adverse effects.Citation257 Notwithstanding, probiotic-induced adverse effects are poorly investigated and less noted in clinical trials.Citation258 It has been recently indicated that the exposure of neonates to probiotic species is associated with a higher risk of oral, respiratory, and gastrointestinal infection throughout the lifespan.Citation259 Furthermore, probiotic co-supplemented therapies, especially with Lactobacillus species, might be correlated with a delayed and incomplete post-antibiotic recovery of normal host–microbiome balance resulting in a long-term gut dysbiosis.Citation260
Although fecal microbial composition may not exactly indicate the intestinal mucosa-adherent microbiome,Citation261 only a limited number of clinical trials have investigated the influence of probiotic consumption on the gastrointestinal microbiota in situ. Thus, till the performance of more accurate studies, comparing the microbiota profile of patient’s stool sample following probiotic interventions and various other conditions may roughly represent the long-term safety and efficacy of probiotic supplementation. In this regard, C. butyricum CBM 588 co-supplementationCitation99 may inhibit the replication of commensal bacteria and cause metabolic disorder, meanwhile reducing the risk of colorectal cancer and preventing the overgrowth of certain opportunistic pathogens. Furthermore, consumption of single-strain probiotic Biofermin-RCitation188 may potentially promote the colonization of commensal bacteria.
Concerning multi-strain probiotic supplementation, results from the study by Guillemard et al.Citation190 indicate the possibility of developing Parkinson’s disease and depletion of key butyrate-producing bacteria. However, this study may further point out the beneficial effect of probiotic supplementation through regulating the host isoflavone, fat, and energy metabolism, and reducing the risk of developing digestive disorders, gastrointestinal inflammation, and colorectal carcinoma. On the other hand, B. subtilis and E. faecium administrationCitation184,Citation185 can inhibit the colonization of some opportunistic pathogens and potentially prevent respiratory tract, urinary tract, and bloodstream infections, as well as gastrointestinal inflammation. Gastrointestinal microbiota alteration following probiotic supplementation in the Tang et al.Citation189 study further indicates the capacity of probiotic strains to prevent intestinal inflammation, as well as the development of UC, IBD, NAFLD, and CDI. However, the persistence of pathogenic genera in the probiotic supplemented group may provoke the emergence of severe gastrointestinal and extra-gastrointestinal diseases. Moreover, Wu et al.Citation186 reported the enrichment of pathogenic bacteria in both the probiotic and the antibiotic groups. This might indicate limited effectiveness for the consumed probiotic strains in modulating drug-related adverse events.
Data from the study by Yang et al.Citation191 may demonstrate the potential capacity of paraprobiotic consumption in promoting the host immune response, preventing intestinal inflammation and IBD development, and improving nutritional availability. Likewise, paraprobiotic administrationCitation192 may also promote the replication of SCFA-producing bacteria and prevent colorectal cancer, and preserve the integrity of the gut barrier. This might further inhibit pathogen colonization and lower the risk of developing bloodstream infection, metabolic syndrome, and IBS.
Limitations and outstanding questions
Extensive complexity might describe the foremost characteristic of the host-microbiota multifaceted interplay. The individual gut microbiota composition at deep resolution levels and enormous structural diversity affecting bacterial functionality remain the challenge of grasping a profound knowledge in the field of host health and probiotic supplementation. This complexity leads to major limitations in determining the source of metabolites as host, probiotic, or indigenous microbiota;Citation54 understanding the whole spectrum of condition-dependent and dose-dependent influence of probiotics; and exploring probiotic-host interaction in cell-culture systems or animal models.Citation131 These limitations are frequently intermingled in ways that force conceptual and statistical interpretation toward substantial challenges. Nonetheless, several cohort studies with probiotic-oriented approaches guided experimental reductionism to elucidate mechanistic comprehension regarding probiotic involvement in human health and disease. Furthermore, the introduction of specific microbiome including probiotic strains into organoids by microinjection is a novel strategy to investigate the accurate cause and effect interaction between the host and microbiome.Citation262
Recent advances in the knowledge about microbiota and the presentation of innovative experimental techniques would enable the integration of strain-specific features of probiotics and consideration of biologically related notions to accelerate the development of tailored therapeutics. The clinically controlled trials are the most practical way toward probiotic strain selection, in which a mechanism-oriented strategy should be pursued and certain questions should be contemplated. Is the probiotic interaction with the host mediated through the secretion of metabolites and alteration of the gut microbiota or colonization of the intestinal surface or other possible contact-dependent interactions? Should a next-generation probiotic strain be considered safe to provide a medical advantage in therapeutic interventions? What are the long-term consequences of probiotic-mediated alteration of the host microbiome? These critically important questions might be resolved by the novel paradigms of microbiome-on-a-chip technology, which can provide the real-time assessment of the host-microbiome interaction and explore the emergence of microbiome-related therapeutics.Citation263
Conclusions and outlook
The oral supplementation of a narrow diversity of Gram-positive bacteria demonstrated distinct amelioration in H. pylori-related clinical symptoms; however, the identified alteration in the gut microbiota demonstrates the possibility of intestinal or extra-gastrointestinal disease development later in life. Considering the enriched and depleted genera in the stool samples of probiotic-supplemented individuals, oral administration of multi-strain probiotics and paraprobiotics than single-strain probiotics might reduce the incidence of developing metabolic disorders. Yet, various characteristics of different strains in a common genus require innovative clinical approaches with high-throughput sequencing technology to determine gut microbiota alteration at the strain level.
Recent studies have expressed the intention of seeking next-generation probiotics and genetically modified microorganisms to promote the beneficial effect of probiotic supplementation in clinical outcomes. Concerning current probiotic strains, two main strategies are suggested for developing next-generation probiotics. One way is to identify the presence or absence of particular strains within the disease condition and investigate the efficiency of supplementing those strains to recover the health state. Another strategy is to harness a well-characterized probiotic strain to express a particular metabolites such as AMPs.Citation264 Recent discoveries in biotechnology will accelerate the emergence of novel candidate probiotic strains and facilitate the transition from empiric into target-oriented interventions. Furthermore, the integration of nanotechnology with microencapsulation strategies may efficiently enhance the probiotic delivery system and thereby provide a regulatory framework to reduce the metabolic consequences of probiotic supplementation. Large-scale population studies with broad-spectrum antibiotic regimens and probiotic strains, as well as germ-free mice modeled by the human microbiome, will shed light on the long-term outcome of probiotic supplementation and elucidate unconventional ways to leverage diet and clinical interventions and personalize them to the subjects’ biology and microbiota.
Author contributions
ANR and AY contributed significantly to the literature review and wrote the draft of the manuscript. ANR and AY designed and illustrated the figures. AY worked on the concept and design of the study and interpreted the collected information. AS, HAA, and MRZ provided clinical advice and guidance for the improvement of the manuscript. AY and SMS critically revised the final version of the manuscript. All authors have seen and approved the final version of the manuscript and the author list.
Acknowledgments
We sincerely thank all the members of the Foodborne and Waterborne Diseases Research Center at the Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this work.
Additional information
Funding
References
- Miller AK, Williams SM. Helicobacter pylori infection causes both protective and deleterious effects in human health and disease. Genes Immun. 2021;22(4):218–34. doi:10.1038/s41435-021-00146-4.
- Nabavi-Rad A, Azizi M, Jamshidizadeh S, Sadeghi A, Aghdaei HA, Yadegar A, Zali MR. The effects of vitamins and micronutrients on helicobacter pylori pathogenicity, survival, and eradication: a crosstalk between micronutrients and immune system. J Immunol Res. 2022;2022:4713684. doi:10.1155/2022/4713684.
- Senchukova MA, Tomchuk O, Shurygina EI. Helicobacter pylori in gastric cancer: features of infection and their correlations with long-term results of treatment. World J Gastroenterol. 2021;27(37):6290–6305. doi:10.3748/wjg.v27.i37.6290.
- Suzuki S, Gotoda T, Kusano C, Ikehara H, Ichijima R, Ohyauchi M, Ito H, Kawamura M, Ogata Y, Ohtaka M, et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: a multicentre randomised trial in Japan. Gut. 2020;69(6):1019. doi:10.1136/gutjnl-2019-319954.
- Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66(1):6–30. doi:10.1136/gutjnl-2016-312288.
- Liu WZ, Xie Y, Lu H, Cheng H, Zeng ZR, Zhou LY, Chen Y, Wang JB, Du YQ, Lu NH, et al. Fifth Chinese national consensus report on the management of Helicobacter pylori infection. Helicobacter. 2018;23(2):e12475. doi:10.1111/hel.12475.
- Nyssen OP, Bordin D, Tepes B, Pérez-Aisa Á, Vaira D, Caldas M, Bujanda L, Castro-Fernandez M, Lerang F, Leja M, et al. European registry on Helicobacter pylori management (Hp-EuReg): patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21 533 patients. Gut. 2021;70(1):40. doi:10.1136/gutjnl-2020-321372.
- McNicholl AG, Molina-Infante J, Lucendo AJ, Calleja JL, Pérez‐Aisa Á, Modolell I, Aldeguer X, Calafat M, Comino L, Ramas M, et al. Probiotic supplementation with lactobacillus plantarum and pediococcus acidilactici for Helicobacter pylori therapy: a randomized, double-blind, placebo-controlled trial. Helicobacter. 2018;23(5):e12529. doi:10.1111/hel.12529.
- Cunningham M, Azcarate-Peril MA, Barnard A. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021;29(8):667–685.
- Goderska K, Agudo Pena S, Alarcon T. Helicobacter pylori treatment: antibiotics or probiotics. Appl Microbiol Biotechnol. 2018;102(1):1–7. doi:10.1007/s00253-017-8535-7.
- Ferraris C, Elli M, Tagliabue A. Gut microbiota for health: how can diet maintain a healthy gut microbiota? Nutrients. 2020;12(11):3596. doi:10.3390/nu12113596.
- de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71(5):1020–1032. doi:10.1136/gutjnl-2021-326789.
- Hillman ET, Lu H, Yao T, Nakatsu CH. Microbial ecology along the gastrointestinal tract. Microbes Environ. 2017;32(4):300–313. doi:10.1264/jsme2.ME17017.
- Shkoporov AN, Hill C. Bacteriophages of the human gut: the “known unknown” of the microbiome. Cell Host Microbe. 2019;25(2):195–209. doi:10.1016/j.chom.2019.01.017.
- O’Riordan KJ, Collins MK, Moloney GM, Knox EG, Aburto MR, Fülling C, Morley SJ, Clarke G, Schellekens H, Cryan JF, et al. Short chain fatty acids: microbial metabolites for gut-brain axis signalling. Mol Cell Endocrinol. 2022;546:111572. doi:10.1016/j.mce.2022.111572.
- Engevik M, Versalovic J. Taking a closer look at the biogeography of the human gastrointestinal microbiome. Gastroenterology. 2019;157(4):927–929. doi:10.1053/j.gastro.2019.08.006.
- Herath M, Hosie S, Bornstein JC, Franks AE, Hill-Yardin EL. The role of the gastrointestinal mucus system in intestinal homeostasis: implications for neurological disorders. Front Cell Infect Microbiol. 2020;10:248. doi:10.3389/fcimb.2020.00248.
- Martens EC, Neumann M, Desai MS. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol. 2018;16(8):457–470. doi:10.1038/s41579-018-0036-x.
- Mowat AM. To respond or not to respond - a personal perspective of intestinal tolerance. Nat Rev Immunol. 2018;18(6):405–415. doi:10.1038/s41577-018-0002-x.
- Yoo JY, Groer M, Dutra SVO, Sarkar A, McSkimming DI. Gut microbiota and immune system interactions. Microorganisms. 2020;8(10):1587. doi:10.3390/microorganisms8101587.
- Le Noci V, Bernardo G, Bianchi F, Tagliabue E, Sommariva M, Sfondrini L. Toll like receptors as sensors of the tumor microbial dysbiosis: implications in cancer progression. Front Cell Dev Biol. 2021;9:732192. doi:10.3389/fcell.2021.732192.
- Ferrand A, Al Nabhani Z, Tapias NS, Mas E, Hugot JP, Barreau F. NOD2 expression in intestinal epithelial cells protects toward the development of inflammation and associated carcinogenesis. Cell Mol Gastroenterol Hepatol. 2019;7(2):357–369. doi:10.1016/j.jcmgh.2018.10.009.
- Moltzau Anderson J, Lipinski S, Sommer F, Pan W-H, Boulard O, Rehman A, Falk-Paulsen M, Stengel ST, Aden K, Häsler R, et al. NOD2 influences trajectories of intestinal microbiota recovery after antibiotic perturbation. Cell Mol Gastroenterol Hepatol. 2020;10(2):365–389. doi:10.1016/j.jcmgh.2020.03.008.
- Fernández-García V, González-Ramos S, Martín-Sanz P, Portillo FG-D, Laparra JM, Boscá L. NOD1 in the interplay between microbiota and gastrointestinal immune adaptations. Pharmacological Research. 2021;171:105775. doi:10.1016/j.phrs.2021.105775.
- Irving AT, Mimuro H, Kufer TA, Lo C, Wheeler R, Turner L, Thomas B, Malosse C, Gantier M, Casillas L, et al. The immune receptor NOD1 and kinase RIP2 interact with bacterial peptidoglycan on early endosomes to promote autophagy and inflammatory signaling. Cell Host Microbe. 2014;15(5):623–635. doi:10.1016/j.chom.2014.04.001.
- Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30(6):492–506. doi:10.1038/s41422-020-0332-7.
- Zong X, Fu J, Xu B, Wang Y, Jin M. Interplay between gut microbiota and antimicrobial peptides. Animal Nutrition. 2020;6(4):389–396. doi:10.1016/j.aninu.2020.09.002.
- Lueschow SR, McElroy SJ. The paneth cell: the curator and defender of the immature small intestine. Front Immunol. 2020;11:587. doi:10.3389/fimmu.2020.00587.
- Liang W, Enee E, Andre-Vallee C, Falcone M, Sun J, Diana J. Intestinal cathelicidin antimicrobial peptide shapes a protective neonatal gut microbiota against pancreatic autoimmunity. Gastroenterology. 2021;162(4):1288–1302.e16. doi:10.1053/j.gastro.2021.12.272.
- Healy DB, Ryan CA, Ross RP, Stanton C, Dempsey EM. Clinical implications of preterm infant gut microbiome development. Nat Microbiol. 2022;7(1):22–33. doi:10.1038/s41564-021-01025-4.
- Lycke NY, Bemark M. The regulation of gut mucosal IgA B-cell responses: recent developments. Mucosal Immunol. 2017;10(6):1361–1374. doi:10.1038/mi.2017.62.
- Pabst O, Slack E. IgA and the intestinal microbiota: the importance of being specific. Mucosal Immunol. 2020;13(1):12–21. doi:10.1038/s41385-019-0227-4.
- Abokor AA, McDaniel GH, Golonka RM, Campbell C, Brahmandam S, Yeoh BS, Joe B, Vijay-Kumar M, Saha P. Immunoglobulin A, an active liaison for host-microbiota homeostasis. Microorganisms. 2021;9(10):2117. doi:10.3390/microorganisms9102117.
- Krishnaswamy JK, Alsen S, Yrlid U, Eisenbarth SC, Williams A. Determination of t follicular helper cell fate by dendritic cells. Front Immunol. 2018;9:2169. doi:10.3389/fimmu.2018.02169.
- Agus A, Clément K, Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut. 2021;70(6):1174–1182. doi:10.1136/gutjnl-2020-323071.
- Michaudel C, Sokol H. The gut microbiota at the service of immunometabolism. Cell Metab. 2020;32(4):514–523. doi:10.1016/j.cmet.2020.09.004.
- Liu KH, Owens JA, Saeedi B, Cohen CE, Bellissimo MP, Naudin C, Darby T, Druzak S, Maner-Smith K, Orr M, et al. Microbial metabolite delta-valerobetaine is a diet-dependent obesogen. Nature Metabolism. 2021;3(12):1694–1705. doi:10.1038/s42255-021-00502-8.
- Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17(4):223–237. doi:10.1038/s41575-019-0258-z.
- Portincasa P, Bonfrate L, Vacca M, De Angelis M, Farella I, Lanza E, Khalil M, Wang DQH, Sperandio M, Di Ciaula A, et al. Gut microbiota and short chain fatty acids: implications in glucose homeostasis. Int J Mol Sci. 2022;23(3):1105. doi:10.3390/ijms23031105.
- Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019;16(8):461–478. doi:10.1038/s41575-019-0157-3.
- Nugent SG, Kumar D, Rampton DS, Evans DF. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut. 2001;48(4):571–577. doi:10.1136/gut.48.4.571.
- Traxinger BR, Richert-Spuhler LE, Lund JM. Mucosal tissue regulatory T cells are integral in balancing immunity and tolerance at portals of antigen entry. Mucosal Immunol. 2021;15(3):398–407]. doi:10.1038/s41385-021-00471-x.
- Kim CH. Control of lymphocyte functions by gut microbiota-derived short-chain fatty acids. Cell Mol Immunol. 2021;18(5):1161–1171. doi:10.1038/s41423-020-00625-0.
- Liu P, Wang Y, Yang G, Zhang Q, Meng L, Xin Y, Jiang X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol Res. 2021;165:105420. doi:10.1016/j.phrs.2021.105420.
- He J, Zhang P, Shen L, Niu L, Tan Y, Chen L, Zhao Y, Bai L, Hao X, Li X, et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int J Mol Sci. 2020;21(17):6356. doi:10.3390/ijms21176356.
- de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013;17(5):657–669. doi:10.1016/j.cmet.2013.03.013.
- Funabashi M, Grove TL, Wang M, Varma Y, McFadden ME, Brown LC, Guo C, Higginbottom S, Almo SC, Fischbach MA, et al. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature. 2020;582(7813):566–570. doi:10.1038/s41586-020-2396-4.
- Guzior DV, Quinn RA. Review: microbial transformations of human bile acids. Microbiome. 2021;9(1):140. doi:10.1186/s40168-021-01101-1.
- Jia X, Lu S, Zeng Z, Liu Q, Dong Z, Chen Y, Zhu Z, Hong Z, Zhang T, Du G, et al. Characterization of gut microbiota, bile acid metabolism, and cytokines in intrahepatic cholangiocarcinoma. Hepatology. 2020;71(3):893–906. doi:10.1002/hep.30852.
- Zhang M, Serna-Salas S, Damba T, Borghesan M, Demaria M, Moshage H. Hepatic stellate cell senescence in liver fibrosis: characteristics, mechanisms and perspectives. Mech Ageing Dev. 2021;199:111572. doi:10.1016/j.mad.2021.111572.
- Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15(2):111–128. doi:10.1038/nrgastro.2017.119.
- Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018;9(1):3294. doi:10.1038/s41467-018-05470-4.
- Peng H, Wang Y, Luo W. Multifaceted role of branched-chain amino acid metabolism in cancer. Oncogene. 2020;39(44):6747–6756. doi:10.1038/s41388-020-01480-z.
- Krautkramer KA, Fan J, Bäckhed F. Gut microbial metabolites as multi-kingdom intermediates. Nat Rev Microbiol. 2021;19(2):77–94. doi:10.1038/s41579-020-0438-4.
- Li X, Hong J, Wang Y, Pei M, Wang L, Gong Z. Trimethylamine-N-Oxide Pathway: a Potential Target for the Treatment of MAFLD. Front Mol Biosci. 2021;8:733507. doi:10.3389/fmolb.2021.733507.
- Brown JM, Hazen SL. Microbial modulation of cardiovascular disease. Nat Rev Microbiol. 2018;16(3):171–181. doi:10.1038/nrmicro.2017.149.
- Dalal N, Jalandra R, Bayal N. Gut microbiota-derived metabolites in CRC progression and causation. J Cancer Res Clin Oncol. 2021;147(11):3141–3155. doi:10.1007/s00432-021-03729-w.
- Li XS, Obeid S, Wang Z, Hazen BJ, Li L, Wu Y, Hurd AG, Gu X, Pratt A, Levison BS, et al. Trimethyllysine, a trimethylamine N-oxide precursor, provides near- and long-term prognostic value in patients presenting with acute coronary syndromes. Eur Heart J. 2019;40(32):2700–2709. doi:10.1093/eurheartj/ehz259.
- McBurney MI, Davis C, Fraser CM, Schneeman BO, Huttenhower C, Verbeke K, Walter J, Latulippe ME. Establishing what constitutes a healthy human gut microbiome: state of the science, regulatory considerations, and future directions. J Nutr. 2019;149(11):1882–1895. doi:10.1093/jn/nxz154.
- Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17(4):219–232. doi:10.1038/nri.2017.7.
- Jochum L, Stecher B. Label or concept - what is a pathobiont? Trends Microbiol. 2020;28(10):789–792. doi:10.1016/j.tim.2020.04.011.
- Zeng MY, Inohara N, Nunez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10(1):18–26. doi:10.1038/mi.2016.75.
- Hiippala K, Jouhten H, Ronkainen A, Hartikainen A, Kainulainen V, Jalanka J, Satokari R. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients. 2018;10(8):988. doi:10.3390/nu10080988.
- Khan R, Petersen FC, Shekhar S. Commensal bacteria: an emerging player in defense against respiratory pathogens. Front Immunol. 2019;10:1203. doi:10.3389/fimmu.2019.01203.
- Carvalho AL, Fonseca S, Miquel-Clopes A, Cross K, Kok K-S, Wegmann U, Gil-Cardoso K, Bentley EG, Al Katy SHM, Coombes JL, et al. Bioengineering commensal bacteria-derived outer membrane vesicles for delivery of biologics to the gastrointestinal and respiratory tract. J Extracell Vesicles. 2019;8(1):1632100. doi:10.1080/20013078.2019.1632100.
- Mosca A, Leclerc M, Hugot JP. Gut microbiota diversity and human diseases: should we reintroduce key predators in our ecosystem? Front Microbiol. 2016;7:455. doi:10.3389/fmicb.2016.00455.
- Roswall J, Olsson LM, Kovatcheva-Datchary P, Nilsson S, Tremaroli V, Simon M-C, Kiilerich P, Akrami R, Krämer M, Uhlén M, et al. Developmental trajectory of the healthy human gut microbiota during the first 5 years of life. Cell Host Microbe. 2021;29(5):765–776 e763. doi:10.1016/j.chom.2021.02.021.
- Pellicano R, Ianiro G, Fagoonee S, Settanni CR, Gasbarrini A. Review: extragastric diseases and Helicobacter pylori. Helicobacter. 2020;25(Suppl 1):e12741. doi:10.1111/hel.12741.
- Ohno H, Satoh-Takayama N. Stomach microbiota, Helicobacter pylori, and group 2 innate lymphoid cells. Exp Mol Med. 2020;52(9):1377–1382. doi:10.1038/s12276-020-00485-8.
- Iino C, Shimoyama T. Impact of Helicobacter pylori infection on gut microbiota. World J Gastroenterol. 2021;27(37):6224–6230. doi:10.3748/wjg.v27.i37.6224.
- Engstrand L, Graham DY. Microbiome and Gastric Cancer. Dig Dis Sci. 2020;65(3):865–873. doi:10.1007/s10620-020-06101-z.
- Chen CC, Liou JM, Lee YC, Hong TC, El-Omar EM, Wu MS. The interplay between Helicobacter pylori and gastrointestinal microbiota. Gut Microbes. 2021;13(1):1–22. doi:10.1080/19490976.2021.1909459.
- Wadhwa R, Song S, Lee JS, Yao Y, Wei Q, Ajani JA. Gastric cancer-molecular and clinical dimensions. Nat Rev Clin Oncol. 2013;10(11):643–655. doi:10.1038/nrclinonc.2013.170.
- Martin-Nuñez GM, Cornejo-Pareja I, Clemente-Postigo M, Tinahones FJ. Gut microbiota: the missing link between helicobacter pylori infection and metabolic disorders? Front Endocrinol (Lausanne). 2021;12(657). doi:10.3389/fendo.2021.639856.
- Martinez JE, Kahana DD, Ghuman S, Wilson HP, Wilson J, Kim SCJ, Lagishetty V, Jacobs JP, Sinha-Hikim AP, Friedman TC, et al. unhealthy lifestyle and gut dysbiosis: a better understanding of the effects of poor diet and nicotine on the intestinal microbiome. Front Endocrinol (Lausanne). 2021;12:667066. doi:10.3389/fendo.2021.667066.
- Gaulke CA, Sharpton TJ. The influence of ethnicity and geography on human gut microbiome composition. Nat Med. 2018;24(10):1495–1496. doi:10.1038/s41591-018-0210-8.
- Vasapolli R, Schutte K, Schulz C, Vital M, Schomburg D, Pieper DH, Vilchez-Vargas R, Malfertheiner P. Analysis of transcriptionally active bacteria throughout the gastrointestinal tract of healthy individuals. Gastroenterology. 2019;157(4):1081–1092 e1083. doi:10.1053/j.gastro.2019.05.068.
- Bik EM, Eckburg PB, Gill SR. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci U S A. 2006;103(3):732–737. doi:10.1073/pnas.0506655103.
- Li XX, Wong GL, To KF, Wong VWS, Lai LH, Chow DKL, Lau JYW, Sung JJY, Ding C. Bacterial microbiota profiling in gastritis without Helicobacter pylori infection or non-steroidal anti-inflammatory drug use. PLoS One. 2009;4(11):e7985. doi:10.1371/journal.pone.0007985.
- Chen X, Zhou X, Liao B, Zhou Y, Cheng L, Ren B. The cross-kingdom interaction between Helicobacter pylori and Candida albicans. PLoS Pathog. 2021;17(5):e1009515–e1009515. doi:10.1371/journal.ppat.1009515.
- Noto JM, Zackular JP, Varga MG, Delgado A, Romero-Gallo J, Scholz MB, Piazuelo MB, Skaar EP, Peek RM. Modification of the gastric mucosal microbiota by a strain-specific helicobacter pylori oncoprotein and carcinogenic histologic phenotype. mBio. 2019;10(3). doi:10.1128/mBio.00955-19.
- Wang L, Xin Y, Zhou J. Gastric mucosa-associated microbial signatures of early gastric cancer. Front Microbiol. 2020;11:1548. doi:10.3389/fmicb.2020.01548.
- Coker OO, Dai Z, Nie Y, Zhao G, Cao L, Nakatsu G, Wu WK, Wong SH, Chen Z, Sung JJY, et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut. 2018;67(6):1024–1032. doi:10.1136/gutjnl-2017-314281.
- Wang L, Zhou J, Xin Y. Bacterial overgrowth and diversification of microbiota in gastric cancer. Eur J Gastroenterol Hepatol. 2016;28(3):261–266. doi:10.1097/MEG.0000000000000542.
- Eun CS, Kim BK, Han DS, Kim SY, Kim KM, Choi BY, Song KS, Kim YS, Kim JF. Differences in gastric mucosal microbiota profiling in patients with chronic gastritis, intestinal metaplasia, and gastric cancer using pyrosequencing methods. Helicobacter. 2014;19(6):407–416. doi:10.1111/hel.12145.
- Aviles-Jimenez F, Vazquez-Jimenez F, Medrano-Guzman R, Mantilla A, Torres J. Stomach microbiota composition varies between patients with non-atrophic gastritis and patients with intestinal type of gastric cancer. Sci Rep. 2014;4(1):4202. doi:10.1038/srep04202.
- Ferreira RM, Pereira-Marques J, Pinto-Ribeiro I, Costa JL, Carneiro F, Machado JC, Figueiredo C. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut. 2018;67(2):226–236. doi:10.1136/gutjnl-2017-314205.
- Dicksved J, Lindberg M, Rosenquist M, Enroth H, Jansson JK, Engstrand L. Molecular characterization of the stomach microbiota in patients with gastric cancer and in controls. J Med Microbiol. 2009;58(Pt 4):509–516. doi:10.1099/jmm.0.007302-0.
- Guo Y, Zhang Y, Gerhard M, Gao -J-J, Mejias-Luque R, Zhang L, Vieth M, Ma J-L, Bajbouj M, Suchanek S, et al. Effect of Helicobacter pylori on gastrointestinal microbiota: a population-based study in Linqu, a high-risk area of gastric cancer. Gut. 2020;69(9):1598–1607. doi:10.1136/gutjnl-2019-319696.
- Yang J, Zhou X, Liu X, Ling Z, Ji F. Role of the gastric microbiome in gastric cancer: from carcinogenesis to treatment. Front Microbiol. 2021;12:641322. doi:10.3389/fmicb.2021.641322.
- Navashenaq JG, Shabgah AG, Banach M, Jamialahmadi T, Penson PE, Johnston TP, Sahebkar A. The interaction of Helicobacter pylori with cancer immunomodulatory stromal cells: new insight into gastric cancer pathogenesis. Semin Cancer Biol. 2021. doi:10.1016/j.semcancer.2021.09.014.
- Bakhti SZ, Latifi-Navid S. Interplay and cooperation of Helicobacter pylori and gut microbiota in gastric carcinogenesis. BMC Microbiol. 2021;21(1):258. doi:10.1186/s12866-021-02315-x.
- Gao JJ, Zhang Y, Gerhard M, Mejias-Luque R, Zhang L, Vieth M, Ma J-L, Bajbouj M, Suchanek S, Liu W-D, et al. Association between gut microbiota and helicobacter pylori-related gastric lesions in a high-risk population of gastric cancer. Front Cell Infect Microbiol. 2018;8:202. doi:10.3389/fcimb.2018.00202.
- He C, Peng C, Wang H, Ouyang Y, Zhu Z, Shu X, Zhu Y, Lu N. The eradication of Helicobacter pylori restores rather than disturbs the gastrointestinal microbiota in asymptomatic young adults. Helicobacter. 2019;24(4):e12590. doi:10.1111/hel.12590.
- Frost F, Kacprowski T, Ruhlemann M, Bang C, Franke A, Zimmermann K, Nauck M, Völker U, Völzke H, Biffar R, et al. Helicobacter pylori infection associates with fecal microbiota composition and diversity. Sci Rep. 2019;9(1):20100. doi:10.1038/s41598-019-56631-4.
- Lapidot Y, Reshef L, Cohen D, Muhsen K. Helicobacter pylori and the intestinal microbiome among healthy school-age children. Helicobacter. 2021;26(6):e12854. doi:10.1111/hel.12854.
- Heimesaat MM, Fischer A, Plickert R, Wiedemann T, Loddenkemper C, Göbel UB, Bereswill S, Rieder G. Helicobacter pylori induced gastric immunopathology is associated with distinct microbiota changes in the large intestines of long-term infected mongolian gerbils. PLOS ONE. 2014;9(6):e100362. doi:10.1371/journal.pone.0100362.
- Dash NR, Khoder G, Nada AM, Al Bataineh MT. Exploring the impact of Helicobacter pylori on gut microbiome composition. PLoS One. 2019;14(6):e0218274. doi:10.1371/journal.pone.0218274.
- Chen L, Xu W, Lee A, He J, Huang B, Zheng W, Su T, Lai S, Long Y, Chu H, et al. The impact of Helicobacter pylori infection, eradication therapy and probiotic supplementation on gut microenvironment homeostasis: an open-label, randomized clinical trial. EBioMedicine. 2018;35:87–96. doi:10.1016/j.ebiom.2018.08.028.
- Iino C, Shimoyama T, Chinda D, Sakuraba H, Fukuda S, Nakaji S. Influence of helicobacter pylori infection and atrophic gastritis on the gut microbiota in a Japanese population. Digestion. 2020;101:422–432.
- Cornejo-Pareja I, Martin-Nunez GM, Roca-Rodriguez MM. H. pylori eradication treatment alters gut microbiota and GLP-1 secretion in humans. J Clin Med. 2019;8(4). doi:10.3390/jcm8040451.
- Wang D, Li Y, Zhong H, Ding Q, Lin Y, Tang S, Zong Y, Wang Q, Zhang X, Yang H, et al. Alterations in the human gut microbiome associated with helicobacter pylori infection. FEBS Open Bio. 2019;9(9):1552–1560. doi:10.1002/2211-5463.12694.
- Yang L, Zhang J, Xu J, Wei X, Yang J, Liu Y, Li H, Zhao C, Wang Y, Zhang L, et al. Helicobacter pylori infection aggravates dysbiosis of gut microbiome in children with gastritis. Front Cell Infect Microbiol. 2019;9:375. doi:10.3389/fcimb.2019.00375.
- Zhou Y, Ye Z, Lu J, Miao S, Lu X, Sun H, Wu J, Wang Y, Huang Y. Long-term changes in the gut microbiota after 14-day bismuth quadruple therapy in penicillin-allergic children. Helicobacter. 2020;25(5):e12721. doi:10.1111/hel.12721.
- Munoz-Ramirez ZY, Pascoe B, Mendez-Tenorio A, Mourkas E, Sandoval-Motta S, Perez-Perez G, Morgan DR, Dominguez RL, Ortiz-Princz D, Cavazza ME, et al. A 500-year tale of co-evolution, adaptation, and virulence: helicobacter pylori in the Americas. ISME J. 2021;15(1):78–92. doi:10.1038/s41396-020-00758-0.
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi:10.1038/nature11053.
- Lopetuso LR, Scaldaferri F, Franceschi F, Gasbarrini A. The gastrointestinal microbiome – functional interference between stomach and intestine. Best Pract Res Clin Gastroenterol. 2014;28(6):995–1002. doi:10.1016/j.bpg.2014.10.004.
- Kienesberger S, Cox LM, Livanos A, Zhang X-S, Chung J, Perez-Perez G, Gorkiewicz G, Zechner E, Blaser M. Gastric Helicobacter pylori infection affects local and distant microbial populations and host responses. Cell Rep. 2016;14(6):1395–1407. doi:10.1016/j.celrep.2016.01.017.
- Takahashi-Kanemitsu A, Knight CT, Hatakeyama M. Molecular anatomy and pathogenic actions of Helicobacter pylori CagA that underpin gastric carcinogenesis. Cell Mol Immunol. 2020;17(1):50–63. doi:10.1038/s41423-019-0339-5.
- Baj J, Forma A, Sitarz M, Portincasa P, Garruti G, Krasowska D, Maciejewski R. Helicobacter pylori virulence factors-mechanisms of bacterial pathogenicity in the gastric microenvironment. Cells. 2020;10(1):27. doi:10.3390/cells10010027.
- Huang Y, Ding Y, Xu H, Shen C, Chen X, Li C. Effects of sodium butyrate supplementation on inflammation, gut microbiota, and short-chain fatty acids in Helicobacter pylori-infected mice. Helicobacter. 2021;26(2):e12785. doi:10.1111/hel.12785.
- Zhang Z, Tang H, Chen P, Xie H, Tao Y. Demystifying the manipulation of host immunity, metabolism, and extraintestinal tumors by the gut microbiome. Signal Transduct Target Ther. 2019;4:41. doi:10.1038/s41392-019-0074-5.
- Wibowo H, Harbuwono DS, Tahapary DL, Kartika R, Pradipta S, Larasati RA. Impact of sodium butyrate treatment in LPS-stimulated peripheral blood mononuclear cells of poorly controlled type 2 DM. Front Endocrinol (Lausanne). 2021;12:652942. doi:10.3389/fendo.2021.652942.
- Campos-Perez W, Martinez-Lopez E. Effects of short chain fatty acids on metabolic and inflammatory processes in human health. Biochimica Et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2021;1866(5):158900. doi:10.1016/j.bbalip.2021.158900.
- Postler TS, Ghosh S. Understanding the Holobiont: how Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab. 2017;26(1):110–130. doi:10.1016/j.cmet.2017.05.008.
- Sit WY, Chen YA, Chen YL, Lai CH, Wang WC. Cellular evasion strategies of Helicobacter pylori in regulating its intracellular fate. Semin Cell Dev Biol. 2020;101:59–67. doi:10.1016/j.semcdb.2020.01.007.
- Cuomo P, Papaianni M, Sansone C, Iannelli A, Iannelli D, Medaglia C, Paris D, Motta A, Capparelli R. an in vitro model to investigate the role of helicobacter pylori in type 2 diabetes, obesity, alzheimer’s disease and cardiometabolic disease. Int J Mol Sci. 2020;21(21):8369. doi:10.3390/ijms21218369.
- Farhangi MA, Vajdi M. Novel findings of the association between gut microbiota-derived metabolite trimethylamine N-oxide and inflammation: results from a systematic review and dose-response meta-analysis. Crit Rev Food Sci Nutr. 2020;60(16):2801–2823. doi:10.1080/10408398.2020.1770199.
- Wu D, Cao M, Peng J, Li N, Yi S, Song L, Wang X, Zhang M, Zhao J. The effect of trimethylamine N-oxide on Helicobacter pylori-induced changes of immunoinflammatory genes expression in gastric epithelial cells. Int Immunopharmacol. 2017;43:172–178. doi:10.1016/j.intimp.2016.11.032.
- Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375(24):2369–2379. doi:10.1056/NEJMra1600266.
- Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19(1):55–71. doi:10.1038/s41579-020-0433-9.
- Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H. Antibiotics as major disruptors of gut microbiota. Front Cell Infect Microbiol. 2020;10(731). doi:10.3389/fcimb.2020.572912.
- Blaser MJ. Antibiotic use and its consequences for the normal microbiome. Science. 2016;352(6285):544–545. doi:10.1126/science.aad9358.
- Palleja A, Mikkelsen KH, Forslund SK, Kashani A, Allin KH, Nielsen T, Hansen TH, Liang S, Feng Q, Zhang C, et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat Microbiol. 2018;3(11):1255–1265. doi:10.1038/s41564-018-0257-9.
- Zarrinpar A, Chaix A, Xu ZZ, Chang MW, Marotz CA, Saghatelian A, Knight R, Panda S. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat Commun. 2018;9(1):2872. doi:10.1038/s41467-018-05336-9.
- Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, Brochado AR, Fernandez KC, Dose H, Mori H, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555(7698):623–628. doi:10.1038/nature25979.
- Rashidi A, Ebadi M, Rehman TU, Elhusseini H, Nalluri H, Kaiser T, Holtan SG, Khoruts A, Weisdorf DJ, Staley C, et al. Gut microbiota response to antibiotics is personalized and depends on baseline microbiota. Microbiome. 2021;9(1):211. doi:10.1186/s40168-021-01170-2.
- Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EMM, Sanders ME, Shamir R, Swann JR, Szajewska H, et al. The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021;18(9):649–667. doi:10.1038/s41575-021-00440-6.
- Veiga P, Suez J, Derrien M, Elinav E. Moving from probiotics to precision probiotics. Nat Microbiol. 2020;5(7):878–880. doi:10.1038/s41564-020-0721-1.
- Gao J, Li X, Zhang G, Sadiq FA, Simal‐Gandara J, Xiao J, Sang Y. Probiotics in the dairy industry-advances and opportunities. Compr Rev Food Sci Food Saf. 2021;20(4):3937–3982. doi:10.1111/1541-4337.12755.
- Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25(5):716–729. doi:10.1038/s41591-019-0439-x.
- Wieërs G, Belkhir L, Enaud R, Leclercq S, Philippart de Foy J-M, Dequenne I, de Timary P, Cani PD. How probiotics affect the microbiota. Front Cell Infect Microbiol. 2020;9(454). doi:10.3389/fcimb.2019.00454.
- Hou Q, Zhao F, Liu W, Lv R, Khine WWT, Han J, Sun Z, Lee Y-K, Zhang H. Probiotic-directed modulation of gut microbiota is basal microbiome dependent. Gut Microbes. 2020;12(1):1736974. doi:10.1080/19490976.2020.1736974.
- Singh TP, Natraj BH. Next-generation probiotics: a promising approach towards designing personalized medicine. Crit Rev Microbiol. 2021;47(4):479–498. doi:10.1080/1040841X.2021.1902940.
- Zhai Q, Feng S, Arjan N, Chen W. A next generation probiotic, Akkermansia muciniphila. Crit Rev Food Sci Nutr. 2019;59(19):3227–3236. doi:10.1080/10408398.2018.1517725.
- Keshavarz Azizi Raftar S, Abdollahiyan S, Azimirad M, Yadegar A, Vaziri F, Moshiri A, Siadat SD, Zali MR. The anti-fibrotic effects of heat-killed akkermansia muciniphila muct on liver fibrosis markers and activation of hepatic stellate cells. Probiotics Antimicrob Proteins. 2021;13(3):776–787. doi:10.1007/s12602-020-09733-9.
- Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, et al. Supplementation with akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25(7):1096–1103. doi:10.1038/s41591-019-0495-2.
- De Palma G, Nadal I, Medina M, Donat E, Ribes-Koninckx C, Calabuig M, Sanz Y. Intestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in children. BMC Microbiol. 2010;10(1):63. doi:10.1186/1471-2180-10-63.
- Rajilic-Stojanovic M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S, de Vos WM. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141(5):1792–1801. doi:10.1053/j.gastro.2011.07.043.
- Balamurugan R, Rajendiran E, George S, Samuel GV, Ramakrishna BS. Real-time polymerase chain reaction quantification of specific butyrate-producing bacteria, desulfovibrio and Enterococcus faecalis in the feces of patients with colorectal cancer. J Gastroenterol Hepatol. 2008;23(8 Pt 1):1298–1303. doi:10.1111/j.1440-1746.2008.05490.x.
- Furet J-P, Kong L-C, Tap J, Poitou C, Basdevant A, Bouillot J-L, Mariat D, Corthier G, Doré J, Henegar C, et al. Differential adaptation of human gut microbiota to bariatric surgery–induced weight loss. Diabetes. 2010;59(12):3049–3057. doi:10.2337/db10-0253.
- Martin R, Miquel S, Benevides L, Bridonneau C, Robert V, Hudault S, Chain F, Berteau O, Azevedo V, Chatel JM, et al. Functional characterization of novel faecalibacterium prausnitzii strains isolated from healthy volunteers: a step forward in the use of F. prausnitzii as a next-generation probiotic. Front Microbiol. 2017;8:1226. doi:10.3389/fmicb.2017.01226.
- Fei Y, Chen Z, Han S, Zhang S, Zhang T, Lu Y, Berglund B, Xiao H, Li L, Yao M, et al. Role of prebiotics in enhancing the function of next-generation probiotics in gut microbiota. Crit Rev Food Sci Nutr. pp.1–18. 2021. doi:10.1080/10408398.2021.1958744
- Min S, Kim S, Cho SW. Gastrointestinal tract modeling using organoids engineered with cellular and microbiota niches. Exp Mol Med. 2020;52(2):227–237. doi:10.1038/s12276-020-0386-0.
- Shah SC, Iyer PG, Moss SF. AGA clinical practice update on the management of refractory Helicobacter pylori infection: expert review. Gastroenterology. 2021;160(5):1831–1841. doi:10.1053/j.gastro.2020.11.059.
- Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, Jones NL, Render C, Leontiadis GI, Moayyedi P, et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016;151(1):51–69.e14. doi:10.1053/j.gastro.2016.04.006.
- Liou JM, Chen CC, Chang CM, Fang Y-J, Bair M-J, Chen P-Y, Chang C-Y, Hsu Y-C, Chen M-J, Chen -C-C, et al. Long-term changes of gut microbiota, antibiotic resistance, and metabolic parameters after Helicobacter pylori eradication: a multicentre, open-label, randomised trial. Lancet Infect Dis. 2019;19(10):1109–1120. doi:10.1016/S1473-3099(19)30272-5.
- Ford AC, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut. 2020;69(12):2113–2121. doi:10.1136/gutjnl-2020-320839.
- Tshibangu-Kabamba E, Yamaoka Y. Helicobacter pylori infection and antibiotic resistance - from biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2021;18(9):613–629. doi:10.1038/s41575-021-00449-x.
- Cheung KS, Chan EW, Wong AYS, Chen L, Wong ICK, Leung WK. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study. Gut. 2018;67(1):28–35. doi:10.1136/gutjnl-2017-314605.
- Yuan J, He Q, Nguyen LH, Wong MCS, Huang J, Yu Y, Xia B, Tang Y, He Y, Zhang C, et al. Regular use of proton pump inhibitors and risk of type 2 diabetes: results from three prospective cohort studies. Gut. 2021;70(6):1070–1077. doi:10.1136/gutjnl-2020-322557.
- Ye Q, Shao X, Shen R, Chen D, Shen J. Changes in the human gut microbiota composition caused by Helicobacter pylori eradication therapy: a systematic review and meta-analysis. Helicobacter. 2020;25(4):e12713. doi:10.1111/hel.12713.
- Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179.
- Handa O, Naito Y, Osawa M. Nutrients and probiotics: current trends in their use to eradicate Helicobacter pylori. J Clin Biochem Nutr. 2020;67(1):26–28. doi:10.3164/jcbn.20-51.
- Montassier E, Valdes-Mas R, Batard E. Probiotics impact the antibiotic resistance gene reservoir along the human GI tract in a person-specific and antibiotic-dependent manner. Nat Microbiol. 2021;6(8):1043–1054. doi:10.1038/s41564-021-00920-0.
- Cifuentes SG, Prado MB, Fornasini M, Cohen H, Baldeon ME, Cardenas PA. Saccharomyces boulardii CNCM I-745 supplementation modifies the fecal resistome during Helicobacter pylori eradication therapy. Helicobacter. 2022;27(2):e12870. doi:10.1111/hel.12870.
- Zhang M, Zhang C, Zhao J, Zhang H, Zhai Q, Chen W. Meta-analysis of the efficacy of probiotic-supplemented therapy on the eradication of H. pylori and incidence of therapy-associated side effects. Microb Pathog. 2020;147:104403. doi:10.1016/j.micpath.2020.104403.
- Losurdo G, Cubisino R, Barone M, Principi M, Leandro G, Ierardi E, Leo AD. Probiotic monotherapy and Helicobacter pylori eradication: a systematic review with pooled-data analysis. World J Gastroenterol. 2018;24(1):139–149. doi:10.3748/wjg.v24.i1.139.
- Kori M, Daugule I, Urbonas V. Helicobacter pylori and some aspects of gut microbiota in children. Helicobacter. 2018;23(Suppl 1):e12524. doi:10.1111/hel.12524.
- Feng JR, Wang F, Qiu X, McFarland LV, Chen P-F, Zhou R, Liu J, Zhao Q, Li J. Efficacy and safety of probiotic-supplemented triple therapy for eradication of Helicobacter pylori in children: a systematic review and network meta-analysis. Eur J Clin Pharmacol. 2017;73(10):1199–1208. doi:10.1007/s00228-017-2291-6.
- Kamiya S, Yonezawa H, Osaki T. Role of probiotics in eradication therapy for Helicobacter pylori infection. Adv Exp Med Biol. 2019;1149:243–255.
- Fang HR, Zhang GQ, Cheng JY, Li ZY. Efficacy of lactobacillus-supplemented triple therapy for Helicobacter pylori infection in children: a meta-analysis of randomized controlled trials. Eur J Pediatr. 2019;178(1):7–16. doi:10.1007/s00431-018-3282-z.
- McElrath C, Espinosa V, Lin JD. Critical role of interferons in gastrointestinal injury repair. Nat Commun. 2021;12(1):2624. doi:10.1038/s41467-021-22928-0.
- George S, Lucero Y, Torres JP, Lagomarcino AJ, O’Ryan M. Gastric damage and cancer-associated biomarkers in Helicobacter pylori-infected children. Front Microbiol. 2020;11(90). doi:10.3389/fmicb.2020.00090.
- Paone P, Cani PD. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2020;69(12):2232–2243. doi:10.1136/gutjnl-2020-322260.
- Ghosh S, Whitley CS, Haribabu B, Jala VR. Regulation of intestinal barrier function by microbial metabolites. Cell Mol Gastroenterol Hepatol. 2021;11(5):1463–1482. doi:10.1016/j.jcmgh.2021.02.007.
- Qureshi N, Li P, Gu Q. Probiotic therapy in Helicobacter pylori infection: a potential strategy against a serious pathogen? Appl Microbiol Biotechnol. 2019;103(4):1573–1588. doi:10.1007/s00253-018-09580-3.
- Azimirad M, Krutova M, Yadegar A, Shahrokh S, Olfatifar M, Aghdaei HA, Fawley WN, Wilcox MH, Zali MR. Clostridioides difficile ribotypes 001 and 126 were predominant in Tehran healthcare settings from 2004 to 2018: a 14-year-long cross-sectional study. Emerg Microbes Infect. 2020;9(1):1432–1443. doi:10.1080/22221751.2020.1780949.
- Cristofori F, Dargenio VN, Dargenio C, Miniello VL, Barone M, Francavilla R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: a door to the body. Front Immunol. 2021;12:578386. doi:10.3389/fimmu.2021.578386.
- Marques MS, Costa AC, Osório H, Pinto ML, Relvas S, Dinis-Ribeiro M, Carneiro F, Leite M, Figueiredo C. Helicobacter pylori PqqE is a new virulence factor that cleaves junctional adhesion molecule A and disrupts gastric epithelial integrity. Gut Microbes. 2021;13(1):1921928. doi:10.1080/19490976.2021.1921928.
- Rose EC, Odle J, Blikslager AT, Ziegler AL. Probiotics, prebiotics and epithelial tight junctions: a promising approach to modulate intestinal barrier function. Int J Mol Sci. 2021;22(13):6729. doi:10.3390/ijms22136729.
- Llewellyn A, Foey A. Probiotic Modulation of Innate Cell Pathogen Sensing and Signaling Events. Nutrients. 2017;9(10):1156. doi:10.3390/nu9101156.
- Ji J, Yang H. Using probiotics as supplementation for helicobacter pylori antibiotic therapy. Int J Mol Sci. 2020;21(3):1136. doi:10.3390/ijms21031136.
- Feng T, Wang J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: a systematic review. Gut Microbes. 2020;12(1):1801944. doi:10.1080/19490976.2020.1801944.
- van Zyl WF, Deane SM, Dicks LMT. Molecular insights into probiotic mechanisms of action employed against intestinal pathogenic bacteria. Gut Microbes. 2020;12(1):1831339. doi:10.1080/19490976.2020.1831339.
- Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol. 2019;16(10):605–616. doi:10.1038/s41575-019-0173-3.
- van Baarlen P, Troost FJ, van Hemert S, van der Meer C, de Vos WM, de Groot PJ, Hooiveld GJEJ, Brummer RJM, Kleerebezem M. Differential NF-κB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc Natl Acad Sci U S A. 2009;106(7):2371–2376. doi:10.1073/pnas.0809919106.
- Fong W, Li Q, Yu J. Gut microbiota modulation: a novel strategy for prevention and treatment of colorectal cancer. Oncogene. 2020;39(26):4925–4943. doi:10.1038/s41388-020-1341-1.
- Hrdý J, Alard J, Couturier-Maillard A, Boulard O, Boutillier D, Delacre M, Lapadatescu C, Cesaro A, Blanc P, Pot B, et al. Lactobacillus reuteri 5454 and Bifidobacterium animalis ssp. lactis 5764 improve colitis while differentially impacting dendritic cells maturation and antimicrobial responses. Sci Rep. 2020;10(1):5345. doi:10.1038/s41598-020-62161-1.
- Lu K, Dong S, Wu X, Jin R, Chen H. Probiotics in Cancer. Front Oncol. 2021;11:638148. doi:10.3389/fonc.2021.638148.
- Tezuka H, Ohteki T. Regulation of IgA Production by Intestinal Dendritic Cells and Related Cells. Front Immunol. 2019;10:1891. doi:10.3389/fimmu.2019.01891.
- Eslami M, Yousefi B, Kokhaei P, Jazayeri Moghadas A, Sadighi Moghadam B, Arabkari V, Niazi Z. Are probiotics useful for therapy of Helicobacter pylori diseases? Comp Immunol Microbiol Infect Dis. 2019;64:99–108. doi:10.1016/j.cimid.2019.02.010.
- Motta JP, Wallace JL, Buret AG, Deraison C, Vergnolle N. Gastrointestinal biofilms in health and disease. Nat Rev Gastroenterol Hepatol. 2021;18(5):314–334. doi:10.1038/s41575-020-00397-y.
- Oh B, Kim BS, Kim JW, Kim JS, Koh S-J, Kim BG, Lee KL, Chun J. The effect of probiotics on gut microbiota during the Helicobacter pylori eradication: randomized controlled trial. Helicobacter. 2016;21(3):165–174. doi:10.1111/hel.12270.
- Oh B, Kim JW, Kim BS. Changes in the functional potential of the gut microbiome following probiotic supplementation during Helicobacter Pylori treatment. Helicobacter. 2016;21:493–503.
- Wu L, Wang Z, Sun G. Effects of anti-H. pylori triple therapy and a probiotic complex on intestinal microbiota in duodenal ulcer. Sci Rep. 2019;9(1):12874. doi:10.1038/s41598-019-49415-3.
- Cárdenas PA, Garcés D, Prado-Vivar B, Flores N, Fornasini M, Cohen H, Salvador I, Cargua O, Baldeón ME. Effect of Saccharomyces boulardii CNCM I-745 as complementary treatment of Helicobacter pylori infection on gut microbiome. Eur J Clin Microbiol Infect Dis. 2020;39(7):1365–1372. doi:10.1007/s10096-020-03854-3.
- Kakiuchi T, Mizoe A, Yamamoto K, Imamura I, Hashiguchi K, Kawakubo H, Yamaguchi D, Fujioka Y, Nakayama A, Okuda M, et al. Effect of probiotics during vonoprazan-containing triple therapy on gut microbiota in Helicobacter pylori infection: a randomized controlled trial. Helicobacter. 2020;25(3):e12690. doi:10.1111/hel.12690.
- Tang B, Tang L, Huang C, Tian C, Chen L, He Z, Yang G, Zuo L, Zhao G, Liu E, et al. The effect of probiotics supplementation on gut microbiota after Helicobacter pylori eradication: a multicenter randomized controlled trial. Infect Dis Ther. 2021;10(1):317–333. doi:10.1007/s40121-020-00372-9.
- Guillemard E, Poirel M, Schäfer F, Quinquis L, Rossoni C, Keicher C, Wagner F, Szajewska H, Barbut F, Derrien M, et al. A randomised, controlled trial: effect of a multi-strain fermented milk on the gut microbiota recovery after helicobacter pylori therapy. Nutrients. 2021;13(9):3171. doi:10.3390/nu13093171.
- Yang C, Liang L, Lv P, Liu L, Wang S, Wang Z, Chen Y. Effects of non-viable Lactobacillus reuteri combining with 14-day standard triple therapy on Helicobacter pylori eradication: a randomized double-blind placebo-controlled trial. Helicobacter. 2021;26(6):e12856. doi:10.1111/hel.12856.
- Yuan Z, Xiao S, Li S, Suo B, Wang Y, Meng L, Liu Z, Yin Z, Xue Y, Zhou L, et al. The impact of Helicobacter pylori infection, eradication therapy, and probiotics intervention on gastric microbiota in young adults. Helicobacter. 2021;26(6):e12848. doi:10.1111/hel.12848.
- Stoeva MK, Garcia-So J, Justice N, Myers J, Tyagi S, Nemchek M, McMurdie PJ, Kolterman O, Eid J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes. 2021;13(1):1–28. doi:10.1080/19490976.2021.1907272.
- Ariyoshi T, Hagihara M, Tomono S, Eguchi S, Minemura A, Miura D, Oka K, Takahashi M, Yamagishi Y, Mikamo H, et al. Clostridium butyricum MIYAIRI 588 modifies bacterial composition under antibiotic-induced dysbiosis for the activation of interactions via lipid metabolism between the gut microbiome and the host. Biomedicines. 2021;9(8):1065. doi:10.3390/biomedicines9081065.
- Wang Y, Huang JM, Zhou YL, Almeida A, Finn RD, Danchin A, He L-S. Phylogenomics of expanding uncultured environmental Tenericutes provides insights into their pathogenicity and evolutionary relationship with Bacilli. BMC Genomics. 2020;21(1):408. doi:10.1186/s12864-020-06807-4.
- Yuan X, Chen R, McCormick KL, Zhang Y, Lin X, Yang X. The role of the gut microbiota on the metabolic status of obese children. Microb Cell Fact. 2021;20(1):53. doi:10.1186/s12934-021-01548-9.
- Yeoh YK, Chen Z, Wong MCS, Hui M, Yu J, Ng SC, Sung JJY, Chan FKL, Chan PKS. Southern Chinese populations harbour non-nucleatum Fusobacteria possessing homologues of the colorectal cancer-associated FadA virulence factor. Gut. 2020;69(11):1998–2007. doi:10.1136/gutjnl-2019-319635.
- Harrandah AM, Chukkapalli SS, Bhattacharyya I, Progulske-Fox A, Chan EKL. Fusobacteria modulate oral carcinogenesis and promote cancer progression. J Oral Microbiol. 2020;13:1849493.
- Harding CM, Hennon SW, Feldman MF. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat Rev Microbiol. 2018;16(2):91–102. doi:10.1038/nrmicro.2017.148.
- Song WY, Kim HJ. Current biochemical understanding regarding the metabolism of acinetobactin, the major siderophore of the human pathogen Acinetobacter baumannii, and outlook for discovery of novel anti-infectious agents based thereon. Nat Prod Rep. 2020;37(4):477–487. doi:10.1039/C9NP00046A.
- Zhao X, Zhong X, Liu X, Wang X, Gao X. Therapeutic and improving function of lactobacilli in the prevention and treatment of cardiovascular-related diseases: a novel perspective from gut microbiota. Frontiers in Nutrition. 2021;8(299). doi:10.3389/fnut.2021.693412.
- Hanchi H, Mottawea W, Sebei K, Hammami R. The genus enterococcus: between probiotic potential and safety concerns—an update. Front Microbiol. 2018;9(1791). doi:10.3389/fmicb.2018.01791.
- Liu X, Mao B, Gu J, Wu J, Cui S, Wang G, Zhao J, Zhang H, Chen W. Blautia —a new functional genus with potential probiotic properties? Gut Microbes. 2021;13(1):1–21. doi:10.1080/19490976.2021.1875796.
- Xiang H, Gan J, Zeng D, Li J, Yu H, Zhao H, Yang Y, Tan S, Li G, Luo C, et al. Specific microbial taxa and functional capacity contribute to chicken abdominal fat deposition. Front Microbiol. 2021;12:643025. doi:10.3389/fmicb.2021.643025.
- Goris T, Cuadrat RRC, Braune A. Flavonoid-modifying capabilities of the human gut microbiome-an in silico study. Nutrients. 2021;13(8):2688. doi:10.3390/nu13082688.
- Engevik MA, Danhof HA, Ruan W, Engevik AC, Chang-Graham AL, Engevik KA, Shi Z, Zhao Y, Brand CK, Krystofiak ES, et al. Fusobacterium nucleatum Secretes outer membrane vesicles and promotes intestinal inflammation. mBio. 2021;12(2). doi:10.1128/mBio.02706-20.
- Ranjbar M, Salehi R, Haghjooy Javanmard S, Rafiee L, Faraji H, Jafarpor S, Ferns GA, Ghayour-Mobarhan M, Manian M, Nedaeinia R, et al. The dysbiosis signature of fusobacterium nucleatum in colorectal cancer-cause or consequences? A systematic review. Cancer Cell Int. 2021;21(1):194. doi:10.1186/s12935-021-01886-z.
- Murros KE, Huynh VA, Takala TM, Saris PEJ. Desulfovibrio bacteria are associated with parkinson’s disease. Front Cell Infect Microbiol. 2021;11:652617. doi:10.3389/fcimb.2021.652617.
- Rosario D, Bidkhori G, Lee S, Bedarf J, Hildebrand F, Le Chatelier E, Uhlen M, Ehrlich SD, Proctor G, Wüllner U, et al. Systematic analysis of gut microbiome reveals the role of bacterial folate and homocysteine metabolism in Parkinson’s disease. Cell Rep. 2021;34(9):108807. doi:10.1016/j.celrep.2021.108807.
- Shao J, Li Z, Gao Y, Zhao K, Lin M, Li Y, Wang S, Liu Y, Chen L. Construction of a “Bacteria-Metabolites” co-expression network to clarify the anti-ulcerative colitis effect of flavonoids of sophora flavescens aiton by regulating the “Host-Microbe” interaction. Front Pharmacol. 2021;12:710052. doi:10.3389/fphar.2021.710052.
- Caulier S, Nannan C, Gillis A, Licciardi F, Bragard C, Mahillon J. Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front Microbiol. 2019;10:302. doi:10.3389/fmicb.2019.00302.
- Martin RM, Bachman MA. Colonization, infection, and the accessory genome of Klebsiella pneumoniae. Front Cell Infect Microbiol. 2018;8:4. doi:10.3389/fcimb.2018.00004.
- Dong S, Jiao J, Jia S, Li G, Zhang W, Yang K, Wang Z, Liu C, Li D, Wang X, et al. 16S rDNA full-length assembly sequencing technology analysis of intestinal microbiome in polycystic ovary syndrome. Front Cell Infect Microbiol. 2021;11:634981. doi:10.3389/fcimb.2021.634981.
- Yu X, Åvall-Jääskeläinen S, Koort J, Lindholm A, Rintahaka J, von Ossowski I, Palva A, Hynönen U. A comparative characterization of different host-sourced lactobacillus ruminis strains and their adhesive, inhibitory, and immunomodulating functions. Front Microbiol. 2017;8(657). doi:10.3389/fmicb.2017.00657.
- Yang J, Li Y, Wen Z, Liu W, Meng L, Huang H. Oscillospira - a candidate for the next-generation probiotics. Gut Microbes. 2021;13(1):1987783. doi:10.1080/19490976.2021.1987783.
- Bartley A, Yang T, Arocha R, Malphurs WL, Larkin R, Magee KL, Vickroy TW, Zubcevic J. Increased abundance of lactobacillales in the colon of beta-adrenergic receptor knock out mouse is associated with increased gut bacterial production of short chain fatty acids and reduced IL17 expression in circulating CD4+ immune cells. Front Physiol. 2018;9(1593). doi:10.3389/fphys.2018.01593.
- Chen Z, Xie Y, Zhou F, Zhang B, Wu J, Yang L, Xu S, Stedtfeld R, Chen Q, Liu J, et al. Featured Gut Microbiomes Associated With the Progression of Chronic Hepatitis B Disease. Front Microbiol. 2020;11:383. doi:10.3389/fmicb.2020.00383.
- Zhou Y, Chen C, Yu H, Yang Z. Fecal microbiota changes in patients with postpartum depressive disorder. Front Cell Infect Microbiol. 2020;10:567268. doi:10.3389/fcimb.2020.567268.
- Nagao-Kitamoto H, Leslie JL, Kitamoto S, Jin C, Thomsson KA, Gillilland MG, Kuffa P, Goto Y, Jenq RR, Ishii C, et al. Interleukin-22-mediated host glycosylation prevents clostridioides difficile infection by modulating the metabolic activity of the gut microbiota. Nat Med. 2020;26(4):608–617. doi:10.1038/s41591-020-0764-0.
- Tito RY, Cypers H, Joossens M, Varkas G, Van Praet L, Glorieus E, Van den Bosch F, De Vos M, Raes J, Elewaut D, et al. Brief report: dialister as a microbial marker of disease activity in spondyloarthritis. Arthritis & Rheumatology. 2017;69(1):114–121. doi:10.1002/art.39802.
- Kaakoush NO. Sutterella species, iga-degrading bacteria in ulcerative colitis. Trends Microbiol. 2020;28(7):519–522. doi:10.1016/j.tim.2020.02.018.
- Astbury S, Atallah E, Vijay A, Aithal GP, Grove JI, Valdes AM. Lower gut microbiome diversity and higher abundance of proinflammatory genus Collinsella are associated with biopsy-proven nonalcoholic steatohepatitis. Gut Microbes. 2020;11(3):569–580. doi:10.1080/19490976.2019.1681861.
- Bailen M, Bressa C, Martinez-Lopez S, González-Soltero R, Montalvo Lominchar MG, San Juan C, Larrosa M. Microbiota features associated with a high-fat/low-fiber diet in healthy adults. Front Nutr. 2020;7:583608. doi:10.3389/fnut.2020.583608.
- Zhang X, Coker OO, Chu ES, Fu K, Lau HCH, Wang Y-X, Chan AWH, Wei H, Yang X, Sung JJY, et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021;70(4):761–774. doi:10.1136/gutjnl-2019-319664.
- Yuan C, Yin Z, Wang J, Qian C, Wei Y, Zhang S, Jiang L, Liu B. Comparative genomic analysis of citrobacter and key genes essential for the pathogenicity of Citrobacter koseri. Front Microbiol. 2019;10:2774. doi:10.3389/fmicb.2019.02774.
- Ma X, Ma L, Wang Z, Liu Y, Long L, Ma X, Chen H, Chen Z, Lin X, Si L, et al. Clinical features and gut microbial alterations in anti-leucine-rich glioma-inactivated 1 encephalitis-a pilot study. Front Neurol. 2020;11:585977. doi:10.3389/fneur.2020.585977.
- Cerqueira FM, Photenhauer AL, Pollet RM, Brown HA, Koropatkin NM. Starch Digestion by Gut Bacteria: crowdsourcing for Carbs. Trends Microbiol. 2020;28(2):95–108. doi:10.1016/j.tim.2019.09.004.
- Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, Shulzhenko N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. doi:10.1016/j.ebiom.2019.11.051.
- Schirmer M, Garner A, Vlamakis H, Xavier RJ. Microbial genes and pathways in inflammatory bowel disease. Nat Rev Microbiol. 2019;17(8):497–511. doi:10.1038/s41579-019-0213-6.
- Lordan C, Thapa D, Ross RP, Cotter PD. Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut Microbes. 2020;11(1):1–20. doi:10.1080/19490976.2019.1613124.
- Tomova A, Bukovsky I, Rembert E, Yonas W, Alwarith J, Barnard ND, Kahleova H. The effects of vegetarian and vegan diets on gut microbiota. Front Nutr. 2019;6:47. doi:10.3389/fnut.2019.00047.
- Shimokawa C, Kato T, Takeuchi T, Ohshima N, Furuki T, Ohtsu Y, Suzue K, Imai T, Obi S, Olia A, et al. CD8(+) regulatory T cells are critical in prevention of autoimmune-mediated diabetes. Nat Commun. 2020;11(1):1922. doi:10.1038/s41467-020-15857-x.
- Carey MA, Medlock GL, Alam M, Kabir M, Uddin MJ, Nayak U, Papin J, Faruque ASG, Haque R, Petri WA, et al. Megasphaera in the stool microbiota is negatively associated with diarrheal cryptosporidiosis. Clin Infect Dis. 2021;73(6):e1242–e1251. doi:10.1093/cid/ciab207.
- Liu F, Li J, Guan Y, Lou Y, Chen H, Xu M, Deng D, Chen J, Ni B, Zhao L, et al. Dysbiosis of the gut microbiome is associated with tumor biomarkers in lung cancer. Int J Biol Sci. 2019;15(11):2381–2392. doi:10.7150/ijbs.35980.
- Vascellari S, Palmas V, Melis M, Pisanu S, Cusano R, Uva P, Perra D, Madau V, Sarchioto M, Oppo V, et al. Gut microbiota and metabolome alterations associated with parkinson’s disease. mSystems. 2020;5(5). doi:10.1128/mSystems.00561-20.
- Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, Schiweck C, Kurilshikov A, Joossens M, Wijmenga C, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol. 2019;4(4):623–632. doi:10.1038/s41564-018-0337-x.
- Yin Z, Zhang S, Wei Y, Wang M, Ma S, Yang S, Wang J, Yuan C, Jiang L, Du Y, et al. Horizontal gene transfer clarifies taxonomic confusion and promotes the genetic diversity and pathogenicity of plesiomonas shigelloides. mSystems. 2020;5(5). doi:10.1128/mSystems.00448-20.
- Suez J, Zmora N, Elinav E. Probiotics in the next-generation sequencing era. Gut Microbes. 2020;11(1):77–93. doi:10.1080/19490976.2019.1586039.
- Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, Kotler E, Zur M, Regev-Lehavi D, Brik RBZ, et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 2018;174(6):1388–1405.e1321. doi:10.1016/j.cell.2018.08.041.
- Zafar H, Saier MH Jr. Gut bacteroides species in health and disease. Gut Microbes. 2021;13(1):1–20. doi:10.1080/19490976.2020.1848158.
- Zou Y, Lin X, Xue W. Characterization and description of Faecalibacterium butyricigenerans sp. nov. and F. longum sp. nov., isolated from human faeces. Sci Rep. 2021;11(1):11340. doi:10.1038/s41598-021-90786-3.
- Van Hul M, Le Roy T, Prifti E, Dao MC, Paquot A, Zucker J-D, Delzenne NM, Muccioli GG, Clément K, Cani PD, et al. From correlation to causality: the case of subdoligranulum. Gut Microbes. 2020;12(1):1–13. doi:10.1080/19490976.2020.1849998.
- Zhang X, Li N, Chen Q, Qin H. Fecal microbiota transplantation modulates the gut flora favoring patients with functional constipation. Front Microbiol. 2021;12:700718. doi:10.3389/fmicb.2021.700718.
- Ashida H, Suzuki T, Sasakawa C. Shigella infection and host cell death: a double-edged sword for the host and pathogen survival. Curr Opin Microbiol. 2021;59:1–7. doi:10.1016/j.mib.2020.07.007.
- Guerrant RL, Bolick DT, Swann JR. Modeling enteropathy or diarrhea with the top bacterial and protozoal pathogens: differential determinants of outcomes. ACS Infect Dis. 2021;7(5):1020–1031. doi:10.1021/acsinfecdis.0c00831.
- Diling C, Longkai Q, Yinrui G, Yadi L, Xiaocui T, Xiangxiang Z, Miao Z, Ran L, Ou S, Dongdong W, et al. CircNF1-419 improves the gut microbiome structure and function in AD-like mice. Aging (Albany NY). 2020;12(1):260–287. doi:10.18632/aging.102614.
- Mukherjee A, Lordan C, Ross RP, Cotter PD. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes. 2020;12(1):1802866. doi:10.1080/19490976.2020.1802866.
- van Trijp MPH, Schutte S, Esser D. Minor changes in the composition and function of the gut microbiota during a 12-week whole grain wheat or refined wheat intervention correlate with liver fat in overweight and obese adults. J Nutr. 2021;151(3):491–502. doi:10.1093/jn/nxaa312.
- Wang Y, Zhang C, Hou S, Wu X, Liu J, Wan X. Analyses of potential driver and passenger bacteria in human colorectal cancer. Cancer Manag Res. 2020;12:11553–11561. doi:10.2147/CMAR.S275316.
- Fan X, Alekseyenko AV, Wu J, Peters BA, Jacobs EJ, Gapstur SM, Purdue MP, Abnet CC, Stolzenberg-Solomon R, Miller G, et al. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut. 2018;67(1):120–127. doi:10.1136/gutjnl-2016-312580.
- Schoilew K, Ueffing H, Dalpke A, Wolff B, Frese C, Wolff D, Boutin S. Bacterial biofilm composition in healthy subjects with and without caries experience. J Oral Microbiol. 2019;11(1):1633194. doi:10.1080/20002297.2019.1633194.
- Costa D, Iraola G. Pathogenomics of Emerging Campylobacter Species. Clin Microbiol Rev. 2019;32(4). doi:10.1128/CMR.00072-18.
- Gurwara S, Ajami NJ, Jang A, Hessel F, Chen L, Plew S, Wang Z, Graham D, Hair C, White D, et al. Dietary nutrients involved in one-carbon metabolism and colonic mucosa-associated gut microbiome in individuals with an endoscopically normal colon. Nutrients. 2019;11(3):613. doi:10.3390/nu11030613.
- Liu Y, Li W, Yang H. Leveraging 16S rRNA microbiome sequencing data to identify bacterial signatures for irritable bowel syndrome. Front Cell Infect Microbiol. 2021;11:645951. doi:10.3389/fcimb.2021.645951.
- Kim JM, Rim JH, Kim DH. Microbiome analysis reveals that ralstonia is responsible for decreased renal function in patients with ulcerative colitis. Clin Transl Med. 2021;11(3):e322. doi:10.1002/ctm2.322.
- Suárez-Jaramillo A, Baldeón ME, Prado B, Fornasini M, Cohen H, Flores N, Salvador I, Cargua O, Realpe J, Cárdenas PA, et al. Duodenal microbiome in patients with or without Helicobacter pylori infection. Helicobacter. 2020;25(6):e12753. doi:10.1111/hel.12753.
- Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol. 2010;7(9):503–514. doi:10.1038/nrgastro.2010.117.
- Sniffen JC, McFarland LV, Evans CT, Goldstein EJC. Choosing an appropriate probiotic product for your patient: an evidence-based practical guide. PLoS One. 2018;13(12):e0209205. doi:10.1371/journal.pone.0209205.
- Quin C, Estaki M, Vollman DM, Barnett JA, Gill SK, Gibson DL. Probiotic supplementation and associated infant gut microbiome and health: a cautionary retrospective clinical comparison. Sci Rep. 2018;8(1):8283. doi:10.1038/s41598-018-26423-3.
- Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, Zur M, Regev-Lehavi D, Ben-Zeev Brik R, Federici S, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174(6):1406–1423.e1416. doi:10.1016/j.cell.2018.08.047.
- Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 2016;14(1):20–32. doi:10.1038/nrmicro3552.
- Lau HCH, Kranenburg O, Xiao H, Yu J. Organoid models of gastrointestinal cancers in basic and translational research. Nat Rev Gastroenterol Hepatol. 2020;17(4):203–222. doi:10.1038/s41575-019-0255-2.
- Jalili-Firoozinezhad S, Gazzaniga FS, Calamari EL, Camacho DM, Fadel CW, Bein A, Swenor B, Nestor B, Cronce MJ, Tovaglieri A, et al. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat Biomed Eng. 2019;3(7):520–531. doi:10.1038/s41551-019-0397-0.
- O’Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2(5):17057. doi:10.1038/nmicrobiol.2017.57.
