ABSTRACT
Shigella spp. are the causative agents of bacterial dysentery and shigellosis, mainly in children living in developing countries. The study of Shigella entire life cycle in vivo and the evaluation of vaccine candidates’ protective efficacy have been hampered by the lack of a suitable animal model of infection. None of the studies evaluated so far (rabbit, guinea pig, mouse) allowed the recapitulation of full shigellosis symptoms upon Shigella oral challenge. Historical reports have suggested that dysentery and scurvy are both metabolic diseases associated with ascorbate deficiency. Mammals, which are susceptible to Shigella infection (humans, non-human primates and guinea pigs) are among the few species unable to synthesize ascorbate. We optimized a low-ascorbate diet to induce moderate ascorbate deficiency, but not scurvy, in guinea pigs to investigate whether poor vitamin C status increases the progression of shigellosis. Moderate ascorbate deficiency increased shigellosis symptom severity during an extended period of time (up to 48 h) in all strains tested (Shigella sonnei, Shigella flexneri 5a, and 2a). At late time points, an important influx of neutrophils was observed both within the disrupted colonic mucosa and in the luminal compartment, although Shigella was able to disseminate deep into the organ to reach the sub-mucosal layer and the bloodstream. Moreover, we found that ascorbate deficiency also increased Shigella penetration into the colon epithelium layer in a Gulo−/− mouse infection model. The use of these new rodent models of shigellosis opens new doors for the study of both Shigella infection strategies and immune responses to Shigella infection.
Introduction
Bacillary dysentery or shigellosis remains a major disease burden especially in developing countries; in 2010 annual shigellosis mortality was estimated to 123,000 deaths worldwide among 88.4 million cases, mainly among children under the age of five.Citation1 In 2017, Shigella was included in the WHO list of the 12 antibiotic-resistant «priority pathogens» that pose the greatest threat to human health.Citation2 Shigellosis is associated with fever, abdominal cramps and rectal inflammation. Dysenteric stools characteristically contain erythrocytes, polymorphonuclear neutrophils (PMNs) and mucus. Shigellosis is characterized by the specific invasion and destruction of the human colonic mucosa by Shigella spp., which are transmitted via the feco-oral route. To date, no animal reservoirs have been reported. Although the etiologic agent, Shigella spp., has been identified during the last century, shigellosis represents a major threat to public health since no licensed vaccine is available.Citation3
The main risk factors of shigellosis are poor sanitation and limited access to clean water.Citation4 Malnutrition has also been shown to correlate with an increased risk of shigellosis.Citation5,Citation6 It has been shown that Vitamin ACitation7 or zinc supplementationCitation8 reduce the severity of acute shigellosis in malnourished children in Bangladesh and has been recommended by the WHO as supportive care in association with antimicrobials.Citation9
In addition to these prophylactic treatments, the development of a pan-Shigella vaccine remains urgently needed. The lack of a suitable animal model of shigellosis, which recapitulates the entire disease process from an oral challenge to the specific infection and destruction of the colonic mucosa, represents a major roadblock for the development of safe and efficacious vaccine candidates, but also to fully understand the Shigella virulence cycle. Over the past few decades, several animal models of shigellosis have been proposed, including monkeys, rabbits, mice, and guinea pigs. All of them have their own limitations, while none of them recapitulates all shigellosis symptoms.Citation2 The infection of the colonic mucosa by Shigella following an oral challenge was reported in rhesus macaques (Macaca mulatta).Citation10 Rare occurrence of bacteria in the lamina propria was observed in zinc-deficient mice.Citation11 Clinical features of shigellosis were observed in orally infected NAIP – NLRC4-deficient mice, however shigellosis symptoms remain transient and animal recovery occurred after a few days.Citation12 A transient colonic infection has been reported in young guinea pigs (Hartley strain) upon intrarectal inoculation.Citation13 In other proposed models, including the sereny test (guinea pig), the ileal loop model (rabbit) or the intravenous or intranasal infections (mouse), the targeted organs are different, making it difficult to interpret the results. It is important to highlight that conventional mice are not susceptible to Shigella infection for reasons that remain unclear.
A potent humoral immunological response to Shigella infection (including IgA and IgG secretion) has been reported in shigellosis patients and has been observed in several animal models (monkeys, mice, rabbit, guinea pigs).Citation14 It has been reported to be serotype-specific and mainly directed toward Shigella lipopolysaccharide (LPS-associated O-antigen repeats).Citation15 However, no direct correlation between the induced immune response and potential protection against shigellosis has been confirmed. Consequently, vaccine candidates are currently evaluated in clinical trials (phases I-III) only based on their immunogenicity (assessed in preclinical models, mainly mice), with no proof of protection efficacy. This strategy is time-consuming, risky, expensive, and should be optimized to improve the chances of success; this includes the validation of a suitable animal model of shigellosis.Citation16
In this study, we aimed to identify a novel susceptibility factor for shigellosis to develop and validate new animal models of shigellosis, that would faithfully reproduce all physiopathologal and clinical symptoms. We hypothesized that ascorbate deficiency may be an unknown susceptibility risk factor for shigellosis based on physiological reasons and historical reports. First, acute shigellosis has only been reported in humans, non-human primates, and guinea pigs, which are among the few mammalian species unable to synthesize ascorbate, due to the lack of a functional L-gulonolactone oxidase enzyme (GULO), which converts L-gulono-1,4-lactone to L-ascorbic acid (ascorbate or Vitamin C). Conversely, conventional mice express functional GULO and synthesize ascorbate. Mice are not susceptible to Shigella infection, regardless of the infection route,Citation17 suggesting that optimal ascorbate supply may limit shigellosis severity. Honjo et al. showed that shigellosis symptoms are more severe in ascorbate-deficient monkeys (cynomolgus monkeys) upon oral challenge, suggesting a possible link between ascorbate status and disease susceptibility.Citation18
Second, the association of scurvy (caused by severe and prolonged ascorbate deficiency) and dysentery was first reported by Lind in 1753.Citation19 Since then, it was hypothesized that both scurvy and chronic diarrhea may be nutritional deficiency syndromes, affecting populations suffering from malnutrition (ie. soldiers, seamen).Citation20 Diarrhea was considered as a “symptom” or “part” of scurvy and characterized as “scorbutic fluxes” or “scorbutic diarrhea”. Importantly, ascorbate supplementation allowed the treatment of both scurvy and diarrhea symptoms.Citation19 Severe ascorbate deficiency has been confirmed as the main cause of scurvy. However, no other clinical symptoms or diseases have been associated with moderate ascorbate deficiency. Our recent study demonstrated that infants with hypovitaminosis C are at a significantly higher risk of bacterial infectious diseases, including shigellosis.Citation21
In this study, we developed and validated an ascorbate-deficient guinea pig model of shigellosis, which recapitulates shigellosis symptoms upon oral challenge with S. flexneri 2a and 5a and S. sonnei. We showed that Shigella penetration into the colon mucosa was also increased in ascorbate-deficient Gulo−/− mice compared to conventional mice. Taken together, these results demonstrate that ascorbate deficiency accelerates the progression of shigellosis symptoms and severity in these infection models.
Results
Optimization of the guinea pig diet to induce a moderate ascorbate deficiency without scurvy symptom
The guinea pig model of shigellosis reporting the colonization and destruction of the colonic mucosa by Shigella was described by Shim and colleaguesCitation13 and consists of an intrarectal challenge of young guinea pigs (2-week) with S. flexneri 2a and S. flexneri 5a. It was reported that severe and acute colitis was induced 8 h post-infection (p.i.), and that symptoms disappeared by 24 h-48 h p.i., thus representing an important limitation of the model. In addition, it was shown that older animals (5-week and more) are no longer susceptible to Shigella infection.Citation13 We confirmed these results in our laboratory and used this model in routine studies to characterize Shigella virulence mechanisms in vivo.Citation22–24
In this study, we aimed to evaluate the impact of moderate ascorbate deficiency on the severity of shigellosis in guinea pigs. Animals were fed with specific diets containing sub-optimal ascorbate amounts for 2 weeks (). The amount of ascorbate in the diet allowing for an optimal supply to guinea pigs was 400 mg ascorbate/kg (see Methods). We evaluated the impact of diets containing 50, 10, and 0 mg ascorbate/kg () on guinea pig plasma ascorbate concentration () and animal weight () and reported the absence of scurvy symptoms ().
Figure 1. Optimization of diet ascorbate content to induce guinea pig moderate ascorbate deficiency. (a) Young guinea pigs (3-week) were fed for 2 weeks with diets containing various ascorbate concentration to induce moderate ascorbate deficiency without inducing scurvy, caused by severe ascorbate deficiency. Commercial diet contains 400 mg ascorbate/kg (Safe); suboptimal ascorbate supply was obtained with diets containing 50 mg, 10 mg and 0 mg ascorbate/kg. (c) Guinea pig ascorbate deficiency was confirmed by dosing plasma ascorbate concentration. Results are expressed as mean ± S.D., **** indicates t-test p < .0001 (n > 10 animals per group). (c-d) Moderate ascorbate deficiency induction did not lead to a significant reduction of weight gain, animals were weighted regularly (day 1, 6, 10, 13 and 17) during this period of time. Results are expressed as mean ± S.D., ‘ns’ indicates t-test p > .05, **** indicates p < 0.0001 (n > 10 animals per group). (e) the absence of scurvy symptoms (as indicated) was confirmed in young animals (3-week) and after 2-week feeding with diets containing 400, 50, 10 and 0 mg ascorbate/kg. ‘+’ indicates the corresponding scurvy symptom has been observed in at least one animal of the group.
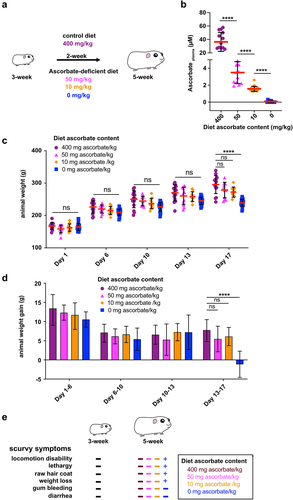
The guinea pig plasma ascorbate concentration upon optimal ascorbate supply was 36.4 ± 14.1 µM, confirming results from other studies,Citation25 which is in the same range although lower compared to human plasma ascorbate concentration (49.5 ± 14.2 µM).Citation26 When guinea pig diet was devoid of ascorbate (0 mg/kg), the plasma ascorbate concentration was 0.1 ± 0.1 µM, as anticipated, confirming that a 2-week diet was sufficient to induce deficiency. A moderate ascorbate deficiency was induced when guinea pigs were fed a diet containing 50 mg ascorbate/kg (3.5 ± 1.3 µM) or 10 mg/kg (1.6 ± 0.3 µM) (). We further aimed to define the most suitable low-ascorbate diet that ensured a significant reduction in guinea pig plasma ascorbate concentration while preventing scurvy.
The animal weight of all groups increased for 13 days and was not significantly different at any time point (). However, between days 13 and 17, significant weight loss was observed in the absence of ascorbate (, 0 mg ascorbate/kg, p < .001), but not in diets containing sub-optimal ascorbate amounts (). In addition, no scurvy symptoms (locomotion disability, lethargy, raw hair coat, or weight loss) were observed in animals fed this diet, as opposed to those devoid of ascorbate ().
In conclusion, a diet containing 10 mg ascorbate/kg induced a significant decrease in plasma ascorbate concentration (), without affecting animal growth () or causing scurvy (), and was further used in this study.
Moderate ascorbate deficiency increases shigellosis severity upon shigella intrarectal challenge
In humans, shigellosis symptoms appear between 24 h to 48 h p.i. As mentioned above, guinea pigs fed an optimal diet did not develop such long-term infectious processes (Shigella was cleared by the immune system 24 h p.i.). In addition, only young guinea pigs (2-week, just after weaning) were susceptible to Shigella infection inoculated intrarectally at high-dose: older animals (5-week or more) were not.Citation13 An oral Shigella challenge did not lead to infection and destruction of the colonic mucosa, as observed in humans (transmission through the fecal-oral route).
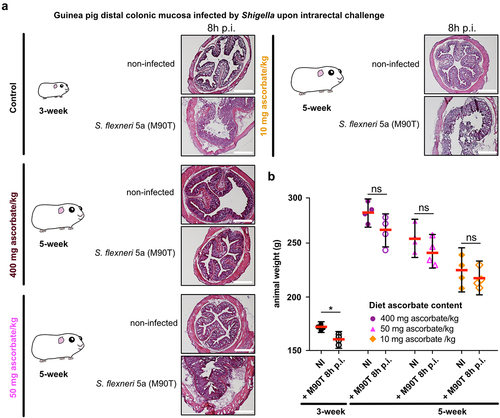
Using S. flexneri 5a as a model, we first confirmed that the colonic mucosa was disrupted 8 h p.i. by Shigella upon intrarectal challenge in young animals (3-week) fed a high-ascorbate diet (400 mg ascorbate/kg) (). We confirmed that older animals (5-week) fed a similar diet were not susceptible to Shigella infection. Conversely, we observed that ascorbate-deficient guinea pigs (5-week) remained susceptible to S. flexneri 5a infection (colonic mucosa destruction, leukocyte infiltration, edema), and this phenotype was observed in animals fed both low-ascorbate diets (50 and 10 mg ascorbate/kg) (). However, no significant weight loss was observed at 8 h p.i. in 5-week guinea pigs (due to high variability of the results), as observed in young animals (, p < .05) and previously reported.Citation13
Figure 2. Moderate ascorbate deficiency increases the susceptibility of adult guinea pigs to Shigella infection upon intrarectal challenge. (a) Young (3-week) and older guinea pigs fed with 400, 50 and 10 mg ascorbate/kg diets (5-week) were challenged intrarectally with 1010 CFU S. flexneri 5a (M90T). 8 h p.I. animals were sacrificed, and distal colonic samples were collected, stained with haematoxylin eosin and compared to non-infected tissues. Scale bars are 30 mm. (b) Animal weight was measured before and after infection. Results are expressed as mean ± S.D., * indicates t-test p < .05. Representative images are presented of at least 5 slices from 3 different animals.
We hypothesized that weight loss might be observed in 5-week guinea pigs during long-term infection. To address this question, the Shigella infection course was studied in ascorbate-deficient guinea pigs over longer infection periods using various Shigella species.
Prolonged infection processes are induced by S. flexneri 5a,S. flexneri 2a and S. sonnei in ascorbate-deficient guinea pigs
We evaluated the severity of Shigella infection 30 h p.i. upon intrarectal infection in 5-week ascorbate-deficient guinea pigs compared to non-infected controls. As previously reported,Citation13 we did not observe severe symptoms of shigellosis 30 h p.i.in guinea pigs fed with a 400 mg ascorbate/kg diet (). Infected animals kept gaining weight during the infection period (30 h, p > .05), and to a lesser extent than non-infected animals (), confirming that a transitory infection occurred at an early time point, e.g. 8 h p.i. ().
Figure 3. Shigella spp. cause destruction of the colonic mucosa and induce weight loss in ascorbate-deficient guinea pigs for extended period of time. (a) 5 weeks old guinea pigs (400 mg ascorbate/kg) and (c) ascorbate-deficient guinea pigs (10 mg ascorbate/kg diet) were infected intrarectally with S. flexneri 5a (wt and ∆mxiD strains), S. flexneri 2a and S. sonnei. 30 h p.I. animals were sacrificed, and distal colonic samples were collected, stained with haematoxylin eosin and compared to non-infected tissues. Scale bars are 30 mm. On the right panel, x3 magnification of white squares (left panel) are shown. (b and d) Animals were weighted before infection and 30 h p.I. Results are expressed as mean ± S.D., ‘ns’ indicates t-test p > .05, * indicates p < .05, ** indicates p < .01 (4 animals per group). Representative images are presented of at least 5 slices from 3 different animals.
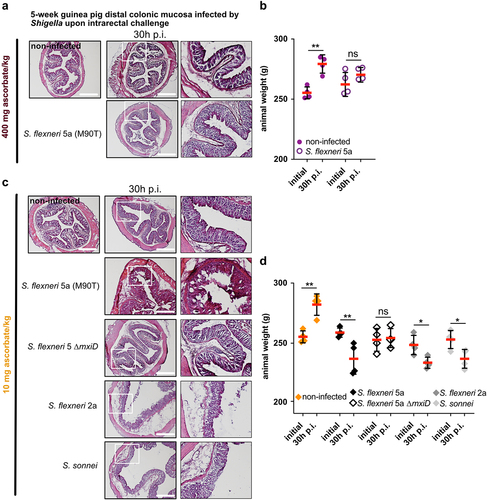
Conversely, severe Shigella infections were observed 30 h p.i. (intrarectal challenge) in 5-week ascorbate-deficient guinea pigs (colonic mucosa destruction, leukocyte infiltration, edema), using S. flexneri 5a, S. flexneri 2a, or S. sonnei, but not with an avirulent plasmid-cured S. flexneri 5a ∆mxiD mutant (). If non-infected guinea pigs gained weight during this period, a significant weight loss was induced by Shigella infection in all groups, but not with S. flexneri 5a ∆mxiD ().
These results demonstrate that ascorbate deficiency increases the severity of Shigella spp. infection in guinea pigs. We previously reported that Shigella infection induced a significant decrease in human plasma ascorbate concentration,Citation26 through a mechanism that remains to be defined. Here, we further investigated in a complementary approach the abundance of ascorbate within different colonic mucosa layers and its modulation during Shigella infection.
Shigella infection induces a systemic ascorbate concentration decrease and occurs in low-ascorbate microenvironments
First, we confirmed that in control animals (fed a high-ascorbate diet) a decrease in the plasma ascorbate concentration upon S. flexneri 5a infection (30 h p.i., intrarectal challenge) (). A significant decrease was observed in ascorbate-deficient guinea pigs upon S. flexneri 5a infection (30 h p.i., intrarectal challenge), but not in S. flexneri 5a ∆mxiD mutant, despite the fact that the plasma ascorbate concentration was already low under basal conditions.
Figure 4. Ascorbate deficiency is associated with reduction of ascorbate in plasma and within colonic mucosa layers. (a) Ascorbate concentration in plasma was measured by HPLC and expressed as mean ± S.D., * indicates t-test p < .05, ** indicates p < .01. (b) Ascorbate abundance was quantified within the different layers of the colonic mucosa by imaging mass spectrometry in tissues from animals fed with 400 mg ascorbate/kg and 10 mg ascorbate/kg diets, infected or not with indicated strains for 30 h (intrarectal challenge). (c-d) Quantification in different layers are averaged from three areas designed on three independent samples and expressed as mean ± S.D., * indicates t-test p < .05, ** indicates p < .01, FOI – foci of infection. Representative images are presented of at least 5 slices from 3 different animals.
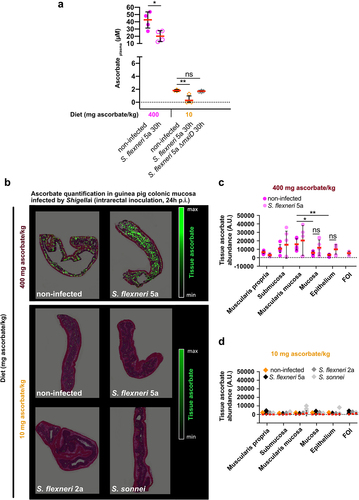
The determination of local ascorbate abundance remains difficult, and only a few quantitative data are available in the colon.Citation27 Ascorbate abundance was further quantified within the colonic mucosa layers using mass imaging spectrometryCitation28,Citation29 in the same models of Shigella infection (). We first observed that under basal conditions, the ascorbate distribution was heterogeneous between layers. In control animals (fed with high-ascorbate diet), higher amounts of ascorbate were detected within the submucosa and muscularis mucosa than in the mucosa or epithelium (). In ascorbate-deficient guinea pigs, ascorbate was almost undetectable in any layer under basal conditions (), showing for the first time that ascorbate deficiency is induced, and not only the plasma ascorbate concentration but also its local distribution within the colon is strongly decreased. No effect of Shigella infection on ascorbate abundance within the colonic layers was observed in control or ascorbate-deficient animals. However, ascorbate was not detected within foci of infection in either control or ascorbate-deficient animals ()). Since Shigella colonies specifically the colonic mucosa, the distribution of ascorbate with the small intestine has not been examined in this study. Further investigations will be required to address this point, since ascorbate is absorbed in this section of the gastrointestinal tract.
As we confirmed that ascorbate deficiency increased shigellosis symptom severity, we further aimed to evaluate whether the overall life cycle of Shigella, from an oral challenge to colon colonization, could be recapitulated in this new shigellosis animal model during extended periods of infection.
Efficient colonization of the colonic mucosa by shigella is observed in ascorbate-deficient guinea pigs
To recapitulate Shigella life cycle, control and ascorbate-deficient guinea pigs were challenged orally with S. flexneri 5a pGFP and long-term infections (24 h and 48 h) were performed (), animals were weighted and colonic mucosa colonization and destruction by Shigella were then evaluated (). As a major sign of shigellosis severity, we found that ascorbate-deficient animal weight continuously decreased during the time of infection, reaching 20% weight-loss 48 h p.i. upon oral and intrarectal challenge (); this phenotype was not observed in control animals. We observed comparable inflammation and infection symptoms within the colonic mucosa at 24 h and 48 h p.i. upon intrarectal and oral challenge major: edema was observed until late time points (48 h p.i.), and the formation of large and purulent ulcerative abscesses were formed with the colonic submucosa, in association with the destruction of the tissue (). No signs of tissue destruction or inflammation were observed in the control animals (fed a high-ascorbate diet) using similar experimental procedures. In addition, we showed that Shigella could reach the blood circulation in ascorbate-deficient animals (oral and intrarectal challenge) but not in control animals (). Shigella colonized deep colonic layers, such as the submuscularis mucosa and mucosa, in ascorbate-deficient animals. Additionally, an important recruitment of neutrophils was observed within infected tissues 48 h p.i., with both challenges, while the colonization of the colonic mucosa by Shigella was more pronounced 24 h p.i. upon intrarectal challenge, although no changes in Shigella distribution was observed between challenges (). Moreover, large populations of both Shigella and neutrophils were detected within the luminal compartment, as reported in patients with shigellosis, confirming the efficient dissemination of the bacteria during long-term infection. Conversely, in control animals, no tissue destruction was observed 24 h or 48 h p.i., with limited colonization of the mucosa by Shigella and only a few neutrophils were recruited (Figure S1).
Figure 5. Ascorbate-deficient guinea pigs are susceptible to Shigella infection, upon intrarectal or oral challenge, over extended periods of time. (a) ascorbate-deficient guinea pigs (10 mg ascorbate/kg diet) and control animals (400 mg ascorbate/kg diet) were infected intrarectally or orally with 10Citation10 CFU S. flexneri 5a. 24 h or 48 h p.I. (b) animals were sacrificed, and distal colonic samples were collected, stained with haematoxylin eosin and compared to non-infected tissues. (c) weight loss in guinea pigs infected by intrarectal and oral challenge is expressed as mean ± S.D., * indicates t-test p < .05, ** indicates p < .01. (d) S. flexneri 5a potential translocation to the bloodstream was assessed by plating blood samples on TSA culture plates. Results are expressed as mean ± S.D., ‘ns’ indicates t-test p > .05, **** indicates p < .0001 (4 animals per group). Representative images are presented of at least 5 slices from 3 different animals.
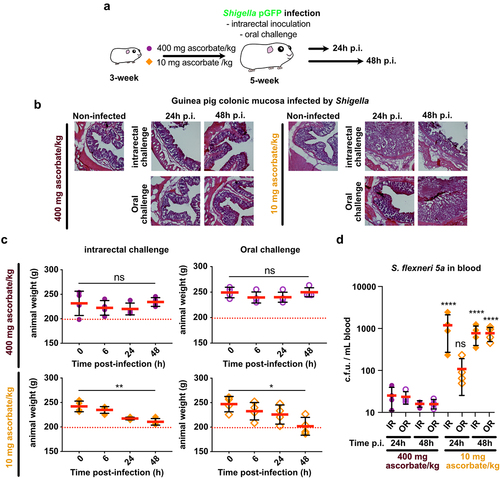
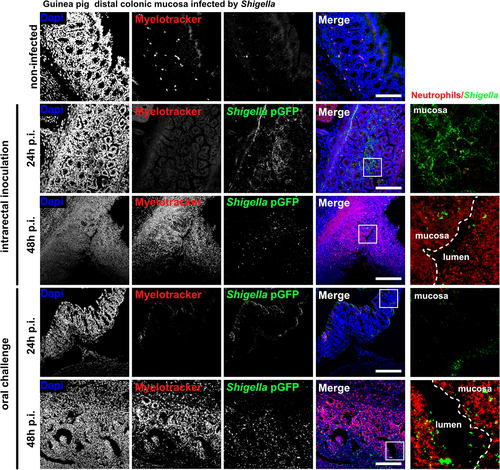
Figure 6. S. flexneri 5a colonize and attract neutrophils into infection foci in ascorbate-deficient guinea pigs. S. flexneri 5a pGFP (green) and neutrophils (red) were detected by immunofluorescence within the distal colonic mucosa of ascorbate-deficient guinea pigs (10 mg ascorbate/kg diet) challenged orally or intrarectally for 24 and 48 h with 1010 CFU. Nuclei were stained with DAPI (blue), neutrophils were stained with myelotracker-Cy3 (red). Scale bars are 150 µm. On the right panel, x5 magnification of merged images (white squares) are shown : S. flexneri 5a (green) and neutrophils (red). Detection of S. flexneri pGFP and neutrophils in tissues from control animals (400 mg ascorbate/kg diet) is shown in fig. S1. Representative images are presented of at least 5 slices from 3 different animals.
Collectively, these findings confirmed that ascorbate deficiency increased the severity of shigellosis symptoms during extended periods of infection in guinea pigs. This infection model of shigellosis allowed for the first time to follow Shigella life cycle from oral challenge to the colonization and destruction of the colonic mucosa, despite innate immune response induction, as reported in humans. We further investigated the effect of ascorbate deficiency on Shigella infection susceptibility in a murine model.
Invasion of colonic mucosa by Shigella is increased in young ascorbate-deficient Gulo−/− mice
To evaluate the impact of ascorbate deficiency on the susceptibility of mice to Shigella infection, Gulo−/− mice were used.Citation30 First, ascorbate deficiency was induced in mice just after weaning (3-weeks old) by adding 0.01% ascorbate to drinking water for 5 weeks; 0.4% ascorbate corresponded to the control condition (Figure S2(A)). Ascorbate deficiency was confirmed by analyzing plasma ascorbate using HPLC (Figure S2(B)); however, no scurvy symptoms were observed. S. flexneri 2a infection was performed in 8-week mice by oral or intrarectal administration, after disruption of the microbiota with ampicillin treatment, because it stabilized the Shigella population in stools for one week after infection (Figure S2(C)). Although mice lost approximately 10% of their initial weight at 24 h p.i., they recovered at 3 days p.i. (Figure S2(D)). In contrast to ascorbate-deficient guinea pigs, ascorbate-deficient 8-week mice had no visible signs of shigellosis, and no bacterial colonization of the colonic mucosa was observed.
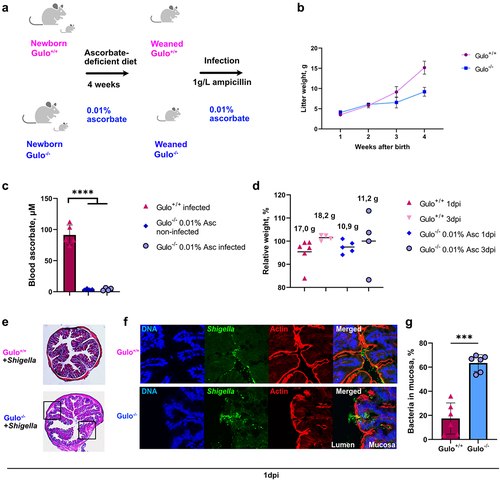
Previously, it was reported that younger mice were more susceptible to Shigella infection.Citation13 Thus, ascorbate deficiency was further induced just after birth for four weeks (). No scurvy symptoms were observed in young Gulo−/− mice; however, significant mouse growth retardation was observed compared to the same age in Gulo+/+ mice (). Plasma ascorbate levels in Gulo−/− mice fed a low-ascorbate diet were significantly lower than those in Gulo+/+ mice (). In these young ascorbate-deficient mice, no difference in relative weight loss upon S. flexneri 2a infection or visible signs of shigellosis were observed (). However, inflammation sites were observed in the colonic submucosal compartments of Gulo−/− mice (). Increased S. flexneri 2a penetration into the colon mucosa was confirmed in ascorbate-deficient Gulo−/− mice compared to Gulo+/+ mice (). Although these results confirmed that ascorbate deficiency increased the susceptibility of young mice to Shigella infection, additional specific defense mechanisms seemed to limit Shigella dissemination in this animal model.
Figure 7. Early ascorbate deficiency induction in young mice leads to increased colon colonization by Shigella upon intrarectal administration. (a) Lactating mothers Gulo−/− mice with new-born litters were treated with water containing 0.01% ascorbate after the day of birth. Mice were weaned 4 weeks later. Three days before and during infection all mice received water containing 0.01% ascorbate and 1 g/L ampicillin. (b) the weights of Gulo−/− and Gulo+/+ mice were measured each week after birth. Dots represent means ± S.D. (c) Ascorbate concentration in mice plasma before and after Shigella infection was measured by HPLC. Columns indicate means ± S.E. Each dot represents one mouse. Significance was calculated by one-way ANOVA, ****p < .0001. (d) Mice relative weight loss was compared to the initial weight (100%) one and three days after infection (1 dpi and 3 dpi). Each dot represents an individual mouse, black solid lines demonstrate means. Average weights of mice in the groups indicated above the columns. (e) HE staining of colons of Gulo+/+ and Gulo−/− mice 24 h after infection by S. flexneri 2a. Black squares indicate inflamed areas in colon from Gulo−/− mice. (f) Representative pictures of murine colon mucosa colonization 24 h after infection by S. flexneri 2a in 4 weeks-old ascorbate-deficient Gulo−/− and Gulo+/+ mice was visualized by immunofluorescent staining with DAPI for DNA (blue), anti-LPS serum for S. flexneri 2a (green) and phalloidin for actin (red). (g) Mucosa infection by S. flexneri 2a in Gulo−/− mice was compared to Gulo+/+ mice. Individual bacteria were counted in the lumen and mucosa. Percentages of penetrated bacteria compared to total count of bacteria are represented as means ± S.D., *** indicates t-test p < .001.
Discussion
While severe ascorbate deficiency is known to cause scurvy, we demonstrated here that moderate ascorbate deficiency is an important risk factor for shigellosis, which has so far been largely neglected and underestimated. This new insight raises the question of the definition of moderate ascorbate deficiency, which is currently not well characterized in humans, probably due to the difficulty in routinely administering plasma ascorbate. In guinea pigs, our results show that plasma ascorbate concentrations from 1.6 ± 0.3 µM to 3.5 ± 1.3 µM (vs 36.4 µM in animals fed with a high-ascorbate diet) are associated with increased shigellosis symptom severity. Further studies are required to define the upper values of moderate ascorbate deficiency defined in this study. In humans, an adequate ascorbate plasma concentration is estimated around 50 µM (confirmed in our previous work (49.5 ± 14.2 µM),Citation26 while plasma ascorbate concentrations associated with insufficiency, moderate deficiency and severe deficiency are currently based on definitions rather than on clinical manifestations.Citation31 Determining the relationship between ascorbate status and shigellosis development and severity requires further clinical studies, especially in children under the age of 5, in developing countries. In a previous study, it was reported that 20% of children in Puerto Rico were moderately deficient (<30 μM), with no correlation with diarrhea episodes.Citation32 However, recent data from Central Africa obtained by our group demonstrated that infants with hypovitaminosis C were at a significantly higher risk of having a positive stool culture for various pathogenic bacteria including Shigella.Citation21 Conversely, further investigations are required to evaluate the impact of shigellosis and other enteric diseases on ascorbate uptake and transport systems.
Vitamin C deficiency has been studied intensively in guinea pigs, but not in relation to shigellosis.Citation33,Citation34 In the present study, we validated a new animal model of shigellosis, the ascorbate-deficient guinea pig, which allowed us to follow for the first time the overall Shigella life cycle from an oral challenge to the colonization and destruction of the colonic mucosa. Until now, only transient infections have been observed, associated with foci of infection formation within the upper part of the colonic submucosa, Shigella being further cleared during the immune response in this model.Citation24,Citation35 The ascorbate-deficient guinea pig model of shigellosis can be a useful tool for the investigation of the immune response and dissemination of Shigella within the colonic mucosa, like we demonstrated with the influx of neutrophils into infection site. In addition, this model can be used to better understand the molecular bases of the subversion and antimicrobial activity of Shigella mediating its efficient dissemination within the infected tissues and to the bloodstream, as reported here. More specifically, this new shigellosis model will allow us to take a fresh look at the role of mucosal IgA in preventing Shigella disseminationCitation36,Citation37 and at Shigella capacity to subvert immune cell function and antimicrobial activity, which has been described previously in vitro or in vivo in other models (macrophage and B-lymphocyte apoptosis inductionCitation38,Citation39 or T-cell migration.Citation40,Citation41 Most of these subversion mechanisms are dependent on Shigella Type Three Secretion System (T33S). As we have shown in a previous study that this secretion apparatus is mainly inactive within hypoxic foci of infection,Citation24 alternative virulence mechanisms are expected to be involved, and may be identified in this new model of shigellosis. In addition, the ascorbate-deficient guinea pig model of shigellosis described in this study will allow investigation of the composition, structural organization, and role of isolated lymphoid follicles (belonging to the Gut-associated Lymphoid Tissue (GALT), specifically disseminated within the colonic mucosa) during Shigella infection, which has not yet been characterized.
In addition to the guinea pig infection model, we tested whether ascorbate-deficient Gulo−/− mice could also be susceptible to Shigella colonization. Although we did not observe any shigellosis symptoms in the low-ascorbate murine model, we confirmed that ascorbate deficiency at the early stages of mouse development could determine more effective bacterial penetration into the colonic mucosa. Since vitamin C plays a key role in collagen production, higher colonization rates could be explained by reduced collagen production in 0.01% ascorbate mice, leading to more pervious intestines for Shigella infection. Several recent studies have demonstrated important differences in the cellular response to Shigella infection comparing murine and human cells.Citation12,Citation42 These studies showed that cellular responses associated with human-specific pyroptosis are important factors related to Shigella-sensitivity. Moreover, Shigella can specifically target gasdermin D and prevent pyroptosis in humans but not in murine cells.Citation42 This could explain why we did no marked shigellosis symptoms were observed in mice even under high bacterial colonization.
Defining the detailed mechanisms involved in the increased susceptibility of ascorbate-deficient animals to Shigella infection requires further investigations. It has been previously shown that infection by enteropathogenic E. coli dysregulates the expression of L-ascorbic acid transporter SVCT1 and SVCT2.Citation43 Low amount of ascorbate may impair the protective properties of epithelium barrier and tissue repair, while more global effects are anticipated on the oxidative stress level and the immune system function, since ascorbate is very important for the activity of both innate and adaptive immune cells. These cells tend to accumulate intracellular ascorbate; lymphocytes, monocytes and neutrophils contain ∼3.5, ∼3 and ∼1.5 mM of ascorbate, respectively, compared to 50 µM in plasma.Citation44 Ascorbate stimulates neutrophil migration toward infectious sites and promotes phagocytosis. Ascorbate also sustains the differentiation and maturation of both B- and T-lymphocytes, particularly NK cells.Citation45 It was recently shown that ascorbate boost the immunogenic properties of dendritic cells, through the NF-κB-dependent increase of TNFβ production.Citation46 Further investigations will be required to evaluate the impact of ascorbate deficiency on the function of immune cells during shigellosis. It would be additionally of a great interest to evaluate the impact of ascorbate supplementation on the course of the infection disease in ascorbate-deficient animals.
Overall, we drew attention to ascorbate deficiency as an important factor determining shigellosis in both young guinea pig and mouse infection models. We presented a new Shigella infection model in young low-ascorbate guinea pigs, which allows the analysis of the entire cycle of Shigella colonization. This new model may boost further research on Shigella virulence mechanisms and vaccine development. We also demonstrated that early ascorbate deficiency induction in mice sensitized the animals to infection. Low ascorbate guinea pigs and Gulo−/− mouse infection models will allow analysis of how immune cells in the colonic mucosa react to infectious Shigella in vivo.
These results are in agreement with our recent study in a mother-infant cohort showing that infants with hypovitaminosis C are at significantly higher risk of having a positive stool bacterial culture.Citation21 The fact that ascorbate deficiency in young animals and children is a risk factor for Shigella infection but not in adults could be related to processes of immunity development in new-borns that are not yet fully understood, raising the question of the importance of ascorbate supplementation in population suffering from endemic diarrheal diseases, especially during childhood.
Materials and methods
Bacteria
Unless otherwise noted, S. flexneri 5a (M90T, wt, and ∆mxiD mutant) and 2a strains were grown at 37°C in Tryptic Soy Broth (TSB) with shaking or TSB agar plates supplemented with 0.01% Congo Red (Sigma-Aldrich) and ampicillin (100 μg/ml) when bacteria were transformed with the pGFP plasmid. S. sonnei was acquired from the Institut Pasteur strain collection (CIP 106,347), a clinical isolate from a 1999 Paris infection.Citation47 The strain was grown in TSB supplemented with ampicillin (100 μg/ml) to maintain the pMW211 plasmid.
Ascorbate-deficient guinea pig model of shigellosis
Young conventional guinea pigs (3 weeks old; female; Dunkin – Hartley; <150 g) were used to study Shigella infection severity. Guinea pigs were fed for 15 days with a standard diet allowing for an optimal ascorbate supply (400 mg ascorbate/kg, Safe ref. 106), as previously describedCitation48 or an ascorbate-deficient diet specifically designed and produced by the Safe (0, 10 mg, or 50 mg ascorbate/kg). Ascorbate deficiency was assessed based on its plasma concentration (see below). Guinea pig infection with Shigella infection was achieved with S. flexneri 5a wild-type and ∆mxiD strains, S. flexneri 2a and S. sonnei wild-type strains was performed by intrarectal challengeCitation24 or oral challenge of animals with 1010 CFU exponentially grown, as indicated. In each experiment, at least three animals were used. Infections proceeded for 8 h, 24 h, 30 h or 48 h, as indicated, before the animals were sacrificed. All experimental procedures were approved by the Institute of Pasteur Ethics Committee (auth. n°190127).
The distal colon was sectioned (7 cm) and either flash-frozen in liquid nitrogen for tissue ascorbate abundance quantification (imaging mass spectrometry, see below) or fixed in 4% PFA in 1× PBS for 1–2 h, and then incubated in 1× PBS/glycine (100 mM) for 30 min to quench the PFA (for tissue labeling and imaging). PFA-fixed tissues were immersed successively in 15% and 30% sucrose at 4°C overnight. Tissue transversal sections (1 cm) were embedded in Tissue-Tek O.C.T. compound (Sakura) using a flash-freeze protocol and frozen at − 80°C. For histological and standard immunofluorescence staining, 10-μm sections were obtained using a CM-3050 cryostat (Leica Biosystems).
To quantify the abundance of bacteria in the bloodstream, 100 µL blood samples were collected in the presence of EDTA and plated on GTCS + ampicillin agar plates. Bacteria were counted after overnight culturing at 37°C.
Ascorbate-deficient mouse model of shigellosis
Mice were fed irradiated Safe A03-A04 granules containing no ascorbate. Late ascorbate deficiency was induced by adding 0.01% ascorbate to mice drinking water for 5 weeks starting with 3 weeks-old Gulo−/− mice. Early ascorbate deficiency was induced by adding 0.01% ascorbate for 4 weeks, starting on the first day of birth, while litters were still together with their mother. No symptoms of scurvy were observed. Three days before infection, 1 g/L ampicillin was added to the mice’s drinking water. Shigella infection was performed in 8 weeks-old mice or young 4 weeks-old just weaned mice by oral or intrarectal administration with 109 infection dose per mouse. A low-ascorbate diet was also administered during the infection period. The appearance and weight of the mice were monitored daily. Infections proceeded for 24 h or 72 h, as indicated, before the animals were sacrificed. The experimental procedures were authorized by the Ministry of Higher Education, Research, and Innovation Ethics Committee (auth. n°APAFIS#29175–2021010711306157v1). Experiments with mice were performed in animal facilities at the Chronobiotron, an animal facility part of the National Research Infrastructure CELPHEDIA (Creation, Breeding, Phenotyping, Distribution and Archiving of model organisms). Murine colons were treated and analyzed as indicated in the ascorbate-deficient guinea pig model of shigellosis.
Plasma ascorbate dosage
Blood samples were collected via intracardiac puncture in the presence of EDTA. Following centrifugation for 5 min at 2,000 × g, plasma was acidified with an equal volume of 10% (w/v) metaphosphoric acid (MPA) containing 2 mmol/L of disodium-EDTA. Ascorbate concentration was quantified by high-performance liquid chromatography with coulometric detection, as described previously.Citation26 Results were averaged from the dosage performed on at least four animals per condition.
Tissue ascorbate quantification
Imaging mass spectrometry was performed in collaboration with the team of Axel Walch (Helmholtz Zentrum München) on flash-frozen colonic samples. Each indicated ascorbate quantification was averaged from three areas designed on three independent tissues (three animals per condition). Metabolite mapping was performed using frozen guinea pig colonic tissue samples (12 μm, Leica Microsystems, CM1950, Germany) mounted onto indium tin oxide (ITO)-coated glass slides (Bruker Daltonik, Bremen, Germany) pretreated with 1:1 poly-Lysine (Sigma Aldrich, Munich, Germany) and 0.1% Nonidet P-40 (Sigma). Air-dried tissue sections were spray-coated with 10 mg/ml 9-aminoacridine hydrochloride monohydrate matrix (Sigma-Aldrich, Munich, Germany) in 70% methanol using a SunCollect™ sprayer (Sunchrom, Friedrichsdorf, Germany). Spray-coating of the matrix was conducted in eight passes (ascending flow rates of 10 µL/min, 20 µL/min, and 30 µL/min for layers 1–3, and 40 µL/min for layers 4–8), utilizing a 2 mm line distance and a spray velocity of 900 mm/min. Metabolites were detected in the negative-ion mode on a 7T Solarix XR Fourier-transform ion cyclotron resonance (FTICR) mass spectrometer (Bruker Daltonik) equipped with a dual ESI-MALDI source and a smartbeam-II Nd:YAG (355 nm) laser. Data acquisition parameters were specified in ftmsControl software 2.2 and flexImaging (v. 5.0) (Bruker Daltonik). Mass spectra were acquired in negative-ion mode covering ⁄ 75–1100, with a 1 M transient (0.367 s duration) and an estimated resolving power of 49,000 at m/z 200,000. The laser was operated at a frequency of 1,000 Hz utilizing 200 laser shots per pixel with a pixel resolution of 15 μm. L-arginine was used for external calibration in the ESI mode. On-tissue MS/MS was conducted on guinea pig colonic tissue sections using continuous accumulation of the selected ion mode and collision-induced dissociation (CID) in the collision cell. MS/MS spectra were analyzed using Bruker Compass DataAnalysis 5.0 (Build 203.2.3586).
Tissue labelling and imaging
For histological studies, sectioned colonic samples were stained with hematoxylin-eosin following standard procedures.Citation49 Labeled tissues were imaged with a transmission light microscope (Axioskop 2, Zeiss) using x10 or x20 objectives. For immunofluorescence imaging, PFA-fixed sectioned tissues were washed thrice in PBS + 0.1% saponin prior to immunolabelling in the same buffer for 1 h. Nuclei were stained with DAPI (1 mg/mL, Life Technologies,1:1000), Alexa Fluor 555 Phalloidin (Invitrogen, 1:40) for actin, or Myelotracker-Cy3 (1 mg/mL, 1:1000), a specific neutrophil marker designed and validated in our laboratory.Citation22 After three additional washes in PBS + 0.1% saponin, three times in PBS, three times in deionized H20 coverslips were mounted with Prolong gold mounting media (Thermo Fisher Scientific). Immunolabelled guinea pig colons infected with Shigella spp. (pGFP-positive or stained with 1:500 dilution of anti-LPS serum from rabbits) strains were imaged using a laser-scanning TCS SP8 confocal microscope (Leica). Z-stack images were taken with 1 μM step size increments. The obtained Z-stack images were processed using Fiji software.
Statistics
Data were analyzed using Prism 8 software (GraphPad). ANOVA or Student’s t-test were performed to analyze the different datasets.
Author contributions
B.S.M. designed research, J.S., C.M., A.C.A., M.C.A., L.I., J.L., and S.R.F. performed research, J.S., A.B., V.M.P., A.K.W., and P.J.S. analyzed data, ML provided expertise, B.S.M. and J.S. wrote the paper.
Supplemental Material
Download Zip (3.6 MB)Acknowledgments
We thank Patricia Flamant from the Institut Pasteur Histology Platform for processing the colonic tissues of guinea pigs. We are very grateful to the team of the Institut Pasteur Animalerie Centrale for their constant support with this project (particularly Serge Hedan, Marion Berard, and Myriam Mattei).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. The data supporting the findings of this study are available from the corresponding author, B.S.M., upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2023.2271597.
Additional information
Funding
References
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2095–18. doi:10.1016/S0140-6736(12)61728-0.
- Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2017;18(3):318–327. doi:10.1016/S1473-3099(17)30753-3.
- Marteyn BS. Shigella vaccine development: the model matters. JSM Trop Med Res. 2016;2:1011–1014.
- Water and Sanitation-Related Diseases and the Environment: Challenges, Interventions, and Preventive Measures. Edited by Janine M. H. Selendy. Hoboken (New Jersey): Wiley-Blackwell. $139.95 (paper). xvii + 497 p. + 16 pl.; ill.; index. ISBN: 978-0-470-52785-6. [Two DVDs are included.] 2011. Q Rev Biology 2013; 88:238–238.
- Mahbub MM, Ahsan CR, Yasmin M, Nessa J. Analysis of different prognostic indicators for malnutrition and Shigella flexneri infection among the children in Bangladesh. Indian J Microbiol. 2012;52(3):400–405. doi:10.1007/s12088-012-0247-1.
- Ferdous F, Das SK, Ahmed S, Farzana FD, Latham JR, Chisti MJ, Ud-Din AIMS, Azmi IJ, Talukder KA, Faruque ASG. Severity of diarrhea and malnutrition among under five-year-old children in rural Bangladesh. Am J Tropical Medicine Hyg. 2013;89(2):223–228. doi:10.4269/ajtmh.12-0743.
- Hossain S, Biswas R, Kabir I, Sarker S, Dibley M, Fuchs G, Mahalanabis D. Single dose vitamin a treatment in acute shigellosis in Bangladeshi children: randomised double blind controlled trial. Bmj. 1998;316(7129):422–426. doi:10.1136/bmj.316.7129.422.
- Roy SK, Raqib R, Khatun W, Azim T, Chowdhury R, Fuchs GJ, Sack DA. Zinc supplementation in the management of shigellosis in malnourished children in Bangladesh. Eur J Clin Nutr. 2008;62(7):849–855. doi:10.1038/sj.ejcn.1602795.
- WHO. Guidelines for the control of shigellosis, including epidemics due to Shigella dysenteriae type 1. 2005.
- Fontaine A, Arondel J, Sansonetti PJ. Role of Shiga toxin in the pathogenesis of bacillary dysentery, studied by using a tox- mutant of Shigella dysenteriae 1. Infect Immun. 1988;56(12):3099–3109. doi:10.1128/iai.56.12.3099-3109.1988.
- Medeiros PHQS, Bolick DT, Ledwaba SE, Kolling GL, Costa DVS, Oriá RB, Lima AÂM, Barry EM, Guerrant RL. A bivalent vaccine confers immunogenicity and protection against Shigella flexneri and enterotoxigenic Escherichia coli infections in mice. NPJ Vaccines. 2020;5(1):30. doi:10.1038/s41541-020-0180-y.
- Mitchell PS, Roncaioli JL, Turcotte EA, Goers L, Chavez RA, Lee AY, Lesser CF, Rauch I, Vance RE. NAIP–NLRC4-deficient mice are susceptible to shigellosis. Elife. 2020;9:e59022. doi:10.7554/eLife.59022.
- Shim D-H, Suzuki T, Chang S-Y, Park S-M, Sansonetti PJ, Sasakawa C, Kweon M-N. New animal model of shigellosis in the Guinea pig: its usefulness for protective efficacy studies. J Immunol. 2007;178(4):2476–2482. doi:10.4049/jimmunol.178.4.2476.
- Brunner K, Samassa F, Sansonetti PJ, Phalipon A. Shigella-mediated immunosuppression in the human gut: subversion extends from innate to adaptive immune responses. Hum Vaccines Immunother. 2019;15(6):1317–1325. doi:10.1080/21645515.2019.1594132.
- Levine MM, Kotloff KL, Barry EM, Pasetti MF, Sztein MB. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat Rev Microbiol. 2007;5(7):540–553. doi:10.1038/nrmicro1662.
- Wenzel H, Kaminski RW, Clarkson KA, Maciel M, Smith MA, Zhang W, Oaks EV. Improving chances for successful clinical outcomes with better preclinical models. Vaccine. 2017;35(49):6798–6802. doi:10.1016/j.vaccine.2017.08.030.
- McArthur MA, Maciel M, Pasetti MF. Human immune responses against Shigella and enterotoxigenic E. coli: Current advances and the path forward. Vaccine. 2017;35(49):6803–6806. doi:10.1016/j.vaccine.2017.05.034.
- Honjo S, Takasaka M, Fujiwara T, Imaizumi K, Ogawa H. Shigellosis in cynomolgus monkeys (MACACA IRUS). Jpn J Medical Sci Biol. 1969;22(3):149–162. doi:10.7883/yoken1952.22.149.
- Lind J A treatise of the scurvy in three parts. 1753;
- Bollet AJ. Scurvy and chronic diarrhea in Civil war troops: were they both nutritional deficiency syndromes? J Hist Med All Sci. 1992;47(1):49–67. doi:10.1093/jhmas/47.1.49.
- Moya‐Alvarez V, Koyembi JJ, Kayé LM, Mbecko J, Sanke‐Waîgana H, Djorie SG, Nyasenu YT, Mad‐Bondo D, Kongoma J, Nakib S, et al. Vitamin C levels in a Central‐African mother–infant cohort: does hypovitaminosis C increase the risk of enteric infections? Maternal Child Nutr. 2021;17(4):e13215. doi:10.1111/mcn.13215.
- Anderson MC, Chaze T, Coïc Y-M, Injarabian L, Jonsson F, Lombion N, Selimoglu-Buet D, Souphron J, Ridley C, Vonaesch P, et al. MUB40 binds to lactoferrin and stands as a specific neutrophil marker. Cell Chem Biol. 2018;25(4):483–493.e9. doi:10.1016/j.chembiol.2018.01.014.
- Marteyn BS, Karimova G, Fenton AK, Gazi AD, West N, Touqui L, Prevost M-C, Betton J-M, Poyraz O, Ladant D, et al. Zape is a novel cell division protein interacting with FtsZ and modulating the Z-Ring dynamics. Mbio. 2014;5(2):e00022–14. doi:10.1128/mBio.00022-14.
- Tinevez J-Y, Arena ET, Anderson M, Nigro G, Injarabian L, André A, Ferrari M, Campbell-Valois F-X, Devin A, Shorte SL, et al. Shigella-mediated oxygen depletion is essential for intestinal mucosa colonization. Nat Microbiol. 2019;4(11):2001–2009. doi:10.1038/s41564-019-0525-3.
- Mortensen A, Hasselholt S, Tveden-Nyborg P, Lykkesfeldt J. Guinea pig ascorbate status predicts tetrahydrobiopterin plasma concentration and oxidation ratio in vivo. Nutr Res New York N Y. 2013;33(10):859–867. doi:10.1016/j.nutres.2013.07.006.
- Injarabian L, Scherlinger M, Devin A, Ransac S, Lykkesfeldt J, Marteyn BS. Ascorbate maintains a low plasma oxygen level. Sci Rep-Uk. 2020;10(1):10659. doi:10.1038/s41598-020-67778-w.
- Padayatty SJ, Levine M. Vitamin C: the known and the unknown and goldilocks. Oral Dis. 2016;22(6):463–493. doi:10.1111/odi.12446.
- Buck A, Aichler M, Huber K, Walch A. Chapter five in situ metabolomics in cancer by mass spectrometry imaging. Adv Cancer Res. 2017;134:117–132.
- Aichler M, Walch A. MALDI imaging mass spectrometry: current frontiers and perspectives in pathology research and practice. Lab Investig. 2015;95(4):422–431. doi:10.1038/labinvest.2014.156.
- Maeda N, Hagihara H, Nakata Y, Hiller S, Wilder J, Reddick R. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc Natl Acad Sci. 2000;97(2):841–846. doi:10.1073/pnas.97.2.841.
- Carr AC, Lykkesfeldt J. Discrepancies in global vitamin C recommendations: a review of RDA criteria and underlying health perspectives. Crit Rev Food Sci Nutr. 2021;61(5):742–755. doi:10.1080/10408398.2020.1744513.
- Preston AM, Rodríguez C, Rivera CE. Plasma ascorbate in a population of children: influence of age, gender, vitamin C intake, BMI and smoke exposure. P R Health Sci J. 2006;25:137–142.
- Jensen PØ, Lykkesfeldt J, Bjarnsholt T, Hougen HP, Høiby N, Ciofu O. Poor antioxidant status exacerbates oxidative stress and inflammatory response to Pseudomonas aeruginosa lung infection in Guinea pigs. Basic Clin Pharmacol Toxicol. 2012;110(4):353–358. doi:10.1111/j.1742-7843.2011.00822.x.
- Hasselholt S, Tveden-Nyborg P, Lykkesfeldt J. Distribution of vitamin C is tissue specific with early saturation of the brain and adrenal glands following differential oral dose regimens in guinea pigs. Br J Nutr. 2015;113(10):1539–1549. doi:10.1017/S0007114515000690.
- Arena ET, Campbell-Valois F-X, Tinevez J-Y, Nigro G, Sachse M, Moya-Nilges M, Nothelfer K, Marteyn B, Shorte SL, Sansonetti PJ. Bioimage analysis of Shigella infection reveals targeting of colonic crypts. Proc National Acad Sci. 2015;112(25):E3282–90. doi:10.1073/pnas.1509091112.
- Phalipon A, Kaufmann M, Michetti P, Cavaillon JM, Huerre M, Sansonetti P, Kraehenbuhl JP. Monoclonal immunoglobulin a antibody directed against serotype-specific epitope of Shigella flexneri lipopolysaccharide protects against murine experimental shigellosis. J Exp Medicine. 1995;182(3):769–778. doi:10.1084/jem.182.3.769.
- Boullier S, Tanguy M, Kadaoui KA, Caubet C, Sansonetti P, Corthésy B, Phalipon A. Secretory IgA-mediated neutralization of Shigella flexneri prevents intestinal tissue destruction by down-regulating inflammatory circuits. J Immunol Baltim Md 1950. 2009;183(9):5879–5885. doi:10.4049/jimmunol.0901838.
- Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358(6382):167–169. doi:10.1038/358167a0.
- Nothelfer K, Arena ET, Pinaud L, Neunlist M, Mozeleski B, Belotserkovsky I, Parsot C, Dinadayala P, Burger-Kentischer A, Raqib R, et al. B lymphocytes undergo TLR2-dependent apoptosis upon Shigella infection. J Exp Medicine. 2014;211(6):1215–1229. doi:10.1084/jem.20130914.
- Salgado-Pabón W, Celli S, Arena ET, Nothelfer K, Roux P, Sellge G, Frigimelica E, Bousso P, Sansonetti PJ, Phalipon A. Shigella impairs T lymphocyte dynamics in vivo. P Natl Acad Sci USA. 2013;110(12):4458–4463. doi:10.1073/pnas.1300981110.
- Konradt C, Frigimelica E, Nothelfer K, Puhar A, Salgado-Pabon W, di BV, Scott-Algara D, Rodrigues CD, Sansonetti PJ, Phalipon A. The Shigella flexneri type three secretion system effector IpgD inhibits T cell migration by manipulating host phosphoinositide metabolism. Cell Host Microbe. 2011;9(4):263–272. doi:10.1016/j.chom.2011.03.010.
- Luchetti G, Roncaioli JL, Chavez RA, Schubert AF, Kofoed EM, Reja R, Cheung TK, Liang Y, Webster JD, Lehoux I, et al. Shigella ubiquitin ligase IpaH7.8 targets gasdermin D for degradation to prevent pyroptosis and enable infection. Cell Host Microbe. 2021;29(10):1521–1530.e10. doi:10.1016/j.chom.2021.08.010.
- Heskett CW, Teafatiller T, Hennessey C, Gareau MG, Marchant JS, Said HM, Subramanian VS. Enteropathogenic Escherichia coli infection inhibits intestinal ascorbic acid uptake via dysregulation of its transporter expression. Dig Dis Sci. 2021;66(7):2250–2260. doi:10.1007/s10620-020-06389-x.
- Ang A, Pullar JM, Currie MJ, Vissers MCM. Vitamin C and immune cell function in inflammation and cancer. Biochem Soc Trans. 2018;46(5):1147–1159. doi:10.1042/BST20180169.
- Carr AC, Maggini S. Vitamin C and immune function. Nutrients. 2017;9(11):1211. doi:10.3390/nu9111211.
- Morante-Palacios O, Godoy-Tena G, Calafell-Segura J, Ciudad L, Martínez-Cáceres EM, Sardina JL, Ballestar E. Vitamin C enhances NF-κB-driven epigenomic reprogramming and boosts the immunogenic properties of dendritic cells. Nucleic Acids Res. 2022;50(19):10981–10994. doi:10.1093/nar/gkac941.
- Anderson MC, Vonaesch P, Saffarian A, Marteyn BS, Sansonetti PJ. Shigella sonnei encodes a functional T6SS used for interbacterial competition and niche occupancy. Cell Host Microbe. 2017;21(6):769–776.e3. doi:10.1016/j.chom.2017.05.004.
- Monceaux V, Chiche-Lapierre C, Chaput C, Witko-Sarsat V, Prevost M-C, Taylor CT, Ungeheuer M-N, Sansonetti PJ, Marteyn BS. Anoxia and glucose supplementation preserve neutrophil viability and function. Blood. 2016;128(7):993–1002. doi:10.1182/blood-2015-11-680918.
- Marteyn B, West NP, Browning DF, Cole JA, Shaw JG, Palm F, Mounier J, Prévost M-C, Sansonetti P, Tang CM. Modulation of Shigella virulence in response to available oxygen in vivo. Nature. 2010;465(7296):355–358. doi:10.1038/nature08970.
