ABSTRACT
Glyphosate, the active ingredient in the broad-spectrum herbicide RoundupTM, has been a topic of discussion for decades due to contradictory reports of the effect of glyphosate on human health. Glyphosate inhibits the enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) of the shikimic pathway producing aromatic amino acids in plants, a mechanism that suggests that the herbicide would not affect humans as this pathway is not found in mammals. However, numerous studies have implicated glyphosate exposure in the manifestation of a variety of disorders in the human body. This review specifically outlines the potential effect of glyphosate exposure on the composition and functionality of the gut microbiome. Evidence has been building behind the hypothesis that the composition of each individual gut microbiota significantly impacts health. For this reason, the potential of glyphosate to inhibit the growth of beneficial microbes in the gut or alter their functionality is an important topic that warrants further consideration.
Background
Glyphosate is the active ingredient in the broad-spectrum herbicide RoundupTM, which was first produced in 1974. Two decades later, this facilitated the release of genetically modified crops resistant to glyphosate, or “RoundupTM Ready” crops, in 1996.Citation1 In 2010, glyphosate was patented as an antibiotic following decades of use as a herbicide, highlighting the compounds impressive antimicrobial activity (US Patent No. 7771736 B2).Citation2 Glyphosate is one of the most popular herbicides used in the United States (US), with two hundred and eighty million pounds of glyphosate being used annually.Citation3 This high level of production has resulted in the widespread presence of glyphosate in the environment through runoff and subsequent incorporation into water cycles. Glyphosate residues have been found in such places as crops (environmentally acquired and treated)Citation4, drinking water,Citation5 rain,Citation6 animal feedCitation7 and air.Citation8 The herbicide is partially broken down in soil, water, and dead plant material. However, stable carbon-phosphorus bonds in the molecule protect it from complete degradation.Citation9 In 2015, glyphosate was reclassified by the World Health Organization (WHO) as “probably carcinogenic to humans”.Citation10 Despite this, glyphosate is still approved by the European Food Safety Authority (EFSA) and the US Environmental Protection Agency (EPA) because these carcinogenic effects are unlikely at the levels at which glyphosate residues are present in plants (0.02–19 mg/kg).Citation11 However, in 2017, the European Union announced that glyphosate would only be allowed for use until December, 2022.Citation12 This authorization has now been extended to December, 2023.Citation13
Given the widespread presence of glyphosate in the environment, residue exposure/ingestion is a risk factor for humans with potential consequences for the human gut microbiome. The gut microbiome is home to a wide range of microorganisms and has been associated with several essential host functions including immune system development and homeostasis,Citation14 modulation of energy metabolism,Citation15 colonization resistance against pathogensCitation16 and production of bioactive metabolitesCitation17. It has been connected with the functioning of other organs of the body including the skin,Citation18 lungsCitation19 and brain.Citation20
Although the precise composition of a healthy gut microbiome is difficult to fully elucidate due to the individual variances between microbiomes and high functional redundancies between species, it has been shown that disruptions to an individual’s gut microbiome may result in disease.Citation21 This occurrence is sometimes termed “dysbiosis”, in which alterations to the “normal” colonization of the gut is associated with disease.Citation22 Many factors of life result in the individuality of a gut microbiome, these include such things as age, dietCitation23 and lifestyle choices such as exercise frequency.Citation24 Several chronic diseases from gastrointestinal to cardiovascular and metabolic diseases, to neurological and respiratory conditions also impact the gut microbiome.Citation25 Inflammatory bowel disease (IBD) is one example where increases in Proteobacteria and Bacteroidota and a decrease in Bacillota have been observed in fecal samples of patients.Citation26 Similarly, an increase in Prevotella copri and a decrease in Bacteroides species have been associated with the onset of rheumatoid arthritis.Citation26 Samples from multiple sclerosis patients have been seen to have an increase in Bacteroides, Faecalibacterium, Prevotella, Butyricimonas, and Collinsella, with enrichment of Bifidobacterium, Streptococcus, Methanobrevibacter, and Akkermansia muciniphila.Citation27
Glyphosate inhibits the enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) in the shikimate pathway for the production of aromatic amino acids, such as tryptophan, tyrosine or phenylalanine. This pathway is found in plants, fungi, bacteria, protozoa and archaea,Citation4 rendering glyphosate an effective antimicrobial. As the human body does not produce tryptophan, tyrosine or phenylalanine, they must be acquired through diet, as well as through production by microbes in the gut.Citation28 However, disruption to the shikimate pathway due to glyphosate has been shown to reduce the levels of these nutrients in plants and therefore potentially limit their bioavailability to humans who consume them.Citation29 One study demonstrated that treatment with 100 µM of pure glyphosate reduced the levels of tryptophan by 13%, tyrosine by 59% and phenylalanine by 77% in sugarcane crops.Citation30 Furthermore, alkaloids, which are medicinal compounds naturally produced by plants with anti-cancer and anti-inflammation properties, are also made by the shikimate pathway and so these natural compounds cannot be produced by plants following treatment with glyphosate.Citation29,Citation31
In this review, we discuss the possible effect that glyphosate has on the human body with a specific focus on the gut microbiome and the potential consequences for human health.
Mechanisms of microbial resistance to glyphosate
The mechanism of action of glyphosate, as described in , is to inhibit EPSPS in the shikimate pathwayCitation4 and is achieved by glyphosate binding to the EPSPS enzyme. The result of this is a disruption to the production of the aromatic amino acids tryptophan, tyrosine and phenylalanine.Citation32
Figure 1. Shikimate pathway and resistance mechanisms to glyphosate. 1. Glyphosate inhibits the EPSPS enzyme. 2. The epsp gene can be overexpressed to combat EPSPS inhibition. 3. Efflux pumps are used to export glyphosate out of the cell as a form of resistance. 4. Amino acid biomarkers on the EPSPS enzyme determine the sensitivity of that enzyme to glyphosate. Changes in amino acid sequence can confer resistance to glyphosate. Created using Biorender.
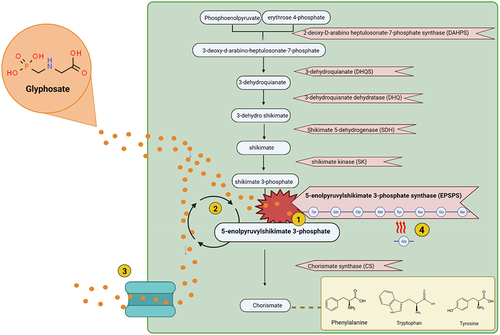
Some species of bacteria have developed mechanisms to overcome the action of glyphosate (as described in ). One such resistance mechanism is target-site resistance, in which amino acid residues found in the EPSPS active site are altered to confer resistance.Citation33 Another mechanism of resistance utilized by some species is non-target site resistance, in which overexpression of the EPSPS gene occurs or efflux pumps are utilized to pump glyphosate out of the cell.Citation34,Citation35 EPSPS enzymes have been categorized into four different classes depending on their sensitivity or resistance.Citation36 Class I includes sensitive versions of the enzyme, while classes II-IV describe glyphosate resistant versions. Classes I, II and IV are similar in that they are all characterized depending on the presence or absence of specific amino acid residues in the EPSPS active site. In contrast to that, class III enzymes contain a run of motifs.Citation37
In general, Bacillota are more resistant to glyphosate when compared to Actinomycetota and Pseudomonadota. Pathogenic bacteria, such as Escherichia coli, Salmonella enterica and Salmonella enterica serotype Typhimurium, showed a higher level of resistance compared to host-associated commensal bacteria.Citation9 One study examining the minimum inhibitory concentrations (MICs) of glyphosate (40% monoisopropylamine salt solution of glyphosate and RoundupTM LB Plus) for pathogenic and commensal bacteria, found that the pathogenic strains had a significantly higher MIC (20–80 mg/mL) than the commensal strains (5–10 mg/mL).Citation38 This suggests that glyphosate ingestion by humans could potentially select for pathogenic bacteria in the gut microbiome. Indeed, one study that examined 101 bacterial species for glyphosate sensitivity/resistance found that 54% of bacterial species commonly found in the gut were sensitive to glyphosate.Citation39 Examples include members of the genera Faecalibacterium, Citrobacter and Bifidobacterium. Whereas 29% of the bacterial species evaluated were potentially resistant. Such species included members of the genera Clostridium, Ruminococcus and Dorea. An increase in these microbes is associated with diseases such as IBD.Citation40 The remaining 17% of bacteria was made up of unclassified bacteria (10%) and bacteria varying in sensitivity (7%). Another study examined 890 bacterial strains from 101 species inhabiting the gut microbiome. They analyzed bacterial DNA sequences coding for the different types of EPSPS and predicted that 12–26% of the bacteria present in the gut microbiome are potentially sensitive to glyphosate.Citation33 This result was a conservative calculation based on the strains tested to establish glyphosate sensitivity in the core gut microbiome, perhaps explaining the significant difference in results between the two studies. Some bacteria have evolved to produce enzymes which can break down glyphosate and subsequently use the glyphosate degradation products (such as phosphate) as a nutrient, an example of this is certain strains of Pseudomonas.Citation41
A study carried out in 2020 examined the functional ability of the shikimic pathway present in bacteria using computational modeling.Citation42 Fecal samples from the IBD Multi-omics Database were in silico screened both on a genomic and transcriptional level for indications of the presence of the shikimic pathway. The pathway was detected in 98% of 734 metagenomic samples however when the corresponding metatranscriptome samples were analyzed they found that only 35% were expressing the shikimic pathway. This suggests that in a large proportion of gut bacteria, the shikimic pathway is transcriptionally inactive and these bacteria are therefore aromatic amino acid auxotrophs. This is presumably due to the ability of gut bacteria to acquire these aromatic amino acids from the diet.
Potential effects of glyphosate on human health
Many human studies have examined the link between glyphosate exposure and various disorders of the reproductive system,Citation43 the central nervous systemCitation44 and the immune system.Citation45 An association between glyphosate exposure and the development of non-Hodgkin’s lymphoma has been reported in multiple studies.Citation46–49 However, these studies were unable to uncover a statistically significant quantitative link between glyphosate exposure and disease development. Similarly, glyphosate exposure has been linked to asthma,Citation50 autism spectrum disorderCitation51, and Parkinson’s disease.Citation44 However, there are also studies which disprove each of these connections.Citation52,Citation53
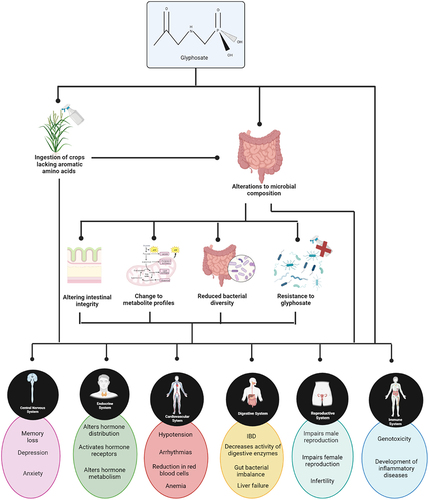
Information for the above-mentioned human studies was gathered via questionnaire and/or telephone interviews. This leaves room for bias in terms of participants subjectively reporting their own glyphosate exposure. In addition, participants were basing their level of glyphosate risk on their exposure to RoundupTM, in terms of both application of the herbicide and proximity to a family member who used the herbicide. While glyphosate is the active ingredient in RoundupTM, the commercial product does contain a number of other chemicals including surfactants which can themselves cause harm independently of glyphosate. There are no human studies to our knowledge with definitively known concentrations of glyphosate exposure. One exception is a study carried out by Zoller et al.Citation54 where a known concentration of glyphosate (196.8 μg) and aminomethylphosphonic acid (AMPA) (1.67 μg) was fed to participants in a “test dish”. Following which, urine output was examined for glyphosate and AMPA concentrations. However, even under these circumstances conclusive concentrations of glyphosate and AMPA are hard to determine, due to the variability of the presence of glyphosate in the diet of participants. Although animal models may not always predict the outcome of human studies, they can be a useful source of information. Animal models have been used to show a link between glyphosate and the development of disorders such as obesity.Citation55 diabetes,Citation56 autism,Citation57 and mental health disorders.Citation58 In animals, glyphosate has been reported to negatively impact the gut,Citation59 the cardiovascular systemCitation60 the endocrine system,Citation61 the reproductive system,Citation62 the central nervous system,Citation63 and immune systemCitation64 (outlined in ). However these studies did not examine the effect of glyphosate on the gut microbiome of their selected animal model. Therefore, in these instances it is impossible to determine if alterations to the gut microbiome were an impacting factor in disease manifestation. It is also worth mentioning the difference between the gut microbiome of humans and animal models and how for this reason results obtained in animal models may not reflect results in humans.
Figure 2. Potential effect of glyphosate exposure on the various bodily systems based on animal studies. Created using Biorender.
A large portion of tryptophan utilized by the host is acquired through diet, however gut bacteria also play a role in the production of tryptophan for host utilization. As glyphosate has been shown to reduce microbially produced tryptophan, this suggests that glyphosate in turn reduces tryptophan availability in the host. Tryptophan and its derivatives have been studied for their role as signaling molecules connecting both the gut microbiota and host cells, subsequently affecting human health and disease.Citation65 There is evidence showing an association between reduced tryptophan levels in the serum of IBD patients, as well as an increased severity of IBD symptoms.Citation66 In addition, tryptophan metabolism results in aryl hydrocarbon receptor agonists which reduce central nervous system inflammation, a cause of multiple sclerosis symptoms, in a mouse model.Citation67 Other properties of tryptophan derivatives include anti-inflammatory,Citation68 increased immune response and barrier function.Citation69,Citation70 Although these disease consequences cannot be directly linked to glyphosate exposure, the large amount of literature linking glyphosate exposure to a reduction in microbially-produced tryptophan suggests this may be a possibility.
Glyphosate and the gut microbiome
As previously discussed, glyphosate is present in the environment due to widespread contamination. This has ultimately led to glyphosate being unknowingly consumed by humans through means of contaminated crops and drinking water, as well as the inhalation of contaminated air.Citation71 Glyphosate was present at concentrations of up to 233 ppb in the urine of 90% of farmers in a study carried out in South Carolina.Citation72 In the USA, 60–95% of the general public have the compound present in their urine at concentrations of 2–3 μg/L, with the same being said for 40–50% of Europeans at concentrations of <1 μg/L.Citation71 Another study examining the effect of glyphosate on kidney function in young children and infants showed that 30% of neonates examined had glyphosate present in their urine at concentrations of <1.06 μg/L.Citation73 This could have occurred as a result of glyphosate present in the breast milk or baby formula,Citation74 or due to the ability of glyphosate to traverse the placenta,Citation75 The neonates had a higher incidence of glyphosate presence in their urine compared to 7.6% of participants in the cohort of infants aged 10–19 months. All of the glyphosate concentrations reported in the above studies do not exceed 5% of the acceptable daily intake (ADI) which is 0.5 mg/kg BW/day in the EU (EFSA),Citation13.
Impact of glyphosate on the gut microbiome composition and functionality
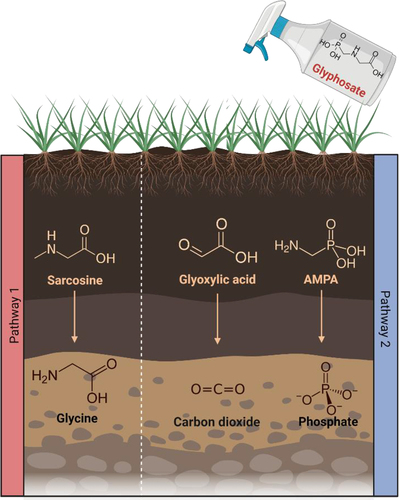
Glyphosate can be degraded in two different ways as described in . The first pathway results in an accumulation of sarcosine, after which the glyphosate C-P bond is cleaved through dephosphorylation resulting in the formation of glycine.Citation76 The second pathway involves the cleavage of the C-N bond via the enzyme glyphosate oxidoreductase resulting in the production of glyoxylate and AMPA.Citation77 Various organisms in nature can degrade glyphosate, including numerous bacteria such as Arthrobacter spp. GLP-1, Geobacillus caldoxylosilyticus T20, Pseudomonas spp., Rhizobium spp., Bacillus megaterium 2BLW and Alcaligenes spp.Citation78,Citation79 Considering the ability of certain bacteria to degrade glyphosate in nature, the possibility that bacteria in the gut microbiome degrade glyphosate has been hypothesized. One study examined glyphosate degradation in the human fecal microbiota by anaerobically incubating pure glyphosate (Sigma-Aldrich) with fecal suspensions from 15 participants.Citation80 The presence of AMPA was not detected following incubation and there was no evidence of degradation of glyphosate by the bacteria present in the fecal samples. This suggests that the human gut microbiome is unable to catabolize glyphosate, and as a result glyphosate is excreted, therefore limiting the potential for bioaccumulation of the compound. Sarcosine, glycine and glyoxylic acid have been shown to be present in urine.Citation81–84 However, this was not demonstrated as part of a study examining degradation of glyphosate and therefore does not provide a conclusion as to how glyphosate metabolites are excreted from the body. Although one study did determine that 1% of all glyphosate (glyphosate, CAS Bioflow® 1071-83-6) initially ingested will be excreted in urine, with 23% of AMPA (AMPA, CAS Pro® 1066-51-9) being excreted in urine.Citation54
Figure 3. Glyphosate degradation pathways and the subsequent products resulting. Created using Biorender.
Information regarding the effect of these metabolites on the host are somewhat limited, with their effect on the gut microbiome even more so. However, there have been studies performed comparing the effect of pure glyphosate (Interchim SS-7701) on the host with its metabolite AMPA (Sigma-Aldrich 324,817), which has shown that AMPA does not have a significant impact on the gut microbiome. This is in contrast to glyphosate which was shown in this study to decrease S. alvi five to 13 fold more than in the untreated control.Citation85 The presence of AMPA in urine samples has also been associated with breast cancer patients, with one study reporting a 38% increase of samples containing AMPA in the breast cancer group compared to healthy controls.Citation81 In a chronic kidney disease rat model, serum levels of glycine conjugated compounds were increased in the untreated group representative of chronic kidney disease, insinuating high levels of glycine are associated with chronic kidney disease. This occurrence was also noted to coincide with an increase in Clostridium, Enterorhabdus, Parasutterella, Blautia, and Escherichia Shigella.Citation86 One study has shown that the presence of glyoxylic acid is of benefit to the host, promoting myogenesis, reducing muscle atrophy, and subsequently metabolizing to amino acid in muscle cells. Although, this study has acknowledged that more in vivo experiments are necessary to fully elucidate to what degree glyoxylic acid may aid the host in this respect.Citation87 An increase of sarcosine is associated with the development of prostate cancer,Citation88 so much so that numerous diagnostic tests have been researched utilizing sarcosine as a biomarker for the disease.Citation89 In contrast, sarcosine has also been used to benefit the host by means of treating schizophrenia, with impressive outcomes when used in conjunction with antipsychotic drugs.Citation90 This area would be worth researching further, as although there is information available outlining the effect these metabolites have on the host, information regarding the effect of these metabolites on the gut microbiome and related biological effects is scarce, and in need of examination.
An increase in the pH of feces has been strongly correlated to the amount of glyphosate (Bioflow®) in the colon.Citation91 Similarly, an increase in glyphosate levels has a negative effect on acetate levels in the cecum.Citation91 This suggests that glyphosate has a disruptive effect on acetate-producing bacteria. Glyphosate has also been shown to affect digestive enzymes such as trypsin, lipase and amylase;Citation92 it is hypothesized that glyphosate inhibits lipase in digesting fats, and trypsin in digesting proteins. This causes intact proteins to enter the colon at which point they are broken down releasing ammonia, subsequently increasing the pH of the colon.
Bifidobacteria are recognized as important members of the human gut microbiota, with many strains proven to provide health-promoting properties.Citation93 Bifidobacteriales is the most abundant bacterial class in the infant gut where its members are associated with infant development and health.Citation94 Some bifidobacteria can metabolize non-digestible carbohydrates such as mucins and human milk oligosaccharides found in breast milk, resulting in the production of short-chain fatty acids (SCFAs) which exert important physiological functions on the host.Citation95 However, bifidobacteria are known to be among the most sensitive bacteria to glyphosate.Citation96 Furthermore, it has been reported that most infant formulas are contaminated with glyphosate. One study reported levels between 0.03 mg kg−1 and 1.08 mg kg−1.Citation97 This could potentially further exacerbate the problem of Bifidobacterium reduction in the infant gut.Citation98
Potential health consequences of glyphosate-associated microbiome alterations based on animal studies
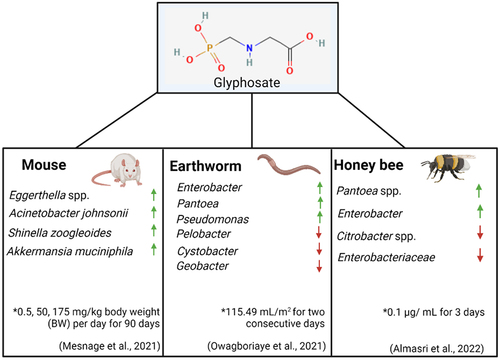
In recent years a variety of studies in animals have examined the effects of glyphosate on the gut microbiome, as seen in . Although it should be noted concentrations of glyphosate used in these studies which resulted in biological consequences are between 3.5 and 500 fold higher than the ADI. These studies have revealed that glyphosate-associated alterations to the gut microbiome are linked to disturbances in a variety of systems around the body. For example, gut dysbiosis resulting from glyphosate exposure has been shown to contribute to male reproductive toxicity in rats.Citation99 Specifically, male rats that ingested feed containing glyphosate (GLY, N-(phosphonomethyl)-glycine, purity > 95%) at levels of 250 mg/kg BW for two months were seen to have a reduction in sperm motility, an increase in sperm malformation and impairments to testes structure. 16S rRNA sequencing of the gut microbiome of the rats showed significant changes to the commensal bacterial population. An increase in Prevotella and Bacteroides was observed in the rats that exhibited reduced sperm quality. These findings support the hypothesis that glyphosate-induced gut dysbiosis may lead to male infertility.
Figure 4. Examples of animal models used to examine the effect of glyphosate on the gut microbiome and the increase and decrease in bacterial species present. * - concentrations of glyphosate used and duration of experiment. Created using Biorender.
The cecum microbiota of rats has been reported to be altered by glyphosate exposure (0.5, 5, 50 mg/kg body weight/day) (Pure glyphosate, RoundupTM Bioflow® and Ranger Pro®). In this case, Bacteroidota were decreased, while Bacillota and Actinomycetota were increased.Citation100 In addition to changes to the composition of the gut microbiota following glyphosate exposure, alterations to intestinal integrity have also been reported in adult male mice. This was confirmed by changes to the circulation of syndecan-1 proteoglycan and the expression of ZO-1 and ZO-2 tight junction effector proteins. In this particular study, Bacteroidota, Pseudomonadota and Desulfobacterota relative levels were altered as a result of glyphosate (Round UpTM) exposure from pregnancy until adulthood at a concentration of 0.075% w/v present in the drinking water.Citation101
Homocysteine is a metabolite which can be converted into cysteine in the presence of specific B vitamins. Deficiencies in vitamins B12, B6 and folic acid have been shown to induce hyperhomocysteinemia (a condition where there is more than 15 μmol/L of homocysteine in the blood). One study examining the effect of pure glyphosate and Roundup® in male pups (1.75 mg/kg bw/day for 49 days), determined that the compound induced hyperhomocysteinemia.Citation60 This is thought to be due to glyphosate reducing the levels of Prevotella, which can biosynthesize B vitamins. This is a conflicting report to that of Liu et al.Citation99 mentioned above, as in that case glyphosate (GLY, N-(phosphonomethyl)-glycine, purity > 95%) treatment increased levels of Prevotella. The difference in results could be due to changes to the dosing, with a reduction in Prevotella abundance associated with a much lower treatment of glyphosate compared to the previously mentioned study. Health issues such as brain damage, memory and cognitive decline and autism have been associated with an increase in homocysteine.Citation60,Citation102 Therefore, it is hypothesized that early exposure to glyphosate may be a contributing factor in the development of such disorders.
Glyphosate has also been heavily correlated with neurodevelopmental disturbances such as autism and encephalopathy (as reviewed by Barnett et al.Citation103). This is potentially due to alterations across the gut-brain axis. The ability of glyphosate to reduce the growth of commensal bacteria such as Ruminococcaceae spp., Bifidobacterium spp. and Lactobacillus spp. probably results in reduced levels of microbial metabolites. These metabolites, including L-glutamate which behaves as a neurotransmitter, and SCFAs involved in neuromodulation, traverse the gut-brain axis. The significance of a reduction in L-glutamate is that it is a precursor in synthesizing GABA, reductions of which are associated with neuropsychiatric disease states, such as autism.Citation104 However, it is worth noting L-glutamate is also readily available in food and so is more commonly taken up through diet. This amino acid is also involved in carbohydrate metabolism in the host.Citation105 As previously mentioned, some bacteria can be resistant to glyphosate including Pseudomonas, Arthrobacter spp. and Geobacillus spp. These bacteria may increase levels of reactive oxygen species and pro-inflammatory cytokines, which may lead to hypothalamic-pituitary-adrenocortical (HPA) activation and increased glucocorticoids with potential effects on neurodevelopment.Citation106 Children with autism have been shown to have increased levels of clostridia compared to children who do not have autism.Citation107 An association between encephalopathy and toxic metabolites produced by clostridia has also been uncovered.Citation108 Clostridia species are more resistant to glyphosate than other bacterial taxa such as bifidobacteria and lactobacilli. This correlation has led to the theory that glyphosate activity on the gut microbiome is leading to changes causing a buildup of toxic metabolites which contribute to brain damage.Citation96
A variety of other animal models have been commonly used to examine the effects of glyphosate on the gut microbiome, including honeybees and earthworms. Three species of earthworms were examined for disturbances to the gut bacterial community following Roundup® (7.20 g/l glyphosate) exposure at a concentration of 115.49 mL/m2 for two consecutive days. The abundance of Pseudomonadota significantly increased to become the dominant phylum.Citation109 The impact of glyphosate on honeybees has been the topic of much research given their significant role in pollination. Honeybees chronically exposed to sublethal doses of pure glyphosate (10 mg/L) (active ingredient, Sigma-Aldrich) and infected with Nosema ceranae (a pathogenic bacterium found in adult bees) for seven days resulted in an altered immune response and inability to resist infection.Citation110 Indeed, the composition of the honeybee microbiome was transformed following glyphosate exposure and N. ceranae infection. Specifically, the abundance of Lactobacillus apis and Snodgrassella alvi decreased, which is a significant observation considering that these bacterial species are core members of the honeybee gut microbiome. Another study examined the effect of pure glyphosate on honeybees at a concentration of 0.1 µg/L (for 3 days). This is more reflective of a concentration that is found naturally in honey. In this case, the honey bee microbiome showed a decrease of only non-core bacterial species.Citation111 It should be noted that this study used a significantly reduced concentration of glyphosate compared to the previously mentioned study.
Only a few studies have utilized advanced molecular profiling to examine the effects of glyphosate on the gut microbiome. To address this knowledge gap, Mesnage et al.Citation112 performed metabolomic and metagenomic sequencing on cecum samples from rats who had been exposed to both glyphosate and MON 52276, a liquid formulation of glyphosate, at concentrations of 0.5, 50, 175 mg/kg body weight (BW) per day for 90 days. As expected, metabolomic studies confirmed that the shikimate pathway in the cecum microbiome of the rat is inhibited by glyphosate.Citation100 With regards to diversity in the gut microbiome, Acinetobacter johnsonii and Eggerthella spp. were increased following glyphosate treatment. While the health implications of increased Acinetobacter remain unknown, some members of Eggerthella spp. such as Eggerthella lenta have been linked to GI tract infections.Citation113 The specific strain of Eggerthella that was increased in the rats used in this study has been associated with liver cirrhosis in humans.Citation114 A previous study spanning over two years was carried out by the same group examining rats following an ultra-low dose of daily RoundupTM (50 ng/L) exposure.Citation115 The rats, in this case, developed nonalcoholic fatty liver disease, further corroborating the hypothesis that glyphosate at low levels negatively impacts the liver.
Rumen models have also been utilized to examine the degree to which glyphosate impacts bacterial communities. One such study, carried out by Ackermann et al.,Citation116 used glyphosate ((N-phosphonomethyl) glycine), at final concentrations of 0, 1, 10, and 100 µg/ml, to treat a rumen fluid fermentation model. Glyphosate was also associated with reductions in specific groups of the microbial population, while increasing certain potentially pathogenic bacteria, however these results were not significant. Riede et al.Citation117 observed minimal alterations caused by glyphosate, in the formultion of PlantacleanR 360 (486 g glyphosate isopropylamine salt per Liter), on a RUSITEC ruminal fermentation. Similarly, minor variations in bacterial community were observed, however there was no indications that glyphosate exposure specifically targeted beneficial commensal bacteria or favored the growth of pathogenic bacteria. Nielsen et al.Citation91 examined the effect of 5–80 mg/mL of Bioflow® 450 PLUS (450 g/L glyphosate acid equivalent) on a sheep rumen fermentation models. These results were also consistent with the previously mentioned studies, in that minimal antimicrobial activity was observed. However, this result was dependant on an environment with sufficient levels of aromatic amino acids.Citation91
Impact of the consumption of glyphosate-treated crops on the gut microbiome
As previously mentioned, the presence of glyphosate residues in crops and livestock feeds has been widely reported. While in past years glyphosate was primarily used as a means of weed control before the seeding of crops, the development of RoundupTM-ready crops has allowed for the use of glyphosate all year round, as the herbicide can be sprayed directly on growing plants. This development has increased the likelihood of glyphosate residues being present in crops for human consumption and crops destined for livestock feed. Monsanto has reported that glyphosate is of minimal risk to mammals and so feed and crops containing glyphosate residues should be of no concern.Citation118 One study that investigated the ruminal microbiome of cows following ingestion of glyphosate-contaminated (73.8 and 84.5 mg/day) feedstuffs for 16 weeks determined there were no adverse effects on the composition of the ruminal microbiome. Preparation of the feedstuff involved treatment with glyphosate pre-harvest.Citation119
Conclusion
Since its release in 1974, glyphosate has increasingly become a part of the environment, particularly with the release of the RoundupTM-ready resistant crops in 1996. This ultimately has led to increased ingestion of glyphosate by humans and livestock through drinking water, crops and inhalation of contaminated air. The widespread use of RoundupTM is correlated with an increase in various disorders affecting almost every aspect of human physiology. It is important to acknowledge this is a correlation, not causation. Other than an increase in studies highlighting the negative effects of glyphosate exposure, there is no study to our knowledge explicitly linking glyphosate distribution to this increase in the prevalence of human disorders.
Animal studies to date convincingly suggest that glyphosate can impact a variety of critical systems in the body. However, research in humans is somewhat contradictory, with some studies indicating a link between glyphosate and cancer development, increase in asthma severity and arthritis, while other studies claim glyphosate has no impact on these disorders. To our knowledge, all human studies examining glyphosate and its interactions with the human body rely solely on self-reporting glyphosate exposure via questionnaire or telephone interview. Given this subjective means of collecting information, the data available on glyphosate exposure in human studies remain somewhat limited. There is a large bank of knowledge on glyphosate exposure in animal models. These studies have shown that glyphosate administered at the ADI that EFSA has determined safe (0.5 mg/kg BW/day) can cause harm. This was seen by Séralini et al.Citation120 who observed kidney and liver dysfunction in rats following exposure to glyphosate below the ADI. However, it is unknown how these studies would translate to humans.
In terms of the gut microbiome, alterations following glyphosate exposure include increases in Bacteroides spp. and a decrease in Bifidobacterium spp., Ruminococcus spp., and Lactobacillus spp. These changes to the gut microbiome are associated with biological effects such as hyperhomocysteinemia, reproductive toxicity, alterations to intestinal integrity, neurodevelopmental disturbances, and buildup of toxic metabolites. However, the concentrations of glyphosate used in these studies were above the ADI. An example of a study which used the EFSA-determined ADI to treat rats reported an increase in Eggerthella, A. johnsonii, and A. muciniphila.Citation112 There were no physiological consequences observed following glyphosate treatment at this concentration. This suggests glyphosate administered at the ADI appears not to cause microbiome disturbances significant enough to result in biological consequences, at least under the precise conditions of this trial. The absence of significant disturbances to the gut microbiome at these concentrations is most likely due to the resistance of these bacteria to glyphosate. Although reports on the sensitivity or resistance of gut bacteria to glyphosate are somewhat contradictory when the focus is on the class of EPSPS gene present, analysis of metadata suggesting that the shikimic pathway is not transcriptionally operational in a large number of gut bacteria is more convincing. This could be due to the environment of the gut providing sufficient levels of aromatic amino acids to the bacteria, therefore minimizing the need for the gut microbiota to produce these aromatic amino acids. As previously mentioned, diet can provide the aromatic amino acids necessary for both host cellular functions as well as function of the bacteria present in the host, provided the diet consumed has not already been depleted of aromatic amino acids as a result of glyphosate exposure.
Although the ADI of glyphosate determined by EFSA is a concentration high enough to cause harm in animal models, it seems unlikely that this is due to disturbances in the gut microbiome. However, there is a significant gap in glyphosate research on humans. Controlled studies treating with known concentrations of glyphosate would be required. While this may not be feasible in terms of ethics, with a lack of such research it is difficult to fully elucidate the consequences glyphosate has on both the gut microbiome and human health in general. EFSA has announced that glyphosate will not be authorized for use in the EU after December of 2023. Nonetheless, associations between glyphosate levels in humans and gut microbiome signatures should be defined. Lastly, knowledge of the impact of glyphosate exposure on microbiome functionality is essential.
Acknowledgments
This publication has emanated from research conducted with the financial support of Science Foundation Ireland (SFI) under Grant Number SFI/12/RC/2273_P2 (APC Phase 2 center grant number APC18753 – Theme 1.1 Microbes to Molecules). Funded by the European Union (ERC, BACtheWINNER, Project No. 101054719). Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Research Council Executive Agency. Neither the European Union nor the granting authority can be held responsible for them.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Benbrook CM. Trends in glyphosate herbicide use in the United States and globally. Environ Sci Europe. 2016;28(1):1–16. doi:10.1186/s12302-016-0070-0.
- Chaturvedi P, Shukla P, Giri BS, Chowdhary P, Chandra R, Gupta P, Pandey A. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ Res. 2021;194:110664. doi:10.1016/j.envres.2020.110664.
- Hawkins C., and Hanson C. Glyphosate: response to comments, usage and benefits. Office of Chemical Safety and Pollution Prevention; 2019.
- Duke SO. Interaction of chemical pesticides and their formulation ingredients with microbes associated with plants and plant pests. J Agr Food Chem. 2018;66(29):7553–7561. doi:10.1021/acs.jafc.8b02316.
- Do MH, Florea A, Farre C, Bonhomme A, Bessueille F, Vocanson F, Tran-Thi N, Jaffrezic-Renault N. Molecularly imprinted polymer-based electrochemical sensor for the sensitive detection of glyphosate herbicide. Int J Environ Anal Chem. 2015;95(15):1489–1501. doi:10.1080/03067319.2015.1114109.
- Majewski MS, Coupe RH, Foreman WT, Capel PD. Pesticides in Mississippi air and rain: a comparison between 1995 and 2007. Environ Toxicol Chem. 2014;33(6):1283–1293. doi:10.1002/etc.2550.
- Liang Y, Sun W, Zhu Y-G, Christie P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: a review. Environ Pollut. 2007;147(2):422–428. doi:10.1016/j.envpol.2006.06.008.
- Chang F, Simcik MF, Capel PD. Occurrence and fate of the herbicide glyphosate and its degradate aminomethylphosphonic acid in the atmosphere. Environ Toxicol Chem. 2011;30(3):548–555. doi:10.1002/etc.431.
- van Bruggen AHC, Finckh MR, He M, Ritsema CJ, Harkes P, Knuth D, Geissen V. Indirect effects of the herbicide glyphosate on plant, animal and human health through its effects on microbial communities. Front Environ Sci. 2021;9:9. doi:10.3389/fenvs.2021.763917.
- Williams GM, Aardema M, Acquavella J, Berry SC, Brusick D, Burns MM, de Camargo JLV, Garabrant D, Greim HA, Kier LD. A review of the carcinogenic potential of glyphosate by four independent expert panels and comparison to the IARC assessment. Crit Rev Toxicol. 2016;46(sup1):3–20. doi:10.1080/10408444.2016.1214677.
- EFSA. Peer review of the pesticide risk assessment of the potential endocrine disrupting properties of glyphosate. EFSA J. 2017;15:e04979.
- Rueda-Ruzafa L, Cruz F, Roman P, Cardona D. Gut microbiota and neurological effects of glyphosate. Neurotoxicology. 2019;75:1–8. doi:10.1016/j.neuro.2019.08.006.
- EFSA. Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J. 2015;13:4302.
- Belkaid Y, Harrison OJ. Homeostatic immunity and the microbiota. Immunity. 2017;46(4):562–576. doi:10.1016/j.immuni.2017.04.008.
- Heiss CN, Olofsson LE. Gut microbiota-dependent modulation of energy metabolism. J Innate Immun. 2018;10(3):163–171. doi:10.1159/000481519.
- Khan I, Bai Y, Zha L, Ullah N, Ullah H, Shah SRH, Sun H, Zhang C. Mechanism of the gut microbiota colonization resistance and enteric pathogen infection. Front Cell Infect Microbiol. 2021;11:1273. doi:10.3389/fcimb.2021.716299.
- Patterson E, Cryan JF, Fitzgerald GF, Ross RP, Dinan TG, Stanton C. Gut microbiota, the pharmabiotics they produce and host health. Proc Nutr Soc. 2014;73(4):477–489. doi:10.1017/S0029665114001426.
- O’Sullivan JN, Rea MC, Hill C, Ross RP. Protecting the outside: biological tools to manipulate the skin microbiota. FEMS Microbiol Ecol. 2020;96(6):fiaa085. doi:10.1093/femsec/fiaa085.
- Dang AT, Marsland BJ. Microbes, metabolites, and the gut–lung axis. Mucosal Immunol. 2019;12(4):843–850. doi:10.1038/s41385-019-0160-6.
- Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013. doi:10.1152/physrev.00018.2018.
- Cénit MC, Matzaraki V, Tigchelaar EF, Zhernakova A. Rapidly expanding knowledge on the role of the gut microbiome in health and disease. Biochim Biophys Acta - Mol Basis Dis. 2014;1842(10):1981–1992. doi:10.1016/j.bbadis.2014.05.023.
- Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26(1):26191. doi:10.3402/mehd.v26.26191.
- Claesson MJ, Jeffery IB, Conde S, Power SE, O’connor EM, Cusack S, Harris H, Coakley M, Lakshminarayanan B, O’sullivan O. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–184. doi:10.1038/nature11319.
- Erlandson KM, Liu J, Johnson R, Dillon S, Jankowski CM, Kroehl M, Robertson CE, Frank DN, Tuncil Y, Higgins J. An exercise intervention alters stool microbiota and metabolites among older, sedentary adults. Ther Adv Infect Dis. 2021;8:20499361211027068. doi:10.1177/20499361211027067.
- Durack J, Lynch SV. The gut microbiome: relationships with disease and opportunities for therapy. J Exp Med. 2019;216(1):20–40. doi:10.1084/jem.20180448.
- Clemente JC, Manasson J, Scher JU. The role of the gut microbiome in systemic inflammatory disease. Bmj. 2018;360:j5145. doi:10.1136/bmj.j5145.
- Mirza A, Mao-Draayer Y. The gut microbiome and microbial translocation in multiple sclerosis. Cl Immunol. 2017;183:213–224. doi:10.1016/j.clim.2017.03.001.
- Lopez MJ, Mohiuddin SS (2020). Biochemistry, essential amino acids.
- Zobiole LHS, Bonini EA, de Oliveira RS, Kremer RJ, Ferrarese-Filho O. Glyphosate affects lignin content and amino acid production in glyphosate-resistant soybean. Acta Physiol Plant. 2010;32(5):831–837. doi:10.1007/s11738-010-0467-0.
- Carbonari CA, Gomes GLGC, Velini ED, Machado RF, Simões PS, de Castro Macedo G. Glyphosate effects on sugarcane metabolism and growth. Amer J Plant Sci. 2014;5(24):3585. doi:10.4236/ajps.2014.524374.
- Casale J, Lydon J. Apparent effects of glyphosate on alkaloid production in coca plants grown in Colombia. J Forensic Sci. 2007;52(3):573–578. doi:10.1111/j.1556-4029.2007.00418.x.
- Steinrücken HC, AMRHEIN N. 5-enolpyruvylshikimate-3-phosphate synthase of Klebsiella pneumoniae. 2. Inhibition by glyphosate [N-(phosphononmethyl)glycine]. Eur J Biochem. 1984;143(2):351–357. doi:10.1111/j.1432-1033.1984.tb08379.x.
- Leino L, Tall T, Helander M, Saloniemi I, Saikkonen K, Ruuskanen S, Puigbo P. Classification of the glyphosate target enzyme (5-enolpyruvylshikimate-3-phosphate synthase) for assessing sensitivity of organisms to the herbicide. J Hazard Mater. 2021;408:124556. doi:10.1016/j.jhazmat.2020.124556.
- Gaines TA, Duke SO, Morran S, Rigon CAG, Tranel PJ, Küpper A, Dayan FE. Mechanisms of evolved herbicide resistance. J Biol Chem. 2020;295(30):10307–10330. doi:10.1074/jbc.REV120.013572.
- Gomes MP, Juneau P. Oxidative stress in duckweed (Lemna minor L.) induced by glyphosate: is the mitochondrial electron transport chain a target of this herbicide? Environ Pollut. 2016;218:402–409. doi:10.1016/j.envpol.2016.07.019.
- Staub JM, Brand L, Tran M, Kong Y, Rogers SG. Bacterial glyphosate resistance conferred by overexpression of an E. coli membrane efflux transporter. J Ind Microbiol Biotechnol. 2012;39(4):641–647. doi:10.1007/s10295-011-1057-x.
- Puigbò P, Leino LI, Rainio MJ, Saikkonen K, Saloniemi I, Helander M. Does glyphosate affect the human microbiota? Life. 2022;12(5):707. doi:10.3390/life12050707.
- Bote K, Pöppe J, Merle R, Makarova O, Roesler U. Minimum inhibitory concentration of glyphosate and of a glyphosate-containing herbicide formulation for Escherichia coli isolates–differences between pathogenic and non-pathogenic isolates and between host species. Front Microbiol. 2019;10:932. doi:10.3389/fmicb.2019.00932.
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi:10.1038/nature08821.
- Rajilić–Stojanović M, Biagi E, Heilig HGHJ, Kajander K, Kekkonen RA, Tims S, de Vos WM. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141(5):1792–1801. doi:10.1053/j.gastro.2011.07.043.
- Jacob GS, Garbow JR, Hallas LE, Kimack NM, Kishore GM, Schaefer J. Metabolism of glyphosate in Pseudomonas sp. strain LBr. Appl. Environ. Micro. 1988;54(12):2953–2958.
- Mesnage R, Antoniou MN. Computational modelling provides insight into the effects of glyphosate on the shikimate pathway in the human gut microbiome. Curr Res Toxicol. 2020;1:25–33. doi:10.1016/j.crtox.2020.04.001.
- Arbuckle TE, Lin Z, Mery LS. An exploratory analysis of the effect of pesticide exposure on the risk of spontaneous abortion in an Ontario farm population. Environ Health Perspect. 2001;109(8):851–857. doi:10.1289/ehp.01109851.
- Caballero M, Amiri S, Denney JT, Monsivais P, Hystad P, Amram O. Estimated residential exposure to agricultural chemicals and premature mortality by parkinson’s disease in Washington state. Int J Env Res Pub He. 2018;15(12):2885. doi:10.3390/ijerph15122885.
- de Roos AJ, Cooper GS, Alavanja MC, Sandler DP. Rheumatoid arthritis among women in the agricultural health study: risk associated with farming activities and exposures. Ann Epidemiol. 2005;15(10):762–770. doi:10.1016/j.annepidem.2005.08.001.
- Cantor KP, Blair A, Everett G, Gibson R, Burmeister LF, Brown LM, Schuman L, Dick FR. Pesticides and other agricultural risk factors for non-hodgkin’s lymphoma among men in Iowa and minnesota. Cancer Res. 1992;52:2447–2455.
- Eriksson M, Hardell L, Carlberg M, Åkerman M. Pesticide exposure as risk factor for non‐Hodgkin lymphoma including histopathological subgroup analysis. Int J Cancer. 2008;123(7):1657–1663. doi:10.1002/ijc.23589.
- Hardell L, Eriksson M. A case–control study of non‐Hodgkin lymphoma and exposure to pesticides. Cancer. 1999;85(6):1353–1360. doi:10.1002/(SICI)1097-0142(19990315)85:6<1353:AID-CNCR19>3.0.CO;2-1.
- McDuffie HH, Pahwa P, McLaughlin JR, Spinelli JJ, Fincham S, Dosman JA, Robson D, Skinnider LF, Choi NW. Non-hodgkin’s lymphoma and specific pesticide exposures in men: cross-Canada study of pesticides and health. Cancer Epidemiol Biomarkers Prev. 2001;10:1155–1163.
- Hoppin JA, Umbach DM, London SJ, Henneberger PK, Kullman GJ, Alavanja MCR, Sandler DP. Pesticides and atopic and nonatopic asthma among farm women in the agricultural health study. Am J Respir Crit Care Med. 2008;177(1):11–18. doi:10.1164/rccm.200706-821OC.
- von Ehrenstein OS, Ling C, Cui X, Cockburn M, Park AS, Yu F, Wu J, Ritz B. Prenatal and infant exposure to ambient pesticides and autism spectrum disorder in children: population based case-control study. Bmj. 2019;364:l962. doi:10.1136/bmj.l962.
- Henneberger PK, Liang X, London SJ, Umbach DM, Sandler DP, Hoppin JA. Exacerbation of symptoms in agricultural pesticide applicators with asthma. Int Arch Occup Environ Health. 2014;87(4):423–432. doi:10.1007/s00420-013-0881-x.
- Kamel F, Tanner CM, Umbach DM, Hoppin JA, Alavanja MC, Blair A, Comyns K, Goldman SM, Korell M, Langston JW. Pesticide Exposure and Self-reported Parkinson's Disease in the Agricultural Health Study. Am. J. Epidemiol. 2006;165(4):364–374. doi:10.1093/aje/kwk024.
- Zoller O, Rhyn P, Zarn JA, Dudler V. Urine glyphosate level as a quantitative biomarker of oral exposure. Int J Hyg Envir Heal. 2020;228:113526. doi:10.1016/j.ijheh.2020.113526.
- De Long NE, Holloway AC. Early-life chemical exposures and risk of metabolic syndrome. DMSO. 2017;Volume 10:101–109. doi:10.2147/DMSO.S95296.
- Prasad M, Gatasheh MK, Alshuniaber MA, Krishnamoorthy R, Rajagopal P, Krishnamoorthy K, Periyasamy V, Veeraraghavan VP, Jayaraman S. Impact of glyphosate on the development of insulin resistance in experimental diabetic rats: role of NFκB signalling pathways. Antioxidants. 2022;11(12):2436. doi:10.3390/antiox11122436.
- Beecham JE, Seneff S. Is there a link between autism and glyphosate-formulated herbicides. J Autism. 2016;3(1):1. doi:10.7243/2054-992X-3-1.
- Aitbali Y, Ba-M’hamed S, Elhidar N, Nafis A, Soraa N, Bennis M. Glyphosate based-herbicide exposure affects gut microbiota, anxiety and depression-like behaviors in mice. Neurotoxicol Teratol. 2018;67:44–49. doi:10.1016/j.ntt.2018.04.002.
- D’Brant J. GMOs, gut flora, the shikimate pathway and cytochrome dysregulation. Nutr Perspect J Council On Nutr. 2014;37(1):5–12.
- Hu J, Lesseur C, Miao Y, Manservisi F, Panzacchi S, Mandrioli D, Belpoggi F, Chen J, Petrick L. Low-dose exposure of glyphosate-based herbicides disrupt the urine metabolome and its interaction with gut microbiota. Sci Rep. 2021;11(1):1–10. doi:10.1038/s41598-021-82552-2.
- Drašar P, Poc P, Stárka L. Glyphosate, an important endocrine disruptor. Diabetol Metab Endokrinol Vyziv. 2018;47:777–780.
- Dai P, Hu P, Tang J, Li Y, Li C. Effect of glyphosate on reproductive organs in male rat. Acta Histochem. 2016;118(5):519–526. doi:10.1016/j.acthis.2016.05.009.
- Gallegos CE, Baier CJ, Bartos M, Bras C, Domínguez S, Mónaco N, Gumilar F, Giménez MS, Minetti A. Perinatal glyphosate-based herbicide exposure in rats alters brain antioxidant status, glutamate and acetylcholine metabolism and affects recognition memory. Neurotox Res. 2018;34(3):363–374. doi:10.1007/s12640-018-9894-2.
- Kreutz LC, Barcellos LJG, de Faria Valle S, de Oliveira Silva T, Anziliero D, dos Santos ED, Pivato M, Zanatta R. Altered hematological and immunological parameters in silver catfish (rhamdia quelen) following short term exposure to sublethal concentration of glyphosate. Fish Shellfish Immunol. 2011;30(1):51–57. doi:10.1016/j.fsi.2010.09.012.
- Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018;9(1):3294. doi:10.1038/s41467-018-05470-4.
- Nikolaus S, Schulte B, Al-Massad N, Thieme F, Schulte DM, Bethge J, Rehman A, Tran F, Aden K, Häsler R. Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology. 2017;153(6):1504–1516. doi:10.1053/j.gastro.2017.08.028.
- Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao C-C, Patel B, Yan R, Blain M. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22(6):586–597. doi:10.1038/nm.4106.
- Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, Ter Horst R, Jansen T, Jacobs L, Bonder MJ. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. 2016;167(4):1125–1136. doi:10.1016/j.cell.2016.10.020.
- Lee J-H, Wood TK, Lee J. Roles of indole as an interspecies and interkingdom signaling molecule. Trends Microbiol. 2015;23(11):707–718. doi:10.1016/j.tim.2015.08.001.
- Roager HM, Hansen L, Bahl MI, Frandsen HL, Carvalho V, Gøbel RJ, Dalgaard MD, Plichta DR, Sparholt MH, Vestergaard H. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat microbiol. 2016;1(9):1–9. doi:10.1038/nmicrobiol.2016.93.
- Gillezeau C, van Gerwen M, Shaffer RM, Rana I, Zhang L, Sheppard L, Taioli E. The evidence of human exposure to glyphosate: a review. Environ Health-Glob. 2019;18(1):1–14. doi:10.1186/s12940-018-0435-5.
- Acquavella JF, Alexander BH, Mandel JS, Burns CJ, Gustin C. Exposure misclassification in studies of agricultural pesticides: insights from biomonitoring. Epidemiology. 2006;17(1):69–74. doi:10.1097/01.ede.0000190603.52867.22.
- Trasande L, Aldana SI, Trachtman H, Kannan K, Morrison D, Christakis DA, Whitlock K, Messito MJ, Gross RS, Karthikraj R. Glyphosate exposures and kidney injury biomarkers in infants and young children. Environ Pollut. 2020;256:113334. doi:10.1016/j.envpol.2019.113334.
- Ehling S, Reddy TM. Analysis of glyphosate and aminomethylphosphonic acid in nutritional ingredients and milk by derivatization with fluorenylmethyloxycarbonyl chloride and liquid chromatography–mass spectrometry. J Agr Food Chem. 2015;63(48):10562–10568. doi:10.1021/acs.jafc.5b04453.
- Aris A, Leblanc S. Maternal and fetal exposure to pesticides associated to genetically modified foods in Eastern Townships of Quebec, Canada. Adv Exp Med Biol. 2011;31(4):528–533. doi:10.1016/j.reprotox.2011.02.004.
- Dick RE, Quinn JP. Glyphosate-degrading isolates from environmental samples: occurrence and pathways of degradation. Appl Microbiol Biotechnol. 1995;43(3):545–550. doi:10.1007/BF00218464.
- Jacob GS, Garbow JR, Hallas LE, Kimack NM, Kishore GM, Schaefer J. Metabolism of glyphosate in Pseudomonas sp. strain LBr. Appl Environ Microb. 1988;54(12):2953–2958. doi:10.1128/aem.54.12.2953-2958.1988.
- Singh BK, Walker A. Microbial degradation of organophosphorus compounds. FEMS Microbiol Rev. 2006;30(3):428–471. doi:10.1111/j.1574-6976.2006.00018.x.
- Singh S, Kumar V, Gill JPK, Datta S, Singh S, Dhaka V, Kapoor D, Wani AB, Dhanjal DS, Kumar M. Herbicide glyphosate: toxicity and microbial degradation. Int J Env Res Pub He. 2020;17(20):7519. doi:10.3390/ijerph17207519.
- Huch M, Stoll DA, Kulling SE, Soukup ST. Metabolism of glyphosate by the human fecal microbiota. Toxicol Lett. 2022;358:1–5. doi:10.1016/j.toxlet.2021.12.013.
- Franke AA, Li X, Shvetsov YB, Lai JF. Pilot study on the urinary excretion of the glyphosate metabolite aminomethylphosphonic acid and breast cancer risk: the multiethnic cohort study. Environ Pollut. 2021;277:116848. doi:10.1016/j.envpol.2021.116848.
- Jentzmik F, Stephan C, Miller K, Schrader M, Erbersdobler A, Kristiansen G, Lein M, Jung K. Sarcosine in urine after digital rectal examination fails as a marker in prostate cancer detection and identification of aggressive tumours. Eur Urol. 2010;58(1):12–18. doi:10.1016/j.eururo.2010.01.035.
- Jones AR. Some observations on the urinary excretion of glycine conjugates by laboratory animals. Xenobiotica. 1982;12(6):387–395. doi:10.3109/00498258209052480.
- Lin ELC, Mattox JK, Daniel FB. Tissue distribution, excretion, and urinary metabolites of dichloroacetic acid in the male Fischer 344 rat. J Toxicol Environ Health, Part A Curr Issues. 1993;38(1):19–32. doi:10.1080/15287399309531697.
- Blot N, Veillat L, Rouzé R, Delatte H, Rueppell O. Glyphosate, but not its metabolite AMPA, alters the honeybee gut microbiota. PloS One. 2019;14(4):e0215466. doi:10.1371/journal.pone.0215466.
- Feng Y-L, Cao G, Chen D-Q, Vaziri ND, Chen L, Zhang J, Wang M, Guo Y, Zhao Y-Y. Microbiome–metabolomics reveals gut microbiota associated with glycine-conjugated metabolites and polyamine metabolism in chronic kidney disease. Cell Mol Life Sci. 2019;76(24):4961–4978. doi:10.1007/s00018-019-03155-9.
- Norikura T, Sasaki Y, Kojima-Yuasa A, Kon A. Glyoxylic acid, an α-keto acid metabolite derived from glycine, promotes myogenesis in C2C12 cells. Nutrients. 2023;15(7):1763. doi:10.3390/nu15071763.
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457(7231):910–914. doi:10.1038/nature07762.
- Mbage B, Li Y, Si H, Zhang X, Li Y, Wang X, Salah A, Zhang K. Fabrication of folate functionalized polyoxometalate nanoparticle to simultaneously detect H2O2 and sarcosine in colorimetry. Sens Actuators B Chem. 2020;304:127429. doi:10.1016/j.snb.2019.127429.
- Lane H-Y, Liu Y-C, Huang C-L, Chang Y-C, Liau C-H, Perng C-H, Tsai GE. Sarcosine (N-methylglycine) treatment for acute schizophrenia: a randomized, double-blind study. Biol Psychiatry. 2008;63(1):9–12. doi:10.1016/j.biopsych.2007.04.038.
- Nielsen LN, Roager HM, Casas ME, Frandsen HL, Gosewinkel U, Bester K, Licht TR, Hendriksen NB, Bahl MI. Glyphosate has limited short-term effects on commensal bacterial community composition in the gut environment due to sufficient aromatic amino acid levels. Environ Pollut. 2018;233:364–376. doi:10.1016/j.envpol.2017.10.016.
- Yang X, Song Y, Zhang C, Pang Y, Song X, Wu M, Cheng Y. Effects of the glyphosate-based herbicide roundup on the survival, immune response, digestive activities and gut microbiota of the Chinese mitten crab, eriocheir sinensis. Aquat Toxicol. 2019;214:105243. doi:10.1016/j.aquatox.2019.105243.
- Hidalgo-Cantabrana C, Delgado S, Ruiz L, Ruas-Madiedo P, Sánchez B, Margolles A. Bifidobacteria and their health‐promoting effects. Bugs As Drugs: Ther Microbes Prev Treat Dis. 2018;79:7628–7638.
- Saturio López S, Nogacka A, Alvarado-Jasso GM, Salazar N, González de Los Reyes-Gavilán C, Gueimonde Fernández M, Arboleya S. Role of bifidobacteria on infant health. Microorganisms. 2021;9(12):2415. doi:10.3390/microorganisms9122415.
- Ruiz L, Delgado S, Ruas-Madiedo P, Sánchez B, Margolles A. Bifidobacteria and their molecular communication with the immune system. Front Microbiol. 2017;8:2345. doi:10.3389/fmicb.2017.02345.
- Shehata AA, Schrödl W, Aldin AA, Hafez HM, Krüger M. The effect of glyphosate on potential pathogens and beneficial members of poultry microbiota in vitro. Curr Microbiol. 2013;66(4):350–358. doi:10.1007/s00284-012-0277-2.
- Rodrigues NR, de Souza APF. Occurrence of glyphosate and AMPA residues in soy-based infant formula sold in Brazil. Food Addit Contam. 2018;35(4):724–731. doi:10.1080/19440049.2017.1419286.
- Walker M. Formula supplementation of breastfed infants: helpful or hazardous? ICAN: Infant, Child, Adolescent Nutr. 2015;7(4):198–207. doi:10.1177/1941406415591208.
- Liu J-B, Chen K, Li Z-F, Wang Z-Y, Wang L. Glyphosate-induced gut microbiota dysbiosis facilitates male reproductive toxicity in rats. Sci Total Environ. 2022;805:150368. doi:10.1016/j.scitotenv.2021.150368.
- Mesnage R, Panzacchi S, Bourne E, Mein CA, Perry MJ, Hu J, Chen J, Mandrioli D, Belpoggi F, Antoniou MN. Glyphosate and its formulations roundup bioflow and rangerpro alter bacterial and fungal community composition in the rat caecum microbiome. bioRxiv. 2021;13.
- Del Castilo I, Neumann AS, Lemos FS, de Bastiani MA, Oliveira FL, Zimmer ER, Rêgo AM, Hardoim CCP, Antunes LCM, Lara FA. Lifelong exposure to a low-dose of the glyphosate-based herbicide RoundUp® causes intestinal damage, gut dysbiosis, and behavioral changes in mice. Int J Mol Sci. 2022;23(10):5583. doi:10.3390/ijms23105583.
- Seshadri S, Shea T. Elevated plasma homocysteine levels: risk factor or risk marker for the development of dementia and alzheimer’s disease? J Alzheimer’s Dis. 2006;9(4):393–398. doi:10.3233/JAD-2006-9404.
- Barnett JA, Bandy ML, Gibson DL. Is the Use of Glyphosate in Modern Agriculture Resulting in Increased Neuropsychiatric Conditions Through Modulation of the Gut-brain-microbiome Axis? Front. Nutr. 2022;9. doi:10.3389/fnut.2022.827384.
- Spencer CM, Alekseyenko O, Hamilton SM, Thomas AM, Serysheva E, Yuva‐Paylor LA, Paylor R. Modifying behavioral phenotypes in Fmr1KO mice: genetic background differences reveal autistic‐like responses. Autism Res. 2011;4(1):40–56. doi:10.1002/aur.168.
- Kondoh T, Mallick HN, Torii K. Activation of the gut-brain axis by dietary glutamate and physiologic significance in energy homeostasis. Am J Clin Nutr. 2009;90(3):832S–837S. doi:10.3945/ajcn.2009.27462V.
- Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75(3):207–246. doi:10.1016/j.pneurobio.2005.02.004.
- Kandeel WA, Meguid NA, Bjørklund G, Eid EM, Farid M, Mohamed SK, Wakeel KE, Chirumbolo S, Elsaeid A, Hammad DY. Impact of Clostridium bacteria in children with autism spectrum disorder and their anthropometric measurements. J Mol Neurosci. 2020;70(6):897–907. doi:10.1007/s12031-020-01482-2.
- Zuo Z, Fan H, Tang X, Chen Y, Xun L, Li Y, Song Z, Zhai H. Effect of different treatments and alcohol addiction on gut microbiota in minimal hepatic encephalopathy patients. Exp Ther Med. 2017;14(5):4887–4895. doi:10.3892/etm.2017.5141.
- Owagboriaye F, Mesnage R, Dedeke G, Adegboyega T, Aladesida A, Adeleke M, Owa S, Antoniou MN. Impacts of a glyphosate-based herbicide on the gut microbiome of three earthworm species (alma millsoni, eudrilus eugeniae and libyodrilus violaceus): a pilot study. Toxicol Rep. 2021;8:753–758. doi:10.1016/j.toxrep.2021.03.021.
- Castelli L, Balbuena S, Branchiccela B, Zunino P, Liberti J, Engel P, Antúnez K. Impact of chronic exposure to sublethal doses of glyphosate on honey bee immunity, gut microbiota and infection by pathogens. Microorganisms. 2021;9(4):845. doi:10.3390/microorganisms9040845.
- Almasri H, Liberti J, Brunet J-L, Engel P, Belzunces LP. Mild chronic exposure to pesticides alters physiological markers of honey bee health without perturbing the core gut microbiota. Sci Rep. 2022;12(1):1–15. doi:10.1038/s41598-022-08009-2.
- Mesnage R, Teixeira M, Mandrioli D, Falcioni L, Ducarmon QR, Zwittink RD, Mazzacuva F, Caldwell A, Halket J, Amiel C. Use of shotgun metagenomics and metabolomics to evaluate the impact of glyphosate or Roundup MON 52276 on the gut microbiota and serum metabolome of Sprague-Dawley rats. Environ Health Perspect. 2021;129(1):017005. doi:10.1289/EHP6990.
- Gardiner BJ, Tai AY, Kotsanas D, Francis MJ, Roberts SA, Ballard SA, Junckerstorff RK, Korman TM, Bourbeau P. Clinical and microbiological characteristics of eggerthella lenta bacteremia. J Clin Microbiol. 2015;53(2):626–635. doi:10.1128/JCM.02926-14.
- Nayfach S, Shi ZJ, Seshadri R, Pollard KS, Kyrpides NC. New insights from uncultivated genomes of the global human gut microbiome. Nature. 2019;568(7753):505–510. doi:10.1038/s41586-019-1058-x.
- Mesnage R, Antoniou MN. Facts and fallacies in the debate on glyphosate toxicity. Front Public Health. 2017;5:316. doi:10.3389/fpubh.2017.00316.
- Ackermann W, Coenen M, Schrödl W, Shehata AA, Krüger M. The influence of glyphosate on the microbiota and production of botulinum neurotoxin during ruminal fermentation. Curr Microbiol. 2015;70(3):374–382. doi:10.1007/s00284-014-0732-3.
- Riede S, Toboldt A, Breves G, Metzner M, Köhler B, Bräunig J, Schafft H, Lahrssen‐Wiederholt M, Niemann L. Investigations on the possible impact of a glyphosate‐containing herbicide on ruminal metabolism and bacteria in vitro by means of the ‘rumen simulation technique. J Appl Microbiol. 2016;121(3):644–656. doi:10.1111/jam.13190.
- Sørensen MT, Poulsen HD, Katholm CL, Højberg O. Feed residues of glyphosate–potential consequences for livestock health and productivity. Animal. 2021;15(1):100026. doi:10.1016/j.animal.2020.100026.
- Billenkamp F, Schnabel K, Hüther L, Frahm J, von Soosten D, Meyer U, Höper D, Beer M, Seyboldt C, Neubauer H. No hints at glyphosate-induced ruminal dysbiosis in cows. npj Biofilm Microbio. 2021;7(1):1–13. doi:10.1038/s41522-021-00198-4.
- Séralini G-E, Clair E, Mesnage R, Gress S, Defarge N, Malatesta M, Hennequin D, de Vendômois JS. Republished study: long-term toxicity of a roundup herbicide and a roundup-tolerant genetically modified maize. Environ Sci Europe. 2014;26(1):1–17. doi:10.1186/s12302-014-0014-5.
