ABSTRACT
The maternal microbiome is essential for the healthy growth and development of offspring and has long-term effects later in life. Recent advances indicate that the maternal microbiome begins to regulate fetal health and development during pregnancy. Furthermore, the maternal microbiome continues to affect early microbial colonization via birth and breastfeeding. Compelling evidence indicates that the maternal microbiome is involved in the regulation of immune and brain development and affects the risk of related diseases. Modulating offspring development by maternal diet and probiotic intervention during pregnancy and breastfeeding could be a promising therapy in the future. In this review, we summarize and discuss the current understanding of maternal microbiota development, perinatal microbial metabolite transfer, mother-to-infant microbial transmission during/after birth and its association with immune and brain development as well as corresponding diseases.
1. Introduction
The human body is colonized by hundreds of millions of commensal microbes, which are essential for maintaining the health of humans. Most of these microbes belong to four phyla: Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria. Citation1,Citation2 In contrast to the relatively stable gut microbiota in adults, the neonatal microbiota undergoes dramatic fluctuation and exhibits high adaptability and plasticity. Recent advances in genome sequencing technologies have expanded our knowledge regarding microbiota colonization during early life and have helped decipher its critical functions in host physiological processes, such as metabolism,Citation3 immunity,Citation4 cognitionCitation5 and intestinal homeostasis.Citation6 Disruption of microbiome establishment during the newborn period can lead to life-threatening diseases such as necrotizing enterocolitis (NEC)Citation7,Citation8 and late-onset sepsis (LOS)Citation9 and might increase the risk of obesity,Citation10 diabetesCitation11 and asthmaCitation12 in adulthood.
Similar to the genome, the microbiome is inherited by the infant from the mother. The seeding of newborn intestinal bacteria is considered to largely occur during the birthing process. After birth, neonatal gut microbes are persistently affected by maternal factors via direct contact with the mother’s skin and breast milk. Full-term vaginal delivery and exclusive breastfeeding of neonates are considered the “golden rules” for optimal microbiota selection in neonates.Citation13 Approximately 11% of maternal-derived bacterial strains (mainly Bacteroides and Bifidobacterium) persist during the first year of life.Citation14 Since the neonatal microbiota is malleable in early-stage newborns, this period is a crucial time window for external intervention (such as with environmental and dietary factors) to regulate neonatal gut dysbiosis and its related diseases. This review focuses on the fluctuation of the maternal microbiome during pregnancy and lactation as well as the impact of the maternal microbiota and its metabolites on infants, including on early gut colonization, immune maturation, and brain development. Insights into the timing and mechanisms by which the maternal gut microbiota regulates the development of neonates can be used to develop gut microbiome-based therapies to prevent and/or treat related diseases in neonates.
2. Maternal microbiome and fetal health and development during gestation
2.1 Fluctuation of maternal gut microbes during pregnancy
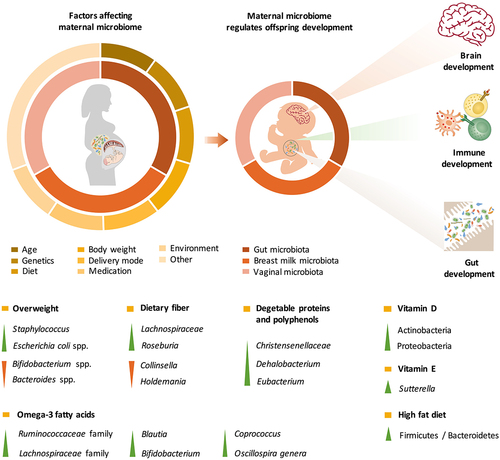
During pregnancy and lactation, maternal gut microbes support the increased energy demand for fetal growth and milk synthesis by digesting carbohydrates that are resistant to host-secreted digestive enzymes. Pregnancy involves hormonal, psychological and immunological changes that regulate gut microbiota variation during different stages of pregnancy. Prior studies have showen that Proteobacteria and Actinobacteria in the maternal gut are enriched during the third trimester of pregnancy, with increases in the levels of proinflammatory cytokines.Citation15 However, recently, a comprehensive overview of the intestinal microbiota from approximately 1500 women indicated that the core microbiota of pregnant women and nonpregnant women was similar during the progression of gestationCitation16 and remained stable during the perinatal period.Citation17 Individual heterogeneity (including in body mass index (BMI), diet, antibiotic treatment, residency status and disease) is the dominant factor that shapes the intestinal microbiota during gestation ().Citation16
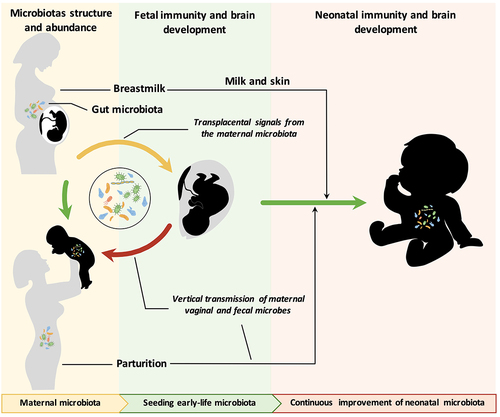
Figure 1. The maternal microbiota and its metabolites regulate fetal development before birth and during lactation. The maternal gut, breast milk and vaginal microbiota are major factors regulating offspring development. The maternal microbiome is influenced by a variety of factors, including age, genetics, diet, body weight, delivery mode, medication use, and the environment. The effects of various factors on the maternal microbiome may be passed on to the offspring through microbes, microbial products or metabolites, which regulate early gut colonization, immune maturation, and brain development in infants.
2.2 Influences on the maternal microbiome during pregnancy
2.2.1 Maternal BMI
Overweight mothers undergo shifts in their intestinal microbiota during gestation, especially between the first and third trimesters.Citation18 Decreased levels of Bifidobacterium spp. and Bacteroides spp. as well as increased levels of Staphylococcus and Escherichia coli spp. are commonly observed in overweight mothers.Citation19,Citation20 In addition, changes in gut microbes regulate maternal metabolism during pregnancy through their modification of metabolic hormones (such as adipokines, insulin and gastrointestinal polypeptides).Citation21 Therefore, the microbial communities in overweight and obese pregnant women might induce inflammation, impair glucose and insulin tolerance, and further lead to gestational diabetes. Although maternal weight status has been found to affect the composition of the offspring microbiome in animal models,Citation22 a meta-analysis of human studies found that maternal weight status had a limited predictive effect on the composition of the microbiome in infants.Citation23
2.2.2 Maternal dietary patterns
Recent evidence indicates that maternal dietary patterns and nutritional compounds are crucial factors that shape the intestinal microbial community (). A higher Firmicutes/Bacteroidetes ratio in the gut during pregnancy was observed in mothers who consumed a high-fat diet.Citation24 Dietary fiber is a predominant factor that regulates the richness and diversity of the gut microbiota.Citation25 Pregnant women who are vegetarians exhibit decreases in the abundances of Collinsella and Holdemania and increases in the abundances of Roseburia and Lachnospiraceae, which are accompanied by an increased production of short-chain fatty acids (SCFAs).Citation26 Conversely, deficiency in dietary fiber intake results in an increased abundance of Collinsella among the intestinal microbes and enhanced lactate fermentation in pregnant women.Citation27 In addition to dietary fiber, plant-derived foods, such as vegetable proteins and polyphenols, increase the number of Christensenellaceae, Dehalobacterium, and Eubacterium Citation28 and the production of butyrate and propionate in the gut.Citation29,Citation30
Recent studies have also uncovered the effects of many other nutrients that shape the gut microbial community during pregnancy. For instance, a higher level of vitamin D intake has been found to decrease alpha diversity and lead to enrichment in Actinobacteria and Proteobacteria in pregnant women, which might result in inflammatory bowel disease, obesity, autism, allergy, and asthma.Citation31 Lower vitamin E consumption is associated with higher levels of Sutterella,Citation31 which can cause gastrointestinal disorders by degrading IgA.Citation32 In addition, Sutterella has been found in greater abundance in infants with autism spectrum disorder.Citation33,Citation34 Omega-3 fatty acid intake increases the abundances of microbes in the Ruminococcaceae and Lachnospiraceae families as well as in the Blautia, Bifidobacterium, Coprococcus, and Oscillospira genera.Citation28
An unhealthy maternal diets has been demonstrated to increase the risk of preterm delivery and impair embryonic growth and development.Citation35–37 However, research on the effects of maternal diet composition on infant gut microbes is in the early stages. Consumption of a high-fat diet (more than 40% of total energy) during pregnancy decreases the number of Bacteroides species in the infant.Citation38 However, the correlations between Bacteroides and the risk of developing obesity are still under intense debate.Citation39,Citation40 In addition, the influence of the mother’s dietary intake during pregnancy on Prevotella abundance is closely related to the risk of food allergy in the infant. The higher the abundance of Prevotella in the maternal gut is, the lower the risk of food allergy in infants at 12 months.Citation41 Future studies need to clarify the optimal dietary formula for the establishment of healthy gut microbes during pregnancy.
2.2.3 Maternal antibiotic exposure
To prevent preterm birth and infection after cesarean (C) section, approximately 40% of women are administered antibiotics during pregnancy.Citation42–44 Broad-spectrum antibiotics, such as cephalosporins, are frequently prescribed to women in developed countries.Citation45,Citation46 Few recently published human studies have evaluated the effects of antibiotic treatment during pregnancy on the maternal microbiome. Maternal exposure to antibiotics during delivery can lead to a reduction in the infant’s gut microbial diversity.Citation23 However, differences in the composition and diversity of the infant gut microbiome due to maternal antibiotic exposure have limited impact and may last only one month.Citation47,Citation48
2.3 Maternal microbiome and fetal health and development
2.3.1 Maternal microbiome regulates fetal health during pregnancy
For over a century, the development of healthy infants was considered to occur in a sterile environment until birth through the vaginal canal.Citation49 Unexpected microbial invasion is commonly associated with intrauterine infections and preterm labor.Citation50,Citation51 During early pregnancy, listeriosis (caused by Listeria monocytogenes) can disrupt the maturation of the fetal immune system and cause neonatal sepsis and death.Citation52,Citation53 Syphilis (caused by Treponema pallidum infection) leads to adverse pregnancy outcomes, and fetal transmission causes congenital diseases such as osteochondritis and meningitis in newborns.Citation54,Citation55 Although the pathophysiology regarding the transplacental transmission of pathogenic bacteria remains unknown, the impact of bacterial infections on congenital malformations and serious diseases in the fetus has been clearly shown.
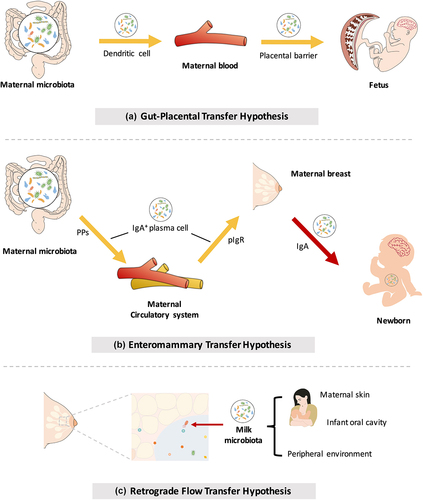
Recently, with the development of 16S rRNA sequencing, this general dogma has been challenged. Bacterial signals have been detected in amniotic fluid,Citation56,Citation57 umbilical cord blood,Citation58 and fetal membranesCitation59 in babies who do not have infection or exhibit inflammation. These findings provide evidence for the transmission of the maternal microbiome to the fetus before birth. Bacteria from the maternal intestine can be harvested by dendritic cells and then migrate to the fetus through the blood (). Although no consensus has been reached regarding the transfer of microbes before birth (discussed in Box 1), metabolites from the maternal microbiota are undoubtedly involved in the regulation of infant health and embryonic development and have long-term effects during postnatal life ().Citation60,Citation61 The physiological mechanisms by which the maternal microbiome affects fetal immunity and brain development are now comparatively well established and are discussed below.
Figure 2. Mother-to-offspring bacterial transmission during pregnancy and lactation. (a) Gut-placental transmission hypothesis. This hypothesis states that transmission of the maternal microbiome to the fetus occurs before birth. Bacteria from the maternal intestine can be harvested by dendritic cells and then migrate to the fetus through the blood. (b) Enteromammary transfer hypothesis. Breast milk functions as the most important postnatal link between infants and mothers. In addition to macronutrients, micronutrients, oligosaccharides and immune factors, maternal milk contains a microbiota. This hypothesis states that the parent microbes combine with IgA in the gut to form a complex (IgA+). IgA+ plasma cells translocate to maternal mammary tissue via intestinal Peyer’s patches (PPs) to release microorganisms and secrete IgA. Breastfeeding newborns acquire nutrients and microorganisms in milk. Microorganisms in milk are transferred to colonize the neonatal gut. (c) Retrograde flow transfer hypothesis. This hypothesis proposes that the microbiota from the maternal skin, fetal oral cavity and environment enter the maternal mammary gland during lactation, thereby composing the milk microbial community.
2.3.2 Microbiota metabolites regulate fetal development during pregnancy
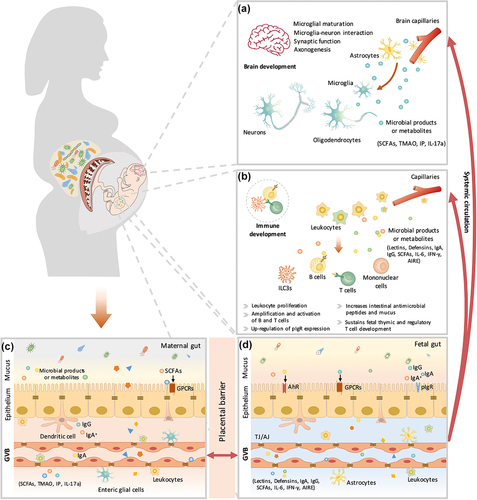
Metabolites from maternal bacteria can be absorbed into the bloodstream and then transported to the fetus through the placenta.Citation62 These metabolites regulate multiple biological functions in the fetus, such as metabolic homeostasis.Citation63 Recently, a growing amount of evidence has indicated that bacterially produced metabolites promote neural development and boost the immune system in the fetus ().
Figure 3. The maternal microbiota or microbiota metabolites regulate fetal immunity and brain development. Bacterial metabolites can be absorbed into the bloodstream and then transported to the fetus during placentation. These metabolites derived from maternal bacteria regulate multiple biological functions of the fetus. Short-chain fatty acids (SCFAs) produced by microbial fermentation of high-fiber diets may regulate microglial maturation, microglial cell-neuron interactions, and synaptic function in the hippocampus by activating fatty acid receptors (GPR41 and GPR43). During pregnancy, maternal microbes also increase axonal numbers and promote thalamocortical axon development in the fetal brain by modulating the levels of metabolites, such as trimethylamine-N-oxide (TMAO) and imidazole propionate (IP). In addition, pathogen infection during pregnancy increases proinflammatory cytokine IL-17a secretion, which is transmitted through the circulation, affects fetal brain development and induces abnormal cortical phenotypes. Maternal microbes are also involved in the regulation of fetal immune establishment during pregnancy. Maternal intestinal Escherichia coli HA107 colonization increases fetal intestinal leukocyte proliferation (that of type 3 innate lymphocytes (Ilc3s) and monocytes) as well as the production of intestinal antimicrobial peptides (C-type lectins and defensins of the REG family) and mucus. Some microbiota-derived compounds are microbial aryl hydrocarbon receptor (AhR) ligands, which promote the amplification of ILC3s. Maternal-derived SCFAs also regulate thymus and T-lymphocyte development. Maternal infection by a pathogen (Yersinia pseudotuberculosis) regulates fetal immunity through an increase in the levels of IL-6, which crosses the placental barrier and promotes intestinal Th17 cell responses and intestinal protective immunity in the fetus. Therefore, maternal microbial metabolites (AhR ligands and SCFAs) and the cytokine-mediated response (IL-6) synergistically regulate the early establishment of immunity before birth. AIRE, autoimmune regulator; AJ, adherens junction; GPCRs, G protein-coupled receptors; GVB, gut vascular barrier; IFN-γ, interferon γ; IgA, immunoglobulin A; IgA+, IgA+ plasma cells; IgG, immunoglobulin G; pIgr, polymeric immunoglobulin receptor; TJ, tight junction.
2.3.2.1 Brain development
Extremely premature birth (gestational age<28 weeks) is associated with high morbidity and incomplete neurodevelopment.Citation64 The third trimester during pregnancy is the predominant period during which the maturation of the human brain and the establishment of cognitive capacities occur.Citation65 Maternal obesity not only affects maternal microbes but also might be associated with neurodevelopmental disorders in offspring, such as deficits in cognition and sociality.Citation66 A high-fiber diet alleviates maternal obesity-induced synaptic impairments in offspring by reshaping maternal gut microbes (increasing the abundances of Bifidobacterium animalis, Prevotella, and Clostridiales).Citation66 The modification of microbes by a high-fiber diet is associated with the enrichment of SCFAs, which further regulate microglial maturation, microglial cell-neuron interactions, and synaptic function in the hippocampus.Citation66 Recently, the biological functions of SCFAs in the fetus have been well characterized. SCFAs are more than an energy source; they also serve as signaling molecules to regulate cellular functions via the activation of GPR41 and GPR43 (G-protein coupled receptors that sense SCFAs). In addition to SCFA production, the process of axonogenesis is promoted by maternal microbes through the modulation of metabolites (such as trimethylamine-N-oxide (TMAO) and imidazole propionate (IP)) during pregnancy, which could increase axon numbers and promote thalamocortical axonogenesis in the fetal brain.Citation61 Furthermore, pathogen infection during pregnancy might negatively affect fetal brain development.Citation67 Bacterial infection during pregnancy can increase the secretion of proinflammatory cytokine interleukin (IL)-17a, which can be transferred through the circulation, affect fetal brain development and induce an abnormal cortical phenotype.Citation68 In rodent models, probiotic supplementation of the maternal diet during pregnancy promotes offspring brain development (). A deep understanding of the maternal microbiota and their metabolites that reach the fetus is crucial for facilitating clinical diagnoses and medical interventions. For instance, some metabolites (e.g., IL-17a) can be used as biomarkers for timely monitoring of fetal developmental brain abnormalities. Furthermore, other metabolites (e.g., SCFAs, TMAO, IP), which are beneficial to fetal brain development, could be used as maternal supplements to reduce neurodevelopmental disorders during pregnancy. Further research is needed to determine their clinical significance. In addition, it is crucial to assess the safety of these metabolites and to identify the optimal time for clinical intervention during pregnancy.
Table 1. Effect of maternal probiotic and prebiotic supplementation during pregnancy on brain development in offspring.
2.3.2.2 Immune developmentt
The intestinal commensal microbiota is crucial for the establishment of the host’s innate and adaptive immunity. Recent evidence indicates that the maternal microbiome is involved in regulating the establishment of fetal immunity during pregnancy. Disruption of immunity might lead to allergic diseases, metabolic disorders, and infection in the fetus.Citation69 A reversible germ-free colonization system was established to decipher the potential role of maternal microbes during pregnancy.Citation70 With this system, transiently colonizing pregnant female mice with a live nonreplicating E. coli HA107 modulates the intestinal mucosal innate immune composition by increasing intestinal group 3 innate lymphoid cells and F4/80+CD11c+ mononuclear cells in their pups.Citation71 These effects are partially dependent on maternal IgG that potentially retains and transmits bacteria-derived AhR ligands to offspring during pregnancy.Citation71
Recently, more metabolites produced by maternal intestinal flora that regulate fetal innate immunity have been identified. Microbiota-derived SCFAs, which play multiple physiological and pharmacological roles, have been reported to boost Treg cell numbers.Citation72 Specifically, bacteria-derived butyrate and propionate have been shown to increase the differentiation of extrathymic Treg cells in a CNS1-dependent manner in a mouse model.Citation72 Acetate could prevent the development of preeclampsia through the promotion of fetal thymic and regulatory T-cell development.Citation73 Furthermore, increased maternal dietary fiber intake during pregnancy has been reported to reduce asthma development in offspring through SCFAs, which might partially be due to the modulation of regulatory T lymphocytes in the fetal lung.Citation74 In addition, the maternal microbiome regulates fetal immunity through the secretion of inflammatory factors. Maternal infection by a pathogen (Yersinia pseudotuberculosis) has been shown to regulate fetal immunity through an increase in the levels of interleukin (IL)-6, which crosses the placental barrier, promotes intestinal Th17 cell responses and enhances intestinal protective immunity in the fetus.Citation75 Taken together, these findings suggest that maternal microbial metabolites (AhR ligands and SCFAs) and inflammatory factors (IL-6) synergistically regulate the early establishment of innate immunity before birth. It is worth noting that these identified microbial metabolites cannot fully explain the complicated molecular mechanism involved in gestational microbial shaping. Targeted and untargeted metabolomic studies are required to further identify other crucial microbial metabolites that participate in the regulation of fetal innate immunity.
To date, whether gestational colonization of the microbiota regulates adaptive immunity is unclear, which might be due to the controversy surrounding the prenatal microbiome. Amplification and activation of B and T cells are triggered mainly by bacterial colonization.Citation76,Citation77 Although colonization only during gestation does not affect adaptive immunity (populations of B or T cells) in infants,Citation78 pregnant mothers who undergo antibiotic treatment have been shown to have lower proportions of both CD4+ and CD8+ T cells.Citation79–81 Future research is needed to verify whether microbes are transferred from mother to fetus during the prenatal period, which could help clarify recent conflicting reports. Despite the inconsistent results regarding adaptive immunity regulation, it is worth noting that some preliminary evidence has indicated that probiotic supplementation of the maternal diet during pregnancy may reduce the risk of immune-mediated disease in infants (). Thus, modulation of the maternal diet, for instance, via direct supplementation with functional metabolites could be an efficient way to program the immunity of neonates.
Table 2. Effect of maternal probiotic and prebiotic supplementation during pregnancy on immune development in offspring.
3. Maternal microbiome and neonatal health and development after birth
After birth, neonates are exposed to commensal microbes from a variety of sources. Maternal oral, gut and vaginal microbes all contribute to the initial bacterial inoculum in the newborn.Citation82 Subsequently, antibiotic use, human milk, solid food, host genetics, and environmental exposure further affect the gut microbiome during early life.Citation83–85 The complicated sources and transmission routes of pioneering microbes in infants make it difficult to systematically understand pioneer colonizers in newborns. In this section, we discuss the current understanding of the influence of the maternal microbiome derived from the maternal gut, vagina, and milk on early microbial colonization in neonates and the role of these microbes in neonatal health and development.
3.1 Maternal-source microbiome
3.1.1 Maternal gut microbiome
The human maternal gut microbiota remains stable over the perinatal period (during late pregnancy and early lactation).Citation17 To the best of our knowledge, change that occurs in the intestinal microbial community of humans during lactation is unknown. As a good model for the human microbiota, swine gut microbes harbor more Firmicutes (Lachnospiraceae, Christensenellaceae, Clostridium, Ruminococcus, and Lactobacillus) and Bacteroidetes (Prevotella and Paraprevotellaceae) during lactation than during gestation, with a corresponding augmentation in SCFA production.Citation86 Similar to the situation that is observed during the gestational period, individual heterogeneity might also be a crucial factor that regulates the composition of the intestinal microbiome during lactation. The maternal gut microbiota regulates neonatal microbes via direct mother-neonate contact during vaginal delivery. In addition, Bifidobacterium, Bacteroides, Parabacteroides and members of Clostridia are shared among neonatal feces, maternal feces and breast milk,Citation87 which indicates that the maternal gut microbiota can be transferred to the neonate through breast milk during lactation (this concept is discussed in the section on the maternal milk microbiome in detail).
3.1.2 Vaginal microbiome
The vaginal microbiota during pregnancy is less diverse and comparatively stable throughout pregnancy.Citation88,Citation89 Five bacterial community state types (CSTs) have been defined in the vagina.Citation90 In Asian and white women, CSTs are dominated by Lactobacillus species (L. crispatus (CST I), L. gasseri (CST II), L. iners (CST III) and L. jensenii (CST V)). CST IV (Lactobacillus spp.) is often observed in black and Hispanic women. This might partially explain why vaginally born infants initially exhibit an enrichment in Lactobacillus spp. in the oral cavity, skin and gut.Citation91 During gestational progression, enriched Lactobacillus species might produce lactic acid and protect the vagina from bacterial vaginosis and pelvic inflammatory disease.Citation89,Citation92 Disruption of the vaginal microbiome during pregnancy is associated with preterm birth. Lactobacillus iners is correlated with the risk of preterm birth,Citation93 while Lactobacillus crispatus dominance has a protective effect against preterm birth.Citation53 Furthermore, preterm birth has been found to be associated with proinflammatory cytokines in vaginal fluid, including eotaxin, IL-1β, IL-6 and macrophage inflammatory protein (MIP)-1β.Citation94
The mode of delivery (C-section-born infants vs. vaginally born infants) considerably affects microbiota colonization in newborns. C-section is usually conducted under antibiotic treatment to prevent infection, which further affects the transmission of maternal microbes to offspring. Thus, neonates born by C-section are associated with an aberrant and distinct gut microbiome that differs from that in vaginally born neonates.Citation95–98 Intriguingly, microbes in vaginally born infants are affected mainly by maternal vaginal and fecal microbes, whereas microbes from C-section-born infants are more likely to be determined by maternal skin microbes.Citation91,Citation99 Infants born by C-section have a decreased abundance of Bacteroides spp. and Lactobacillus. Citation100,Citation101 The shift in the microbial community caused by C-section might subsequently increase the risk of childhood sickness, such as asthma, obesity, allergies, and autoimmunity.Citation83,Citation117,Citation118
Both vaginal seeding and maternal fecal microbiota transplantation can partially restore the microbiota difference caused by C-section delivery.Citation102 However, unlike maternal intestinal strains, maternal vaginal strains seem to contribute to only a small and transient fraction of the microbes compared to the neonatal intestinal microbiota after birth. Oral administration of a maternal vaginal microbiota has a weak effect on the early development of the neonatal gut microbiome after one month.Citation103 This might be because the vaginal environment is remarkably different from the gut environment, which makes the maternal vaginal strains difficult to seed. Currently, the understanding of neonatal microbiome seeding is limited, which prevents comprehensive assessment of the acute and lasting effects of the vaginal microbiome on newborns. Further research is needed to determine whether the maternal microbiota composition is optimal. Understanding the most effective combination of microbes and their metabolites may allow for the development of targeted probiotics that could be used for infants born by C-section.
3.1.3 Milk microbiome
As the optimal nutrient source for infants, breast milk is the most important postnatal link between the mother and infant. In addition to macronutrients (protein, fat, and lactose), micronutrients (minerals and vitamins), oligosaccharides and immune factors, maternal milk contains a microbiota. Breast milk-derived microbes help neonates build a beneficial microbiome.Citation104 The origins of bacteria in breast milk have not been fully elucidated, and these bacteria might be derived from maternal skin,Citation105 the infant oral cavity,Citation106 the peripheral environment,Citation107 and the maternal gut.Citation108 Currently, the neonatal oral cavityCitation109,Citation110 and maternal gastrointestinal tractCitation108,Citation111 are considered two major sources of milk microbes. Researchers have proposed “the enteromammary transfer hypothesis” and “the retrograde flow transfer hypothesis” to interpret the origin of the human milk microbiota ().Citation112,Citation113
Both culture-based and sequence-based work suggest that streptococci and staphylococci are the most predominant taxa in human milk, but results regarding other bacterial taxa (such as lactobacilli) are inconsistent.Citation114 To date, it has been difficult to conclude that the variation in milk microbes is attributed to geographical location or contamination during sampling.Citation115–117 The milk microbiome is affected by lactation stage and influenced by maternal health status and BMI during pregnancy. In human milk, hindmilk (milk produced at the end of breastfeeding) has a higher bacterial load than foremilk (milk produced at the start of breastfeeding).Citation118 Obese mothers have lower numbers of Bifidobacterium and higher numbers of Staphylococcus in their breast milk than normal-weight mothers.Citation119 The effect of breast milk on infants lasts throughout the breastfeeding period. Approximately 33% and 23% of the bacterial taxa (especially Streptococcus, Veillonella and Bifidobacterium spp.) detected in infant feces are similar to those in maternal milk at 5 and 9 months, respectively.Citation118
3.2 Maternal microbiome regulates neonatal health and development
3.2.1 Early microbial colonization regulates neonatal health
Microorganisms in the neonatal gut regulate infant development and immune system maturation. There is a close relationship between intestinal microbial disorders and the development of pediatric diseases, such as obesity and metabolic disease.Citation120,Citation121 NEC and LOS are major causes of neonatal morbidity and mortality, and their occurrence is closely related to early microbial colonization. During early infancy, elevated levels of Enterobacteriaceae may contribute to the development of NEC.Citation122,Citation123 The intestinal microbiota in LOS infants has limited diversity, with low levels of Bacteroides and Bifidobacterium and a predominance of enterobacteria.Citation124 In addition, early microbial colonization can affect asthma.Citation125 The relative abundance of Lachnospira, Veillonella, Faecalibacterium and Rothia in the first 100 days of life has been found to be significantly reduced in children at risk of asthma.Citation125 Furthermore, maternal group B Streptococcus colonization is related to increased aortic intima-media thickness in infants, which may have an impact on cardiovascular risk.Citation126 Currently, it remains unclear how maternal microbes modulate pediatric disease through early microbial colonization. However, there is growing evidence indicating that the maternal microbiome plays an important role in the regulation of neonatal immunity and neurodevelopment.
3.2.2 Maternal microbiome regulates neonatal development
3.2.2.1 Immune development
Beneficial microbes inherited from breast milk contribute to the establishment of the immune system in neonates, especially during the first 3 months of life ().Citation127 Lactobacillus reuteri enrichment in milk promotes the production of IgA through the induction of T cells and type 3 innate lymphocytes (ILC3s) in neonates.Citation127 Milk-derived Bifidobacterium produces indolelactic acid via aromatic lactate dehydrogenase in infants.Citation128 Importantly, indolelactic acid can further induce the immune responses of CD4+ T cells and monocytes through the activation of AhR and hydroxycarboxylic acid receptor 3.Citation128 Human breast milk contains a large amount of indigestible oligosaccharides (human milk oligosaccharides, HMOs), which can be used by commensal organisms as well as probiotics.Citation129–131 Bifidobacterium expressing HMO utilization genes can alleviate inflammatory responses by decreasing proinflammatory Th2 cells and promoting the expansion of anti-inflammatory regulatory T cells.Citation130 Thus, maternal milk-derived HMOs and beneficial microbes can coordinately boost the immunity of neonates.
Figure 4. The maternal microbiota regulates neonatal immunity and brain development. Beneficial microbes inherited from breast milk contribute to the establishment of the immune system in neonates. Beneficial microbes from breast milk promote the production of immunoglobulins (IgA and IgG) and regulate intestinal homeostasis and immune responses (CD4+ T cells and monocytes) through the aryl hydrocarbon receptor (AhR). IgA+ plasma cells are recruited from maternal intestinal Peyer’s patches (PPs) and then translocate through the circulatory system to the mammary gland and secrete IgA. Furthermore, maternal intestinal Ig-coated bacteria can directly translocate to the mammary gland and then be enriched in maternal milk. Immunoglobulins in milk promote the differentiation of neonatal intestinal epithelial cells and the establishment of intestinal barrier function, reducing intestinal inflammation. During this process, immunoglobulins cooperate with gut microbes to regulate innate and adaptive immunity in neonates. In addition, maternal prebiotic intake increases the concentrations of offspring intestinal SCFAs, which may cross the blood – brain barrier and modulate brain function by inhibiting histone deacetylases (HDACs).
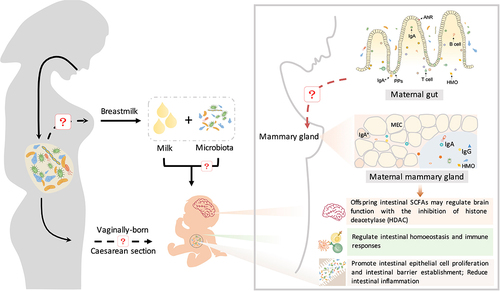
The maternal microbiome also regulates neonatal immunity through the secretion of milk immunoglobulin (Ig). Maternal milk, especially colostrum, is enriched in IgA. In contrast to other organic components in milk, Ig is not synthesized by mammary epithelial cells. In the mammary gland, both pIgR expression and IgA plasma cell accumulation are positively correlated with the production of milk IgA.Citation132 Evidence suggests that milk IgA is either derived from serum or secreted from IgA+ plasma cells that translocate to the mammary gland.Citation133 From pregnancy to the first few days of lactation, intestinal IgA+ plasma cells are significantly enriched, accompanied by a similar increase in the number of IgA+ plasma cells in the mammary gland.Citation134 It is now clear that mammary gland IgA+ plasma cells are recruited mainly from intestinal Peyer’s patches (PPs) but not supramammary lymph nodes (LNs).Citation135 Therefore, maternal gut microbes are involved in the regulation of milk IgA production.Citation135 Maternal milk IgA production in germ-free (GF) mice is markedly lower than that in wild-type mice.Citation135 Bacteroides acidifaciens and Prevotella buccalis cohabiting in the large intestine induce the synthesis of bacterium-specific IgA in milk.Citation135
IgA binds to a variety of microbes, including four major phyla, namely, Firmicutes, Actinobacteria, Bacteroidetes, and Proteobacteria.Citation136 Recently, accumulating evidence has revealed the potential role of IgA in the neonatal gut, which contributes to the establishment of the intestinal barrier and regulates the innate and adaptive immunity of neonates by binding to intestinal bacteria. First, IgA coats harmful bacteria and prevents their colonization and propagation. The enrichment of Proteobacteria, especially Enterobacteriaceae, is a crucial factor that triggers the development of NEC, an inflammatory gastrointestinal disease, in preterm infants.Citation8 Milk IgA binds to Enterobacteriaceae and prevents the development of NEC in human infants.Citation137 Second, IgA facilitates the adherence of beneficial bacteria (such as Bacteroides fragilis) to intestinal epithelial cells and promotes their proliferation.Citation138 Third, IgA coating intestinal bacteria alleviates intestinal inflammation in neonates.Citation139 The IgA coating of the microbiota decreases the proportions of ROR-γt+ Tregs and alleviates mucosal immune induction and inflammatory disease susceptibility.Citation140 Furthermore, maternal intestinal Ig-coated bacteria can directly translocate to the mammary gland and then be enriched in maternal milk.Citation136 IgA-coated Lachnospiraceae bacteria in milk can directly regulate the adaptive immune responses in the intestine.Citation141,Citation142 Due to technical issues, such as those with anti-Ig antibodies (monoclonal or polyclonal) and sequencing methods, it is difficult to identify the dominant Ig-coated community in maternal milk.Citation143–145 In addition to IgA, IgG binds with bacteria and regulates gut immune function. Enteropathogenic E. coli (EPEC) is a foodborne pathogen that causes diarrhea in neonates.Citation146 EPEC induces Ig production through “attaching and effacing” (A/E) lesions located on the intestinal epithelium.Citation147 Interestingly, maternal pathogen-induced production of IgG but not IgA or IgM protects neonates against EPEC infection. Mechanistically, neutrophil phagocytosis is increased when pathogens are specifically coated with IgG.Citation148 Although the origins of milk Igs and their capacity to prevent bacterial pathogenicity are well established, their role in promoting beneficial microbes and regulating immunity needs to be clarified. In addition, future research is required to identify other potential bacteria that can bind with Igs and their role in the regulation of neonatal gut immune function.
Allergic diseases affect the respiratory system, digestive system, skin and other systems. There are potential associations between immune dysfunction (such as an imbalance toward a T-helper-2 response and exaggerated IgE responses) and allergic diseases in infants.Citation149 Atopic eczema is a strong predictor of allergic disease and occurs during childhood.Citation150 During early infancy, a reduction in the abundance of bacteria with the ability to produce butyrate and propionate was observed in infants with atopic eczema.Citation151 The World Allergy Organization (WAO) guideline panel suggests the use of probiotics in pregnant and breasting women with children at high risk for allergies.Citation152 This recommendation could efficiently prevent the development of atopic eczema during childhood. Additionally, initial microbial colonization is associated with other immune-mediated diseases, such as inflammatory bowel disease (IBD)Citation153 and asthma.Citation154 More research is needed to clarify whether and how maternal microbes modulate clinical disease.
3.2.2.2 Brain development
Recent evidence also indicates that the maternal microbiome regulates neonatal brain development (). Maternal prebiotic intake reduces anxiety and promotes brain development. The intestinal microbiome composition in the offspring of mice fed prebiotics differs from that in control offspring, with significant decreases in the abundances of Bacteroides caecimuris, the Barnesiella genus, and the Bifidobacterium genus.Citation155 Importantly, postnatal maternal prebiotic intake significantly increases the concentrations of intestinal SCFAs in offspring, which might cross the blood – brain barrier and regulate brain function via the inhibition of histone deacetylase (HDAC).Citation155 In contrast, maternal infection with E. coli O16:H48 has a negative effect on the growth of neonates in a mouse model.Citation156 E. coli O16:H48 can disrupt the production of precursors of neurotransmitters (such as serotonin), which negatively affects maternal behavior.Citation156 Poor maternal behavior blocks milk availability for neonates and subsequently affects the brain development of neonates.Citation156
During pregnancy, the developing fetus is entirely dependent on maternally derived nutrients. However, during lactation, when maternal breast milk is insufficient, an alternative form of enteral nutrition for preterm or low birth weight (LBW) infants is artificial formula. Currently, artificial formula contains more nutrients than breast milk but might be deficient in some nonnutritive bioactive components. Fortification of artificial formulas with microbiota and functional metabolites such as milk-derived Bifidobacterium and Lactobacillus reuteri as well as indolelactic acid could be beneficial for preterm or LBW infants and might have great potential for alleviating clinical diseases such as IBD and asthma.
4. Conclusion and perspectives
A healthy maternal microbiota is required for the health and development of offspring. During the progression of gestation and lactation, the maternal gut microbe composition is comparatively stable. Individual heterogeneity is the main factor that shapes the maternal intestinal microbiome. These microbes do not act as silent passengers but have long-term effects that reduce the risk of immune-mediated illness and improve the cognitive outcomes of offspring. During pregnancy, maternal microbial metabolites can be transported from the placenta to the fetus to regulate immunity and recognition. Whether a small number of bacteria exist in the fetus before birth is uncertain, and further research is needed.
Microbial colonization of neonates begins at birth. The transfer of microbes from mother to newborn is influenced by many factors, such as birth route and nursing. Vaginal birth, breastfeeding, and skin-to-skin care are considered predominant factors in establishing the close relationship between the mother and infant microbiome. Maternal microbes that originate from the maternal gut but not the vagina contribute mainly to the early colonization of the microbiome. Maternal milk is rich in microbes, oligosaccharides and Igs, which boost immune and brain development. The disruption of maternal-to-child microbiome transfer might lead to short- and/or long-term adverse health issues.
Recently, great interest in modulating the maternal and neonatal microbiomes through therapeutic manipulation has emerged. Maternal probiotic and prebiotic supplementation during pregnancy have been found to influence the regulation of offspring brain and immune development (as shown in ). The analysis and modulation of specific bacterial metabolites, such as IL-17a, AhR ligands and SCFAs, may provide a noninvasive monitoring and intervention strategy to improve adverse prognoses and alleviate clinical symptoms. Further studies are needed to identify other crucial microbial metabolites that participate in regulating fetal immune and brain development using both targeted and untargeted metabolomics.
Outstanding questions
Numerous outstanding questions that deserve to be addressed in the future are listed below.
Is the womb sterile or not?
Are there key molecular, genetic or microbiome-based biomarkers available to test the “gut-placental transmission hypothesis”, “entero-mammary transfer hypothesis” and “retrograde flow transfer hypothesis”?
Which maternal-derived microbes participate in early colonization of the offspring’s gut and what is the potential role of maternal-derived microbes in later microbiota development?
In the milk, are there any functional metabolites synthesized locally in the mammary gland?
Could diet-derived maternal microbial metabolites in milk be modulated by dietary intervention or by direct supplementation with targeted metabolites?
Box 1. Is the womb sterile?
To verify this hypothesis, pregnant mice were inoculated with genetically labeled Enterococcus faecium.Citation157 Notably, genetically labeled Enterococcus faecium was detected in the meconium of fetuses delivered via sterile C-section, which provides primary evidence for maternal microbial transmission to the fetus.Citation157 However, recently, inconsistent results regarding in utero bacterial colonization among different studies indicate that the detection of a prenatal microbiome might just be due to contamination of reagents, sample handling, etc.Citation158 Furthermore, even though many studies have reported that bacterial DNA can be detected in first-pass meconium samples, most of the samples are collected hours to days after birth.Citation159 Meconium collected during cesarean deliveries indicates that healthy infants do not harbor a detectable gut microbiota.Citation159 Thus, the microbiotas previously identified in neonatal meconium might be acquired during and/or after birth. In addition, bacterial DNA does not indicate the existence of live microbes.
Recent evidence supports the ‘sterile womb’ hypothesis, as the existence of C-section-born germ-free animals strongly argues against the existence of microbes in the placenta. However, an intense debate about the existence of microbes in the fetus is ongoing. Recently, although strict contamination controls (environmental control, operator control, PBS buffer control, and reagent control) were carefully applied in an experiment,Citation160 weak but consistent microbial signals were still detected by 16S rRNA sequencing in fetal organs (including the gut, skin, placenta, and lungs) during the 2nd trimester of gestation. More importantly, these microbes were viable and could be cultured and propagated in vitro. These sparsely distributed microbes, such as Staphylococcus and Lactobacillus, induce the activation of memory T cells, which might contribute to the establishment of early immunity.Citation160 Currently, early fetal microbial colonization is thought to be affected by microbes from the maternal vagina or gut. The contradictions in the results regarding the sterile womb necessitate the collection and assessment of additional evidence in the future.
Authors’ contributions
MT and SHZ initiated the idea, scope, and outline of this review paper. MT, SHZ, QHL, THZ, SWY and FC studied and analyzed all of the publications cited in this paper and were involved in manuscript preparation. SHZ and WTG conducted the final editing and proofreading. All authors have read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- D’Argenio V, Salvatore F. The role of the gut microbiome in the healthy adult status. Clin Chim Acta. 2015;451(Pt A):97–24. doi:10.1016/j.cca.2015.01.003.
- Sartor RB. Therapeutic correction of bacterial dysbiosis discovered by molecular techniques. Proc Natl Acad Sci U S A. 2008;105(43):16413–16414. doi:10.1073/pnas.0809363105.
- Kerr CA, Grice DM, Tran CD, Bauer DC, Li D, Hendry P, Hannan GN. Early life events influence whole-of-life metabolic health via gut microflora and gut permeability. Crit Rev Microbiol. 2015;41(3):326–340. doi:10.3109/1040841X.2013.837863.
- Sanidad KZ, Zeng MY. Neonatal gut microbiome and immunity. Curr Opin Microbiol. 2020;56:30–37. doi:10.1016/j.mib.2020.05.011.
- Meckel KR, Kiraly DD. Maternal microbes support fetal brain wiring. Nature. 2020;586(7828):203–205. doi:10.1038/d41586-020-02657-y.
- Koboziev I, Webb CR, Furr KL, Grisham MB. Role of the enteric microbiota in intestinal homeostasis and inflammation. Free Radical Biol Med. 2014;68:122–133. doi:10.1016/j.freeradbiomed.2013.11.008.
- Warner BB, Deych E, Zhou Y, Hall-Moore C, Weinstock GM, Sodergren E, Shaikh N, Hoffmann JA, Linneman LA, Hamvas A, et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet. 2016;387(10031):1928–1936. doi:10.1016/s0140-6736(16)00081-7.
- Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, Gregory KE, Kroll JS, McMurtry V, Ferris MJ, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome. 2017;5(1):31. doi:10.1186/s40168-017-0248-8.
- Stewart CJ, Embleton ND, Marrs ECL, Smith DP, Fofanova T, Nelson A, Skeath T, Perry JD, Petrosino JF, Berrington JE, et al. Longitudinal development of the gut microbiome and metabolome in preterm neonates with late onset sepsis and healthy controls. Microbiome. 2017;5(1):75. doi:10.1186/s40168-017-0295-1.
- Mischke M, Arora T, Tims S, Engels E, Sommer N, van Limpt K, Baars A, Oozeer R, Oosting A, Bäckhed F, et al. Specific synbiotics in early life protect against diet‐induced obesity in adult mice. Diabetes Obes Metab. 2018;20(6):1408–1418. doi:10.1111/dom.13240.
- Livanos AE, Greiner TU, Vangay P, Pathmasiri W, Stewart D, McRitchie S, Li H, Chung J, Sohn J, Kim S, et al. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat Microbiol. 2016;1(11):16140. doi:10.1038/nmicrobiol.2016.140.
- Stokholm J, Blaser MJ, Thorsen J, Rasmussen MA, Waage J, Vinding RK, Schoos AMM, Kunøe A, Fink NR, Chawes BL, et al. Maturation of the gut microbiome and risk of asthma in childhood. Nat Commun. 2018;9(1):141. doi:10.1038/s41467-017-02573-2.
- Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO, Ruan Y. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5(7):e177. doi:10.1371/journal.pbio.0050177.
- Lou YC, Olm MR, Diamond S, Crits-Christoph A, Firek BA, Baker R, Morowitz MJ, Banfield JF. Infant gut strain persistence is associated with maternal origin, phylogeny, and traits including surface adhesion and iron acquisition. Cell Rep Med. 2021;2(9):100393. doi:10.1016/j.xcrm.2021.100393.
- Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Bäckhed HK, Gonzalez A, Werner JJ, Angenent LT, Knight R, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150(3):470–480. doi:10.1016/j.cell.2012.07.008.
- Yang HL, Guo RC, Li SC, Liang F, Tian C, Zhao XQ, Long Y, Liu F, Jiang M, Zhang Y, et al. Systematic analysis of gut microbiota in pregnant women and its correlations with individual heterogeneity. NPJ Biofilms Microbiomes. 2020;6(1):1–12. doi:10.1038/s41522-020-00142-y.
- Jost T, Lacroix C, Braegger C, Chassard C. Stability of the maternal gut microbiota during late pregnancy and early lactation. Curr Microbiol. 2014;68(4):419–427. doi:10.1007/s00284-013-0491-6.
- Collado MC, Isolauri E, Laitinen K, Salminen S. Effect of mother’s weight on infant’s microbiota acquisition, composition, and activity during early infancy: a prospective follow-up study initiated in early pregnancy. Am J Clin Nutr. 2010;92(5):1023–1030. doi:10.3945/ajcn.2010.29877.
- Collado MC, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am J Clin Nutr. 2008;88(4):894–899. doi:10.1093/ajcn/88.4.894.
- Santacruz A, Collado MC, Garcia-Valdes L, Segura MT, Martin-Lagos JA, Anjos T, Marti-Romero M, Lopez RM, Florido J, Campoy C, et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. 2010;104(1):83–92. doi:10.1017/S0007114510000176.
- Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Nitert MD. Connections between the gut microbiome and metabolic hormones in early pregnancy in overweight and obese women. Diabetes. 2016;65(8):2214–2223. doi:10.2337/db16-0278.
- Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070–11075. doi:10.1073/pnas.0504978102.
- Grech A, Collins CE, Holmes A, Lal R, Duncanson K, Taylor R, Gordon A. Maternal exposures and the infant gut microbiome: a systematic review with meta-analysis. Gut Microbes. 2021;13(1):1–30. doi:10.1080/19490976.2021.1897210.
- Mann PE, Huynh K, Widmer G. Maternal high fat diet and its consequence on the gut microbiome: a rat model. Gut Microbes. 2018;9(2):143–154. doi:10.1080/19490976.2017.1395122.
- Röytiö H, Mokkala K, Vahlberg T, Laitinen K. Dietary intake of fat and fibre according to reference values relates to higher gut microbiota richness in overweight pregnant women. Br J Nutr. 2017;118(5):343–352. doi:10.1017/S0007114517002100.
- Barrett HL, Gomez-Arango LF, Wilkinson SA, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M. A vegetarian diet is a major determinant of gut microbiota composition in early pregnancy. Nutrients. 2018;10(7):890. doi:10.3390/nu10070890.
- Gomez-Arango LF, Barrett HL, Wilkinson SA, Callaway LK, McIntyre HD, Morrison M, Dekker Nitert M. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes. 2018;9(3):189–201. doi:10.1080/19490976.2017.1406584.
- García-Mantrana I, Selma-Royo M, González S, Parra-Llorca A, Martínez-Costa C, Collado MC. Distinct maternal microbiota clusters are associated with diet during pregnancy: impact on neonatal microbiota and infant growth during the first 18 months of life. Gut Microbes. 2020;11(4):962–978. doi:10.1080/19490976.2020.1730294.
- Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. 2016;7:979. doi:10.3389/fmicb.2016.00979.
- Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19(1):29–41. doi:10.1111/1462-2920.13589.
- Mandal S, Godfrey KM, McDonald D, Treuren WV, Bjørnholt JV, Midtvedt T, Moen B, Rudi K, Knight R, Brantsæter AL, et al. Fat and vitamin intakes during pregnancy have stronger relations with a pro-inflammatory maternal microbiota than does carbohydrate intake. Microbiome. 2016;4(1):55. doi:10.1186/s40168-016-0200-3.
- Kaakoush NO. Sutterella species, IgA-degrading bacteria in ulcerative colitis. Trends Microbiol. 2020;28(7):519–522. doi:10.1016/j.tim.2020.02.018.
- Williams BL, Hornig M, Parekh T, Lipkin WI, Biron C. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. mBio. 2012;3(1):e00261–11. doi:10.1128/mBio.00261-11.
- Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol Autism. 2013;4(1):42. doi:10.1186/2040-2392-4-42.
- Ami N, Bernstein M, Boucher F, Rieder M, Parker L. Folate and neural tube defects: the role of supplements and food fortification. Paediatr Child Health. 2016;21(3):145–154. doi:10.1093/pch/21.3.145.
- King JC. A Summary of pathways or mechanisms linking preconception maternal nutrition with birth outcomes. J Nutr. 2016;146(7):1437s–1444s. doi:10.3945/jn.115.223479.
- Chia AR, Chen LW, Lai JS, Wong CH, Neelakantan N, Van Dam RM, Chong MF. Maternal dietary patterns and birth outcomes: a systematic review and meta-analysis. Adv Nutr. 2019;10(4):685–695. doi:10.1093/advances/nmy123.
- Chu DM, Antony KM, Ma J, Prince AL, Showalter L, Moller M, Aagaard KM. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med. 2016;8(1):77. doi:10.1186/s13073-016-0330-z.
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi:10.1038/nature05414.
- Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106(7):2365–2370. doi:10.1073/pnas.0812600106.
- Vuillermin PJ, O’Hely M, Collier F, Allen KJ, Tang MLK, Harrison LC, Carlin JB, Saffery R, Ranganathan S, Sly PD, et al. Maternal carriage of Prevotella during pregnancy associates with protection against food allergy in the offspring. Nat Commun. 2020;11(1):1452. doi:10.1038/s41467-020-14552-1.
- De Tejada BM. Antibiotic use and misuse during pregnancy and delivery: benefits and risks. Int J Env Res Public Health. 2014;11(8):7993–8009. doi:10.3390/ijerph110807993.
- Stokholm J, Schjørring S, Pedersen L, Bischoff AL, Følsgaard N, Carson CG, Chawes BL, Bønnelykke K, Mølgaard A, Krogfelt KA, et al. Prevalence and predictors of antibiotic administration during pregnancy and birth. PLoS One. 2013;8(12):e82932. doi:10.1371/journal.pone.0082932.
- Broe A, Pottegård A, Lamont RF, Jørgensen JS, Damkier P. Increasing use of antibiotics in pregnancy during the period 2000–2010: prevalence, timing, category, and demographics. BJOG. 2014;121(8):988–996. doi:10.1111/1471-0528.12806.
- Petersen I, Gilbert R, Evans S, Ridolfi A, Nazareth I. Oral antibiotic prescribing during pregnancy in primary care: uK population-based study. J Antimicrob Chemother. 2010;65(10):2238–2246. doi:10.1093/jac/dkq307.
- Palmsten K, Hernández-Díaz S, Chambers CD, Mogun H, Lai S, Gilmer TP, Huybrechts KF. The most commonly dispensed prescription medications among pregnant women enrolled in the United States Medicaid program. Obstet Gynecol. 2015;126(3):465–473. doi:10.1097/AOG.0000000000000982.
- Nogacka A, Salazar N, Suárez M, Milani C, Arboleya S, Solís G, Fernández N, Alaez L, Hernández-Barranco AM, de Los Reyes-Gavilán CG, et al. Impact of intrapartum antimicrobial prophylaxis upon the intestinal microbiota and the prevalence of antibiotic resistance genes in vaginally delivered full-term neonates. Microbiome. 2017;5(1):93. doi:10.1186/s40168-017-0313-3.
- Aloisio I, Quagliariello A, De Fanti S, Luiselli D, De Filippo C, Albanese D, Corvaglia LT, Faldella G, Di Gioia D. Evaluation of the effects of intrapartum antibiotic prophylaxis on newborn intestinal microbiota using a sequencing approach targeted to multi hypervariable 16S rDNA regions. Appl Microbiol Biotechnol. 2016;100(12):5537–5546. doi:10.1007/s00253-016-7410-2.
- Tissier H. Recherches sur la flore intestinale des nourrissons:(état normal et pathologique). Paris: Master’s Thesis of G.Carre and C.Naud; 1900.
- Goldenberg RL, Hauth JC, Andrews WW, Epstein FH. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–1507. doi:10.1056/NEJM200005183422007.
- Gonçalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8(1):3–13. doi:10.1002/mrdd.10008.
- Thomas J, Govender N, McCarthy KM, Erasmus LK, Doyle TJ, Allam M, Ismail A, Ramalwa N, Sekwadi P, Ntshoe G, et al. Outbreak of listeriosis in South Africa associated with processed meat. N Engl J Med. 2020;382(7):632–643. doi:10.1056/NEJMoa1907462.
- Elinav H, Hershko-Klement A, Valinsky L, Jaffe J, Wiseman A, Shimon H, Braun E, Paitan Y, Block C, Sorek R, et al. Pregnancy-associated listeriosis: clinical characteristics and geospatial analysis of a 10-year period in Israel. Clin Infect Dis. 2014;59(7):953–961. doi:10.1093/cid/ciu504.
- De Santis M, De Luca C, Mappa I, Spagnuolo T, Licameli A, Straface G, Scambia G. Syphilis Infection during pregnancy: fetal risks and clinical management. Infect Dis Obstet Gynecol. 2012;2012:430585. doi:10.1155/2012/430585.
- Rac MW, Bryant SN, McIntire DD, Cantey JB, Twickler DM, Wendel GD Jr., Sheffield JS. Progression of ultrasound findings of fetal syphilis after maternal treatment. Am J Obstet Gynecol. 2014;211(4):426.e1–6. doi:10.1016/j.ajog.2014.05.049.
- Bearfield C, Davenport ES, Sivapathasundaram V, Allaker RP. Possible association between amniotic fluid micro‐organism infection and microflora in the mouth. BJOG Inter J Obstet Gynaecol. 2002;109(5):527–533. doi:10.1111/j.1471-0528.2002.01349.x.
- Rautava S, Collado MC, Salminen S, Isolauri E. Probiotics modulate host-microbe interaction in the placenta and fetal gut: a randomized, double-blind, placebo-controlled trial. Neonatology. 2012;102(3):178–184. doi:10.1159/000339182.
- Jiménez E, Fernández L, Marín ML, Martín R, Odriozola JM, Nueno-Palop C, Narbad A, Olivares M, Xaus J, Rodríguez JM. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr Microbiol. 2005;51(4):270–274. doi:10.1007/s00284-005-0020-3.
- Steel JH, Malatos S, Kennea N, Edwards AD, Miles L, Duggan P, Reynolds PR, Feldman RG, Sullivan MH. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr Res. 2005;57(3):404–411. doi:10.1203/01.PDR.0000153869.96337.90.
- Calatayud M, Koren O, Collado MC. Maternal microbiome and metabolic health program microbiome development and health of the offspring. Trends Endocrinol Metab. 2019;30(10):735–744. doi:10.1016/j.tem.2019.07.021.
- Vuong HE, Pronovost GN, Williams DW, Coley EJL, Siegler EL, Qiu A, Kazantsev M, Wilson CJ, Rendon T, Hsiao EY. The maternal microbiome modulates fetal neurodevelopment in mice. Nature. 2020;586(7828):281–286. doi:10.1038/s41586-020-2745-3.
- Kimura I, Miyamoto J, Ohue-Kitano R, Watanabe K, Yamada T, Onuki M, Aoki R, Isobe Y, Kashihara D, Inoue D, et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science. 2020;367(6481):eaaw8429. doi:10.1126/science.aaw8429.
- Laforest-Lapointe I, Becker AB, Mandhane PJ, Turvey SE, Moraes TJ, Sears MR, Subbarao P, Sycuro LK, Azad MB, Arrieta M-C. Maternal consumption of artificially sweetened beverages during pregnancy is associated with infant gut microbiota and metabolic modifications and increased infant body mass index. Gut Microbes. 2021;13(1):1–15. doi:10.1080/19490976.2020.1857513.
- Adams-Chapman I, Heyne RJ, DeMauro SB, Duncan AF, Hintz SR, Pappas A, Vohr BR, McDonald SA, Das A, Newman JE, et al. Neurodevelopmental impairment among extremely preterm infants in the neonatal research network. Pediatrics. 2018;141(5):e20173091. doi:10.1542/peds.2017-3091.
- Matthews LG, Walsh BH, Knutsen C, Neil JJ, Smyser CD, Rogers CE, Inder TE. Brain growth in the NICU: critical periods of tissue-specific expansion. Pediatr Res. 2018;83(5):976–981. doi:10.1038/pr.2018.4.
- Liu XN, Li X, Xia B, Jin X, Zou QH, Zeng ZH, Zhao WY, Yan SK, Li L, Yuan S, et al. High-fiber diet mitigates maternal obesity-induced cognitive and social dysfunction in the offspring via gut-brain axis. Cell Metab. 2021;33(5):923–938. e6. doi:10.1016/j.cmet.2021.02.002.
- Kalish BT, Kim E, Finander B, Duffy EE, Kim H, Gilman CK, Yim YS, Tong L, Kaufman RJ, Griffith EC, et al. Maternal immune activation in mice disrupts proteostasis in the fetal brain. Nat Neurosci. 2021;24(2):204–213. doi:10.1038/s41593-020-00762-9.
- Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351(6276):933–939. doi:10.1126/science.aad0314.
- Qu WH, Liu LS, Miao LY. Exposure to antibiotics during pregnancy alters offspring outcomes. Expert Opin Drug Metab Toxicol. 2021;17(10):1165–1174. doi:10.1080/17425255.2021.1974000.
- Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328(5986):1705–1709. doi:10.1126/science.1188454.
- Gomez de Agüero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, Steinert A, Heikenwalder M, Hapfelmeier S, Sauer U, et al. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351(6279):1296–1302. doi:10.1126/science.aad2571.
- Arpaia N, Campbell C, Fan X, Dikiy S, Van Der Veeken J, Deroos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. doi:10.1038/nature12726.
- Hu M, Eviston D, Hsu P, Mariño E, Chidgey A, Santner-Nanan B, Wong K, Richards JL, Yap YA, Collier F, et al. Decreased maternal serum acetate and impaired fetal thymic and regulatory T cell development in preeclampsia. Nat Commun. 2019;10(1):3031. doi:10.1038/s41467-019-10703-1.
- Gray LEK, O’Hely M, Ranganathan S, Sly PD, Vuillermin P. The maternal diet, gut bacteria, and bacterial metabolites during pregnancy influence offspring asthma. Front Immunol. 2017;8:365. doi:10.3389/fimmu.2017.00365.
- Lim AI, McFadden T, Link VM, Han S-J, Karlsson R-M, Stacy A, Farley TK, Lima-Junior DS, Harrison OJ, Desai JV, et al. Prenatal maternal infection promotes tissue-specific immunity and inflammation in offspring. Science. 2021;373(6558):eabf3002. doi:10.1126/science.abf3002.
- Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34(5):794–806. doi:10.1016/j.immuni.2011.03.021.
- Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol. 2007;19(2):59–69. doi:10.1016/j.smim.2006.10.002.
- Nyangahu DD, Lennard KS, Brown BP, Darby MG, Wendoh JM, Havyarimana E, Smith P, Butcher J, Stintzi A, Mulder N et al. Disruption of maternal gut microbiota during gestation alters offspring microbiota and immunity. Microbiome. 2018;6(1):124. doi:10.1186/s40168-018-0511-7.
- Hu YJ, Peng J, Tai NW, Hu CY, Zhang XJ, Wong FS, Wen L. Maternal antibiotic treatment protects offspring from diabetes development in nonobese diabeticmice by generation of tolerogenic APCs. J Immunol. 2015;195(9):4176-4184. doi:10.4049/jimmunol.1500884.
- Gonzalez-Perez G, Hicks AL, Tekieli TM, Radens CM, Williams BL, Lamousé-Smith ES. Maternal antibiotic treatment impacts development of the neonatal intestinal microbiome and antiviral immunity. J Immunol. 2016;196(9):3768–3779. doi:10.4049/jimmunol.1502322.
- Gonzalez-Perez G, Lamousé-Smith ES. Gastrointestinal microbiome dysbiosis in infant mice alters peripheral CD8+ T cell receptor signaling. Front Immunol. 2017;8:265. doi:10.3389/fimmu.2017.00265.
- Ferretti P, Pasolli E, Tett A, Asnicar F, Gorfer V, Fedi S, Armanini F, Truong DT, Manara S, Zolfo M, et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host & Microbe. 2018;24(1):133–145. e5. doi:10.1016/j.chom.2018.06.005.
- Hamilton MK, Ronveaux CC, Rust BM, Newman JW, Hawley M, Barile D, Mills DA, Raybould HE. Prebiotic milk oligosaccharides prevent development of obese phenotype, impairment of gut permeability, and microbial dysbiosis in high fat-fed mice. Am J Physiol Gastrointest Liver Physiol. 2017;312(5):G474–487. doi:10.1152/ajpgi.00427.2016.
- Gregory KE, Samuel BS, Houghteling P, Shan G, Ausubel FM, Sadreyev RI, Walker WA. Influence of maternal breast milk ingestion on acquisition of the intestinal microbiome in preterm infants. Microbiome. 2016;4(1):68. doi:10.1186/s40168-016-0214-x.
- Dong TS, Gupta A. Influence of early life, diet, and the environment on the microbiome. Clin Gastroenterol Hepatol. 2019;17(2):231–242. doi:10.1016/j.cgh.2018.08.067.
- Liu HB, Hou CL, Li N, Zhang XY, Zhang GL, Yang FY, Zeng XF, Liu ZH, Qiao SY. Microbial and metabolic alterations in gut microbiota of sows during pregnancy and lactation. FASEB J. 2019;33(3):4490–4501. doi:10.1096/fj.201801221RR.
- Jost T, Lacroix C, Braegger CP, Rochat F, Chassard C. Vertical mother–neonate transfer of maternal gut bacteria via breastfeeding. Environ Microbiol. 2014;16(9):2891–2904. doi:10.1111/1462-2920.12238.
- DiGiulio DB, Callahan BJ, McMurdie PJ, Costello EK, Lyell DJ, Robaczewska A, Sun CL, Goltsman DS, Wong RJ, Shaw G, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc Natl Acad Sci U S A. 2015;112(35):11060–11065. doi:10.1073/pnas.1502875112.
- Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, Galuppi M, Lamont RF, Chaemsaithong P, Miranda J, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014;2(1):1–19. doi:10.1186/2049-2618-2-10.
- Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4680–4687. doi:10.1073/pnas.1002611107.
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971–11975. doi:10.1073/pnas.1002601107.
- Walther-António MR, Jeraldo P, Berg Miller ME, Yeoman CJ, Nelson KE, Wilson BA, White BA, Chia N, Creedon DJ, Gilbert JA. Pregnancy’s Stronghold on the Vaginal Microbiome. PLoS One. 2014;9(6):e98514. doi:10.1371/journal.pone.0098514.
- Kindinger LM, Bennett PR, Lee YS, Marchesi JR, Smith A, Cacciatore S, Holmes E, Nicholson JK, Teoh TG, MacIntyre DA. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome. 2017;5(1):6. doi:10.1186/s40168-016-0223-9.
- Fettweis JM, Serrano MG, Brooks JP, Edwards DJ, Girerd PH, Parikh HI, Huang B, Arodz TJ, Edupuganti L, Glascock AL, et al. The vaginal microbiome and preterm birth. Nat Med. 2019;25(6):1012–1021. doi:10.1038/s41591-019-0450-2.
- Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host & Microbe. 2015;17(5):690–703. doi:10.1016/j.chom.2015.04.004.
- Shao Y, Forster SC, Tsaliki E, Vervier K, Strang A, Simpson N, Kumar N, Stares MD, Rodger A, Brocklehurst P, et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature. 2019;574(7776):117–121. doi:10.1038/s41586-019-1560-1.
- Wampach L, Heintz-Buschart A, Fritz JV, Ramiro-Garcia J, Habier J, Herold M, Narayanasamy S, Kaysen A, Hogan AH, Bindl L, et al. Birth mode is associated with earliest strain-conferred gut microbiome functions and immunostimulatory potential. Nat Commun. 2018;9(1):5091. doi:10.1038/s41467-018-07631-x.
- Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, Aagaard KM. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med. 2017;23(3):314–326. doi:10.1038/nm.4272.
- Tun HM, Bridgman SL, Chari R, Field CJ, Guttman DS, Becker AB, Mandhane PJ, Turvey SE, Subbarao P, Sears MR, et al. Roles of birth mode and infant gut microbiota in intergenerational transmission of overweight and obesity from mother to offspring. JAMA Pediatr. 2018;172(4):368–377. doi:10.1001/jamapediatrics.2017.5535.
- Korpela K, Helve O, Kolho K-L, Saisto T, Skogberg K, Dikareva E, Stefanovic V, Salonen A, Andersson S, de Vos WM. Maternal fecal microbiota transplantation in cesarean-born infants rapidly restores normal gut microbial development: a proof-of-concept study. Cell. 2020;183(2):324–334.e5. doi:10.1016/j.cell.2020.08.047.
- Stencel‐gabriel K, Gabriel I, Wiczkowski A, Paul M, Olejek A. Prenatal priming of cord blood T lymphocytes by microbiota in the maternal vagina. Am J Reprod Immunol. 2009;61(3):246–252. doi:10.1111/j.1600-0897.2009.00687.x.
- Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A, Bokulich NA, Song SJ, Hoashi M, Rivera-Vinas JI, et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med. 2016;22(3):250–253. doi:10.1038/nm.4039.
- Wilson BC, Butler ÉM, Grigg CP, Derraik JGB, Chiavaroli V, Walker N, Thampi S, Creagh C, Reynolds AJ, Vatanen T, et al. Oral administration of maternal vaginal microbes at birth to restore gut microbiome development in infants born by caesarean section: a pilot randomised placebo-controlled trial. EBioMedicine. 2021;69:103443. doi:10.1016/j.ebiom.2021.103443.
- Parigi SM, Eldh M, Larssen P, Gabrielsson S, Villablanca EJ. Breast milk and solid food shaping intestinal immunity. Front Immunol. 2015;6:415. doi:10.3389/fimmu.2015.00415.
- Kordy K, Gaufin T, Mwangi M, Li F, Cerini C, Lee DJ, Adisetiyo H, Woodward C, Pannaraj PS, Tobin NH, et al. Contributions to human breast milk microbiome and enteromammary transfer of Bifidobacterium breve. PLoS One. 2020;15(1):e0219633. doi:10.1371/journal.pone.0219633.
- Williams JE, Carrothers JM, Lackey KA, Beatty NF, Brooker SL, Peterson HK, Steinkamp KM, York MA, Shafii B, Price WJ, et al. Strong multivariate relations exist among milk, oral, and fecal microbiomes in mother-infant dyads during the first six months postpartum. J Nutr. 2019;149(6):902–914. doi:10.1093/jn/nxy299.
- Moossavi S, Sepehri S, Robertson B, Bode L, Goruk S, Field CJ, Lix LM, de Souza RJ, Becker AB, Mandhane PJ, et al. Composition and variation of the human milk microbiota are influenced by maternal and early-life factors. Cell Host & Microbe. 2019;25(2):324–335.e4. doi:10.1016/j.chom.2019.01.011.
- Rodríguez JM. The origin of human milk bacteria: is there a bacterial entero-mammary pathway during late pregnancy and lactation? Adv Nutr. 2014;5(6):779–784. doi:10.3945/an.114.007229.
- Biagi E, Quercia S, Aceti A, Beghetti I, Rampelli S, Turroni S, Faldella G, Candela M, Brigidi P, Corvaglia L. The bacterial ecosystem of mother’s milk and infant’s mouth and gut. Front Microbiol. 2017;8:1214. doi:10.3389/fmicb.2017.01214.
- Ramsay DT, Kent JC, Owens RA, Hartmann PE. Ultrasound imaging of milk ejection in the breast of lactating women. Pediatrics. 2004;113(2):361–367. doi:10.1542/peds.113.2.361.
- Perez PF, Doré J, Leclerc M, Levenez F, Benyacoub J, Serrant P, Segura-Roggero I, Schiffrin EJ, Donnet-Hughes A. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics. 2007;119(3):e724–732. doi:10.1542/peds.2006-1649.
- McGuire MK, McGuire MA. Got bacteria? The astounding, yet not-so-surprising, microbiome of human milk. Curr Opin Biotechnol. 2017;44:63–68. doi:10.1016/j.copbio.2016.11.013.
- Damaceno QS, Souza JP, Nicoli JR, Paula RL, Assis GB, Figueiredo HC, Azevedo V, Martins FS. Evaluation of potential probiotics isolated from human milk and colostrum. Probiotics Antimicrob Proteins. 2017;9(4):371–379. doi:10.1007/s12602-017-9270-1.
- Sakwinska O, Bosco N. Host microbe interactions in the lactating mammary gland. Front Microbiol. 2019;10:1863. doi:10.3389/fmicb.2019.01863.
- Kumar H, du Toit E, Kulkarni A, Aakko J, Linderborg KM, Zhang Y, Nicol MP, Isolauri E, Yang B, Collado MC, et al. Distinct patterns in human milk microbiota and fatty acid profiles across specific geographic locations. Front Microbiol. 2016;7:1619. doi:10.3389/fmicb.2016.01619.
- Gomez-Gallego C, Garcia-Mantrana I, Salminen S, Collado MC. The human milk microbiome and factors influencing its composition and activity. Semin Fetal Neonatal Med. 2016;21(6):400–405. doi:10.1016/j.siny.2016.05.003.
- Ding M, Qi C, Yang Z, Jiang S, Bi Y, Lai J, Sun J. Geographical location specific composition of cultured microbiota and Lactobacillus occurrence in human breast milk in China. Food Funct. 2019;10(2):554–564. doi:10.1039/c8fo02182a.
- Laursen MF, Pekmez CT, Larsson MW, Lind MV, Yonemitsu C, Larnkjær A, Mølgaard C, Bode L, Dragsted LO, Michaelsen KF, et al. Maternal milk microbiota and oligosaccharides contribute to the infant gut microbiota assembly. ISME Commun. 2021;1(1):1–13. doi:10.1038/s43705-021-00021-3.
- Collado MC, Laitinen K, Salminen S, Isolauri E. Maternal weight and excessive weight gain during pregnancy modify the immunomodulatory potential of breast milk. Pediatric Res. 2012;72(1):77–85. doi:10.1038/pr.2012.42.
- Indiani CMDSP, Rizzardi KF, Castelo PM, Ferraz LFC, Darrieux M, Parisotto TM. Childhood obesity and Firmicutes/Bacteroidetes ratio in the gut microbiota: a systematic review. Child Obes. 2018;14(8):501–509. doi:10.1089/chi.2018.0040.
- Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19(1):55–71. doi:10.1038/s41579-020-0433-9.
- Arboleya S, Binetti A, Salazar N, Fernández N, Solís G, Hernández-Barranco A, Margolles A, de Los Reyes-Gavilán CG, Gueimonde M. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol Ecol. 2012;79(3):763–772. doi:10.1111/j.1574-6941.2011.01261.x.
- Arboleya S, Sánchez B, Milani C, Duranti S, Solís G, Fernández N, de Los Reyes-Gavilán CG, Ventura M, Margolles A, Gueimonde M. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr. 2015;166(3):538–544. doi:10.1016/j.jpeds.2014.09.041.
- Mai V, Torrazza RM, Ukhanova M, Wang X, Sun Y, Li N, Shuster J, Sharma R, Hudak ML, Neu J, et al. Distortions in development of intestinal microbiota associated with late onset sepsis in preterm infants. PLoS One. 2013;8(1):e52876. doi:10.1371/journal.pone.0052876.
- Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ, Britton HM, Lefebvre DL, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7(307):307ra152. doi:10.1126/scitranslmed.aab2271.
- McCloskey K, Vuillermin P, Carlin JB, Cheung M, Skilton MR, Tang ML, Allen K, Gilbert GL, Ranganathan S, Collier F, et al. Perinatal microbial exposure may influence aortic intima-media thickness in early infancy. Int J Epidemiol. 2017;46(1):209–218. doi:10.1093/ije/dyw042.
- Mu Q, Swartwout BK, Edwards M, Zhu J, Lee G, Eden K, Cabana-Puig X, McDaniel DK, Mao J, Abdelhamid L, et al. Regulation of neonatal IgA production by the maternal microbiota. Proc Natl Acad Sci U S A. 2021;118(9). doi:10.1073/pnas.2015691118.
- Laursen MF, Sakanaka M, von Burg N, Mörbe U, Andersen D, Moll JM, Pekmez CT, Rivollier A, Michaelsen KF, Mølgaard C, et al. Bifidobacterium species associated with breastfeeding produce aromatic lactic acids in the infant gut. Nat Microbiol. 2021;6(11):1367–1382. doi:10.1038/s41564-021-00970-4.
- Sela DA, Mills DA. Nursing our microbiota: molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol. 2010;18(7):298–307. doi:10.1016/j.tim.2010.03.008.
- Henrick BM, Rodriguez L, Lakshmikanth T, Pou C, Henckel E, Arzoomand A, Olin A, Wang J, Mikes J, Tan Z, et al. Bifidobacteria-mediated immune system imprinting early in life. Cell. 2021;184(15):3884–3898.e11. doi:10.1016/j.cell.2021.05.030.
- Bode L. The functional biology of human milk oligosaccharides. Early Hum Dev. 2015;91(11):619–622. doi:10.1016/j.earlhumdev.2015.09.001.
- Boumahrou N, Chevaleyre C, Berri M, Martin P, Bellier S, Salmon H. An increase in milk IgA correlates with both pIgr expression and IgA plasma cell accumulation in the lactating mammary gland of PRM/Alf mice. J Reprod Immunol. 2012;96(1–2):25–33. doi:10.1016/j.jri.2012.08.001.
- Johansen F-E, Braathen R, Brandtzaeg P. The J chain is essential for polymeric Ig receptor-mediated epithelial transport of IgA. J Immunol. 2001;167(9):5185–5192. doi:10.4049/jimmunol.167.9.5185.
- Wilson E, Butcher EC. CCL28 controls immunoglobulin (Ig) a plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate. J Exp Med. 2004;200(6):805–809. doi:10.1084/jem.20041069.
- Usami K, Niimi K, Matsuo A, Suyama Y, Sakai Y, Sato S, Fujihashi K, Kiyono H, Uchino S, Furukawa M, et al. The gut microbiota induces Peyer’s-patch-dependent secretion of maternal IgA into milk. Cell Rep. 2021;36(10):109655. doi:10.1016/j.celrep.2021.109655.
- Ding MF, Yang B, Ross RP, Stanton C, Zhao JX, Zhang HX, Chen W. Crosstalk between sIgA-Coated bacteria in infant gut and early-life health. Trends Microbiol. 2021;29(8):725–735. doi:10.1016/j.tim.2021.01.012.
- Dunne-Castagna VP, Taft DH. Mother’s Touch: milk IgA and Protection from Necrotizing Enterocolitis. Cell Host & Microbe. 2019;26(2):147–148. doi:10.1016/j.chom.2019.07.013.
- Donaldson GP, Ladinsky MS, Yu KB, Sanders JG, Yoo B, Chou W-C, Conner M, Earl A, Knight R, Bjorkman P, et al. Gut microbiota utilize immunoglobulin a for mucosal colonization. Science. 2018;360(6390):795–800. doi:10.1126/science.aaq0926.
- Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host & Microbe. 2007;2(5):328–339. doi:10.1016/j.chom.2007.09.013.
- Zimmermann J, Macpherson AJ. Breast Milk Modulates Transgenerational Immune Inheritance. Cell. 2020;181(6):1202–1204. doi:10.1016/j.cell.2020.05.030.
- Wu W, Liu HP, Chen F, Liu H, Cao AT, Yao S, Sun M, Evans‐marin HL, Zhao Y, Zhao Q, et al. Commensal A4 bacteria inhibit intestinal Th2‐cell responses through induction of dendritic cell TGF‐β production. Eur J Immunol. 2016;46(5):1162–1167. doi:10.1002/eji.201546160.
- Liu X, Zeng B, Zhang J, Li W, Mou F, Wang H, Zou Q, Zhong B, Wu L, Wei H, et al. Role of the gut microbiome in modulating arthritis progression in mice. Sci Rep. 2016;6(1):30594. doi:10.1038/srep30594.
- D’Auria G, Peris-Bondia F, Džunková M, Mira A, Collado MC, Latorre A, Moya A. Active and secreted IgA-coated bacterial fractions from the human gut reveal an under-represented microbiota core. Sci Rep. 2013;3(1):3515. doi:10.1038/srep03515.
- Planer JD, Peng Y, Kau AL, Blanton LV, Ndao IM, Tarr PI, Warner BB, Gordon JI. Development of the gut microbiota and mucosal IgA responses in twins and gnotobiotic mice. Nature. 2016;534(7606):263–266. doi:10.1038/nature17940.
- Palm NW, De Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, Degnan PH, Hu J, Peter I, Zhang W, et al. Immunoglobulin a coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158(5):1000–1010. doi:10.1016/j.cell.2014.08.006.
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–222. doi:10.1016/S0140-6736(13)60844-2.
- Mundy R, MacDonald TT, Dougan G, Frankel G, Wiles S. Citrobacter rodentium of mice and man. Cell Microbiol. 2005;7(12):1697–1706. doi:10.1111/j.1462-5822.2005.00625.x.
- Caballero-Flores G, Sakamoto K, Zeng MY, Wang Y, Hakim J, Matus-Acuña V, Inohara N, Núñez G. Maternal immunization confers protection to the offspring against an attaching and effacing pathogen through delivery of IgG in breast milk. Cell Host & Microbe. 2019;25(2):313–323.e4. doi:10.1016/j.chom.2018.12.015.
- Umetsu DT, DeKruyff RH. The regulation of allergy and asthma. Immunol Rev. 2006;212(1):238–255. doi:10.1111/j.0105-2896.2006.00413.x.
- Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387(10023):1109–1122. doi:10.1016/s0140-6736(15)00149-x.
- Ta LDH, Chan JCY, Yap GC, Purbojati RW, Drautz-Moses DI, Koh YM, Tay CJX, Huang CH, Kioh DYQ, Woon JY, et al. A compromised developmental trajectory of the infant gut microbiome and metabolome in atopic eczema. Gut Microbes. 2020;12(1):1–22. doi:10.1080/19490976.2020.1801964.
- Fiocchi A, Pawankar R, Cuello-Garcia C, Ahn K, Al-Hammadi S, Agarwal A, Beyer K, Burks W, Canonica GW, Ebisawa M, et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): probiotics. World Allergy Organ J. 2015;8(1):4. doi:10.1186/s40413-015-0055-2.
- López-Serrano P, Pérez-Calle JL, Pérez-Fernández MT, Fernández-Font JM, Boixeda de Miguel D, Fernández-Rodríguez CM. Environmental risk factors in inflammatory bowel diseases. Investigating the hygiene hypothesis: a Spanish case-control study. Scand J Gastroenterol. 2010;45(12):1464–1471. doi:10.3109/00365521.2010.510575.
- Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrländer C, Heederik D, Piarroux R, von Mutius E. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364(8):701–709. doi:10.1056/NEJMoa1007302.
- Hebert JC, Radford-Smith DE, Probert F, Ilott N, Chan KW, Anthony DC, Burnet PW. Mom’s diet matters: maternal prebiotic intake in mice reduces anxiety and alters brain gene expression and the fecal microbiome in offspring. Brain Behav Immun. 2021;91:230–244. doi:10.1016/j.bbi.2020.09.034.
- Lee YM, Mu A, Wallace M, Gengatharan JM, Furst AJ, Bode L, Metallo CM, Ayres JS. Microbiota control of maternal behavior regulates early postnatal growth of offspring. Sci Adv. 2021;7(5):eabe6563. doi:10.1126/sciadv.abe6563.
- Jiménez E, Marín ML, Martín R, Odriozola JM, Olivares M, Xaus J, Fernández L, Rodríguez JM. Is meconium from healthy newborns actually sterile? Res Microbiol. 2008;159(3):187–193. doi:10.1016/j.resmic.2007.12.007.
- Blaser MJ, Devkota S, McCoy KD, Relman DA, Yassour M, Young VB. Lessons learned from the prenatal microbiome controversy. Microbiome. 2021;9(1):8. doi:10.1186/s40168-020-00946-2.
- Kennedy KM, Gerlach MJ, Adam T, Heimesaat MM, Rossi L, Surette MG, Sloboda DM, Braun T. Fetal meconium does not have a detectable microbiota before birth. Nat Microbiol. 2021;6(7):865–873. doi:10.1038/s41564-021-00904-0.
- Mishra A, Lai GC, Yao LJ, Aung TT, Shental N, Rotter-Maskowitz A, Shepherdson E, Singh GSN, Pai R, Shanti A, et al. Microbial exposure during early human development primes fetal immune cells. Cell. 2021;184(13):3394–3409.e20. doi:10.1016/j.cell.2021.04.039.
- Lebovitz Y, Kowalski EA, Wang X, Kelly C, Lee M, McDonald V, Ward R, Creasey M, Mills W, Basso EKG, et al. Lactobacillus rescues postnatal neurobehavioral and microglial dysfunction in a model of maternal microbiome dysbiosis. Brain Behav Immun. 2019;81:617–629. doi:10.1016/j.bbi.2019.07.025.
- Wang X, Yang J, Zhang H, Yu J, Yao Z. Oral probiotic administration during pregnancy prevents autism‐related behaviors in offspring induced by maternal immune activation via anti‐inflammation in mice. Autism Res. 2019;12(4):576–588. doi:10.1002/aur.2079.
- Cowan CS, Stylianakis AA, Richardson R. Early-life stress, microbiota, and brain development: probiotics reverse the effects of maternal separation on neural circuits underpinning fear expression and extinction in infant rats. Dev Cog Neurosci. 2019;37:100627. doi:10.1016/j.dcn.2019.100627.
- Krishna G, Divyashri G, Prapulla S. A combination supplement of fructo-and xylo-oligosaccharides significantly abrogates oxidative impairments and neurotoxicity in maternal/fetal milieu following gestational exposure to acrylamide in rat. Neurochem Res. 2015;40(9):1904–1918. doi:10.1007/s11064-015-1687-x.
- Cho NA, Nicolucci AC, Klancic T, Wang W, Sharkey KA, Mychasiuk R, Reimer RA. Impaired hypothalamic microglial activation in offspring of antibiotic-treated pregnant/lactating rats is attenuated by prebiotic oligofructose co-administration. Microorganisms. 2020;8(7):1085. doi:10.3390/microorganisms8071085.
- de Oliveira Y, Cavalcante RGS, Neto MPC, Magnani M, de Andrade Braga V, de Souza EL, de Brito Alves JL. Oral administration of Lactobacillus fermentum post-weaning improves the lipid profile and autonomic dysfunction in rat offspring exposed to maternal dyslipidemia. Food Funct. 2020;11(6):5581–5594. doi:10.1039/d0fo00514b.
- Krishna G, Muralidhara. Oral supplements of inulin during gestation offsets rotenone-induced oxidative impairments and neurotoxicity in maternal and prenatal rat brain. Biomed Pharmacother. 2018;104:751–762. doi:10.1016/j.biopha.2018.05.107.
- Krishna G, Muralidhara. Inulin supplementation during gestation mitigates acrylamide-induced maternal and fetal brain oxidative dysfunctions and neurotoxicity in rats. Neurotoxicol Teratol. 2015;49:49–58. doi:10.1016/j.ntt.2015.03.003.
- Selle A, Brosseau C, Dijk W, Duval A, Bouchaud G, Rousseaux A, Bruneau A, Cherbuy C, Mariadassou M, Cariou V, et al. Prebiotic supplementation during gestation induces a tolerogenic environment and a protective microbiota in offspring mitigating food allergy. Front Immunol. 2021;12:745535. doi:10.3389/fimmu.2021.745535.
- Brosseau C, Selle A, Duval A, Misme-Aucouturier B, Chesneau M, Brouard S, Cherbuy C, Cariou V, Bouchaud G, Mincham K, et al. Prebiotic supplementation during pregnancy modifies the gut microbiota and increases metabolites in amniotic fluid, driving a tolerogenic environment in utero. Front Immunol. 2021;12:712614. doi:10.3389/fimmu.2021.712614.
- Terada-Ikeda C, Kitabatake M, Hiraku A, Kato K, Yasui S, Imakita N, Ouji-Sageshima N, Iwabuchi N, Hamada K, Ito T, et al. Maternal supplementation with Bifidobacterium breve M-16V prevents their offspring from allergic airway inflammation accelerated by the prenatal exposure to an air pollutant aerosol. PLoS One. 2020;15(9):e0238923. doi:10.1371/journal.pone.0238923.
- Nakajima A, Kaga N, Nakanishi Y, Ohno H, Miyamoto J, Kimura I, Hori S, Sasaki T, Hiramatsu K, Okumura K, et al. Maternal high fiber diet during pregnancy and lactation influences regulatory T cell differentiation in offspring in mice. J Immunol. 2017;199(10):3516–3524. doi:10.4049/jimmunol.1700248.
- Azagra-Boronat I, Tres A, Massot-Cladera M, À F, Castell M, Guardiola F, Pérez-Cano FJ, Rodríguez-Lagunas MJ. Lactobacillus fermentum CECT5716 supplementation in rats during pregnancy and lactation impacts maternal and offspring lipid profile, immune system and microbiota. Cells. 2020;9(3):575. doi:10.3390/cells9030575.
- Needell JC, Ir D, Robertson CE, Kroehl ME, Frank DN, Zipris D, Mounier C. Maternal treatment with short-chain fatty acids modulates the intestinal microbiota and immunity and ameliorates type 1 diabetes in the offspring. PLoS One. 2017;12(9):e0183786. doi:10.1371/journal.pone.0183786.
- Hogenkamp A, Thijssen S, van Vlies N, Garssen J. Supplementing pregnant mice with a specific mixture of nondigestible oligosaccharides reduces symptoms of allergic asthma in male offspring. J Nutr. 2015;145(3):640–646. doi:10.3945/jn.114.197707.
- Barouei J, Moussavi M, Hodgson DM, Heimesaat MM. Effect of maternal probiotic intervention on HPA axis, immunity and gut microbiota in a rat model of irritable bowel syndrome. PLoS One. 2012;7(10):e46051. doi:10.1371/journal.pone.0046051.
- Ho T, Jahan M, Haque Z, Kracht S, Wynn PC, Du Y, Gunn A, Wang B. Maternal chitosan oligosaccharide intervention optimizes the production performance and health status of gilts and their offspring. Anim Nutr. 2020;6(2):134–142. doi:10.1016/j.aninu.2020.02.001.
- Duan XD, Tian G, Chen DW, Huang LH, Zhang D, Zheng P, Mao XB, Yu J, He J, Huang ZQ, et al. Mannan oligosaccharide supplementation in diets of sow and (or) their offspring improved immunity and regulated intestinal bacteria in piglet. J Anim Sci. 2019;97(11):4548–4556. doi:10.1093/jas/skz318.
- Li Y, Liu HY, Zhang LJ, Yang Y, Lin Y, Zhuo Y, Fang ZF, Che LQ, Feng B, Xu SY, et al. Maternal dietary fiber composition during gestation induces changes in offspring antioxidative capacity, inflammatory response, and gut microbiota in a sow model. Int J Mol Sci. 2019;21(1):31. doi:10.3390/ijms21010031.
- Shang QH, Liu HS, Liu SJ, He TF, Piao XS. Effects of dietary fiber sources during late gestation and lactation on sow performance, milk quality, and intestinal health in piglets. J Anim Sci. 2019;97(12):4922–4933. doi:10.1093/jas/skz278.
- Cheng CS, Wei HS, Xu CJ, Xie XW, Jiang SW, Peng J, Björkroth J. Maternal soluble fiber diet during pregnancy changes the intestinal microbiota, improves growth performance, and reduces intestinal permeability in piglets. Appl Environ Microbiol. 2018;84(17):e01047–18. doi:10.1128/AEM.01047-18.
- Duan XD, Chen DW, Zheng P, Tian G, Wang JP, Mao XB, Yu J, He JF, Li B, Huang ZQ, et al. Effects of dietary mannan oligosaccharide supplementation on performance and immune response of sows and their offspring. Anim Feed Sci Technol. 2016;218:17–25. doi:10.1016/j.anifeedsci.2016.05.002.
- Le Bourgot C, Ferret-Bernard S, Le Normand L, Savary G, Menendez-Aparicio E, Blat S, Appert-Bossard E, Respondek F, Le Huërou-Luron I, Blachier F. Maternal short-chain fructooligosaccharide supplementation influences intestinal immune system maturation in piglets. PLoS One. 2014;9(9):e107508. doi:10.1371/journal.pone.0107508.
- Leonard S, Sweeney T, Bahar B, O’Doherty J. Effect of maternal seaweed extract supplementation on suckling piglet growth, humoral immunity, selected microflora, and immune response after an ex vivo lipopolysaccharide challenge. J Anim Sci. 2012;90(2):505–514. doi:10.2527/jas.2010-3243.