ABSTRACT
The present report summarizes the United States Department of Veterans Affairs (VA) field-based meeting titled “Modulating microbiome-immune axis in the deployment-related chronic diseases of Veterans.” Our Veteran patient population experiences a high incidence of service-related chronic physical and mental health problems, such as infection, irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), various forms of hematological and non-hematological malignancies, neurologic conditions, end-stage organ failure, requiring transplantation, and posttraumatic stress disorder (PTSD). We report the views of a group of scientists who focus on the current state of scientific knowledge elucidating the mechanisms underlying the aforementioned disorders, novel therapeutic targets, and development of new approaches for clinical intervention. In conclusion, we dovetailed on four research areas of interest: 1) microbiome interaction with immune cells after hematopoietic cell and/or solid organ transplantation, graft-versus-host disease (GVHD) and graft rejection, 2) intestinal inflammation and its modification in IBD and cancer, 3) microbiome-neuron-immunity interplay in mental and physical health, and 4) microbiome-micronutrient-immune interactions during homeostasis and infectious diseases. At this VA field-based meeting, we proposed to explore a multi-disciplinary, multi-institutional, collaborative strategy to initiate a roadmap, specifically focusing on host microbiome-immune interactions among those with service-related chronic diseases to potentially identify novel and translatable therapeutic targets.
Meeting background
The Department of Veterans Affairs (VA) Field-Based meeting, which took place in Chicago on May 5, 2023, was organized to facilitate discussions on microbiota-immune interactions in both homeostasis and disease, with the goal of potentially developing a multidisciplinary research collaboration in which state-of-the-art approaches would be used to investigate deployment-related chronic diseases affecting Veterans. Specific investigators and potential collaborators from the VA-funded space were invited to share novel ideas and visions related to foregoing topics, which are expected to initiate fruitful collaborations.
Members of our Veteran patient population experience a high incidence of service-related chronic illnesses. These include infections, irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), various forms of hematological and non-hematological malignancies, end-stage organ failure requiring transplantation, and mental health and neurologic disorders associated with the gut-brain axis, including posttraumatic stress disorder (PTSD). Many of these diseases can be triggered by severe stress, exposure to environmental toxins (e.g., agent orange), and altered composition of the microbiome. The human microbiome includes bacteria, viruses, and fungi that reside in our body. In the meeting, we emphasized the role of the microbiome not only in the intestine, but also in other organs, such as mouth, nose, lung, blood, and tumor. Environmental exposures, e.g., burn pit, jet fuel, may occur while individuals are deployed to diverse geographies nearby or overseas, particularly in the context of combat conditions. The altered composition and function of the gut microbiome and associated metabolites in deployed military personnel may also occur in areas with suboptimal hygienic conditions. The service connections of many of these disorders have been extensively investigated and established, particularly since the Gulf War. Previous studies have documented links between service-related conditions and environmental factors, including the causative link between agent orange exposure and hematological malignancies in Vietnam War Veterans. These service-related disorders can be lethal (e.g., cancer), and many of them are painful, disabling, and chronic. Long after deployment, service members can experience ongoing symptoms and disease sequelae, demonstrate organ failure, or develop malignant disorders that require either hematopoietic cell transplantation or solid organ transplantation. Transplantation can result in lethal and devastating graft-versus-host disease or graft rejection.
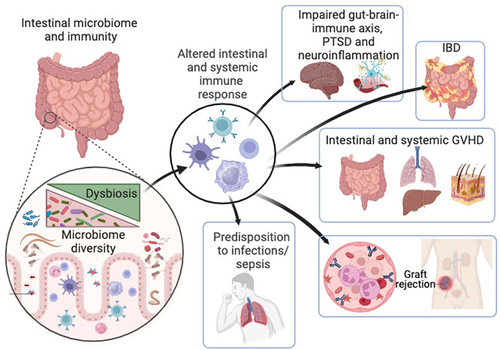
Recent scientific discoveries have greatly enhanced our understanding of the complex crosstalk between microbiome and host immunity. Altered composition and function of the microbiome can lead to various disorders. Altered microbiome composition and metabolic activities thereof, also called dysbiosis not only affects intestinal homeostasis but also extraintestinal organs, including the brain, lung, liver, and skin (). Dysregulation of microbiome-immune interactions may manifest in various organ pathologies, including IBD, cancer, and neuroinflammatory diseases. Crosstalk between the microbiome and immune cells can also affect the outcome of treatment, as observed in Veterans undergoing hematopoietic cell transplantation (HCT) to treat hematological malignancies, where gut dysbiosis is highly associated with the development of lethal and devastating GVHD. Many unknowns and challenges remain to be elucidated by disentangling the underlying mechanisms involving microbiome-immunity interplay in hosts during homeostasis and disease.
Focused topics
The goals of our VA Field-Based meeting were to further define the significance of microbiota and associated metabolites that drive the fate of host immunity and to collaboratively elucidate potentially involved mechanisms in the microbiome and associated metabolites in both, health and disease, and the consequences thereafter. To identify fruitful research targets and promote collaboration between VA-based researchers/clinicians and non-VA affiliated researchers, short talks/idea exchanges were presented that centered on the areas specified below. Such collaborative efforts are expected to utilize multiomic approaches, apply machine learning, establish novel genetically engineered animal models, and employ organoids from biopsies of Veterans to interrogate mechanisms implicated in diseases and the potential link to microbiome–host interactions. Furthermore, the meeting highlighted various aspects of the current knowledge gaps and discussed the molecular mechanisms potentially driving local and peripheral disease progression. Moreover, at the conference, investigators vividly discussed the dysregulation of microbiome-immune interactions in various human diseases, and the challenges and limitations in achieving a causal understanding of host microbiome-immune interplay to obtain critical insights into these areas of interest, which can translate toward future development of microbiome-targeted therapeutic interventions. The focus was on novel and personalized management possibilities for these systemic inflammatory diseases. These disorders are grouped in 4 focus areas and include:
Focus 1: microbiome interactions with the immune system after hematopoietic cell and/or solid organ transplantation (GVHD and graft rejection)
Each year, more than 20,000 hematopoietic cells and more than 35,000 solid organ transplantations are performed in the United States. Hematopoietic cell transplantation (HCT) is performed in most cases as a curative treatment for hematological malignancies, such as leukemia or myeloma, whereas solid organ transplantation (SOT) is a state-of-the-art therapeutic practice to restore the function of a failing organ, such as the kidney, liver, or heart. In certain cases, the combined transplantation of hematopoietic cells and solid organs is performed to treat hematological malignancies and organ failure.
Veterans constitute an important group of these patients because they are prone to develop hematological malignancies due to exposure to different toxins during their service, such as agent orange. Veterans are more likely to develop end-stage organ failure. The frequency of kidney disease was higher among Veterans. Furthermore, more than 40,000 Veterans with end-stage renal disease are enrolled in the VA Health System and receive treatment via dialysis or transplantation. The outcome of transplantation is adversely affected by graft-versus-host disease (GVHD) after HCT, and organ rejection after SOT. In this context, studies suggest that intestinal microbiota is dysregulated after HCT and in patients who develop GVHD,Citation1 and monitoring and remodeling of microbiota after HCT can lead to the development of novel diagnostic tools, such as new disease markers or novel therapeutics in the form of elements of gut microbiota.Citation2 Similarly, dysbiosis occurs after SOTCitation3, and it is believed to be caused by immunosuppressive therapy, which while preventing rejection, predisposes the host to various complications, such as life-threatening infections, cancer, or metabolic syndrome. Achieving immune tolerance after solid organ transplantation without the use of immunosuppressive medications is an ideal endpoint in clinical transplantation and is being investigated by inducing in vivo tolerogenic states, such as mixed chimerism.Citation4 Whether targeting intestinal microbiota modulates graft rejection is an important question in transplantation medicine,Citation5 which the Focus 1 group asked during the Field-Based Meeting in an attempt to synergize research efforts in collaboration with other focus groups.
Focus 2: intestinal inflammation and immune dysfunction (IBD and cancer therapy)
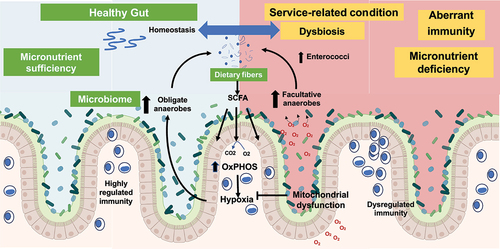
Research into the pathogenesis of IBD has extended across broad landscapes to identify important insights into the roles of the intestinal microbiome and mucosal metabolism in the regulation of intestinal adaptive and innate immune responses. The data presented at this meeting illustrate the role of intestinal epithelial mitochondrial respiration (reduced in IBD)Citation6 on barrier function as well as the composition of the intestinal microbiomeCitation7–9 (). Further data highlighted the role of goblet cell-associated antigen passages (GAPs) that deliver luminal substances to the underlying lamina propria (LP) antigen-presenting cells (APCs). A dysbiotic microbiome adversely affects GAP responses needed to maintain regulation of mucosal T cell populations.Citation10 The importance of genetic dysregulation of innate regulatory pathways in IBD pathogenesis was highlightedCitation11 in the discussion. Ranjan et al. identified IBD-associated genetic variants that confer disease risk through both excessive and inadequate innate immune responses. This work shows the utility of defining the functional implications of pathways identified through genetic associations as a means of understanding disturbances in immune regulation underlying disease activity in patients with IBD.Citation11 Using a powerful tool, Focus Group 2 also presented results from a T cell receptor transgenic mouse that was specific for the CBir flagellin antigen. These results indicate that restoration of a healthy microbiome may improve wound healing by enhancing short chain fatty acid (SCFA) induction of IL-22 production.Citation12 Overall, a multilayered approach to IBD pathogenesis has raised a multitude of potential targets for novel therapeutic discovery. A similar multilayered approach could be applied to research on colitis-associated colon cancer and other chronic diseases, as discussed in other focus groups. A working model of the microbiome and immunity has been proposed for mechanistic studies ().
Figure 2. Mechanism of intestinal microbial architecture and immunity in health and disease.
Focus 3: microbiome-neuron-immunity axis (mental and physical health of Veterans)
Owing to genetic and environmental factors, imbalances in microbiota-immune interactions in a susceptible host can trigger severe inflammation and initiate acute and chronic disorders. In this context, neurological and psychiatric disorders are associated with specific molecular and microbial profiles.Citation13–18 In the case of posttraumatic stress disorder (PTSD), which can occur after a life-threatening experience, such as combat on the battle field. Individuals develop symptoms, which include severe anxiety, cognitive problems, and challenges with functioning due to psychological distress. Although often conceptualized as a disorder primarily associated with the brain, recent research suggests that PTSD is associated with dysbiosis of the gut and oral microbiomes.Citation19 While the cause and effect remain unproven, the relationship between PTSD and the gut microbiome is likely to be bi-directional: alterations in commensal bacteria may modulate PTSD symptoms via gut-derived neurohormonal and immune pathways, while at the same time, trauma and stressor exposure-associated dysautonomia, through the enteric nervous system, may affect gut microbial composition.Citation20 Besides the PTSD, military Veterans, regardless of the branch of service, the era in which they served, or whether they served during a time of peace or war, are at a greater risk of succumbing to death from the devastating motor neuron disease, amyotrophic lateral sclerosis (ALS) than if they had not served in the military. For reasons yet unknown, Veterans are twice as likely to be diagnosed with ALS compared to the general population. A study by Martin et al. highlighted the novel role of the microbiome in the development and progression of ALS.Citation18 Targeting the intestinal microbiome and immunity could be a novel strategy for ALS.Citation18,Citation21 As such, the gut microbiome-immune axis constituted the focus of our Field-Based meeting. Specifically, we discussed biological phenomena associated with conditions of interest, as well as the mechanisms underlying these disorders, with the goal of identifying novel therapeutic targets and developing new approaches for clinical intervention. Some work in this area focused on addressing symptoms associated with mild traumatic brain injury and/or PTSD, which can improve after administration of immunomodulatory probiotics, and these studies have already commencedCitation15 and were presented. This Focus Group identified a gap in the mechanistic studies of microbiome dysbiosis and immune dysfunction in Veterans affected by PTSD (undernutrition, neuroinflammation, infections).Citation15–17 A key direction of these collaborative efforts is to investigate the pivotal role of dysbiosis in modulating the interaction between gut and brain immunity, the impact of induced life-threatening stress, and stress mediators, which potentially ravage barrier integrity and neuronal inflammation. These efforts are expected to facilitate the development of a roadmap to design novel research projects and at the end to provide much-needed strategies or therapeutic approaches, which can promote the homeostatic effects of microbes on host microbiome-immune axis. Same strategies are also anticipated to materialize in microbiome transplant experiments in humans and rodents, including germ-free and gnotobiotic animals.
Focus 4: microbe-immune-micronutrition interactions in infection and inflammation
This group discussed the critical micronutrients that reprogram the functions of phagocytic cells to resist pathogen-induced inflammation. Multilayered mechanisms implicated in neuroinflammatory diseases such as Alzheimer’s disease (AD) require further investigation. However, tauopathy-associated neuroinflammation can be increasingly induced by risk factors, including micronutrient deficiencies or pathogen infection.Citation22–32 Induced pathogenic neuroinflammationCitation29,Citation33 may then functionally derail microglia that disrupt synaptic functionsCitation34–37 and hijack cerebral homeostasis, potentially contributing to cognitive deficits.Citation27,Citation37–39 Compelling evidence highlights the significance of functional microbiome and the associated metabolites in controlling vagus nerve activity, to tonically transmit bidirectional signals from the viscera to the brain that regulate inflammation via neuronal motor efferents.Citation40–44 Furthermore, microbiome-associated metabolites also tightly support the maintenance of gut-resident macrophages (gMacs),Citation45 strategically situated in the muscularis externa, to interact with enteric neurons (ENs) that critically initiate gut motility.Citation46,Citation47 Hence, it is plausible to recognize the detrimental impact of pathogen-induced neuroinflammation on critically deteriorating host-brain homeostasis that may profoundly contribute to hypertauopathy and the consequences thereafter. Thus, considering pathogenic infection inciting neuroinflammation and how this can be reprogrammed through microbiome-associated metabolites serving as micronutrients and functioning as crucial factors that contribute to regulated genomic programs to potentially mitigate neurodegenerative diseases (e.g., AD) represent a novel line of inquiry and may warrant further investigations.Citation27 To further delve into vitamin physiology, we posit that vitamin B12 controls the transcriptomic and metabolomic machinery of phagocytic cells (e.g., microglia, gMacs) and critically sustains healthy microbiomes and the associated metabolites to synergistically limit pathogen-induced neuroinflammation that may otherwise contribute to host memory loss.
Micronutrients play a pivotal role in maintaining overall health and facilitating optimal functioning of the human body. Among these, vitamin D is notable for its unique function as a potent immunomodulator. Sepsis is a complex and potentially life-threatening condition that stems from an excessive immune response to infection, resulting in organ dysfunction. Numerous studies have highlighted the potential correlation between inadequate vitamin D levels and an increased risk of sepsis among critically ill patients. However, clinical trials have shown that the early administration of high-dose enteral vitamin D3 during critical illness can rapidly correct vitamin D deficiency. Yet, this correction does not appear to yield discernible benefits in terms of mortality or other clinically significant outcomes,Citation48.Citation49 and the underlying reasons for this phenomenon remain elusive. The effects of vitamin D are primarily mediated through its receptor, known as the vitamin D receptor (VDR), which is distributed across various tissues in the body, including the intestines, kidneys, skin, immune cells, and different organ systems. VDR is known to exert immunomodulatory effects that influence the body’s response to infections and inflammatory processes.Citation50,Citation51 Vitamin D, in conjunction with VDR, can regulate the expression of numerous genes related to immune responses, impacting the microbiome/virome and host immunity.Citation52,Citation53 However, currently, there is limited information regarding the association between VDR and sepsis. Therefore, unraveling the intricate interplay among micronutrients, microbiome, and immune responses in the Veterans is crucial.
Roadmap and gaps
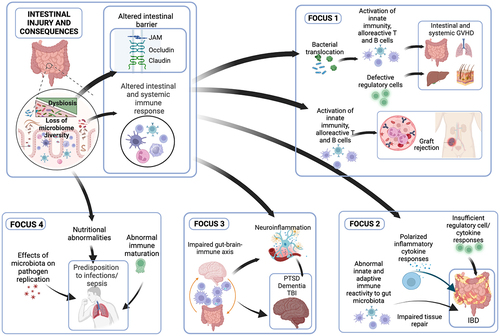
From meeting discussions, specific four Focuses are developed and tightly centered on defining translational and basic research platforms for reshaping the microbiome, detecting prognosticates, and treating prevalent chronic diseases in the Veteran population (). Our central hypothesis was that dysregulation of the microbiome and immune interactions contributes to deployment-related diseases, including chronic intestinal, mental health, and neurologic disorders. An improved understanding of the microbiome and the relevance of critical micronutrients may provide in-depth insights into the underlying mechanisms implicated in the deficiency of these crucial cofactors and whether these vitamins can reprogram the molecular machinery of cells to resist pathogen-induced inflammation. The outcomes are anticipated to lead to the development of novel therapeutic interventions through pathways such as micronutrients, mitochondria, and microbiome functions.
Figure 3. Focus areas of deployment-related diseases and their synergy in mechanistic studies for mechanisms and innovative treatments.
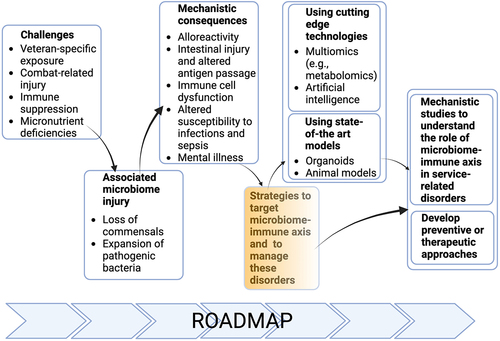
We agreed that for progress to be made, efforts should be directed to design mechanistic studies that address the interplay between the microbiome and immune system in pathogenesis of alloreactivity, intestinal and neuronal inflammation as well as local and systemic response to pathogen infection. Successful address of these mechanistic questions could benefit from the development and use of novel tools, such as organoids or novel preclinical animal models, or cutting-edge technologies, such as large-scale sample analysis using multiomics or application of artificial intelligence (AI) (). To better understand the etiopathogenesis of these disorders in the context of microbiome-immune axis and to develop novel preventive or therapeutic approaches, we propose to develop and apply novel tools, such as organoids or humanized animal models, and cutting edge technologies, like spatial analysis, machine learning, multiomic approaches to microbiome and its metabolic activities. Of benefit will be the centralized and collaborative use of microbiome and metabolomic research, as well as the above-mentioned novel tools and cutting-edge technologies. To accomplish these goals and reach our strategic endpoints, we also propose to establish collaborative networks supported by efforts to centralize the data, share resources and develop core facilities/laboratories. Our efforts detailed on our roadmap are expected to help design novel mechanistic studies and develop novel therapeutic or preventative approaches to manage the clinical conditions discussed in four focus groups affecting Veterans.
Figure 4. Roadmap of modulating microbiome-immune axis in the deployment-related chronic diseases of Veterans.
The conference was based on delineating the commonalities and differences between conditions impacted by changes in regulated immunity and the microbiome and the associated metabolites in hosts. In response to the request for applications of collaborative merit review awards by the BLRD and CSRD of the Department of Veterans Affairs (RFA: BX23–007) and to investigate these commonalities and differences, we propose to submit several merit award applications, developed in synergy between focus groups despite the diversities among these groups, to address mutual aspects of service-related injuries and the consequences that may impact digestive physiology and extraintestinal organs.
Conclusion and future plans
In summary, during this VA field-based meeting, we discussed specific diseases related to immunity and the microbiome. We identified four special focuses (). Attendees started to establish the foundations of future research proposals during their presentations at the meeting, which highlighted the importance of mechanistic links between the microbiome and immune system, between the microbiome and diseases that the different focuses of the aforementioned research groups and between immune dysregulation and these diseases. At conclusion of the meeting, we developed a strategy on how to target these mechanistic links. The short-term goal is to develop a synergistic approach between grant applications before submission and structure innovative approaches that involve cutting edge technologies like machine learning to facilitate progress in this endeavor. In long-term goal, we are determined to characterize the functions of intestinal and extra-intestinal microbiomes, such as the oral microbiome, nasal microbiome, and lung microbiome, in health and disease, address-specific immune disorders prevalent in the Veteran population and the impact of dysbiosis on these diseases, and explore microbe-host immune interactions in the pathogenesis of these diseases and the role of microbiome/metabolite-based therapeutic approaches.
We expect our roadmap () to enable the researchers develop collaborative merit award applications in synergy to potentially identify novel and translatable therapeutic targets for the management of these disorders associated with deployment. This work could potentially be translatable to Veterans in other countries and more broadly to the worldwide population for disease prevention and treatment.
Grant support
Sponsored by Veterans Affairs Central Office through a Field-Based Planning Meeting Award (J.S. and M.N.I).
Author contributions
All authors contributed to the planning and presentations during the meeting and were involved in the drafting and finalizing of the manuscript.
Availability of data and material
No new data were generated during the meetings.
Acknowledgments
We thank Drs. Masood Khan and Arun Sharma from the Veterans Affairs Central Office and Office of Research and Development for advising the investigators about the VA Collaborative Merit application process.
Disclosure statement
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
JS, MNI, CA, YZ, RD, PKD, DE, TSG, PSH, AH, TKK, NK, JL, MM, EM, IR, CST, XT, JW, and ZZ declare no conflict of interest.
TAB is a co-inventor of the U.S. Nonprovisional Application Serial No. PCT/US21/43774 Accelerating the repair of mucosal injury using gold(III) compounds.
RDN is a co-inventors on U.S. Nonprovisional Application Serial No. 15/880,658 Compositions And Methods For Modulation Of Dietary And Microbial Exposure
TKK is a consultant for Agenus and Immunobiome.
LAB reports grants from the VA, DOD, NIH, and the State of Colorado; editorial remuneration from Wolters Kluwer and the Rand Corporation; and royalties from the American Psychological Association and Oxford University Press. In addition, she consulted sports leagues through her university affiliation.
KI reports grants from the VA, NIH, Office of Naval Research (DOD), Marfan Foundation, and support from the University of Iowa (The Iowa Aging Initiative and Professorship in Cardiovascular Medicine). He is a co-inventor of U.S. Provisional Patent Application # 18/165,809 Methods and Compositions Comprising Therapeutic Gamma Peptide Nucleic Acid-based Molecules.
JUP reports research funding, intellectual property fees, and travel reimbursement from Seres Therapeutics and consulting fees from DaVolterra, CSL Behring, and MaaT Pharma. He serves on an advisory board and holds equity in Postbiotics Plus Research. He filed intellectual property applications related to the microbiome (reference numbers #62/843,849, #62/977,908, and 15/756,845). The Memorial Sloan Kettering Cancer Center (MSK) has financial interests relative to Seres Therapeutics.
Additional information
Funding
References
- Hong T, Wang R, Wang X, Yang S, Wang W, Gao Q, Zhang X. Interplay between the intestinal microbiota and acute graft-versus-host disease: experimental evidence and clinical significance. Front Immunol. 2021;12:644982. doi:10.3389/fimmu.2021.644982.
- Sofi MH, Wu Y, Ticer T, Schutt S, Bastian D, Choi HJ, Tian L, Mealer C, Liu C, Westwater C, et al. A single strain of bacteroides fragilis protects gut integrity and reduces GVHD. JCI Insight. 2021;6(3):6. doi:10.1172/jci.insight.136841.
- Gabarre P, Loens C, Tamzali Y, Barrou B, Jaisser F, Tourret J. Immunosuppressive therapy after solid organ transplantation and the gut microbiota: bidirectional interactions with clinical consequences. Am J Transplant. 2022;22(4):1014–11. doi:10.1111/ajt.16836.
- Podesta MA, Sykes M. Chimerism-based tolerance to kidney allografts in humans: novel insights and future perspectives. Front Immunol. 2021;12:791725. doi:10.3389/fimmu.2021.791725.
- Pirozzolo I, Sepulveda M, Chen L, Wang Y, Lei YM, Li Z, Li R, Sattar H, Theriault B, Belkaid Y, et al. Host-versus-commensal immune responses participate in the rejection of colonized solid organ transplants. J Clin Invest. 2022;132(17):132. doi:10.1172/JCI153403.
- Sosnovski KE, Braun T, Amir A, Moshel D, BenShoshan M, VanDussen KL, Levhar N, Abbas-Egbariya H, Beider K, Ben-Yishay R, et al. GATA6-AS1 regulates intestinal epithelial mitochondrial functions, and its reduced expression is linked to intestinal inflammation and less favourable disease course in ulcerative colitis. J Crohns Colitis. 2023;17(6):960–971. doi:10.1093/ecco-jcc/jjad006.
- Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson K, Glover L, Kominsky D, Magnuson A, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host & Microbe. 2015;17(5):662–671. doi:10.1016/j.chom.2015.03.005.
- Litvak Y, Byndloss MX, Baumler AJ. Colonocyte metabolism shapes the gut microbiota. Sci. 2018;362(6418):eaat9076. doi:10.1126/science.aat9076.
- Tiffany CR, Baumler AJ. Dysbiosis: from fiction to function. Am J Physiol Gastrointest Liver Physiol. 2019;317(5):G602–G608. doi:10.1152/ajpgi.00230.2019.
- Knoop KA, Kulkarni DH, McDonald KG, Gustafsson JK, Davis JE, Floyd AN, Newberry RD. In vivo labeling of epithelial cell–associated antigen passages in the murine intestine. Lab Anim (NY). 2020;49(3):79–88. doi:10.1038/s41684-019-0438-z.
- Ranjan K, Hedl M, Sinha S, Zhang X, Abraham C. Ubiquitination of ATF6 by disease-associated RNF186 promotes the innate receptor-induced unfolded protein response. J Clin Invest. 2021;131(17):131. doi:10.1172/JCI145472.
- Yang W, Yu T, Huang X, Bilotta AJ, Xu L, Lu Y, Sun J, Pan F, Zhou J, Zhang W, et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat Commun. 2020;11(1):4457. doi:10.1038/s41467-020-18262-6.
- MacKay M, Yang BH, Dursun SM, Baker GB. The gut-brain axis and the microbiome in anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder. Curr Neuropharmacol. 2023;21. doi:10.2174/1570159X21666230222092029.
- Flux MC, Lowry CA. Finding intestinal fortitude: integrating the microbiome into a holistic view of depression mechanisms, treatment, and resilience. Neurobiol Dis. 2020;135:104578. doi:10.1016/j.nbd.2019.104578.
- Brenner LA, Forster JE, Stearns-Yoder KA, Stamper CE, Hoisington AJ, Brostow DP, Mealer M, Wortzel HS, Postolache TT, Lowry CA. Evaluation of an immunomodulatory probiotic intervention for Veterans with co-occurring mild traumatic brain injury and posttraumatic stress disorder: a pilot study. Front Neurol. 2020;11:1015. doi:10.3389/fneur.2020.01015.
- Brenner LA, Hoisington AJ, Stearns-Yoder KA, Stamper CE, Heinze JD, Postolache TT, Hadidi DA, Hoffmire CA, Stanislawski MA, Lowry CA. Military-related exposures, social determinants of health, and dysbiosis: the United States-Veteran Microbiome Project (US-VMP). Front Cell Infect Microbiol. 2018;8:400. doi:10.3389/fcimb.2018.00400.
- Brenner LA, Stamper CE, Hoisington AJ, Stearns-Yoder KA, Stanislawksi MA, Brostow DP, Hoffmire CA, Forster JE, Schneider AL, Postolache TT, et al. Microbial diversity and community structures among those with moderate to severe TBI: a United States-Veteran microbiome project study. J Head Trauma Rehabil. 2020;35(5):332–341. doi:10.1097/HTR.0000000000000615.
- Martin S, Battistini C, Sun J. A gut feeling in amyotrophic lateral sclerosis: microbiome of mice and men. Front Cell Infect Microbiol. 2022;12:839526. doi:10.3389/fcimb.2022.839526.
- Levert-Levitt E, Shapira G, Sragovich S, Shomron N, Lam JCK, Li VOK, Heimesaat MM, Bereswill S, Yehuda AB, Sagi-Schwartz A, et al. Oral microbiota signatures in post-traumatic stress disorder (PTSD) veterans. Mol Psychiatry. 2022;27(11):4590–4598. doi:10.1038/s41380-022-01704-6.
- Sumner JA, Nishimi KM, Koenen KC, Roberts AL, Kubzansky LD. Posttraumatic stress disorder and inflammation: untangling issues of bidirectionality. Biol Psychiatry. 2020;87(10):885–897. doi:10.1016/j.biopsych.2019.11.005.
- Boddy SL, Giovannelli I, Sassani M, Cooper-Knock J, Snyder MP, Segal E, Elinav E, Barker LA, Shaw PJ, McDermott CJ. The gut microbiome: a key player in the complexity of amyotrophic lateral sclerosis (ALS). BMC Med. 2021;19(1):13. doi:10.1186/s12916-020-01885-3.
- Sato H, Takado Y, Toyoda S, Tsukamoto-Yasui M, Minatohara K, Takuwa H, Urushihata T, Takahashi M, Shimojo M, Ono M, et al. Neurodegenerative processes accelerated by protein malnutrition and decelerated by essential amino acids in a tauopathy mouse model. Sci Adv. 2021;7(43):eabd5046. doi:10.1126/sciadv.abd5046.
- Edwards G 3rd, Zhao J, Dash PK, Soto C, Moreno-Gonzalez I. Traumatic brain injury induces tau aggregation and spreading. J Neurotrauma. 2020;37(1):80–92. doi:10.1089/neu.2018.6348.
- Sobinoff AP, Dando SJ, Redgrove KA, Sutherland JM, Stanger SJ, Armitage CW, Timms P, McLaughlin EA, Beagley KW. Chlamydia muridarum infection-induced destruction of male germ cells and sertoli cells is partially prevented by chlamydia major outer membrane protein-specific immune CD4 cells. Biol Reprod. 2015;92(1):27. doi:10.1095/biolreprod.114.124180.
- Whitson HE, Colton C, El Khoury J, Gate D, Goate A, Heneka MT, Kaddurah-Daouk R, Klein RS, Shinohara ML, Sisodia S, et al. Infection and inflammation: new perspectives on Alzheimer’s disease. Brain Behav Immun Health. 2022;22:100462. doi:10.1016/j.bbih.2022.100462.
- Vigasova D, Nemergut M, Liskova B, Damborsky J. Multi-pathogen infections and Alzheimer’s disease. Microb Cell Fact. 2021;20(1):25. doi:10.1186/s12934-021-01520-7.
- Lotz SK, Blackhurst BM, Reagin KL, Funk KE. Microbial infections are a risk factor for neurodegenerative diseases. Front Cell Neurosci. 2021;15:691136. doi:10.3389/fncel.2021.691136.
- Sy M, Kitazawa M, Medeiros R, Whitman L, Cheng D, Lane TE, LaFerla FM. Inflammation induced by infection potentiates tau pathological features in transgenic mice. Am J Pathol. 2011;178(6):2811–2822. doi:10.1016/j.ajpath.2011.02.012.
- Langworth-Green C, Patel S, Jaunmuktane Z, Jabbari E, Morris H, Thom M, Lees A, Hardy J, Zandi M, Duff K. Chronic effects of inflammation on tauopathies. Lancet Neurol. 2023;22(5):430–442. doi:10.1016/S1474-4422(23)00038-8.
- Cardoso BR, Cominetti C, Cozzolino SM. Importance and management of micronutrient deficiencies in patients with Alzheimer’s disease. Clin Interv Aging. 2013;8:531–542. doi:10.2147/CIA.S27983.
- Fei HX, Qian CF, Wu XM, Wei YH, Huang JY, Wei LH. Role of micronutrients in Alzheimer’s disease: review of available evidence. World J Clin Cases. 2022;10(22):7631–7641. doi:10.12998/wjcc.v10.i22.7631.
- Fink A, Doblhammer G, Tamguney G, Tyas S. Recurring gastrointestinal infections increase the risk of dementia. J Alzheimers Dis. 2021;84(2):797–806. doi:10.3233/JAD-210316.
- Lauer AA, Grimm HS, Apel B, Golobrodska N, Kruse L, Ratanski E, Schulten N, Schwarze L, Slawik T, Sperlich S, et al. Mechanistic link between vitamin B12 and Alzheimer’s disease. Biomolecules. 2022;12(1):12. doi:10.3390/biom12010129.
- Vogels T, Murgoci AN, Hromadka T. Intersection of pathological tau and microglia at the synapse. Acta Neuropathol Commun. 2019;7(1):109. doi:10.1186/s40478-019-0754-y.
- Leyns CEG, Holtzman DM. Glial contributions to neurodegeneration in tauopathies. Mol Neurodegener. 2017;12(1):50. doi:10.1186/s13024-017-0192-x.
- Ardura-Fabregat A, Boddeke E, Boza-Serrano A, Brioschi S, Castro-Gomez S, Ceyzeriat K, Dansokho C, Dierkes T, Gelders G, Heneka MT, et al. Targeting neuroinflammation to treat Alzheimer’s disease. Cns Drugs. 2017;31(12):1057–1082. doi:10.1007/s40263-017-0483-3.
- Sarlus H, Heneka MT. Microglia in Alzheimer’s disease. J Clin Invest. 2017;127(9):3240–3249. doi:10.1172/JCI90606.
- Ahmad MA, Kareem O, Khushtar M, Akbar M, Haque MR, Iqubal A, Haider MF, Pottoo FH, Abdulla FS, Al-Haidar MB, et al. Neuroinflammation: a potential risk for dementia. Int J Mol Sci. 2022;23(2):23. doi:10.3390/ijms23020616.
- Hicks AJ, James AC, Spitz G, Ponsford JL. Traumatic brain injury as a risk factor for dementia and Alzheimer disease: critical review of study methodologies. J Neurotrauma. 2019;36(23):3191–3219. doi:10.1089/neu.2018.6346.
- Fulling C, Dinan TG, Cryan JF. Gut microbe to brain signaling: what happens in Vagus…. Neuron. 2019;101(6):998–1002. doi:10.1016/j.neuron.2019.02.008.
- Goehler LE, Gaykema RP, Opitz N, Reddaway R, Badr N, Lyte M. Activation in vagal afferents and central autonomic pathways: early responses to intestinal infection with Campylobacter jejuni. Brain Behav Immun. 2005;19(4):334–344. doi:10.1016/j.bbi.2004.09.002.
- Han W, Tellez LA, Perkins MH, Perez IO, Qu T, Ferreira J, Ferreira TL, Quinn D, Liu Z-W, Gao X-B, et al. A neural circuit for gut-induced reward. Cell. 2018;175(3):887–888. doi:10.1016/j.cell.2018.10.018.
- Kaelberer MM, Buchanan KL, Klein ME, Barth BB, Montoya MM, Shen X, Bohórquez DV. A gut-brain neural circuit for nutrient sensory transduction. Sci. 2018;361(6408):361. doi:10.1126/science.aat5236.
- Suarez AN, Hsu TM, Liu CM, Noble EE, Cortella AM, Nakamoto EM, Hahn JD, de Lartigue G, Kanoski SE. Gut vagal sensory signaling regulates hippocampus function through multi-order pathways. Nat Commun. 2018;9(1):2181. doi:10.1038/s41467-018-04639-1.
- Chen Q, Nair S, Ruedl C. Microbiota regulates the turnover kinetics of gut macrophages in health and inflammation. Life Sci Alliance. 2022;5(1):e202101178. doi:10.26508/lsa.202101178.
- Viola MF, Boeckxstaens G. Niche-specific functional heterogeneity of intestinal resident macrophages. Gut. 2021;70(7):1383–1395. doi:10.1136/gutjnl-2020-323121.
- Muller PA, Koscso B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li X-M, Mucida D, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158(5):1210. doi:10.1016/j.cell.2014.08.002.
- Blood Institute PCTN, National Heart L, Ginde AA, Brower RG, Caterino JM, Finck L. Early high-dose vitamin D(3) for critically Ill, vitamin D-Deficient patients. N Engl J Med. 2019;381:2529–2540.
- Amrein K, Schnedl C, Holl A, Riedl R, Christopher KB, Pachler C, Urbanic Purkart T, Waltensdorfer A, Münch A, Warnkross H, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically Ill patients with vitamin D deficiency. JAMA. 2014;312(15):1520–1530. doi:10.1001/jama.2014.13204.
- Sun J, Zhang YG. Vitamin D receptor Influences intestinal Barriers in health and disease. Cells. 2022;11(7):11. doi:10.3390/cells11071129.
- Ogbu D, Xia E, Sun J. Gut instincts: vitamin D/vitamin D receptor and microbiome in neurodevelopment disorders. Open Biol. 2020;10(7):200063. doi:10.1098/rsob.200063.
- Wang J, Thingholm LB, Skieceviciene J, Rausch P, Kummen M, Hov JR, Degenhardt F, Heinsen F-A, Rühlemann MC, Szymczak S, et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet. 2016;48(11):1396–1406. doi:10.1038/ng.3695.
- Zhang J, Zhang Y, Xia Y, Sun J. Imbalance of the intestinal virome and altered viral-bacterial interactions caused by a conditional deletion of the vitamin D receptor. Gut Microbes. 2021;13(1):1957408. doi:10.1080/19490976.2021.1957408.