ABSTRACT
Delivery by cesarean section (CS) is associated with an altered gut microbiota (GM) colonization and a higher risk of later chronic inflammatory diseases. Studies investigating the association between CS and atopic dermatitis (AD) are contradictive and often biased by confounding factors. The aim of this study was therefore to provide experimental evidence for the association between CS and AD in a mouse model and clarify the role of the GM changes associated with CS. It was hypothesized that CS-delivered mice, and human CS-GM transplanted mice develop severe dermatitis due to early dysbiosis. BALB/c mice delivered by CS or vaginally (VD) as well as BALB/c mice transplanted with GM from CS or VD human donors were challenged with oxazolone on the ear. The severity of dermatitis was evaluated by ear thickness and clinical and histopathological assessment which were similar between all groups. The immune response was assessed by serum IgE concentration, local cytokine response, and presence of immune cells in the draining lymph node. Both CS-delivered mice and mice inoculated with human CS-GM had a higher IgE concentration. A higher proportion of Th2 cells were also found in the CS-GM inoculated mice, but no differences were seen in the cytokine levels in the affected ears. In support of the experimental findings, a human cohort analysis from where the GM samples were obtained found that delivery mode did not affect the children’s risk of developing AD. In conclusion, CS-GM enhanced a Th2 biased immune response, but had no effect on oxazolone-induced dermatitis in mice.
Introduction
Delivery by CS has progressively increased in recent decades, mainly due to a rise in elective CS performed without strict medical indication.Citation1–3 The consequences of CS are given much attention since a growing number of epidemiological studies have associated CS with an increased risk of allergic diseases,Citation4 inflammatory bowel disease,Citation5–7 obesity,Citation8,Citation9 neurodegenerative disorders,Citation10–13 among other chronic diseases. Due to the high CS rates this presents a significant public health concern, and in the search of potential underlying mechanisms and new preventative strategies, the focus has mainly been on the critical role of the GM for maturation of the immune system.Citation14–19
CS changes the initial intestinal colonization often resulting in a higher abundance of e.g. Clostridium spp. and Enterococcus spp. and lower abundance of e.g. Bacteroides spp. and Bifidobacterium spp. compared to vaginally delivered (VD) infants.Citation14,Citation15,Citation20 Many of these are well-known gut-colonizing bacteria with substantial impact on the immune system.Citation21 CS-delivered mice also show microbiota-mediated immune disturbances with lower proportions of regulatory T cells together with an increased sensitivity to experimental colitis.Citation22,Citation23 The early GM is crucial for shifting the immune system from Th2 to Th1 biased pathways and induction of regulatory immunity.Citation24–27 Dysbiosis in CS-delivered infants are therefore thought to result in insufficient maturation of the immune system with a skewed balance of Th1/Th2 cells which facilitates an increased risk of allergic diseases.
Atopic dermatitis (AD) is a common chronic inflammatory skin disease, often with early childhood onset. It is a widespread disease with an average prevalence of 20% among children in Europe.Citation28,Citation29 The skin inflammation is dominated by Th2 cells in the acute response with a higher expression of IL-4 and IL-13 and enhancement of serum IgE production.Citation30 An imbalance in the GM diversity and composition is evident in patients with AD already before any manifestations of the disease occur,Citation31–33 strongly indicating that the early GM is crucial for disease development. This has been supported by experimental evidence in an oxazolone-induced dermatitis mouse model, in which the sensitivity to disease was transferable to germ-free mice with fecal transplant of their respective microbiomes.Citation34 However, contradictive results on whether CS is associated with AD exist as some studies found an increased relative risk in CS-delivered children,Citation35–38 while others point to confounding factors to account for these findings.Citation4,Citation39–44 The aim of this study was therefore to clarify the importance of CS-induced dysbiosis in the development of AD.
Results
Two experimental approaches were taken. Sensitivity to an induced model of AD was tested either in mice born by CS or VD or in mice transplanted with human fecal microbiomes from children born by CS or VD. The oxazolone-induced dermatitis mouse model was used due to its sensitivity to early microbial dysbiosis.Citation34,Citation45
CS delivery enhanced the allergic immune response, but had no effect on the degree of oxazolone-induced dermatitis
Oxazolone-induced dermatitis was assessed in barrier-bred mice delivered either by CS or VD (). The concentration of IL-2, IL-6, CXCL-1, and IL-10 in the inflamed ear tissue was higher, while TNF-α was lower, in CS delivered mice compared to VD mice (). Also CS-delivered mice had a higher concentration of serum IgE after oxazolone challenge compared to VD mice (), and the proportion of CD8+ T cells in ALN was higher (Figure S1A). A lower proportion of CD4+ T helper cells in the spleen and of TCRγδ+ cells in ALN were present in CS delivered mice (Figure S1B and S1D). Hence, the immune response was increased in CS delivered pups, but not for Th2 cell markers in general, except for the high IgE levels.
Figure 1. Cesarean section in mice induced a higher IgE response but had no effect on dermatitis. a) sensitivity to oxazolone-induced dermatitis was measured and compared in BALB/c mice delivered by vaginal delivery (VD) or cesarean section (CS). At 8 weeks of age, all mice offspring were sensitized with 0.8% oxazolone on the ear and after 1 week challenged with 0.4% every second day for a total of 5 times. b) multiplex mesoscale results of cytokine concentration (pg/ml/mg tissue) in the inflamed ear after oxazolone challenge. c) ELISA results showing serum concentration (ng/ml) of IgE in serum after oxazolone challenge. d) ear thickness of the inflamed ear in mm measured after oxazolone challenge. e) total histopathology score of hematoxylin and eosin staining cross section of the inflamed ear tissue after oxazolone challenge calculated as the sum of f) dermal infiltration, epidermal infiltration, spongiosis, epidermal thickness, and mast cells present which all were given a severity score from 0 to 3. g) Representative histological images of H&E stained inflamed ear section with mild spongiosis (orange arrow), epidermal infiltrations (black arrow), and dermal infiltration (green arrow), as well as Giemsa stained inflamed ear section with mast cells (yellow arrow). h) volcano plot based on the total transcriptome of the inflamed ear tissue. The red dot represents the Cd163l1 gene for which gene counts are shown in (i). Bars represent mean. p*<.05, p**<.01, p****<.0001. The experiment was repeated in four litters per group reaching a total of VD, n = 18 and CS, n = 14 pups which are all shown. There were no litter/round effects in the statistical analyses.
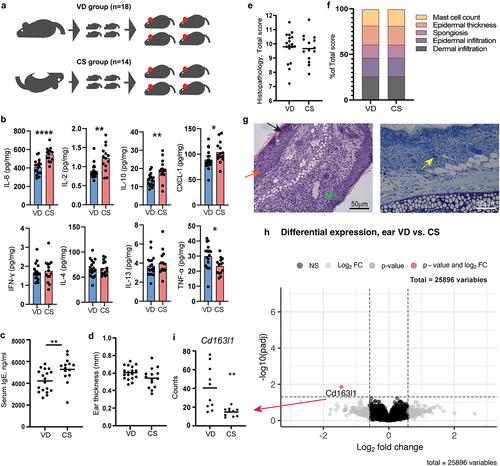
In addition, CS had no effect on ear thickness () or histopathological manifestations in the inflamed ear (). Furthermore, no significant differential gene expressions were shown by RNA sequencing of the total transcriptome of the inflamed ear, with the exception of a single gene Cd163l1 (), which verified the lack of change in AD specific inflammatory markers. 16S rRNA gene amplicon sequencing of feces sampled before oxazolone sensitization (8 weeks of age) revealed no long-term differences in GM composition related to delivery mode (Figure S1G-H).
Human GM was successfully transferred to recipient germ-free mice
Selected human feces samples from CS and VD infants were inoculated into germ-free pregnant mice and the offspring were induced with experimental dermatitis (). Human CS donor samples (n = 4) were characterized by a lower abundance of Bacteroides spp., Parabacteroides spp., Bilophila spp., Collinsella spp., order of Bacteroidales, and family of Lachnospiraceae and Ruminococcaceae, together with a higher abundance of the family of Enterobacteriaceae, Enterococcus spp., and Streptococcus spp. compared to VD donor samples (n = 4) ().
Figure 2. Cesarean section induced gut microbial changes were successfully transferred from human donors to germ-free recipient mice. a) illustration of study setup and how fecal gut microbiota (GM) samples from selected human donors delivered either by cesarean section (CS) or vaginal delivery (VD) were pooled and transferred into a germ-free dam and her offspring for a total of four times per group. Experimental dermatitis was induced in female offspring at the age of 8 weeks by sensitizing with 0.8% oxazolone and after 1 week challenge on both sides of the ear with 0.4% oxazolone every second day for a total of 6 times. b) Shannon α-diversity plot of taxa detected in feces samples from human GM donors (1 month of age, n = 4 per group) and GM transplanted mice (5 weeks of age, n = 14 per group). c) violin plot showing the top 20 overlapping genera in feces samples from human GM donors (1 month of age) and GM transplanted mice (5 weeks of age). Significant p values marked in bold indicate a difference in relative abundance according to delivery mode.
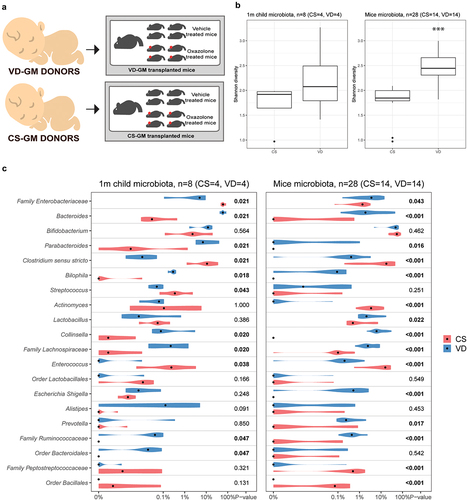
16S gene amplicon sequencing of feces samples obtained from the transplanted mice (CS-GM, n = 14 and VD-GM, n = 14) at 5 weeks of age revealed that the GM from recipient mice resembled their respective donor GM. illustrates the top 20 most abundant genera within the overlapping genera between donor and recipient samples, in which the abundance of Bacteroides spp, Parabacteroides spp., Bilophila spp., Collinsella spp., Enterococcus spp and family of Lachnospiraceae and Ruminococcaceae are different according to delivery mode in both human donors and recipient mice. Moreover, the α-diversity Shannon index was higher in both the VD donors and VD-GM mice compared to the CS donors and CS-GM mice respectively (), though only significant in the mice due to the low sample size of the donors. QIIME2 analysis showed that CS-GM and VD-GM recipient mice clustered separately (Figure S2A-C), and ANCOM analysis revealed 88 genera being significantly different in abundance. Significantly different bacterial genera with a relative abundance above 1% in at least one of the two groups has been listed in supplementary Figure S2D.
Inoculation with human CS-associated GM enhanced IgE sensitization, but had only minor effects on oxazolone-induced dermatitis
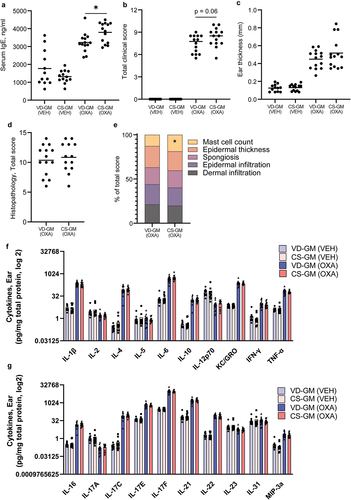
Similar to the CS delivered mice, oxazolone treated mice inoculated with CS-GM had a significantly higher concentration of serum IgE compared to VD-GM (). There was also a tendency (p =.06) toward a higher clinical score in the CS-GM mice compared to the VD-GM mice (), but no differences were observed in ear thickness (). There were also no differences in the majority of the histopathological manifestations (), except significantly more mast cells in the CS-GM mice compared to the VD-GM mice ().
Figure 3. Mice transplanted with human gut microbiota from cesarean section delivered donors had a higher IgE response than vaginally delivered donors, but there was no effect on features of oxazolone-induced dermatitis. a) ELISA results of serum concentration (ng/ml) of IgE after oxazolone challenge. b) total dermatitis score calculated as the sum of flare hemorrhage, edema, excoriation and erosion, and incrustation and xerosis which were all given a score from 0 to 3 corresponding to 0 = no sign; 1 = mild; 2 = moderate; or 3 = severe of the ear of cesarean section gut microbiota (CS-GM) and vaginally delivered gut microbiota (VD-GM) associated mice with oxazolone-induced dermatitis (OXA) or vehicle treated (VEH). c) ear thickness of the inflamed ear in mm measured after oxazolone challenge. d) total histopathology score of hematoxylin and eosin stained cross section of the inflamed ear tissue after oxazolone challenge calculated as the sum of (e) dermal infiltration, epidermal infiltration, spongiosis, epidermal thickness, and mast cells present which all were given a severity score from 0 to 3. f+g) bar plots illustrate mean cytokine concentrations (pg/mg total protein) in the inflamed ear tissue of CS-GM and VD-GM mice with oxazolone-induced dermatitis. Bars represent mean. p*<.05. The experiment was repeated in four litters per group reaching a total of n = 14 pups per OXA group and n = 12–13 per VEH group which are all shown. There were no litter/round effects in the statistical analyses.
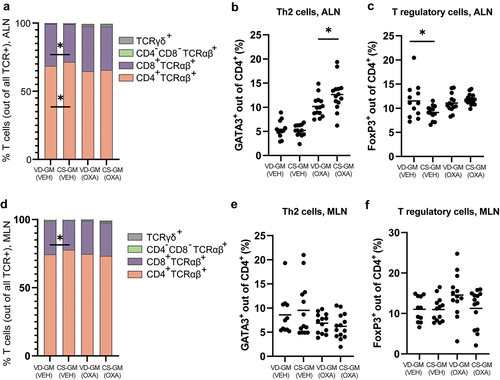
Oxazolone-induced dermatitis mice inoculated with CS-GM had more Th2 cells in the auricular lymph node (ALN), but the cytokine production was unaffected by CS-associated GM
Vehicle treated mice inoculated with CS-GM had a higher proportion of CD4+TCRαβ+ Th cells and a lower proportion of cytotoxic CD8+ TCRαβ+ T cells in ALN compared to vehicle mice inoculated with VD-GM (). All mice with oxazolone-induced dermatitis had increased proportions of GATA3+ Th2 cells compared to the vehicle treated mice, CS-GM mice had a significantly higher proportion of Th2 cells compared to VD-GM mice (). FoxP3+ regulatory T cells were not affected by dermatitis induction and independent of GM (), except for the vehicle treated CS-GM mice which had a lower proportion of regulatory T cells in ALN compared to vehicle treated VD-GM mice (). Similar to ALN, a lower proportion of cytotoxic CD8+ TCRαβ+ T cells in vehicle treated CS-GM mice was also observed in MLN (), but despite that, no differences in T cell distribution were present in MLN according to GM (). Hence, the CS-GM induced a Th2 biased immune response in the draining lymph node of the oxazolone treated mice, possibly due to a microbiota-driven reduction in regulatory immunity as observed in the ALN of vehicle treated mice.
Figure 4. More Th2 and less regulatory T cells in mice transplanted with human gut microbiota from cesarean section delivered donors compared to vaginally delivered donors. a+d) distribution of TCRαβ+, TCRγδ+, CD4+, and CD8+ T cells, (b+e) GATA3+ Th2 cells, and (c+f) FoxP3+ T regulatory cells isolated from the auricular lymph node (ALN) and mesenteric lymph node (MLN) from cesarean section gut microbiota (CS-GM) and vaginally delivered gut microbiota (VD-GM) associated mice with oxazolone-induced dermatitis (OXA) or vehicle treated (VEH). Bars represent mean. p*<0.05. The experiment was repeated in four litters per group reaching a total of n = 14 pups per OXA group and n = 12–13 per VEH group which are all shown. There were no litter/round effects in the statistical analyses.
A broad panel of cytokines were measured in the ear tissue of both vehicle and oxazolone treated mice. All mice with oxazolone-induced dermatitis had a dramatic increase in IL-1β, IL-4, IL-6, IL-10, TNF-α, IFN-γ, IL-16, IL-17C, IL-17E, IL-17F, IL-21, IL-22, and MIP-3a, whereas the concentrations of IL-12p70, IL-2, IL-17A, and IL-23 were lower in dermatitis mice (). There were, however, no differences in the cytokine response between the two inoculated groups CS-GM and VD-GM () supporting the lack of difference found in ear thickness and histological analysis.
Human CS delivery and CS-associated GM at 1 month of age did not associate with later risk of AD in children
As both CS delivery mode and inoculation with CS-GM from human donors had no effect on the degree of oxazolone-induced dermatitis in the mice, we examined possible associations in the human COPSAC2010 cohort, from where the donor fecal samples originated. Among the 662 children with follow up to age 6 years, 210 children (32%) were diagnosed with AD. Of these, the 139 children born by CS (21%) were not in higher risk of developing AD during the first 6 years of life compared to VD children (odds ratio 1.04, 95% CI [0.95–1.13], p = 0.42). This was also found when evaluating the risk of AD based on our CS microbial score (degree of microbial perturbationCitation14 at 1 month of age (n = 557), which is the time point and score used for selecting the human donor samples (odds ratio 1.02, 95% CI [0.98–1.06], p = .31).
Discussion
CS has with considerable evidence been associated with increased risk of chronic diseases such as allergy and asthma, but contradictive results and challenges with numerous confounding factors present a major limitation in demonstrating causality. There is a growing scientific consensus that infants born by CS experience disturbances in their early life microbiome,Citation15–17 which may have long-standing consequences on the developing immune system of the children, and hence play an instrumental role in determining their health and disease.Citation14,Citation46,Citation47 In support, it has been shown in the COPSAC2010 cohort that the increased risk of asthma found in CS delivered children was only present among children, whose microbiome perturbation did not normalize through the first year of life.Citation14 In this study, we investigated whether CS and the associated GM dysbiosis, which was not normalized in the first year of life, were associated with changes in the immune response and sensitivity to oxazolone-induced dermatitis in mice.
CS-delivered mice experienced increased serum IgE levels and increased cytokine response in the oxazolone treated ears, but besides that, CS had no effects on the clinical and histopathological phenotype. Especially the IgE response is influenced by early microbial perturbations and low diversity,Citation48–50 and it is independent on the later microbiota composition.Citation45,Citation51 It has previously been shown that CS in mice has significant effects on the GM, however, the composition of bacteria often vary between studies,Citation22,Citation23,Citation52–54 making it difficult to pinpoint certain causative bacteria and translatability to human findings that also vary. Some of the repeated findings in CS-delivered mice across studies are an underrepresentation of Bacteroides spp., Lactobacillus spp. and Ruminococcaceae members, together with an overrepresentation of Prevotella spp. and S24–7 members.Citation23,Citation52–54 The low abundance of Bacteroides spp. is translational to the CS-induced GM changes often seen in human infants.Citation14,Citation55,Citation56 In this study, no changes were found in the GM composition at 8 weeks of age in barrier-bred CS-delivered mice. However, CS-induced GM changes have previously shown to normalize with growing age in both humansCitation57 and mice,Citation23,Citation52 so it is not unlikely that CS-associated GM changes were present if feces sampling had been done earlier. The high IgE level in CS delivered mice indicates that early dysbiosis was present, though other factors independent of the GM can also alter IgE levels.
In fecal microbiota transplant studies from human to mice the GM of the recipient mice will never resemble the donors 100% due to microbe-host specificity.Citation58 Also in the current study, Bifidobacterium spp. e.g. propagated to a higher abundance than observed in the donors. However, it is of course essential that the differences in the GM between the groups are transferred. In this study, the donor samples were pooled to avoid the large individual variation between human samples that may transfer differences to the mice not representative to the two groups in the cohort, and the main GM differences observed between the two groups were successfully transferred from human donors to recipient mice with most taxa being present in a proportion resembling the donors.
In a longitudinal study of fecal microbiota maturation during childhood by Galazzo and colleagues,Citation33 the initial microbiota around 1 month of age was mainly determined by birth mode. The selection of samples from 1-month-old infants in the current study was significantly enriched by Bacteroides spp. in the vaginally born group as in the study by Galazzo et al. AD patients show a low relative abundance of intestinal Bacteroides spp. compared to healthy children,Citation20,Citation59–61 and lack of Bacteroides spp. is strongly associated with an immune phenotype characterized by less regulatory T cells,Citation62 which was also observed in the CS-GM transplanted vehicle treated mice. Perinatal microbial interventions with e.g. probiotic in experimental animal models for AD that enhances Bacteroides species also show alleviating effects on the symptoms,Citation63–65 whereas others show the opposite association.Citation66,Citation67 Differences in Bacteroides strains, synergistic interactions with other bacteria, choice of animal model etc. can explain the varying results. Nonetheless, the CS-GM was not sufficient to exert any major effects on the severity of oxazolone-induced dermatitis in the recipient mice.
Inoculation with CS-GM did, however, induce a Th2 biased immune response with a higher proportion of Th2 cells in ALN, high systemic IgE levels, and increased presence of mast cells in the inflamed ears. The involvement of GM in the pathogenesis of AD is not fully understood, but Bacteroides spp. has been shown to suppress cell surface expression of the high-affinity IgE receptor FcεRI on mast cells important for allergen sensitivity.Citation68 Moreover, Bacteroides spp. can correct the Th1/Th2 imbalance found in germ-free mice via polysaccharide A-activated dendritic cell signaling.Citation69 Similar pathways may be involved in the CS-GM-induced changes in the offspring’s immune response. In our study, Cd163l1 was downregulated in the CS-GM inoculated mice, which is a gene that encodes a scavenger receptor mainly found on immune cells and only slightly expressed in human skin.Citation70 While CD163 molecule-like 1, that the gene encodes, has not been associated with AD, CD163 in the same family and a marker for alternatively activated macrophages is increased in mouse models and human patients with AD.Citation71,Citation72 This is interesting as the functional relevance of CD163 in dermatitis seems to be related to its immunoregulatory role and antimicrobial defense against staphylococcus aureus, a common microorganism isolated from skin of AD patients.Citation73 A prominent Th2 cell response, as observed in the current study, is equivalent to acute stage AD and considering the possible involvement of Cd163l1 in the initial stages of the disease, the dysbiosis induced by CS is not irrelevant for AD. However, it seems that additional mechanisms are required to predispose the children and mice born by CS to a higher risk of developing AD.
When the disease progresses into more chronic stages, the immune response is constituted of a mixed Th2/Th1/Th22 response,Citation74–76 which is also present in the oxazolone-induced dermatitis mice in this study. Repeated oxazolone induces a strong Th2 response with elevated IL-4, IgE levels, and number of Th2 cells compared to vehicle treated mice and induces histopathological characteristic resembling AD in humans.Citation77 However, the prominent Th2 response induced by CS related dysbiosis was not sufficient to increase the sensitivity of oxazolone-induced dermatitis in mice, and we were therefore not able to prove an association between CS and AD. In concordance with our findings, analysis of the human cohort used in this study did also not find any indications that CS delivery or CS-associated microbial dysbiosis enhance the risk of AD later in life.
It is important to note, that while some human cohort studies find less Bifidobacterium spp. related to birth more,Citation57 we and others did not find such an association.Citation33 Bifidobacterium spp. has never been associated with higher AD risk in CS delivered children, and hence the importance of Bifidobacterium spp. for such a link is highly questionable. Nonetheless, considering the fact that fecal microbiota compositions low in Bifidobacterium spp. has previously been associated with development of AD and use of probiotics shown to be advantageous,Citation78 it would be interesting to repeat our study with samples from human cohorts in which lower abundance of Bifidobacterium spp. were detected in CS-delivered children. Differences in GM profiles between human birth cohort studies may explain the varying findings in relation to childhood eczema.
The CS-GM-induced Th2 biased immune response may likely have a more profound impact on allergic diseases driven mainly by Th2 cells and a high IgE response such as allergic asthma and food allergy rather than AD that has a more complex pathogenesis likely requiring more environmental factors involved other than microbiota dependent Th1/Th2 imbalance. In support of this notion, such an association has already been found in the same human cohort between birth by CS and asthma.Citation14 Future studies testing the effect of CS-GM in other models of asthma, food allergy, or other AD models including the effect on late recovery would be of high relevance.
Conclusion
Our aim was to investigate whether GM dysbiosis following birth by CS mitigates the development of AD in the children by combining an experimental approach using two complementary animal models and incorporating human cohort data. CS delivered mice with oxazolone-induced dermatitis had higher cytokine levels in the ear and higher serum IgE compared to VD mice, but these changes were not accompanied by detectable changes in ear thickness, histopathology, or gene expression patterns in the inflamed ear tissue. In the other experiment, germ-free mouse recipients of stool from CS infants, compared to VD infants, had slightly higher serum IgE, more mast cells in the ear, and more Th2 cells in the ALN. However, the majority of the measured features of oxazolone-induced dermatitis were unchanged between the groups despite significant changes in especially Bacteroides spp. in their fecal GM. Thus, birth mode and its associated GM seem to induce a Th2 skewed immune response, but do not alter features in an oxazolone-induced mouse model of AD. These results were further supported with an analysis of the human COPSAC2010 cohort, which also found that neither delivery mode nor the associated GM profiles of the donor samples affected the children’s risk of AD. In light of this work, preventative microbiota-directed interventions aimed specifically at reducing the incidence of AD in children born by CS seems irrelevant. However, clinical studies with the focus of restoring the GM of CS delivered children are still needed to prevent the microbiota-mediated Th2 biased immune response that may increase the risk of other allergic disorders, possibly more sensitive to the microbial changes following birth by CS.
Materials and methods
See supplementary material for detailed description of ethics, and details on material and methods.
Experimental setup
The aim was tested in two experimental setups. Either in mice delivered by VD or CS, or by fecal microbial transplant methods from CS-delivered human infant donors to germ-free recipients as a humanized GM mouse model. In the first experiment, barrier-bred BALB/c mice (Taconic, Lille Skensved, DK) were time-mated for 48 hours and CS was performed at pregnancy day 20 and pups transferred to a foster-mom within 30 minutes. VD pups were transferred to a foster-mom at day 0. In the second experiment, germ-free BALB/c mice (Taconic, Germantown, NY) were inoculated with feces obtained from 1-month-old CS and VD infants, which were selected based on 16S rRNA gene amplicon sequencing data of the COPSAC2010 cohort published by Stokholm et al.Citation14 All donors were breastfed, and in addition to being born by CS or VD, the infants had a high or low CS microbial score respectively (as described in Stokholm et al, Citation14 both at 1 month and 1 year of age, to ensure that the fecal microbial composition in CS children was not restored to a normal microbial trajectory within the first year of life. See supplementary material and methods for further information regarding the COPSAC2010 cohort, selection criteria, inoculation procedures, and animal housing.
Only female pups were used for model induction due to space limitations in the isolators and the practical infeasibility of single housing males that frequently fight when the oxazolone model is induced. All dermatitis-induced mice were sensitized at 8 weeks of age with 100 µl 0.8% (w/v) oxazolone (Sigma-Aldrich, St. Louis, MO) dissolved 4:1 in acetone:olive oil on the abdomen. After one week the mice were challenged repeatedly every second day with 10 μl on both sides of the left ear with 0.4% (w/v) oxazolone dissolved 4:1 in acetone:olive oil for a total of 5 challenges for the barrier-bred mice and 6 challenges for the human GM transplanted mice. Euthanization was performed 10 hours after the last challenge. Vehicle mice were treated with the vehicle only in the human GM transplanted mice. In the first experiment vehicle animals were not included due to the lack of significant differences in any of the readouts between the two groups of oxazolone treated mice.
Clinical and histological scoring of dermatitis
Before euthanasia, the inflamed ear of the human GM transplanted mice was scored blinded by two scientists for each of the clinical signs (1) hemorrhage (2) edema (3) excoriation and (4) incrustation as follows: 0 = no sign; 1 = mild; 2 = moderate; or 3 = severe,Citation79 and added to a total dermatitis score. Ear thickness was measured using a micrometer (Mitutoyo Low Force Caliper Series 573, Aurora, Illinois); each measurement was repeated and the calculated mean used.
After euthanasia, the inflamed ear was split into three parts, and the same parts from each mouse were used for either histology, cytokines or RNA seq. Ear tissue for histology embedded in paraffin was cut in 1–2 µm sections using a histiotome MICROM HM355S (Sakura Finetek, Brøndby, Denmark), stained with hematoxylin/eosin and Giemsa, blinded and evaluated by two scientists and given a score from 0 to 3 according to dermal cell infiltration and epidermal spongiosis where 0 = no sign, 1 = mild infiltration/spongiosis, 2 = moderate infiltration/spongiosis, and 3 = severe infiltration/spongiosis. Epithelial thickness were measured and given a score where 0 = <25 μm, 1 = 25 μm-49 μm, 2 = 50 μm-74 μm, and 3 = >75 μm. The amount of mast cells was counted in 6*High power field (HPF) (40X) and the average number was given a score from 0 to 3, where 0 = <10 mast cells/HPF, 1 = 11–20 mast cells/HPF, 2 = 21–30 mast cells/HPF, and 3 = >30 mast cells/HPF. All scores were summarized to a total histopathological score.
High throughput sequencing of the GM
Feces samples for sequencing were obtained from barrier-bred mice (8 weeks) and human GM transplanted mice (5 weeks). See supplementary material and methods for detailed information on DNA extraction, PCR amplification of 16S rRNA, and sequencing using the Oxford Nanopore GridION x 5 sequencing platform (Oxford Nanopore Technologies, Oxford, UK).
Flow cytometry
Single cell suspension from the auricular lymph node (ALN), mesenteric lymph nodes (MLN), and spleen were prepared as previously described,Citation80 and stained for selected T cell subpopulations for 30 min, 4°C in dark. See supplementary material and methods for antibody information.
Cytokine analysis
Pre-weighted ear tissue was homogenized in 400 µl lysis buffer (stock solution: 10 ml Tris lysis buffer, 100 µl phosphatase inhibitor 1, 100 µl phosphatase inhibitor 2, and 200 µl protease inhibitor (MSD inhibitor pack, Mesoscale Discovery, Rockville, MD) using a tissue blender (POLYTRON PT 1200 E, Kinematica, Luzern, Switzerland), and centrifuged (7500 g; 4°C; 5 min). Samples were diluted 1:2 and analyzed for IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, KC/GRO, and TNF-α with V-PLEX Proinflammatory Panel 1 Mouse kit (Mesoscale Discovery) and for MIP-3α, IL-16, IL-17A, IL-17C, IL-17E, IL-17F, IL-21, IL-22, IL-23, and IL-31 with V-PLEX Th17 Panel 1 Mouse (Mesoscale Discovery) according to manufacturer’s instructions. Measurements out of detection range were assigned the value of lower or upper detection limit. Concentrations were extrapolated from a standard curve and normalized to total protein measured with Pierce Detergent Compatible Bradford Assay kit according to manufacturer’s protocol.
Serum IgE
Serum IgE concentrations were measured in serum collected at euthanasia using the Mouse IgE ELISA Kit (Bethyl Laboratories, Montgomery, TX) as previously described.Citation45
Gene expression analysis
RNA from oxazolone treated ears was purified using RNeasy Lipid kit (Qiagen, Hilden, Germany) and send to RNA sequencing at Novogene (Beijing, China) as previously described.Citation81 See supplementary material and methods for RNA extraction and sequencing details. Data was analyzed in R (v 4.0.5) using DeSeq2 (v 1.30.1) for differential expression analysis. All gene counts for the individual mice are provided in the Supplementary Table S1.
Statistics
Statistical analysis was conducted in GraphPad Prism version 9.3.1 (GraphPad Software, San Diego, CA) in regards to clinical output, cytokines, IgE, flow cytometry, and histopathology. For the barrier-bred mice, significance between the two groups (CS vs. VD) was tested by student´s t-test for parametric data and Mann Whitney´s U-test for non-parametric data. D’Agostino-Pearson omnibus test was used for normality test. For the human GM transplanted mice, significance was tested by two-way ANOVA with GM (CS-GM vs. VD-GM) and treatment (oxazolone vs. vehicle) as variables and groups were compared by Šídák’s multiple comparisons test. Histopathology scores from were compared with Mann Whitney´s U-test. p values <.05 were considered significant. See supplementary material and methods for statistics used for high throughput data.
Author contributions
Study conception and design: LFZ, MBBE, JS, CHFH; Acquisition of data: LFZ, MBBE, CMJM, LD, LK, JS, CHFH; Analysis and interpretation of data; LFZ, MBBE, CMJM, LD, LK, DNS, JS, CHFH; Drafting of manuscript: LFZ, CMJM, LD, LK, JS, CHFH; Critical revision: LFZ, AKH, DSN, TLH, PT, CHFH. Final approval: LFZ, MBBE, CMJM, LD, LK, DSN, JS, CHFH.
Supplemental Material
Download Zip (2.1 MB)Acknowledgments
Data was generated through accessing research infrastructure at University of Copenhagen, including FOODHAY (Food and Health Open Innovation Laboratory, Danish Roadmap for Research Infrastructure).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The GM 16S sequencing dataset generated for this study can be found in the BioProject database with ID: PRJNA890556. https://eur02.safelinks.protection.outlook.com/?url=https%3A%2F%2Fwww.ncbi.nlm.nih.gov%2Fsra%2FPRJNA890556&data=05%7C01%7Ckrych%40food.ku.dk%7C583eaab1d1694aee1d4b08daaf3fdc60%7Ca3927f91cda14696af898c9f1ceffa91%7C0%7C0%7C638014986071254893%7CUnknown%7CTWFpbGZsb3d8eyJWIjoiMC4wLjAwMDAiLCJQIjoiV2luMzIiLCJBTiI6Ik1haWwiLCJXVCI6Mn0%3D%7C3000%7C%7C%7C&sdata=5ifUQY8qHZEIoHy%2B6k8depDvB33WZfwKu2LfaJyfAi0%3D&reserved=0
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2023.2271151
Additional information
Funding
References
- Betran AP, Ye J, Moller AB, Zhang J, Gulmezoglu AM, Torloni MR, Zeeb H. The increasing trend in caesarean section rates: global, regional and national estimates: 1990-2014. PloS One. 2016;11(2):e0148343. doi:10.1371/journal.pone.0148343.
- Stjernholm YV, Petersson K, Eneroth E. Changed indications for cesarean sections. Acta Obstet Gynecol Scand. 2010;89(1):49–15. doi:10.3109/00016340903418777.
- Betran AP, Torloni MR, Zhang JJ, Gülmezoglu AM. WHO statement on caesarean section rates. Bjog. 2016;123(5):667–670. doi:10.1111/1471-0528.13526.
- Bager P, Wohlfahrt J, Westergaard T. Caesarean delivery and risk of atopy and allergic disease: meta-analyses. Clin Exp Allergy. 2008;38(4):634–642. doi:10.1111/j.1365-2222.2008.02939.x.
- Bager P, Simonsen J, Nielsen NM, Frisch M. Cesarean section and offspring’s risk of inflammatory bowel disease: a national cohort study. Inflamm Bowel Dis. 2012;18(5):857–862. doi:10.1002/ibd.21805.
- Sevelsted A, Stokholm J, Bonnelykke K, Bisgaard H. Cesarean section and chronic immune disorders. Pediatrics. 2015;135(1):e92–98. doi:10.1542/peds.2014-0596.
- Andersen V, Möller S, Jensen PB, Møller FT, Green A. Caesarean delivery and risk of chronic inflammatory diseases (inflammatory bowel disease, rheumatoid arthritis, coeliac disease, and diabetes mellitus): a population based registry study of 2,699,479 births in Denmark during 1973-2016. Clin Epidemiol. 2020;12:287–293. doi:10.2147/clep.S229056.
- Blustein J, Attina T, Liu M, Ryan AM, Cox LM, Blaser MJ, Trasande L. Association of caesarean delivery with child adiposity from age 6 weeks to 15 years. Int J Obes (Lond). 2013;37(7):900–906. doi:10.1038/ijo.2013.49.
- Blustein J, Liu J. Time to consider the risks of caesarean delivery for long term child health. Bmj. 2015;350(jun09 3):h2410. doi:10.1136/bmj.h2410.
- Yip BHK, Leonard H, Stock S, Stoltenberg C, Francis RW, Gissler M, Gross R, Schendel D, Sandin S. Caesarean section and risk of autism across gestational age: a multi-national cohort study of 5 million births. Int J Epidemiol. 2017;46(2):429–439. doi:10.1093/ije/dyw336.
- Sucksdorff M, Lehtonen L, Chudal R, Suominen A, Gissler M, Sourander A. Lower apgar scores and Caesarean sections are related to attention-deficit/hyperactivity disorder. Acta Paediatr. 2018;107(10):1750–1758. doi:10.1111/apa.14349.
- Chudal R, Sourander A, Polo-Kantola P, Hinkka-Yli-Salomaki S, Lehti V, Sucksdorff D, Gissler M, Brown AS. Perinatal factors and the risk of bipolar disorder in Finland. J Affect Disord. 2014;155:75–80. doi:10.1016/j.jad.2013.10.026.
- Polidano C, Zhu A, Bornstein JC. The relation between cesarean birth and child cognitive development. Sci Rep. 2017;7(1):11483–11483. doi:10.1038/s41598-017-10831-y.
- Stokholm J, Thorsen J, Blaser MJ, Rasmussen MA, Hjelmsø M, Shah S, Christensen ED, Chawes BL, Bønnelykke K, Brix S, et al. Delivery mode and gut microbial changes correlate with an increased risk of childhood asthma. Sci Transl Med. 2020;12(569):eaax9929. doi:10.1126/scitranslmed.aax9929.
- Stokholm J, Thorsen J, Chawes BL, Schjørring S, Krogfelt KA, Bønnelykke K, Bisgaard H. Cesarean section changes neonatal gut colonization. J Allergy Clin Immunol. 2016;138(3):881–889.e2. doi:10.1016/j.jaci.2016.01.028.
- Reyman M, van Houten MA, van Baarle D, Bosch A, Man WH, Chu M, Arp K, Watson RL, Sanders EAM, Fuentes S, et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat Commun. 2019;10(1):4997. doi:10.1038/s41467-019-13014-7.
- Shao Y, Forster SC, Tsaliki E, Vervier K, Strang A, Simpson N, Kumar N, Stares MD, Rodger A, Brocklehurst P, et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature. 2019;574(7776):117–121. doi:10.1038/s41586-019-1560-1.
- Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, Björkstén B, Engstrand L, Andersson AF. Decreased gut microbiota diversity, delayed bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63(4):559–566. doi:10.1136/gutjnl-2012-303249.
- Francino MP. Birth mode-related differences in gut microbiota colonization and immune system development. Ann Nutr Metab. 2018;Suppl 73(Suppl. 3):12–16. doi:10.1159/000490842.
- Penders J, Gerhold K, Stobberingh EE, Thijs C, Zimmermann K, Lau S, Hamelmann E. Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J Allergy Clin Immunol. 2013;132(3):601–607.e8. doi:10.1016/j.jaci.2013.05.043.
- Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Sci. 2016;352(6285):539–544. doi:10.1126/science.aad9378.
- Zachariassen LF, Hansen AK, Krych L, Nielsen DS, Holm TL, Tougaard P, Hansen CHF. Cesarean section increases sensitivity to oxazolone-induced colitis in C57BL/6 mice. Mucosal Immunol. 2019;12(6):1348–1357. doi:10.1038/s41385-019-0207-8.
- Zachariassen LF, Krych L, Rasmussen SH, Nielsen DS, Kot W, Holm TL, Hansen AK, Hansen CHF. Cesarean section induces microbiota-regulated immune disturbances in C57BL/6 mice. J Immunol. 2019;202(1):142–150. doi:10.4049/jimmunol.1800666.
- Oyama N, Sudo N, Sogawa H, Kubo C. Antibiotic use during infancy promotes a shift in the T(H)1/T(H)2 balance toward T(H)2-dominant immunity in mice. J Allergy Clin Immunol. 2001;107(1):153–159. doi:10.1067/mai.2001.111142.
- Berger A. Science commentary: Th1 and Th2 responses: what are they? BMJ. 2000;321(7258):424. doi:10.1136/bmj.321.7258.424.
- Jiang HQ, Bos NA, Cebra JJ, Clements JD. Timing, localization, and persistence of colonization by segmented filamentous bacteria in the neonatal mouse gut depend on immune status of mothers and pups. Infect Immun. 2001;69(6):3611–3617. doi:10.1128/IAI.69.6.3611-3617.2001.
- Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997;159(4):1739–1745. doi:10.4049/jimmunol.159.4.1739.
- Bylund S, Kobyletzki LB, Svalstedt M, Svensson Å. Prevalence and incidence of atopic dermatitis: a systematic review. Acta Derm Venereol. 2020;100(12):adv00160. doi:10.2340/00015555-3510.
- Suaini NHA, Tan CPT, Loo EXL, Tham EH, Eigenmann P. Global differences in atopic dermatitis. Pediatr Allergy Immunol. 2021;32(1):23–33. doi:10.1111/pai.13335.
- Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22(2):125–137. doi:10.5021/ad.2010.22.2.125.
- Kirjavainen PV, Arvola T, Salminen SJ, Isolauri E. Aberrant composition of gut microbiota of allergic infants: a target of bifidobacterial therapy at weaning? Gut. 2002;51(1):51–55. doi:10.1136/gut.51.1.51.
- Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, Adams H, van Ree R, Stobberingh EE. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA birth cohort study. Gut. 2007;56(5):661–667. doi:10.1136/gut.2006.100164.
- Galazzo G, van Best N, Bervoets L, Dapaah IO, Savelkoul PH, Hornef MW, Lau S, Hamelmann E, Penders J, Hutton EK. Development of the microbiota and associations with birth mode, diet, and atopic disorders in a Longitudinal analysis of Stool samples, collected from infancy through early childhood. Gastroenterology. 2020;158(6):1584–1596. doi:10.1053/j.gastro.2020.01.024.
- Zachariassen LF, Krych L, Engkilde K, Nielsen DS, Kot W, Hansen CH, Hansen AK. Sensitivity to oxazolone induced dermatitis is transferable with gut microbiota in mice. Sci Rep. 2017;7(1):44385. doi:10.1038/srep44385.
- Korhonen P, Haataja P, Ojala R, Hirvonen M, Korppi M, Paassilta M, Uotila J, Gissler M, Luukkaala T, Tammela O. Asthma and atopic dermatitis after early-, late-, and post-term birth. Pediatr Pulmonol. 2018;53(3):269–277. doi:10.1002/ppul.23942.
- Gorris A, Bustamante G, Mayer KA, Kinaciyan T, Zlabinger GJ. Cesarean section and risk of allergies in Ecuadorian children: a cross-sectional study. Immun Inflamm Dis. 2020;8(4):763–773. doi:10.1002/iid3.368.
- Yu M, Han K, Kim DH, Nam GE. Atopic dermatitis is associated with Caesarean sections in Korean adolescents, but asthma is not. Acta Paediatr. 2015;104(12):1253–1258. doi:10.1111/apa.13212.
- Gerlich J, Benecke N, Peters-Weist AS, Heinrich S, Roller D, Genuneit J, Weinmayr G, Windstetter D, Dressel H, Range U, et al. Pregnancy and perinatal conditions and atopic disease prevalence in childhood and adulthood. Allergy. 2018;73(5):1064–1074. doi:10.1111/all.13372.
- Richards M, Ferber J, Chen H, Swor E, Quesenberry CP, Li D-K, Darrow LA. Caesarean delivery and the risk of atopic dermatitis in children. Clin Exp Allergy. 2020;50(7):805–814. doi:10.1111/cea.13668.
- Renz-Polster H, David MR, Buist AS, Vollmer WM, O’Connor EA, Frazier EA, Wall MA. Caesarean section delivery and the risk of allergic disorders in childhood. Clin Exp Allergy. 2005;35(11):1466–1472. doi:10.1111/j.1365-2222.2005.02356.x.
- Skajaa N, Nissen TN, Birk NM, Jeppesen DL, Thøstesen LM, Benn CS. Cesarean delivery and risk of atopic dermatitis. Allergy. 2020;75(5):1229–1231. doi:10.1111/all.14093.
- Kolokotroni O, Middleton N, Gavatha M, Lamnisos D, Priftis KN, Yiallouros PK. Asthma and atopy in children born by caesarean section: effect modification by family history of allergies – a population based cross-sectional study. BMC Pediatr. 2012;12(1):179. doi:10.1186/1471-2431-12-179.
- Keag OE, Norman JE, Stock SJ, Myers JE. Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: systematic review and meta-analysis. PLoS Med. 2018;15(1):e1002494. doi:10.1371/journal.pmed.1002494.
- Mubanga M, Lundholm C, Rohlin ES, Rejnö G, Brew BK, Almqvist C. Mode of delivery and offspring atopic dermatitis in a Swedish nationwide study. Pediatr Allergy Immunol. 2023;34(1):e13904. doi:10.1111/pai.13904.
- Arildsen AW, Zachariassen LF, Krych L, Hansen AK, Hansen CHF Delayed gut colonization shapes future allergic responses in a murine model of atopic dermatitis. Original Research. Front Immunol. 2021;12(803):650621. doi:10.3389/fimmu.2021.650621.
- Bisgaard HLN, Bonnelykke K, Chawes BL, Skov T, Paludan-Müller G, Stokholm J, Smith B, Krogfelt KA. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011;128(3):646–652.e5. doi:10.1016/j.jaci.2011.04.060.
- Shreiner AB, Kao JY, Young VB. The gut microbiome in health and in disease. Curr Opin Gastroenterol. 2015;31(1):69–75. doi:10.1097/MOG.0000000000000139.
- Ruohtula T, de Goffau MC, Nieminen JK, Honkanen J, Siljander H, Hämäläinen A-M, Peet A, Tillmann V, Ilonen J, Niemelä O. Maturation of gut microbiota and Circulating regulatory T cells and development of IgE sensitization in early life. Front Immunol. 2019;10:2494–2494. doi:10.3389/fimmu.2019.02494.
- Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014;44(6):842–850. doi:10.1111/cea.12253.
- Wyss M, Brown K, Thomson CA, Koegler M, Terra F, Fan V, Ronchi F, Bihan D, Lewis I, Geuking MB, et al. Using precisely defined in vivo microbiotas to Understand microbial Regulation of IgE. Front Immunol. 2019;10:3107. doi:10.3389/fimmu.2019.03107.
- Cahenzli J, Köller Y, Wyss M, Geuking MB, McCoy KD. Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host & Microbe. 2013;14(5):559–570. doi:10.1016/j.chom.2013.10.004.
- Hansen CH, Andersen LS, Krych L, Metzdorff SB, Hasselby JP, Skov S, Nielsen DS, Buschard K, Hansen LH, Hansen AK. Mode of delivery shapes gut colonization pattern and modulates regulatory immunity in mice. J Immunol. 2014;193(3):1213–1222. doi:10.4049/jimmunol.1400085.
- Martinez KA 2nd, Devlin JC, Lacher CR, Yin Y, Cai Y, Wang J, Dominguez-Bello MG. Increased weight gain by C-section: functional significance of the primordial microbiome. Sci Adv. 2017;3(10):eaao1874. doi:10.1126/sciadv.aao1874.
- Morais LH, Golubeva AV, Moloney GM, Moya-Pérez A, Ventura-Silva AP, Arboleya S, Bastiaanssen TFS, O’Sullivan O, Rea K, Borre Y, et al. Enduring behavioral effects induced by birth by caesarean section in the mouse. Curr Biol. 2020;30(19):3761–3774.e6. doi:10.1016/j.cub.2020.07.044.
- Korpela K, Helve O, Kolho KL, Saisto T, Skogberg K, Dikareva E, Stefanovic V, Salonen A, Andersson S, de Vos WM. Maternal fecal microbiota transplantation in cesarean-born infants rapidly restores normal gut microbial development: a proof-of-concept study. Cell. 2020;183(2):324–334.e5. doi:10.1016/j.cell.2020.08.047.
- Shaterian N, Abdi F, Ghavidel N, Alidost F. Role of cesarean section in the development of neonatal gut microbiota: a systematic review. Open Med (Wars). 2021;16(1):624–639. doi:10.1515/med-2021-0270.
- Rutayisire E, Huang K, Liu Y, Tao F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: a systematic review. BMC Gastroenterol. 2016;16(1):86. doi:10.1186/s12876-016-0498-0.
- Arrieta MC, Walter J, Finlay BB. Human microbiota-associated mice: a model with challenges. Cell Host & Microbe. 2016;19(5):575–578. doi:10.1016/j.chom.2016.04.014.
- Petersen EBM, Skov L, Thyssen JP, Jensen P. Role of the gut microbiota in atopic dermatitis: a systematic review. Acta Derm Venereol. 2019;99(1):5–11. doi:10.2340/00015555-3008.
- Kirjavainen PV, Apostolou E, Arvola T, Salminen SJ, Gibson GR, Isolauri E. Characterizing the composition of intestinal microflora as a prospective treatment target in infant allergic disease. FEMS Immunol Med Microbiol. 2001;32(1):1–7. doi:10.1111/j.1574-695X.2001.tb00526.x.
- Nylund L, Satokari R, Nikkilä J, Rajilić-Stojanović M, Kalliomäki M, Isolauri E, Salminen S, de Vos WM. Microarray analysis reveals marked intestinal microbiota aberrancy in infants having eczema compared to healthy children in at-risk for atopic disease. BMC Microbiol. 2013;13(1):12. doi:10.1186/1471-2180-13-12.
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107(27):12204–12209. doi:10.1073/pnas.0909122107.
- Qi C, Tu H, Zhao Y, Zhou J, Chen J, Hu H, Yu R, Sun J. Breast milk-derived Limosilactobacillus reuteri prevents atopic dermatitis in mice via activating retinol absorption and metabolism in Peyer’s patches. Mol Nutr Food Res. 2023;67(2):e2200444. doi:10.1002/mnfr.202200444.
- Ikarashi N, Fujitate N, Togashi T, Takayama N, Fukuda N, Kon R, Sakai H, Kamei J, Sugiyama K. Acacia polyphenol ameliorates atopic dermatitis in trimellitic anhydride-induced model mice via changes in the gut microbiota. Foods. 2020;9(6):773. doi:10.3390/foods9060773.
- Kim IS, Lee SH, Kwon YM, Adhikari B, Kim JA, Yu DY, Kim GI, Lim JM, Kim SH, Lee SS, et al. Oral administration of β-glucan and lactobacillus plantarum Alleviates atopic dermatitis-like Symptoms. J Microbiol Biotechnol. 2019;29(11):1693–1706. doi:10.4014/jmb.1907.07011.
- Kim DY, Jung DH, Song EJ, Jang AR, Park JY, Ahn JH, Lee TS, Kim YJ, Lee YJ, Seo IS, et al. D-galactose intake alleviates atopic dermatitis in mice by modulating intestinal microbiota. Front Nutr. 2022;9:895837. doi:10.3389/fnut.2022.895837.
- Zhao H, Zhou J, Lu H, Xi A, Luo M, Wang K, Lv H, Wang H, Wang P, Miao J. Azithromycin pretreatment exacerbates atopic dermatitis in trimellitic anhydride-induced model mice accompanied by correlated changes in the gut microbiota and serum cytokines. Int Immunopharmacol. 2022;102:108388. doi:10.1016/j.intimp.2021.108388.
- Fukatsu S, Horinouchi H, Nagata S, Kamei R, Tanaka D, Hong W, Kazami Y, Fujimori M, Itoh K, Momose Y, et al. Post-translational suppression of the high affinity IgE receptor expression on mast cells by an intestinal bacterium. Immunobiology. 2021;226(2):152056. doi:10.1016/j.imbio.2021.152056.
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122(1):107–118. doi:10.1016/j.cell.2005.05.007.
- Fagerberg L, Hallström BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S, Danielsson A, Edlund K, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics: MCP. 2014;13(2):397–406. doi:10.1074/mcp.M113.035600.
- Vestergaard C, Just H, Baumgartner Nielsen J, Thestrup-Pedersen K, Deleuran M. Expression of CCR2 on monocytes and macrophages in chronically inflamed skin in atopic dermatitis and psoriasis. Acta Derm Venereol. 2004;84(5):353–358. doi:10.1080/00015550410034444.
- Kim M, Lee S-H, Kim Y, Kwon Y, Park Y, Lee H-K, Jung HS, Jeoung D. Human adipose tissue-derived mesenchymal stem cells attenuate atopic dermatitis by Regulating the expression of MIP-2, miR-122a-SOCS1 Axis, and Th1/Th2 responses. Original Research. Front Pharmacol. 2018;9:1175. doi:10.3389/fphar.2018.01175.
- Fischer-Riepe L, Daber N, Schulte-Schrepping J, Véras De Carvalho BC, Russo A, Pohlen M, Fischer J, Chasan AI, Wolf M, Ulas T, et al. CD163 expression defines specific, IRF8-dependent, immune-modulatory macrophages in the bone marrow. J Allergy Clin Immunol. 2020;146(5):1137–1151. doi:10.1016/j.jaci.2020.02.034.
- Peng W, Novak N. Pathogenesis of atopic dermatitis. Clin Exp Allergy. 2015;45(3):566–574. doi:10.1111/cea.12495.
- Suárez-Fariñas M, Tintle SJ, Shemer A, Chiricozzi A, Nograles K, Cardinale I, Duan S, Bowcock AM, Krueger JG, Guttman-Yassky E. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol. 2011;127(4):954–964.e644. doi:10.1016/j.jaci.2010.12.1124.
- Gittler JK, Shemer A, Suárez-Fariñas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, Mitsui H, Cardinale I, de Guzman Strong C, Krueger JG, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130(6):1344–1354. doi:10.1016/j.jaci.2012.07.012.
- Laigaard A, Krych L, Zachariassen LF, Ellegaard-Jensen L, Nielsen DS, Hansen AK, Hansen CHF. Dietary prebiotics promote intestinal Prevotella in association with a low-responding phenotype in a murine oxazolone-induced model of atopic dermatitis. Sci Rep. 2020;10(1):21204. doi:10.1038/s41598-020-78404-0.
- Sestito S, D’Auria E, Baldassarre ME, Salvatore S, Tallarico V, Stefanelli E, Tarsitano F, Concolino D, Pensabene L. The role of prebiotics and probiotics in prevention of allergic diseases in infants. Front Pediatr. 2020;8:583946. doi:10.3389/fped.2020.583946.
- Ohmura T, Konomi A, Satoh Y, Hayashi T, Tsunenari I, Kadota T, Panzenbeck MJ, Satoh H. Suppression of atopic-like dermatitis by treatment with antibody to lymphocyte function-associated antigen-1 in NC/Nga mouse. Eur J Pharmacol. 2004;504(1–2):113–137. doi:10.1016/j.ejphar.2004.09.035.
- Hansen CH, Krych L, Nielsen DS, Vogensen FK, Hansen LH, Sorensen SJ, Buschard K, Hansen AK. Early life treatment with vancomycin propagates akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia. 2012;55(8):2285–2294. doi:10.1007/s00125-012-2564-7.
- Hansen CHF, Larsen CS, Zachariassen LF, Mentzel CMJ, Laigaard A, Krych L, Nielsen DS, Gobbi A, Haupt-Jorgensen M, Buschard K, et al. Gluten-free diet reduces autoimmune diabetes mellitus in mice across multiple generations in a microbiota-independent manner. J Autoimmun. 2022;127:102795. doi:10.1016/j.jaut.2022.102795.
