ABSTRACT
An optimally operating microbiome supports protective, metabolic, and immune functions, but disruptions produce metabolites and toxins which can be involved in many conditions. Probiotics have the potential to manage these. However, their use in vulnerable people is linked to possible safety concerns and maintaining their viability is difficult. Interest in postbiotics is therefore increasing. Postbiotics contain inactivated microbial cells or cell components, thus are more stable and exert similar health benefits to probiotics. To review the evidence for the clinical benefits of postbiotics in highly prevalent conditions and consider future potential areas of benefit. There is growing evidence revealing the diverse clinical benefits of postbiotics in many prevalent conditions. Postbiotics could offer a novel therapeutic approach and may be a safer alternative to probiotics. Establishing interaction mechanisms between postbiotics and commensal microorganisms will improve the understanding of potential clinical benefits and may lead to targeted postbiotic therapy.
Introduction
The human microbiome is the catalog of all microorganisms inhabiting the human body and their genetic complement.Citation1 When operating optimally, the microbiome plays an important role in human health by supporting protective, metabolic, and immune functions.Citation2 Specifically, the evidence suggests that the relationship between the gut microbiome and intestinal epithelial cells supports mucosal and systemic immunity, neuroendocrine function, and intestinal and extra-intestinal health from infancy to adulthood.Citation3–5 When the microbiome is disrupted, metabolites and toxins are produced and are involved with both intestinal and extraintestinal diseases, including chronic digestive disorders, chronic inflammatory disorders, autoimmunity, allergies, and metabolic syndromes.Citation6,Citation7 Microbiome disruption can also influence disease development in distal organs including the brain, liver, lung, and adipose tissue.Citation7
In recent years, it has been suggested that live microorganisms with bioactive properties (probiotics or live biotherapeutics) have therapeutic potential for various immune, neurological, and physiological pathologies.Citation2,Citation8 However, there have been some concerns about administering live microbial therapeutics to immunocompromised or critically ill individuals, those with intestinal barrier dysfunction, or neonates and young children.Citation9 These concerns include the risk of translocation from the gut into the blood, the risk of acquiring and transferring antibiotic resistance genes and the risk of interfering with normal colonization of neonatal gut microbiota.Citation9,Citation10 Furthermore, live biotherapeutic viability is difficult to maintain and can be unstable at room temperature, shortening shelf-life. These issues have therefore increased the interest in using alternative biotherapeutic products containing inanimate bacteria, microorganism-derived cell components and metabolites which are safer to use in vulnerable populations. These biotherapeutic products are referred to as postbiotics.
Postbiotics are defined as “a preparation of inanimate microorganisms and/or their components that confers a health benefit on the host”Citation11 and are produced from inactivated commensal bacteria. They include inactivated microbial cells, cell-free supernatants, and key components, commonly inactivated by heat. Although inanimate, they exert similar, and sometimes more, health benefits compared with probiotics, a phenomenon that has been referred to as the “probiotic paradox”.Citation12
Their efficacy is thought to be derived from interactions between the host and products generated by the microbiome during microbial growth following alterations caused by the postbiotic. These products include microbial metabolites, proteins, lipids, carbohydrates, vitamins, organic acids, cell wall components or other complex molecules.Citation13,Citation14 It is also possible that active molecules in the postbiotic preparation may pass through the mucus layers and stimulate epithelial cells more directly.Citation9 Furthermore, the loss of viability and cell lysis could potentially produce more complex beneficial effects such as immunomodulation.Citation9
Being inanimate, their efficacy is not dependent on cell viability meaning they can be used in combination with antimicrobials without losing efficacy.Citation10,Citation15 In addition, their inanimate nature means they are less sensitive to environmental conditions resulting in a longer shelf-life and enabling storage and transportation at ambient temperatures.Citation3,Citation10,Citation11,Citation16,Citation17 Postbiotics have the potential to provide novel therapeutic approaches and may pave the way towards increasing the potency of active microorganisms or provide a means to convert them into functional ingredients.Citation18
This paper aims to review the available evidence for the clinical benefits of postbiotics in highly prevalent conditions and consider further potential areas of benefit.
Overview of knowledge about mechanism of action
Postbiotics are a complex preparation containing many bioactive compounds with multiple mechanisms of action. The mechanisms of action by which postbiotics exert their benefit and their role in human health are not clearly understood in most instances. These mechanisms may occur independently or in combination and in some cases could be similar to known probiotic mechanisms of action.Citation11,Citation19,Citation20 Two of the major mechanisms by which postbiotics could potentiate a clinical benefit are immune system modulation and enhancing intestinal barrier function (). We will only provide a brief overview of mechanisms of action because they are covered in detail in other publications. Citation11
Figure 1. Postbiotic mechanism of action. Postbiotics could act in many ways, four of which are illustrated here. Postbiotics could enhance barrier function, through the stimulation of tight junctions, or by stimulating mucous production. Postbiotics could also act through changes in the microbiome and could modulate the immune response. Created with BioRender.
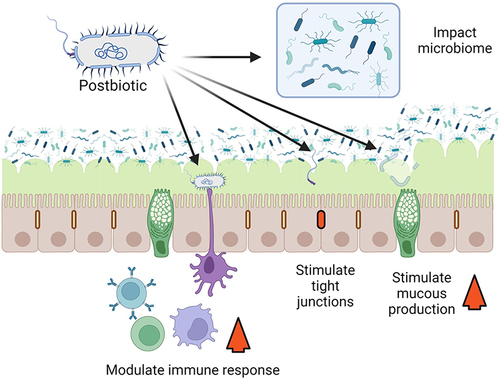
Local and systemic immunomodulation generally occurs by stimulating various cytokine and immune modulator expression and microorganism-associated molecular patterns interacting with specific immune cell receptors such as C-type lectins and toll-like receptors (TLR).Citation21 Other immunomodulatory microbial metabolites including histamine, branched chain fatty acids and short-chain fatty acids (SCFA), and cell wall components including peptidoglycans and muramic acid may be contained in postbiotics and can influence various immune responses.Citation22,Citation23
Intestinal barrier function can be enhanced by exopolysaccharides, including those from Bifidobacterium spp.Citation24 Some Bifidobacterium spp. have also been shown to promote tight junction function.Citation25 Furthermore, if present in sufficient amounts, SCFAs can potentially modify barrier function and protect against lipopolysaccharide-induced disruption.Citation26 Additionally, postbiotics may modulate the intestinal microbiome itself. For example, lactic acid and bacteriocins can have a direct antimicrobial effect,Citation27,Citation28 while indirect microbiome modulation can occur through mechanisms such as carrying lactic acid that is consumed by microbiome microorganisms resulting in SCFAs and butyrate production which both have beneficial roles.Citation29
Postbiotic preparations could also antagonize intestinal pathogens by delivering antimicrobial compounds (metabolites and bacteriocins) that may prevent biofilm formation and inactivate specific microorganisms.Citation11,Citation30
Table 1. Clinical evidence for postbiotics as microbial therapeutics through intestinal barrier function enhancement.
Overview of clinical benefit evidence organized by mechanism of action
Enhancing intestinal barrier function ()
Evidence of efficacy in gastrointestinal disorders
Pediatric population
Acute diarrhea is a significant cause of childhood morbidity and mortality in developing countriesCitation31 and rotavirus is the most common pathogen causing 29% to 45% of severe diarrhea cases.Citation32 Acute gastroenteritis has been found to cause large-scale alterations of the intestinal microbiome.Citation33 Enteric bacterial infections markedly reduce the intestinal microbiome richness and diversity which can last up to 14 weeks post-infection.Citation34 Similar changes are observed with viral diarrhea.Citation35 Microbiome alterations are more significant in children with a “failure to thrive” and these children also take longer to recover from diarrheal illness.Citation36 Postbiotics may help to mitigate these alterations and preserve a balanced microbiome during and after diarrheal illnesses. Although live Lactobacillus has been shown to be effective against viral diarrhea,Citation37,Citation38 studies have also demonstrated that heat-treated Lactobacillus LB can promote faster recovery, reduce morbidity and reduce hospitalization duration.Citation39–41 Furthermore, well-controlled studies have shown that heat-treated Lactobacillus paracasei helps prevent diarrhea by significantly reducing the number of diarrhea episodes compared with a placebo.Citation42,Citation43
Antibiotics can also alter the intestinal microbiome leading to antibiotic-associated diarrhea. Although, probiotic efficacy is widely documented with diarrhea and has been suggested to treat antibiotic-associated diarrhea, there is a perception that antibiotics may reduce probiotic viability.Citation44 Postbiotics may reduce the risk of antibiotic-associated diarrhea as shown by an exploratory study revealing that in patients receiving antibiotics, there was less diarrhea in the heat-treated Lactobacillus LB group.Citation44
Adult population
Chronic diarrhea is commonly caused by chronic functional diarrhea and chronic parasitic and bacterial infections in developing countriesCitation45 while in developed countries, irritable bowel syndrome (IBS) is the most common cause affecting up to 15% of adults.Citation46,Citation47 Treatment often includes antibiotics and antimotility drugs, but they can be ineffective and cause adverse effects. Postbiotics could be a possible alternative. A recent randomized-controlled study showed that heat-treated Lactobacillus LB significantly improved chronic diarrhea and clinical symptoms compared with live lactobacilli (p < 0.05).Citation48 Non-viable Bifidobacterium bifidum MIMBb75 has been found to substantially alleviate IBS and its symptoms compared with the placebo (p = 0 · 0007).Citation49 Similarly, inactivated Lactobacillus LB plus fermented culture medium significantly decreased the number of weekly stools (p < 0.0001) and improved abdominal pain, bloating and quality of life in patients with IBS (p < 0.0001).Citation50
Postbiotics may also help improve the efficacy of standard treatment for Helicobacter pylori infections. Lactobacilli has been shown to inhibit the attachment of Helicobacter pylori to gastric epithelial cells in in vitro studies.Citation51 Furthermore, L. acidophilus LB spent culture supernatant decreases H. pylori viability, regardless of pH and lactic acid levels, in vitro and in vivo.Citation51 Heat stabilized L. acidophilus LB was given to in addition to the standard treatment to H. pylori positive patients in an open-label, prospective, randomized trial. It was demonstrated that adding L. acidophilus to the standard treatment significantly increases eradication rates compared to standard treatment alone.Citation52
Table 2. Clinical evidence for postbiotics as microbial therapeutics through immune system modulation.
Immune system modulation ()
Evidence of efficacy in immunity and allergies
Live Lactobacillus spp. have been shown to improve immune function and health-related quality of life (HRQoL), and control allergies. This may be due to its ability to skew the immune system away from T helper 2 (Th2) responses towards T helper 1 (Th1) responses.Citation53 This may be beneficial in Westernized societies where good public hygiene and fewer infections reduce this response, increasing the risk of developing allergies.Citation54 Poor immunity also increases the risk of pathogenic infections and can reduce HRQoL.Citation55,Citation56
There is also well-controlled clinical evidence demonstrating that heat-treated Lactobacilli plantarum (HK-LP) may also improve Th-1 related immune function. Compared with a placebo, the change in Con A-induced proliferation and Th1:Th2 ratio was greater (p = 0.036 and p = 0.002, respectively) and HRQoL improved more in the HK-LP group (p = 0.049 at week 8, p = 0.092 at week 12).Citation58
Similarly, live Lactobacillus paracasei 33 (LP33) has been shown to effectively and safely improve QoL for patients with house-dust mite induced perennial allergic rhinitis.Citation59 A subsequent randomized-controlled trial revealed that heat-treated LP33 had similar efficacy to the live variant since patients taking either the live or heat-treated LP33 had improved QoL scores for frequency (p < 0.0001) and level of bother (p = 0.004) compared with the placebo.Citation60 Furthermore, inactivated Lactobacillus LB and non-pathogenic E. coli have been shown to modulate allergic immune response in grass pollen allergies, both clearly through Th1-dominated responses.Citation61
Allergic rhinitis requires long-term management so the efficacy, safety, low cost, and ease of storage may mean that postbiotics are a good treatment option. It has also been suggested that postbiotics could potentially improve food allergies.Citation62
Evidence of efficacy in upper respiratory tract infections
Animal and clinical data support the use of three postbiotic strains (Lactobacillus plantarum, Pediococcus acidilactici, and Lactobacillus pentosus) in preventing upper respiratory tract infections (URTI).
In mice, heat-treated Lactobacillus plantarum L-137 has been shown to stimulate type 1 interferon production thus enhancing protection against the influenza virusCitation63 which has also been observed in humans.Citation64 It has been suggested that high levels of psychological stress increase the risk of acute respiratory illness.Citation65 Well-controlled clinical evidence suggests daily heat-treated Lactobacillus plantarum L-137 (HK L-137) intake can decrease URTI incidence in healthy people with high levels of psychological stress, possibly through immune function augmentation. The 12-week randomized-controlled study found URTI incidence was significantly lower in the HK L-137 group vs the control group (p = 0.011). There was also a significant negative correlation between HK L-137 intake duration and URTI incidence, duration, severity, and medication duration. The concanavalin A-induced proliferation of peripheral blood mononuclear cells was significantly greater in the HK L-137 group than in the control group (p = 0.048).Citation66 This means HK L-137 intake could potentially reduce this URTI risk.
Similarly, studies have evaluated the possibility of preventing respiratory tract infections in children. Lactobacillus paracasei CBA L74 was found to reduce the number of cases of pharyngitis, laryngitis and tracheitis in two well-controlled trials including children aged 12–48 months.Citation42,Citation43 Furthermore, Pediococcus acidilactici K15 was found to support anti-infectious immune systems in children who ate less fermented foods and maintained salivary secretory IgA levels in all subjects in a randomized-controlled study focusing on respiratory tract infection prevention in pre-school children. The four-month study also found that in children eating little fermented food, K15 significantly reduced fever duration compared with the placebo.Citation67
Salivary IgA levels decrease with ageCitation68–70 suggesting that elderly adults may be more susceptible to upper respiratory tract infections. Lactobacillus pentosus b240 has been shown to increase salivary IgA levelsCitation71 and a well-controlled trial demonstrated that b240 intake significantly reduces common cold incidence rates in elderly adults, possibly improving infection resistance through mucosal immunity. This study found that common cold incidence rates were lower (log-rank test, p = 0.034) and general health perceptions, determined using SF-36 w, dose-dependently increased (p for trend = 0.016).Citation72
Table 3. Clinical evidence for postbiotics as microbial therapeutics through multiple mechanisms of action.
Multiple mechanisms of action ()
Evidence for efficacy in stress and neurological conditions
The gut-brain axis has been shown to have a crucial role in maintaining intestinal homeostasis and brain function.Citation73,Citation74 The intestinal microbiome affects communication between the intestines and the brain through immune, endocrine and neural pathways,Citation75 and evidence suggests that it significantly impacts brain function affecting mood, recognition and behavior.Citation76
Probiotics have been shown to modulate hippocampus-mediated negative feedback regulation of the hypothalamic–pituitary–adrenal axis, mitigate stress-induced pain and behavior,Citation77,Citation78 transduce signals to the brain via the afferent vagal nerve and relieve mood disturbances.Citation79,Citation80 Research is underway to investigate the effects of heat-treated bacteria.
Well-controlled clinical results show that long-term Lactobacillus gasseri CP2305 use may improve the mental state, sleep quality and gut microbiome of healthy adults under stressful conditions. The 24-week randomized-controlled study included students preparing for national medical examinations and daily CP2305 significantly reduced anxiety, sleep disturbance and salivary chromogranin A levels compared with the placebo (p < 0.05). CP2305 also attenuated the stress-induced decline of Bifidobacterium spp. and the stress-induced elevation of Streptococcus spp.Citation81
Postbiotics have uses in other nervous system-related conditions. For example, it has been demonstrated in mice that daily intake of plasmalogens, a component of postbiotics, can inhibit memory loss by inhibiting glial activation.Citation82 Furthermore, a well-controlled study revealed that plasmalogen may improve cognitive function in patients with mild Alzheimer’s disease.Citation83
Potential uses include new anti-depressant approaches since depression has been linked with intestinal microbiome alterations.Citation84,Citation85 Furthermore, multiple sclerosis pathogenesis has also been linked with microbiome alterationsCitation86 including lower levels of SCFA-producing bacteriaCitation87 which could be a potential target for postbiotics.
Efficacy in cardiac and vascular disorders
Oxidative damage can lead to inflammation and cardiovascular disease. Molecules with antioxidant properties found in postbiotic preparations could therefore potentially reduce this.Citation88 Inflammation and endothelial dysfunction occurring with chronic kidney disease (CKD) may cause cardiac damage; however, postbiotic preparations have been identified as a potential prophylaxis for CKD-associated cardiac damage.Citation89 Further data suggest that bacterial metabolites and components may prevent or slow the progression of CKD and hypertension.Citation89
Evidence for efficacy in metabolic syndrome
Metabolic syndrome is characterized by various comorbidities predisposing individuals to cardiovascular pathologies and type 2 diabetes mellitus.Citation90 Obesity-related disorder onset can be linked to the intestinal microbiome.Citation91 Modulating the intestinal microbiome with postbiotics has been shown to facilitate weight reductionCitation92 and may reduce cholesterol, as demonstrated in one study where B. longum administered to rats had a cholesterol-lowering effect.Citation93 Moreover, muramyl dipeptide, a bacterial wall component, can modulate GLP-1 secretion thereby increasing insulin sensitivity and improving glucose tolerance.Citation94
In rodents, live Akkermansia muciniphila reduced obesity, glucose intolerance, insulin resistance, steatosis, and gut permeability.Citation95–97 Subsequently, it was discovered that pasteurization enhances its effect on adiposity, insulin resistance and glucose tolerance.Citation96 Clinical data from a randomized-controlled study including overweight/obese, insulin-resistant individuals show that pasteurized A. muciniphila reduces liver dysfunction and inflammation blood marker levels while leaving the overall gut microbiome structure unaffected. The three-month study found that pasteurized A. muciniphila was safe and well tolerated, improved insulin sensitivity (p = 0.002), and reduced insulinemia (p = 0.006), plasma total cholesterol (p = 0.02) and body weight (p = 0.091) compared with the placebo. It also reduced fat mass (p = 0.092) and hip circumference (p = 0.091) compared with baseline.Citation98
Similarly, the intestinal microbiome has been shown to also influence appetite,Citation99 meaning postbiotics could potentially be used as an appetite regulator.
Future potential
There are areas where postbiotics have potential efficacy, for example the liver. The gut–liver axis is a bidirectional relationship between the gut, its microbiome, and the liver. The portal vein carries gut-derived products to the liver and the liver feedbacks via bile and antibody secretion into the intestine. A healthy microbiome maintains gut-liver axis homeostasis. Liver cirrhosis is associated with a profoundly altered microbiome and damaged intestinal barrier. There is increasing evidence that microorganism-derived metabolites including trimethylamine, SCFAs and ethanol have a pathogenic role in non-alcoholic fatty liver disease. Postbiotics are considered a potential new therapeutic avenue for these liver diseases, but more research is needed to confirm their benefit.Citation100
Probiotic genome editing already existsCitation101 and could potentially be used with postbiotics to modify the precursor bacteria, thus generating new postbiotic interventions. Furthermore, advancements in microbiome metagenomic mapping can further elucidate interactions between commensal microorganisms and the intestine as well as strengthen the evidence for postbiotics. This could even be extended to individualized microbiome phenotyping to prevent disease, though this is a long way off.
Establishing the interaction mechanisms between postbiotics and commensal microorganisms will improve the understanding of the potential clinical benefits and possibly lead to targeted postbiotic therapy.
Conclusion
Postbiotics are safe and stable with a long shelf-life enabling easy storage and transportation and can be administered during antibiotic treatment without affecting efficacy, making them an appealing alternative to probiotics. There is growing evidence for the clinical benefits of postbiotics in the management of highly prevalent conditions including gastrointestinal, dermatological, and neurological disorders as well as respiratory infections and metabolic syndrome. Postbiotics may offer a novel therapeutic approach for these conditions and could be a safer alternative to probiotics, particularly in vulnerable populations such as pediatrics. Additional randomized, placebo-controlled clinical trials are necessary to further verify the clinical benefits of postbiotics.
Acknowledgments
The authors thank Charlotte Wright BVM&S MRCVS DipTrans for providing medical writing support in the drafting and writing of the manuscript and Amy Whereat BSc Physiology & Pharmacology MMktg MGSM for editorial support, both from Speak the Speech Consulting.
Disclosure statement
All authors are on the ADARE advisory board. AM is involved with PileJe, Biogaia, Danone, Havea, and Biocodex. ATA consults for Carnot Mexico, Médix Mexico, Sanofi México and Latin America, Alfasigma Mexico. ATA is a speaker for Alfasigma Mexico and Italy, Sanofi Global, Menarini Mexico, Médix Mexico, Takeda Mexico, Carnot Mexico, Adare France, Columbia Mexico, Abbott México and Latin America, Sanofi Mexico, Latin America and Global, ABiotics Spain, Axon Pharma Chili, Mayoli Spindler, Mexico and France, Biocodex Mexico and France, Tecnoquímicas Colombia, MD Pharma Colombia, Medix – Healthcare – Instituto Rosell Mexico, Menarini, Mexico, Ferrer Mexico, Takeda, Mexico, and Latin America, Columbia, Mexico, FAES FARMA Mexico, Ecuador, Falk Institute Mexico, Instituto de Nutrición y Salud Kellogg´s, Instituto Danone Mexico. JT has given has given scientific advice to AlfaWassermann, Arena, Bayer, Christian Hansen, Clasado, Danone, Devintec, Falk, FitForMe, Grünenthal, Ironwood, Janssen, Kiowa Kirin, Menarini, Mylan, Neurogastrx, Neutec, Novartis, Nutricia, Reckitt Benckiser, Ricordati, Shionogi, Takeda, Truvion, Tsumura, Zealand and Zeria pharmaceuticals, has received research support from Biohit, Shire, Sofar and Takeda, and has served on the Speaker bureau for Abbott, Allergan, AstraZeneca, FitForMe, Janssen, Kyowa Kirin, Mayoly, Menarini, Mylan, Novartis, Schwabe Pharmaceuticals, Takeda, Wellspect and Zeria. JT is funded by a Methusalem grant from Leuven University. TVHN has been on expert panels for expert panels organized by Friesland Campina, Sanofi, AstraZeneca, Abbott, and Nestle. CH conducts research funded by ADARE Biome.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
References
- Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev. 2012;70(Suppl 1):S38–14. doi:10.1111/j.1753-4887.2012.00493.x.
- Belkaid Y, Harrison OJ. Homeostatic immunity and the microbiota. Immunity. 2017;46(4):562–576. doi:10.1016/j.immuni.2017.04.008.
- Kataria J, Li N, Wynn JL, Neu J. Probiotic microbes: do they need to be alive to be beneficial? Nutr Rev. 2009;67(9):546–550. doi:10.1111/j.1753-4887.2009.00226.x.
- Neu J, Douglas-Escobar M, Lopez M. Microbes and the developing gastrointestinal tract. Nutr Clin Pract. 2007;22(2):174–182. doi:10.1177/0115426507022002174.
- Huffnagle GB. Gi microbiota and regulation of the immune system. New York: Springer-Verlag New York. Advances in experimental medicine and biology
- Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol. 2010;7(9):503–514. doi:10.1038/nrgastro.2010.117.
- Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17(4):219–232. doi:10.1038/nri.2017.7.
- Peluzio M, Martinez JA, Milagro FI. Postbiotics: metabolites and mechanisms involved in microbiota-host interactions. Trends Food Sci Technol. 2021;108(11):11–26. doi:10.1016/j.tifs.2020.12.004.
- Piqué N, Berlanga M, Miñana-Galbis D. Health benefits of heat-killed (tyndallized) probiotics: an overview. Int J Mol Sci. 2019;20(10):2534. doi:10.3390/ijms20102534.
- Warda AK, de Almeida Bettio PH, Hueston CM, Di Benedetto G, Clooney AG, Hill C. Oral administration of heat-treated lactobacilli modifies the murine microbiome and reduces citrobacter induced colitis. Front Microbiol. 2020;11(69). doi:10.3389/fmicb.2020.00069.
- Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EMM, Sanders ME, Shamir R, Swann JR, Szajewska H, et al. The international scientific association of probiotics and prebiotics (isapp) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021. doi:10.1038/s41575-021-00440-6.
- Adams CA. The probiotic paradox: live and dead cells are biological response modifiers. Nutr Res Rev. 2010;23(1):37–46. doi:10.1017/s0954422410000090.
- Konstantinov SR, Kuipers EJ, Peppelenbosch MP. Functional genomic analyses of the gut microbiota for CRC screening. Nat Rev Gastroenterol Hepatol. 2013;10(12):741–745. doi:10.1038/nrgastro.2013.178.
- Aguilar-Toalá JE, Garcia-Varela R, Garcia HS, Mata-Haro V, González-Córdova AF, Vallejo-Cordoba B, Hernández-Mendoza A. Postbiotics: an evolving term within the functional foods field. Trends Food Sci Technol. 2018;75(105):105–114. doi:10.1016/j.tifs.2018.03.009.
- Warda AK, Clooney AG, Ryan F, de Almeida Bettio PH, Di Benedetto G, Ross RP, Hill C. A postbiotic consisting of heat-treated lactobacilli has a bifidogenic effect in pure culture and in human fermented faecal communities. Appl Environ Microbiol. 2021;87(8). doi:10.1128/aem.02459-20.
- Deshpande G, Athalye-Jape G, Patole S. Para-probiotics for preterm neonates-the next frontier. Nutrients. 2018;10(7):871. doi:10.3390/nu10070871.
- Wegh CAM, Geerlings SY, Knol J, Roeselers G, Belzer C. Postbiotics and their potential applications in early life nutrition and beyond. Int J Mol Sci. 2019;20(19):4673. doi:10.3390/ijms20194673.
- Ouwehand AC, Tölkkö S, Kulmala J, Salminen S, Salminen E. Adhesion of inactivated probiotic strains to intestinal mucus. Lett Appl Microbiol. 2000;31(1):82–86. doi:10.1046/j.1472-765x.2000.00773.x.
- Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. doi:10.1038/nrgastro.2014.66.
- Lebeer S, Bron PA, Marco ML, Van Pijkeren JP, Motherway OM, Hill C, Pot B, Roos S, Klaenhammer T. Identification of probiotic effector molecules: present state and future perspectives. Curr Opin Biotechnol. 2018;49(217):217–223. doi:10.1016/j.copbio.2017.10.007.
- Lebeer S, Vanderleyden J, De Keersmaecker SC. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol. 2010;8(3):171–184. doi:10.1038/nrmicro2297.
- Thomas CM, Hong T, van Pijkeren JP, Hemarajata P, Trinh DV, Hu W, Britton RA, Kalkum M, Versalovic J. Histamine derived from probiotic lactobacillus reuteri suppresses tnf via modulation of PK and Erk signaling. PLoS One. 2012;7(2):e31951. doi:10.1371/journal.pone.0031951.
- Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, Mellinger JD, Smith SB, Digby GJ, Lambert NA, et al. Gpr109a is a g-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009;69(7):2826–2832. doi:10.1158/0008-5472.Can-08-4466.
- Schiavi E, Gleinser M, Molloy E, Groeger D, Frei R, Ferstl R, Rodriguez-Perez N, Ziegler M, Grant R, Moriarty TF, et al. The surface-associated exopolysaccharide of bifidobacterium longum 35624 plays an essential role in dampening host proinflammatory responses and repressing local th17 responses. Appl Environ Microbiol. 2016;82(24):7185–7196. doi:10.1128/aem.02238-16.
- Engevik MA, Luk B, Chang-Graham AL, Hall A, Herrmann B, Ruan W, Endres BT, Shi Z, Garey KW, Hyser JM, et al. Bifidobacterium dentium fortifies the intestinal mucus layer via autophagy and calcium signaling pathways. mBio. 2019;10(3). doi:10.1128/mBio.01087-19
- Feng Y, Wang Y, Wang P, Huang Y, Wang F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of nlrp3 inflammasome and autophagy. Cell Physiol Biochem. 2018;49(1):190–205. doi:10.1159/000492853.
- Sun Z, Harris HM, McCann A, Guo C, Argimón S, Zhang W, Yang X, Jeffery IB, Cooney JC, Kagawa TF, et al. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat Commun. 2015;6(1). doi:10.1038/ncomms9322
- Corr SC, Li Y, Riedel CU, O’Toole PW, Hill C, Gahan CG. Bacteriocin production as a mechanism for the antiinfective activity of lactobacillus salivarius ucc118. Proc Natl Acad Sci U S A. 2007;104(18):7617–7621. doi:10.1073/pnas.0700440104.
- Laverde Gomez JA, Mukhopadhya I, Duncan SH, Louis P, Shaw S, Collie-Duguid E, Crost E, Juge N, Flint HJ. Formate cross-feeding and cooperative metabolic interactions revealed by transcriptomics in co-cultures of acetogenic and amylolytic human colonic bacteria. Environ Microbiol. 2019;21(1):259–271. doi:10.1111/1462-2920.14454.
- Hernández-Granados MJ, Franco-Robles E. Postbiotics in human health: possible new functional ingredients? Food Res Int. 2020;137(109660):109660. doi:10.1016/j.foodres.2020.109660.
- Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81:197–204.
- Parashar UD, Gibson CJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12(2):304–306. doi:10.3201/eid1202.050006.
- Pop M, Walker AW, Paulson J, Lindsay B, Antonio M, Hossain MA, Oundo J, Tamboura B, Mai V, Astrovskaya I, et al. Diarrhea in young children from low-income countries leads to large-scale alterations in intestinal microbiota composition. Genome Biol. 2014;15(6):R76. doi:10.1186/gb-2014-15-6-r76.
- Singh P, Teal TK, Marsh TL, Tiedje JM, Mosci R, Jernigan K, Zell A, Newton DW, Salimnia H, Lephart P, et al. Intestinal microbial communities associated with acute enteric infections and disease recovery. Microbiome. 2015;3(1). doi:10.1186/s40168-015-0109-2
- Ma C, Wu X, Nawaz M, Li J, Yu P, Moore JE, Xu J. Molecular characterization of fecal microbiota in patients with viral diarrhea. Curr Microbiol. 2011;63(3):259–266. doi:10.1007/s00284-011-9972-7.
- Rouhani S, Griffin NW, Yori PP, Gehrig JL, Olortegui MP, Salas MS, Trigoso DR, Moulton LH, Houpt ER, Barratt MJ, et al. Diarrhea as a potential cause and consequence of reduced gut microbial diversity among undernourished children in Peru. Clin Infect Dis. 2020;71(4):989–999. doi:10.1093/cid/ciz905.
- Van Niel CW, Feudtner C, Garrison MM, Christakis DA. Lactobacillus therapy for acute infectious diarrhea in children: a meta-analysis. Pediatrics. 2002;109(4):678–684. doi:10.1542/peds.109.4.678.
- Collinson S, Deans A, Padua-Zamora A, Gregorio GV, Li C, Dans LF, Allen SJ. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2020;12(12):Cd003048. doi:10.1002/14651858.CD003048.pub4.
- Simakachorn N, Pichaipat V, Rithipornpaisarn P, Kongkaew C, Tongpradit P, Varavithya W. Clinical evaluation of the addition of lyophilized, heat-killed lactobacillus acidophilus lb to oral rehydration therapy in the treatment of acute diarrhea in children. J Pediatr Gastroenterol Nutr. 2000;30(1):68–72. doi:10.1097/00005176-200001000-00020.
- Liévin-Le Moal V, Sarrazin-Davila LE, Servin AL. An experimental study and a randomized, double-blind, placebo-controlled clinical trial to evaluate the antisecretory activity of lactobacillus acidophilus strain lb against nonrotavirus diarrhea. Pediatrics. 2007;120(4):e795–803. doi:10.1542/peds.2006-2930.
- Salazar-Lindo E, Figueroa-Quintanilla D, Caciano MI, Reto-Valiente V, Chauviere G, Colin P. Effectiveness and safety of lactobacillus lb in the treatment of mild acute diarrhea in children. J Pediatr Gastroenterol Nutr. 2007;44(5):571–576. doi:10.1097/MPG.0b013e3180375594.
- Nocerino R, Paparo L, Terrin G, Pezzella V, Amoroso A, Cosenza L, Cecere G, De Marco G, Micillo M, Albano F, et al. Cow’s milk and rice fermented with lactobacillus paracasei cba l74 prevent infectious diseases in children: a randomized controlled trial. Clin Nutr. 2017;36(1):118–125. doi:10.1016/j.clnu.2015.12.004.
- Corsello G, Carta M, Marinello R, Picca M, De Marco G, Micillo M, Ferrara D, Vigneri P, Cecere G, Ferri P, et al. Preventive effect of cow’s milk fermented with lactobacillus paracasei cba l74 on common infectious diseases in children: a multicenter randomized controlled trial. Nutrients. 2017;9(7):669. doi:10.3390/nu9070669.
- Yap SKJ, Gwee KA, Chen Z, Tai BC, Wong ML. Lacteol fort treatment reduces antibiotic associated diarrhea. Singapore Fam Physician. 2010;36:46–49.
- Altuntaş B, Gül H, Yarali N, Ertan U. Etiology of chronic diarrhea. Indian J Pediatr. 1999;66(5):657–661. doi:10.1007/bf02726245.
- Lovell RM, Ford AC. Prevalence of gastro-esophageal reflux-type symptoms in individuals with irritable bowel syndrome in the community: a meta-analysis. Am J Gastroenterol. 2012 quiz 1802.10.1038/ajg.2012.336;107(12):1793–1801. doi:10.1038/ajg.2012.336.
- Müller-Lissner SA, Bollani S, Brummer RJ, Coremans G, Dapoigny M, Marshall JK, Muris JW, Oberndorff-Klein Wolthuis A, Pace F, Rodrigo L, et al. Epidemiological aspects of irritable bowel syndrome in Europe and North America. Digestion. 2001;64(3):200–204. doi:10.1159/000048862.
- Xiao SD, Zhang DZ, Lu H, Jiang SH, Liu HY, Wang GS, Xu GM, Zhang ZB, Lin GJ, Wang GL. Multicenter, randomized, controlled trial of heat-killed lactobacillus acidophilus lb in patients with chronic diarrhea. Adv Ther. 2003;20(5):253–260. doi:10.1007/bf02849854.
- Andresen V, Gschossmann J, Layer P. Heat-inactivated bifidobacterium bifidum mimbb75 (syn-hi-001) in the treatment of irritable bowel syndrome: a multicentre, randomised, double-blind, placebo-controlled clinical trial. Lancet Gastroenterol Hepatol. 2020;5(7):658–666. doi:10.1016/s2468-1253(20)30056-x.
- Tarrerias AL, Costil V, Vicari F, Létard JC, Adenis-Lamarre P, Aisène A, Batistelli D, Bonnaud G, Carpentier S, Dalbiès P, et al. The effect of inactivated lactobacillus lb fermented culture medium on symptom severity: observational investigation in 297 patients with diarrhea-predominant irritable bowel syndrome. Dig Dis. 2011;29(6):588–591. doi:10.1159/000332987.
- Coconnier MH, Lievin V, Hemery E, Servin AL. Antagonistic activity against helicobacter infection in vitro and in vivo by the human lactobacillus acidophilus strain lb. Appl Environ Microbiol. 1998;64(11):4573–4580. doi:10.1128/aem.64.11.4573-4580.1998.
- Canducci F, Armuzzi A, Cremonini F, Cammarota G, Bartolozzi F, Pola P, Gasbarrini G, Gasbarrini A. A lyophilized and inactivated culture of lactobacillus acidophilus increases helicobacter pylori eradication rates. Aliment Pharmacol Ther. 2000;14(12):1625–1629. doi:10.1046/j.1365-2036.2000.00885.x.
- Mohamadzadeh M, Olson S, Kalina WV, Ruthel G, Demmin GL, Warfield KL, Bavari S, Klaenhammer TR. Lactobacilli activate human dendritic cells that skew t cells toward t helper 1 polarization. Proc Natl Acad Sci U S A. 2005;102(8):2880–2885. doi:10.1073/pnas.0500098102.
- Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296(5567):490–494. doi:10.1126/science.296.5567.490.
- Hassan IS, Bannister BA, Akbar A, Weir W, Bofill M. A study of the immunology of the chronic fatigue syndrome: correlation of immunologic parameters to health dysfunction. Clin Immunol Immunopathol. 1998;87(1):60–67. doi:10.1006/clin.1997.4512.
- Majeed M, Majeed S, Nagabhushanam K, Mundkur L, Rajalakshmi HR, Shah K, Beede K. Novel topical application of a postbiotic, lactosporin®, in mild to moderate acne: a randomized, comparative clinical study to evaluate its efficacy, tolerability and safety. Cosmetics. 2020;7(3):70. doi:10.3390/cosmetics7030070.
- Rinaldi F, Trink A, Pinto D. Efficacy of postbiotics in a prp-like cosmetic product for the treatment of alopecia area celsi: a randomized double-blinded parallel-group study. Dermatol Ther (Heidelb). 2020;10(3):483–493. doi:10.1007/s13555-020-00369-9.
- Hirose Y, Murosaki S, Yamamoto Y, Yoshikai Y, Tsuru T. Daily intake of heat-killed lactobacillus plantarum l-137 augments acquired immunity in healthy adults. J Nutr. 2006;136(12):3069–3073. doi:10.1093/jn/136.12.3069.
- Wang MF, Lin HC, Wang YY, Hsu CH. Treatment of perennial allergic rhinitis with lactic acid bacteria. Pediatr Allergy Immunol. 2004;15(2):152–158. doi:10.1111/j.1399-3038.2004.00156.x.
- Peng GC, Hsu CH. The efficacy and safety of heat-killed lactobacillus paracasei for treatment of perennial allergic rhinitis induced by house-dust mite. Pediatr Allergy Immunol. 2005;16(5):433–438. doi:10.1111/j.1399-3038.2005.00284.x.
- Rasche C, Wolfram C, Wahls M, Worm M. Differential immunomodulating effects of inactivated probiotic bacteria on the allergic immune response. Acta Derm Venereol. 2007;87(4):305–311. doi:10.2340/00015555-0232.
- Homayouni Rad A, Aghebati Maleki L, Samadi Kafil H, Abbasi A. Postbiotics: a novel strategy in food allergy treatment. Crit Rev Food Sci Nutr. 2021;61(3):492–499. doi:10.1080/10408398.2020.1738333.
- Maeda N, Nakamura R, Hirose Y, Murosaki S, Yamamoto Y, Kase T, Yoshikai Y. Oral administration of heat-killed lactobacillus plantarum l-137 enhances protection against influenza virus infection by stimulation of type i interferon production in mice. Int Immunopharmacol. 2009;9(9):1122–1125. doi:10.1016/j.intimp.2009.04.015.
- Arimori Y, Nakamura R, Hirose Y, Murosaki S, Yamamoto Y, Shidara O, Ichikawa H, Yoshikai Y. Daily intake of heat-killed lactobacillus plantarum l-137 enhances type i interferon production in healthy humans and pigs. Immunopharmacol Immunotoxicol. 2012;34(6):937–943. doi:10.3109/08923973.2012.672425.
- Cohen S, Tyrrell DA, Smith AP. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991;325(9):606–612. doi:10.1056/nejm199108293250903.
- Hirose Y, Yamamoto Y, Yoshikai Y, Murosaki S. Oral intake of heat-killed lactobacillus plantarum l-137 decreases the incidence of upper respiratory tract infection in healthy subjects with high levels of psychological stress. J Nutr Sci. 2013;2:e39. doi:10.1017/jns.2013.35.
- Hishiki H, Kawashima T, Tsuji NM, Ikari N, Takemura R, Kido H, Shimojo N. 1A double-blind, randomized, placebo-controlled trial of heat-killed pediococcus acidilactici k15 for prevention of respiratory tract infections among preschool children. Nutrients. 2020;12(7):1989. doi:10.3390/nu12071989.
- Smith DJ, Joshipura K, Kent R, Taubman MA. Effect of age on immunoglobulin content and volume of human labial gland saliva. J Dent Res. 1992;71(12):1891–1894. doi:10.1177/00220345920710120701.
- Challacombe SJ, Percival RS, Marsh PD. Age-related changes in immunoglobulin isotypes in whole and parotid saliva and serum in healthy individuals. Oral Microbiol Immunol. 1995;10(4):202–207. doi:10.1111/j.1399-302x.1995.tb00143.x.
- Miletic ID, Schiffman SS, Miletic VD, Sattely-Miller EA. Salivary IgA secretion rate in young and elderly persons. Physiol Behav. 1996;60(1):243–248. doi:10.1016/0031-9384(95)02161-2.
- Kotani Y, Shinkai S, Okamatsu H, Toba M, Ogawa K, Yoshida H, Fukaya T, Fujiwara Y, Chaves PH, Kakumoto K, et al. Oral intake of lactobacillus pentosus strain b240 accelerates salivary immunoglobulin a secretion in the elderly: a randomized, placebo-controlled, double-blind trial. Immun Ageing. 2010;7(11). doi:10.1186/1742-4933-7-11
- Shinkai S, Toba M, Saito T, Sato I, Tsubouchi M, Taira K, Kakumoto K, Inamatsu T, Yoshida H, Fujiwara Y, et al. Immunoprotective effects of oral intake of heat-killed lactobacillus pentosus strain b240 in elderly adults: a randomised, double-blind, placebo-controlled trial. Br J Nutr. 2013;109(10):1856–1865. doi:10.1017/s0007114512003753.
- Cryan JF, O’Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23(3):187–192. doi:10.1111/j.1365-2982.2010.01664.x.
- Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12(8):453–466. doi:10.1038/nrn3071.
- Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6(5):306–314. doi:10.1038/nrgastro.2009.35.
- Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–712. doi:10.1038/nrn3346.
- Abildgaard A, Elfving B, Hokland M, Wegener G, Lund S. Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology. 2017;79(40):40–48. doi:10.1016/j.psyneuen.2017.02.014.
- Ait-Belgnaoui A, Payard I, Rolland C, Harkat C, Braniste V, Théodorou V, Tompkins TA. Bifidobacterium longum and lactobacillus helveticus synergistically suppress stress-related visceral hypersensitivity through hypothalamic-pituitary-adrenal axis modulation. J Neurogastroenterol Motil. 2018;24(1):138–146. doi:10.5056/jnm16167.
- Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, et al. The anxiolytic effect of bifidobacterium longum ncc3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23(12):1132–1139. doi:10.1111/j.1365-2982.2011.01796.x.
- Takada M, Nishida K, Kataoka-Kato A, Gondo Y, Ishikawa H, Suda K, Kawai M, Hoshi R, Watanabe O, Igarashi T, et al. Probiotic lactobacillus casei strain shirota relieves stress-associated symptoms by modulating the gut-brain interaction in human and animal models. Neurogastroenterol Motil. 2016;28(7):1027–1036. doi:10.1111/nmo.12804.
- Nishida K, Sawada D, Kuwano Y, Tanaka H, Rokutan K. Health benefits of lactobacillus gasseri cp2305 tablets in young adults exposed to chronic stress: a randomized, double-blind, placebo-controlled study. Nutrients. 2019;11(8):1859. doi:10.3390/nu11081859.
- Hossain MS, Tajima A, Kotoura S, Katafuchi T. Oral ingestion of plasmalogens can attenuate the lps-induced memory loss and microglial activation. Biochem Biophys Res Commun. 2018;496(4):1033–1039. doi:10.1016/j.bbrc.2018.01.078.
- Fujino T, Yamada T, Asada T, Tsuboi Y, Wakana C, Mawatari S, Kono S. Efficacy and blood plasmalogen changes by oral administration of plasmalogen in patients with mild Alzheimer’s disease and mild cognitive impairment: a multicenter, randomized, double-blind, placebo-controlled trial. EBioMedicine. 2017;17(199):199–205. doi:10.1016/j.ebiom.2017.02.012.
- Clarke G. The gut microbiome and depression: finding a way through troubled waters where the river meets the sea. Expert Rev Gastroenterol Hepatol. 2020;14(5):301–304. doi:10.1080/17474124.2020.1754796.
- Flux MC, Lowry CA. Finding intestinal fortitude: integrating the microbiome into a holistic view of depression mechanisms, treatment, and resilience. Neurobiol Dis. 2020;135:104578. doi:10.1016/j.nbd.2019.104578.
- Castillo-Álvarez F, Marzo-Sola ME. Role of intestinal microbiota in the development of multiple sclerosis. Neurologia. 2017;32(3):175–184. doi:10.1016/j.nrl.2015.07.005.
- Melbye P, Olsson A, Hansen TH, Søndergaard HB, Bang Oturai A. Short-chain fatty acids and gut microbiota in multiple sclerosis. Acta Neurol Scand. 2019;139(3):208–219. doi:10.1111/ane.13045.
- Foo HL, Loh T, Abdul Mutalib NE, Rahim RA. Microbiome and metabolome in diagnosis, therapy, and other strategic applications. 1st. The myth and therapeutic potentials of postbiotics. London, UK: Academic Press; p. 234.
- Cosola C, Rocchetti MT, Cupisti A, Gesualdo L. Microbiota metabolites: pivotal players of cardiovascular damage in chronic kidney disease. Pharmacol Res. 2018;130:132–142. doi:10.1016/j.phrs.2018.03.003.
- O’Neill S, O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16(1):1–12. doi:10.1111/obr.12229.
- Cani PD, Van Hul M, Lefort C, Depommier C, Rastelli M, Everard A. Microbial regulation of organismal energy homeostasis. Nat Metab. 2019;1(1):34–46. doi:10.1038/s42255-018-0017-4.
- Vallianou N, Stratigou T, Christodoulatos GS, Tsigalou C, Dalamaga M. Probiotics, prebiotics, synbiotics, postbiotics, and obesity: current evidence, controversies, and perspectives. Curr Obes Rep. 2020;9(3):179–192. doi:10.1007/s13679-020-00379-w.
- Shin HS, Park SY, Lee DK, Kim SA, An HM, Kim JR, Kim MJ, Cha MG, Lee SW, Kim KJ, et al. Hypocholesterolemic effect of sonication-killed bifidobacterium longum isolated from healthy adult Koreans in high cholesterol fed rats. Arch Pharm Res. 2010;33(9):1425–1431. doi:10.1007/s12272-010-0917-7.
- Williams L, Alshehri A, Robichaud B, Cudmore A, Gagnon J. The role of the bacterial muramyl dipeptide in the regulation of glp-1 and glycemia. Int J Mol Sci. 2020;21(15):5252. doi:10.3390/ijms21155252.
- Everard A, Plovier H, Rastelli M, Van Hul M, de Wouters D’oplinter A, Geurts L, Druart C, Robine S, Delzenne NM, Muccioli GG, et al. Intestinal epithelial n-acylphosphatidylethanolamine phospholipase d links dietary fat to metabolic adaptations in obesity and steatosis. Nat Commun. 2019;10(1):457. doi:10.1038/s41467-018-08051-7.
- Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, et al. A purified membrane protein from akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23(1):107–113. doi:10.1038/nm.4236.
- Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, et al. Cross-talk between akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–9071. doi:10.1073/pnas.1219451110.
- Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, et al. Supplementation with akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25(7):1096–1103. doi:10.1038/s41591-019-0495-2.
- Mu C, Yang Y, Zhu W. Gut microbiota: the brain peacekeeper. Front Microbiol. 2016;7:345. doi:10.3389/fmicb.2016.00345.
- Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 2020;72(3):558–577. doi:10.1016/j.jhep.2019.10.003.
- Zuo F, Marcotte H. Advancing mechanistic understanding and bioengineering of probiotic lactobacilli and bifidobacteria by genome editing. Curr Opin Biotechnol. 2021;70:75–82. https://doi.org/10.1016/j.copbio.2020.12.015.
