ABSTRACT
Intestinal bacteria are equipped with an enzyme apparatus that is involved in the active biotransformation of xenobiotics, including drugs. Pharmacomicrobiomics, a new area of pharmacology, analyses interactions between bacteria and xenobiotics. However, there is another side to the coin. Pharmacotherapeutic agents can significantly modify the microbiota, which consequently affects their efficacy. In this review, we comprehensively gathered scientific evidence on the interplay between anticancer therapies and gut microbes. We also underlined how such interactions might impact the host response to a given therapy. We discuss the possibility of modulating the gut microbiota to increase the effectiveness/decrease the incidence of adverse events during tumor therapy. The anticipation of the future brings new evidence that gut microbiota is a target of interest to increase the efficacy of therapy.
Introduction
The gut microbiome has been analyzed in multiple aspects, especially over the last several years. Recently, many communication mechanisms between microorganisms residing in particular parts of the human body and distal organs have been described. It is known as an “axis.” For instance, the gut – brain axis, gut – liver axis, gut – muscle axis, gut – bone axis, and others.Citation1–4 Currently, some data indicate that there is also a bidirectional communication between drugs and the gut microbiome.Citation5 It means that gut microbes have an impact on individuals’ response to drugs, whereas drugs affect the gut microbiome. Overall, these interactions are now collectively known as the term “pharmacomicrobiomics”. Gut microbes are able to change the bioavailability, bioactivity, and toxicity of drugs.Citation5 The gut microbiota interacts with drugs with respect to their pharmacokinetics and pharmacodynamics.Citation6,Citation7 Drug metabolism, which is a part of pharmacokinetics, may be mediated by gut microbes both directly (by converting drugs into active/inactive or toxic metabolites) and indirectly (via microbiota-derived metabolites).Citation6 Gut microbiome may also affect drug-drug interactions and even induce those interactions. Supplementation of probiotics (some of them are registered as drugs depending on country regulations) allows the analysis of gut microbes as drugs. For instance, yeast Saccharomyces boulardii CNCM I-745 is registered as a probiotic drug in Poland. The selected bacteria significantly altered the response to the treatment. Akkermansia muciniphila (next-generation probiotic, postbiotic) may affect the efficiency of anti-cancer treatment regarding immunotherapy through enhancement of CTLA-4 and PD-1/PD-L1 blockade.Citation8–10 Overall, the gut microbiome influences the functioning of immune system both systemically and locally, contributing to the maintenance of intestinal homeostasis.Citation11 Thus, microbial community may affect the response to the immunotherapy in different tumors regarding also breast cancer.Citation8,Citation12,Citation13 The influence of drugs on the gut microbiome is observed in alteration of gut microbiome composition (for instance, by proton pump inhibitors) and its function (by metformin).Citation14 Both the composition and production of microbiota-derived metabolites (for instance, SCFAs – short-chain fatty acids) may be altered during chemotherapy.Citation15
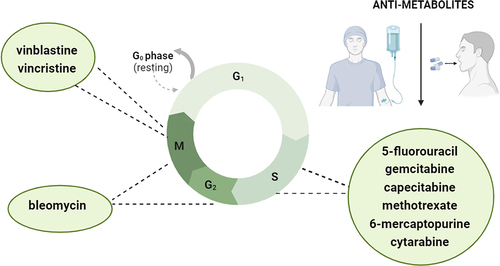
Understanding the bidirectional communication between drugs and the gut microbiome may open new perspectives for more effective pharmacological treatment of health conditions. Most papers describing pharmacomicrobiomics have focused on antibiotics, proton pump inhibitors, and metformin. There is also a bunch of evidence pointing to the interaction between psychotropic drugs and the microbiome.Citation16,Citation17 Nevertheless, recent data indicate that bidirectional interactions between some chemotherapeutic agents and the gut microbiome may affect the response and efficiency of anti-cancer treatment.Citation18 Therefore, in the present review, we mainly concentrate on three main aspects: (1) interaction between the most significant cell-cycle specific anti-cancer drugs () and gut microbiome, (2) mapping of drug metabolism by gut microbiome, and (3) limitations and future perspectives by mentioning key points with their specialist’s justifications and presenting trials that are under investigation.
Anti-metabolites specific for S phase
5-fluorouracil (5-FU)
5-FU (chemical formula: C4H3FN2O2) is used as a chemotherapeutic agent for drugs specific to the S phase of the cell cycle. The side effects of 5-FU include hemorrhagic enteritis and both neurological and hematological toxicity.Citation19 5-FU-induced intestinal mucositis can be reduced by probiotic administration, Streptococcus thermophilus ST4, which has been observed in animal model studies.Citation20 The efficiency of 5-FU is limited by its basic properties, such as short half-life (bolus intravenous − 10–15 min), rapid metabolism, and low bioavailability.Citation21,Citation22 5-FU is metabolized into its inactive metabolite dihydrofluorouracil by Proteobacteria and Firmicutes.Citation23 Recently, Wan et al. investigated the interaction between chemotherapeutic drugs (5-FU – cell-cycle specific drug and oxaliplatin – cell-cycle nonspecific drug) and the gut microbiome during chemotherapy.Citation24 It is noteworthy that the gut microbiome was analyzed by two different methods, that is, 16S rRNA sequencing and shotgun metagenomic sequencing, the latter providing a functional point of view. Based on 16S rRNA sequencing, 5-FU administration decreased the counts of Streptococcus and Bacteroides genera and increased Clostridium hathewayi and Lachnospiraceae abundance. Meanwhile, oxaliplatin was related to the depletion of the Lachnospiraceae family with an increase in Lactobacillus and Streptococcus genera. The results of shotgun metagenomic sequencing showed significant enrichment of Streptococcus salivarius and Ligilactobacillus salivarius after chemotherapy. Additionally, it was demonstrated that the alterations of metabolism in the 5-FU group suggest that gut microbiota can provide NAD+ - nicotinamide adenine dinucleotide to inhibit cancer cell autophagy.Citation24 Since, a response to chemotherapy can be affected by bidirectional interactions between the gut microbiome and drugs24,Citation25; an intra-tumoral microenvironment may also play a significant role in this context. The assessment ‘tumor microenvironment’ is used in terms of both groups of cells, i.e. cancer cells and types of cells (such as immune cells, blood vessels, fibroblasts, mediators – enzymes, cytokines) which surround them.Citation26 Notably, tumor tissues include also bacteria, fungi, viruses, and archaea which overall is assessed as intratumor microbiota.Citation26 Nejman et al. reported that each tumor type presents a distinct composition of microbiome.Citation27 Some studies refer to breast cancer due to the fact that the abundance of microbes building microbiome in this tumor is particularly rich.Citation27,Citation28 Moreover, it was demonstrated that microbial signature is different considering a particular type of breast cancer, i.e. hormone receptor-positive breast cancer and hormone receptor-negative breast cancer.Citation29 Escherichia coli, which is a member of the intra-tumoral microbiota of colorectal cancer tissue, is resistant to 5-FU.Citation30 The most dominant bacterium in colorectal cancer tissue is Fusobacterium nucleatum.Citation30 The modification of response to chemotherapy by F. nucleatum was analyzed in a study by Yu et al. study.Citation31 It was noted that F. nucleatum modulates autophagy, thus promoting resistance to chemotherapy in colorectal cancer. Another study reported that the enhancement of resistance to chemotherapy in colorectal cancer is associated with the ability of F. nucleatum to upregulate the expression of BIRC3 (cellular IAP2 – inhibitors of apoptosis protein).Citation32 Therefore, the measurement of F. nucleatum in both stool and cancer tissue seems to be significant in the effective management of colorectal cancer.Citation31 The association between chemosensitivity and modulation of microbiome has also been investigated in colorectal cancer cell lines (HT-29 and HCT-116).Citation33 In that study, it was demonstrated that the treatment with Lactobacillus plantarum supernatant and chemotherapy based on 5-FU caused cell death through the indication of caspase-3 activity; moreover, the inactivation of Wnt/β-catenin signaling of chemoresistant colorectal cancer cells was noted. Therefore, this combination (L. plantarum supernatant and 5-FU) can increases the chemosensitivity in colorectal cancer cells.Citation33 Similar results were obtained in another study conducting on 5-FU-resistant colorectal cancer cells HCT-116 in which it was shown that L. plantarum can act as a chemosensitizer.Citation34
5-FU causes not only dysbiotic changes in the gut microbiome but also in the oral microbiome, resulting in the development of oral mucositis, which is one of the most common side effects of chemotherapy.Citation35 In a study by Hong et al., it was shown that F. nucleatum and Prevotella oris are enriched during mucositis.Citation35 The pathogenic factors of P. oris origin include immunoglobulin A protease, hyaluronidase, and β-lactamase.Citation36 Notably, P. oris is a gram-negative and anaerobic periodontopathogen that can interact with the major periodontopathic bacterium Porphyromonas gingivalis with numerous virulence factors (mainly gingipains, fimbriae, lipopolysaccharide, outer membrane vesicles, nucleoside diphosphate kinase, and serine phosphatase) belonging to the red complex group of bacteria.Citation36,Citation37 P. gingivalis co-aggregates with F. nucleatum, a pathogen also involved in the development of periodontal diseases.Citation36,Citation37
The efficiency of chemotherapy may be affected by some metabolites that produce the gut microbiota. Urolithin A is a natural metabolite of ellagitannins, which is a dietary polyphenols.Citation38–40 Gut microbiome affects the transformation of ellagitannins into urolithin A; however, its bioavailability depends on the individual’s gut microbiome composition.Citation39,Citation40 This metabolite is characterized by immunomodulatory properties with anti-inflammatory activity.Citation41 Ghosh et al. have reported that urolithin A and UAS03 (its structural analog) chemosensitized colon cancer resistant to 5-FU.Citation42 Therefore, it is worth considering the consumption of urolithin A during 5-FU-based chemotherapy.Citation42
The gut-microbiota-brain axis has been shown to be involved in the pathogenesis of depression.Citation43 In a study by Zhang et al. (n = 20 males, 5-week-old Sprague-Dawley rats; n = 10 – control group, n = 10–5-FU treatment group), the link between gut microbiome changes caused by 5-FU and depressive mood was investigated.Citation44 The gut microbiome was analyzed with 16S rRNA sequencing. Depressive-like behavior was assessed using specific behavioral tests. It was observed that the development of depressive-like behaviors and prefrontal cortex disorders might have been induced by 5-FU and related to gut microbiome alterations following drug use.Citation44 Neuroscientific evidence confirms that prefrontal cortex is involved in depression. The alterations of prefrontal cortex metabolic can be caused by 5-FU and that mechanism is based on the functioning of microbiome-gut-brain axis.Citation44 5-FU changed both the abundance and diversity of the gut microbiome. Moreover, depressive-like behaviors and prefrontal cortex disorders were alleviated by fecal microbiota transplantation (FMT) from healthy controls in rats receiving 5-FU.Citation44 Consequently, these results confirm that 5-FU-induced depressive-like behaviors are strongly associated with the gut microbiome, and an appropriate modification can relieve these symptoms.
Gemcitabine
Gemcitabine is a pyrimidine nucleoside analog that acts as an anti-cancer drug.Citation45 Currently, gemcitabine remains the cornerstone in the treatment of pancreatic cancer.Citation46,Citation47 The intra-tumoral microenvironment in the case of pancreatic cancer may play a significant role in the efficacy of gemcitabine-based chemotherapy ().
Figure 2. Gemcitabine (2’,2’-difluoro-2’-deoxycytidine) is widely used as a chemotherapeutic agent to treat pancreatic cancer. Currently, it is known that tumours are not sterile and intra-tumoral microbes communities exist. Moreover, some bacterium may affect the efficiency of gemcitabine-based chemotherapy. In that context, Gammaproteobacteria is extremely significant which is commonly found in PDAC (pancreatic ductal adenocarcinoma) tumour tissues. Long isoform of bacterial enzyme cytidine deaminase (CDDL) is seen in Gammaproteobacteria. It causes 2’,2’-difluoro-2’-deoxycytidine transformation into its inactivated form, i.e. 2′,2′-difluorodeoxyuridine consequently leading to gemcitabine chemoresistance. Own elaboration based on literature.Citation6,Citation48–50 this figure was created using Biorender.com.
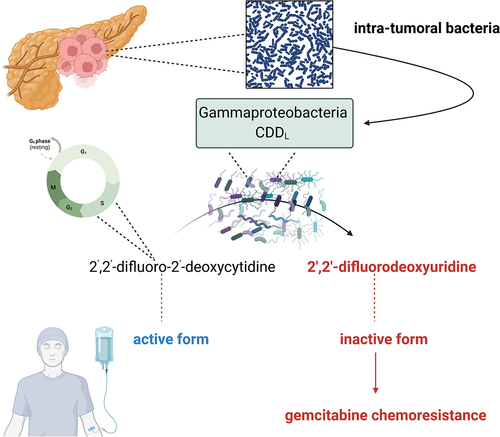
Because the tumor microenvironment mediates the efficacy of gemcitabine-based chemotherapy, the question arises as to whether the elimination of microbes residing in the tumor can alter the response to that treatment. Kang et al. used dual-cascade responsive nanoparticles (sNP@G/IR) with the ability to kill intra-tumoral bacteria and control the release of chemotherapeutic agents was used.Citation51 It included hyaluronic acid shell and glutathione-responsive polymer-core that encapsulates gemcitabine and a photothermal agent. It has been reported that the elimination of intracellular bacteria residing in tumors improves anticancer treatment to a large extent.Citation51
Gemcitabine alters gut microbiota, which has been shown in pancreatic cancer xenograft mice receiving this drug for 3 weeks.Citation52 Gemcitabine reduces the proportion of gram-positive bacteria Firmicutes and Gram-negative Bacteroidetes.Citation52 Microbiota-derived metabolites are also involved in bidirectional interactions between gemcitabine and the gut microbiome. Butyrate (C4), which is a microbial metabolite, provides multiple benefits, such as improvement of intestinal barrier integrity, upregulation of mucin-2 (MUC2) gene expression, inhibition of pro-inflammatory mediators, and others.Citation53 Panebianco et al. investigated the effect of sodium butyrate on response to gemcitabine in both in vitro and in vivo models.Citation54 It was shown that butyrate acts by slowing proliferation and promoting apoptosis in human pancreatic cell lines, leading to enhancement of gemcitabine efficiency. In a pancreatic cancer mouse model, the agent enhanced intestinal integrity and modulated the composition of microbiota by increasing the counts of SCFAs bacterial producers.Citation54
Lipopolysaccharide (LPS) originates from gram-negative bacteria, because it is a major component of their outer membrane.Citation55 It can be considered as a negative predictor for the efficacy of adjuvant gemcitabine in the case of PDAC.Citation56 Recently, it was also shown that LPS can stimulate the growth of breast tumors, which include mainly gram-negative bacteria.Citation57 It was elegantly summarized that such an event might be due to the following mechanisms: (1) the treatment of breast cancer cells using LPS increases S100A7 expression in these cells in vitro; (2) the overexpression of S100A7 downregulates Toll-like receptor 4 (TLR4) whereas upregulates the expression of advanced glycation end product receptor (RAGE) in breast cancer cells; and (3) the novel signaling axis LPS/S100A7/TLR4/RAGE can be involved in the enhancement of tumor growth.Citation57 Considering breast cancer and microbiome aspects, it should be emphasized that there is a difference in bacterial profile in breast tissue in healthy subjects and breast cancer patients.Citation58 Moreover, both local and gut microbial imbalance known commonly as dysbiosis are observed in these patients.Citation59,Citation60 The colonization of breast cancer by F. nucleatum stimulates the growing of tumor as well as progress metastasis.Citation61 In Barroso-Sousa et al. phase II study, it was analyzed whether pembrolizumab in combination with palliative radiation therapy affects the outcome of patients with hormone receptor-positive metastatic breast cancer.Citation62 Pembrolizumab was given intravenously in dose 200 mg 2–7 d prior to radiation (5 treatment, 4 Gy) and on day 1 of repeating 21-d cycles. It was noted that the administration of pembrolizumab together with radiation treatment did not provide an objective response in these patients.Citation62
Capecitabine
Capecitabine is an oral pro-drug of fluorouracil.Citation63,Citation64 The interaction between the gut microbiome and capecitabine was analyzed in a study comprising 33 patients with metastatic colorectal cancer patients.Citation63 Stool samples were collected before, during, and after three cycles of capecitabine. The gut microbiome was analyzed using 16S rRNA sequencing. The gut microbiome was not significantly affected by the three cycles of capecitabine. Moreover, microbial diversity and bacterial abundance were not significantly different between responders and non-responders.Citation63 However, in another study, it was noted that CapeOx (capecitabine plus oxaliplatin) therapy significantly alters gut microbiota of colorectal cancer patients (treated with radical surgery and above mentioned adjuvant therapy).Citation65 Also, in Kaźmierczak-Siedlecka et al. study, it was shown that the proportion between SCFAs is changed in colorectal cancer patients in preoperative period.Citation66 Recently, in 2023 Ziemons et al. investigated the impact of three cycles of capecitabine (± bevacizumab, n = 32) on both SCFAs and branched chain fatty acids (BCFAs) measured from fecal samples in patients (n = 44) with metastatic or unresectable colorectal cancer.Citation67 A significant decrease of valerate and caproate during three cycles of capecitabine was observed. There was no significant association between SCFAs/BCFAs and nutritional status, chemotherapy-related toxicity, or physical performance.Citation67 However, the modulation of gut microbiome by supplementation of probiotic Lactobacillus rhamnosus R0011 improved the efficacy of capecitabine-based chemotherapy, which has been recently shown in an animal model (male Balb/c mice, colon cancer) study.Citation68 The impact of prebiotics (xylo-oligosaccharides) on gut microbiota, side effects, and drug (capecitabine) bioavailability in the case of colorectal cancer patients is under investigation.Citation69 In Guan et al. study including human epidermal growth factor receptor 2 (HER-2) negative metastatic breast cancer patients, it was observed that composition, diversity, and functional structure of gut microbiome were different in participants treated with metronomic chemotherapy (capecitabine) compared to the conventional dose of chemotherapy.Citation64 The different results obtained from the above-mentioned studies may be associated with the following potential reasons: (1) the impact of capecitabine on gut microbiome can be determined by its dose, (2) capecitabine can interact with gut microbiome and vice versa depending on type of cancers (i.e. gastrointestinal cancers vs. hormone-dependent tumors) involving possible other underlying mechanisms, (3) gut microbiota alterations can depend on the combination of chemotherapeutic drugs. Interestingly, in published study protocol the role of intestinal microbiota in the treatment (regarding capecitabine and TAS-102) of cancer as a part of personalized medicine has also been highlighted.Citation70
Methotrexate
Methotrexate (4-amino-4-deoxy-N-10-methylpteroylglutamic acid) is a folic acid antagonist.Citation71,Citation72 At high doses, it inhibits DNA synthesis, repair, and cellular replication.Citation71,Citation73 Methotrexate is used to treat cancer and autoimmune diseases (for instance rheumatoid arthritis) owing to its immunosuppressive function.Citation74,Citation75 Clinical response to methotrexate can be affected by the gut microbiome.Citation76 Additionally, side effects of methotrexate, including gastrointestinal toxicity, might limit its efficacy. In a mouse model study, it was observed that methotrexate caused hepatotoxicity and altered the gut microbiome by increasing Aerococcus, Collinsella, Staphylococcus, Enterococcus, Streptococcus while reducing the levels of Lactobacillus, Bifidobacterium, Ruminococcus, norank_f_Muribaculaceae, unclassified_f_Lachnospiraceae, norank_f_Lachnospiraceae, Eubacterium_xylanophilum_group, Phascolarctobacterium, and Faecalibaculum.Citation77 Nevertheless, only some of these microbes (Streptococcus, Enterococcus, Staphylococcus, Collinsella, Phascolarctobacterium, Faecalibaculum, norank_f_Muribaculaceae) were related to liver injury.Citation77 In the reduction of methotrexate-related intestinal toxicity, Toll-like receptor 2 (TLR2) is involved.Citation78,Citation79 In a Huang et al. study it was observed that methotrexate-induced intestinal toxicity can be reduced by leucovorin (folinic acid) via modulation of gut microbiome.Citation74 Lethal intestinal injury after treatment with high-dose of methotrexate can be alleviated by dietary restrictions, which have been shown in animal model study.Citation71 Short-term dietary restrictions altered gut microbiome by significantly increasing the level of Lactobacillus genus.Citation71 Ferreira et al. assessed whether vitamins C and B2 can reduce methotrexate associated with gastrointestinal mucositis.Citation80 It was noted that in vitro these vitamins increase the growth of Blautia coccoides as well as Roseburia intestinalis, thus they can change the composition of gut microbiota, but their impact on methotrexate-induced mucositis is limited.Citation80 FMT is the most modern method applied to modify gut microbiome; nevertheless, it is still approved only for the treatment of recurrent Clostridioides difficile infection.Citation81,Citation82 Wardill et al. reported that modulation of gut microbiome by FMT after methotrexate treatment has no significant influence on gastrointestinal toxicity.Citation83 Nevertheless, the administration of antibiotics prior to chemotherapy aggravated that toxicity by impairment of mucosal recovery (p < 0.0001), increase of severity of diarrhea (p = 0.0007) and mortality associated with treatment (p = 0.0045). Moreover, restoring the gut microbiome by autologous FMT reversed these effects.Citation83
6-mercaptopurine
6-mercaptopurine is an antiproliferative purine analog and a metabolite of azathioprine. It is not only used to treat inflammatory bowel diseases, non-Hodgkin lymphoma, and lymphoblastic leukemia, but it also has therapeutic potential in solid tumor management.Citation84–87 6-mercaptopurine is characterized by its low bioavailability (16%), short half-life (0.5–1.5 h), and high first-pass effect.Citation84 It is a part of thiopurine metabolic pathway. At the beginning, azathioprine is cleaved to 6-mercaptopurine; next, there are three metabolic pathways in 6-mercaptopurine metabolism, that is, (1) inactive 6-thiouric acid (enzyme – xanthine oxidase), (2) inactive 6-methylmercaptopurine (enzyme – thiopurine methyltransferase), and (3) therapeutic 6-thioguanine nucleotide (enzyme – hypoxanthine phosphoribosyl transferase).Citation88 Oancea et al. reported that an alternative way of thioguanine pro-drug conversion is through bacteria.Citation89 Recently, in a rat model study, it was shown that the pharmacokinetics of the above-mentioned metabolites of azathioprine (i.e., both 6-thioguanine nucleotide and 6-methylmercaptopurine) is altered by gut microbial metabolism.Citation90 Moreover, the efficacy of azathioprine is affected by the synthesis of microbial butyrate, which has been observed in patients with inflammatory bowel diseases.Citation91
Antibiotics specific for G2/M phase
Bleomycin
Bleomycin is a broad-spectrum anticancer drug belonging to the subfamily of glycopeptide antibiotics.Citation92,Citation93 The side effects of bleomycin include lung injury. Bleomycin-induced pulmonary fibrosis has been considered in the context of the gut-lung axis.Citation94 The gut microbiota imbalance was observed in a mouse model with pulmonary fibrosis induced by bleomycin; the amounts of Catenibacterium, Lactobacillus – L. johnsonii and L. gasseri were decreased, whereas the abundance of Verrucomicrobiales and Enterobacteriales was increased.Citation94 Notably, both Catenibacterium and Lactobacillus are probiotics, thus they provide beneficial effects.Citation95,Citation96 The role of gut microbiota and gut-lung axis in case of bleomycin-induced lung injury has also been investigated in Yoon et al. mice model study.Citation97 It was shown that the role of gut microbiota is mostly important in acute phase of bleomycin treatment.Citation97 Bleomycin-induced pulmonary fibrosis can be beneficially affected by phycocyanin, which has been shown in C57BL/6 mice model study.Citation98 It was noted that phycocyanin not only reduces the pro-inflammatory cytokines but also significantly increases both bacterial diversity and SCFAs-bacterial producers as well as decreases inflammation-related bacteria.Citation98 Phycocyanin belongs to the phycobiliprotein family. It is obtained from different species including Spirulina sp., Phormidium sp., Synechococcus sp., and others.Citation99 Phycocyanin is characterized by anti-tumor properties; therefore, it can be considered as an anti-cancer agent.Citation99 Huang et al. reported that phycocyanin suppresses epithelial-mesenchymal transition and affects the Akt/β-catenin pathway, thus inhibiting pancreatic cancer metastasis.Citation100
Although the interactions between bleomycin and the gut microbiome are not fully described and currently most of the presented studies are conducted using animal models, there are promising opportunities to consider bleomycin-related pharmacomicrobiomics in oncology.
Drugs specific for M phase
Vinblastine and vincristine
Vinblastine and vincristine are monoterpene indole alkaloids (MIAs) of Catharanthus (or Vinca)Citation101 origin, also produced using genetic engineering techniques.Citation102 Both of which have proven efficacy in cancer treatment mainly due to mitotic arrest and/or cell death.Citation103–105 Information on the interaction between these agents and the microbiome is scarce. In a study by Rtibi et al.Citation106, it was demonstrated that vinblastine administered to rats diminished the gastrointestinal motility (12.88% compared to vehicle and 24.33% compared to loperamide) and consequently induced constipation (11.16% compared to vehicle and 32.95% compared to loperamide). This proves that the interaction with the microbiome as gut microbes regulate the motility predominantly via their cell wall components bioactive products of their metabolismCitation107,Citation108 and the constipation was proved to be related to gut microbiota alterations.Citation109 These changes were accompanied by alterations in pro- and antioxidant synthesis, which also negatively influenced lipid peroxidation. Again, both were linked to gut microbiota.Citation110 Also, a clinical trial in 2011Citation111 found that the administration of high-dose methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) with or without granulocyte colony-stimulating factor resulted in a relatively high percentage of nausea and/or vomiting. These results suggest, although not directly, that vinblastine has the potential to alter the microbiological niche within the gut, especially as such functional phenotypes are linked to microbiota-gut-brain axis dysregulation.Citation112 Nevertheless, more studies with vinblastine administered alone are needed to verify this hypothesis. In addition, it was historically verified that the administration of peritumoral hyaluronidase may prevent vinblastine-induced local inflammation and simultaneously as a pretreatment can drastically increase the activity of low-dose vinblastine.Citation113,Citation114 One might conclude that the gut microbiota functions might be at least partly responsible for the action of vinblastine. Indeed, hyaluronidases might be of microbiota-origin.Citation115
A study by Peiris and OppenheimCitation116 found that the antimicrobial activity of vincristine was minimal. However, as elegantly demonstrated by López-GómezCitation117 a 10-d administration of vincristine resulted in gastrointestinal motility inhibition and alterations in the digestive wall in the ileum and colon, including villus shortening and inflammatory nodules, respectively. Some of these results were replicated recently.Citation118,Citation119 In the latter oneCitation119, a cannabinoid antagonist was found to counteract dysmotility. As evidenced in the literature, both the structure of the gutCitation120, its motilityCitation107,Citation108 and inflammatory processes taking place there are predominantly driven by microorganisms.Citation121 In addition, gut microbiota structure and functions might shape the response to vincristine. Taper et al. tested the effect of administering inulin or oligofructose to mice with transplantable liver tumors treated with, inter alia, vincristine, and observed a potentiation of anti-carcinogenic effects, confirming that gut microbiota might be an object of interest in anti-tumor therapy.Citation122
Other anti-cancer drugs
Platinum-based drugs
Cisplatin, carboplatin, and oxaliplatin belong to the platinum-based chemotherapeutic which are widely used in oncology.Citation123 There are two mechanisms by which cisplatin acts. First one regards cellular uptake, DNA platination and activation of cellular process leading to apoptosis of cancer cells.Citation123,Citation124 Second mechanism is an alternative effect and it includes short acidification of cytoplasm, disruption of RNA transcription, inhibition of oncogenic proteins and alterations of tumor cells metabolic plasticity.Citation123 Gui et al. in lung cancer mice model study reported that the response to anti-cancer treatment based on cisplatin can be modulate by commensal microbiota.Citation125 It was shown that co-treatment with probiotics – Lactobacillus acidophilus affects the expressions of interferon-γ (IFN-γ), GZMB, PRF1 in CD8+ T cells which were previously decreased by administration of antibiotics (such as vancomycin, ampicillin, neomycin). Additionally, the improvement of survival rate was observed in mice treated with cisplatin and L. acidophilus (p = 0.048) in contrast to mice which received cisplatin and above listed antibiotics.Citation125 The enhancement of anti-tumor effects of chemotherapeutic cisplatin (in combination with gemcitabine) through administration of probiotics (Lactobacillus casei Shirota and Bifidobacterium breve) has also been recently confirmed in urothelial cancer in Miyake et al. study.Citation126 On the other hand, as we previously concerned, some of microbes may enhance chemoresistance. In Liang et al. study it was shown that F. nucleatum promotes cisplatin resistance in case of esophageal squamous cell carcinoma.Citation127 It is based on the ability of F. nucleatum to induction of myeloid-derived suppressor cells by the activation of NOD-like receptor protein 3 (NLRP3).Citation127
Paclitaxel
Paclitaxel is a class of taxanes, and it has an impact on the stabilization of microtubules. It is used as a first-line drug in breast cancer patients.Citation128 It is also used to treat other type of cancers, such as non-small cell lung cancer. Paclitaxel affects microbiome causing dysbiotic alterations.Citation129 Interestingly, some of gut microbial imbalance caused by other disease than cancer can influence the response to paclitaxel, which has been shown in Kesh et al. study.Citation130 They reported that microbiome dysbiosis caused by type 2 diabetes can be associated with chemoresistance (paclitaxel, gemcitabine) in case of pancreatic adenocarcinoma.Citation130
Doxorubicin
Doxorubicin is an anti-cancer agent which induces cancer cell death through many intracellular target inhibition of topoisomerase II or generation of reactive oxygen species.Citation131 It belongs to the nonselective class I anthracycline family.Citation132 The bidirectional interactions between doxorubicin and gut microbiome were investigated in Bawaneh et al. study regarding triple-negative breast cancer (female BALB/c mice).Citation131 Intestinal microbiota was analyzed from fecal samples. It was noted that doxorubicin was associated with increased abundance of A. muciniphila; moreover, doxorubicin responders present an elevated abundance of this bacterium prior to this treatment.Citation133 The results of another study show that neoadjuvant chemotherapy affects breast tumor microbiota.Citation134 Interestingly, doxorubicin can be inactivated by Raoultella planticola via reductive deglycosylation; moreover, doxorubicin may be metabolized into inactive metabolites by Klebsiella pneumoniae and Escherichia coli BW25113.Citation132,Citation135
Mapping of drug metabolism by gut microbiome
Mapping of microbiota-host-drug networks can be a part of personalized medicine.Citation136–139 This is based on the assumption that biotransformation involves microbes chemically transforming drugs.Citation140 It should be emphasized that bacterial metabolism regarding both reduction and hydrolysis results in the generation of nonpolar compounds. Low molecular weight byproducts; therefore, this metabolism is different from liver metabolism, which is mainly based on oxidation and conjugation.Citation141 Nevertheless, some drugs have minimal contact with intestinal bacteria because they are absorbed in the upper gut.Citation141 Javdan et al. reported that personalized microbiomes metabolize drugs in different ways; thus, the interactions between drugs and gut microbiome vary between individuals and, consequently, can be considered as personalized medicine.Citation137 It is also related to the ways of drug administration, that is, oral, sublingual, parenteral. Some of drugs are taken sublingually to avoid first-pass liver metabolism and achieve high bioavailability and rapid effects.Citation142 For instance, oral administration of nitroglycerin provides approximately 10–20% of its bioavailability; thus, sublingual administration is recommended to provide rapid effects in case of angina pectoris.Citation143 Zimmermann et al. have reported that many of oral drugs are modified by microorganisms.Citation144 They measured the ability of 76 diverse human gut bacteria to metabolize 271 oral drugs. Both the systemic and intestinal metabolism of drugs in mice can be influenced by the microbiome (more precisely by microbiome-encoded enzymes). These results indicate that the genomic content of gut bacteria is strongly associated with alterations in drug metabolism.Citation144 In another study, 70 interactions between bacteria and drugs were reported.Citation140 It is noteworthy that some of these interactions were observed by the ability of bacteria to store drugs intracellularly; however, without chemical transformation.Citation140 Therefore, these results indicate the underlying mechanism of this mapping, which regards not only the biotransformation of drugs by microorganisms but also the bioaccumulation of drugs by bacteria.
Perspectives for the future and limitations
Key points:
It is recommended to analyze the influence of cell-cycle specific anti-cancer drugs in combination with cell-cycle phase-nonspecific anti-tumor agents or monoclonal antibodies on the gut microbiome and vice versa.
Justification: Combinations of drugs such as 5-FU + oxaliplatin, capecitabine + bevacizumab, CapeOx (capecitabine + oxaliplatin), and others are used. The different results of the interaction between monotherapy vs. gut microbiome compared to a mixture of anti-tumor agents vs. gut microbiome can be observed. Moreover, monoclonal antibodies, such as bevacizumab, act against vascular endothelial growth factorCitation145 therefore, it presents another mechanism of action.
It is recommended to take into consideration factors which modulate the gut microbiome.
Justification: The gut microbiome is modulated by multiple factors, such as diet and administration of prebiotics, probiotics, synbiotics (prebiotics and probiotics), and postbiotics. Therefore, observation during cycles of chemotherapy should also consider additional factors that may affect the interactions between drugs and microbes.
Individual matching of therapeutic gut microbiome modulators can beneficially affect the restoration of gut microbiome imbalance and, consequently, enhance the efficiency of anti-cancer treatment.
Justification: The modification of the gut microbiome through therapeutic methods, such as administration of prebiotics, probiotics, synbiotics, postbiotics, and FMT, can restore gut microbiome imbalance; however, the introduction of these methods should be based not only on underlying disease but also on additional disorders/conditions as well as drug administration and their interactions.
The interactions between drugs and the gut microbiome should consider not only bacteria but also other components of that community.
Justification: The gut microbiome should be considered a complex community of bacteria, fungi, viruses, archaea, and parasites.Citation146 It would be interesting to analyze the interactions between drugs and fungi. For instance, the fungal probiotic Saccharomyces boulardii CNCM I-745 is resistant to antibiotics because of its natural fungal properties.Citation147
The way of drug administration may affect their interactions with gut microbiome.
Justification: Drugs that are absorbed in the upper part of the gastrointestinal tract exhibit decreased interactions with gut microbes.
Although the impact of the microbiome on the response to chemotherapeutics seems to be clinically significant, there are a limited number of clinical trials that directly analyze its relationship. All the related studies revealed in “Clinicaltrials.gov, clinicaltrialsregister.eu, eortc.org/clinical-trials-database, trialsearch.who.int” are shown in . In the future, new prospective clinical trials should be conducted in this field. There is a need for studies investigating the changes in the microbiome before and after chemotherapeutic treatment. Therefore, the potential of changing the microbiota composition before treatment to obtain a better response should be investigated.
Table 1. The summary of chemotherapeutics which are under investigation based on available clinical trial databases.
Conclusions
Currently, pharmacomicrobiomics is a hot topic that opens new perspectives in personalized cancer management. It is extremely necessary due to the fact that the range of problems affecting oncological patients is wide, that is, from the early stage of cancer with no metastasis (with different responses to the treatment) to non-resectable tumors with multiple metastases. Among others, the transformation of chemotherapeutic drugs into their inactive form by specific microbes and the consequent promotion of chemoresistance confirms that pharmacomicrobiomics should be included in personalized medicine. Despite the fact that this paper is concentrated on cell-cycle specific anti-cancer drugs, it should be mentioned that immune checkpoint inhibitors and overall immunotherapy are strongly involved into bidirectional interactions between them and microbiome. Some of the bacteria, such as A. muciniphila, is able to enhance CTLA-4 as well as PD-1/PD-L1 blockade thus it improves the effects of immunotherapy. The chemical structure of drugs, pharmacodynamics, pharmacokinetics, drug-drug and drug-nutrient interactions, tumor microenvironment, composition of the gut microbiome, and metabolome must be considered. Thus, it is recommended that studies assessing pharmacomicrobiomics be designed and conducted by a multidisciplinary team of specialists, such as oncologists, oncological surgeons, biotechnologists, pharmacists, and nutritionists.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Xie Z, Zhang X, Zhao M, Huo L, Huang M, Li D, Zhang S, Cheng X, Gu H, Zhang C, et al. The gut-to-brain axis for toxin-induced defensive responses. Cell. 2022;185(23):4298–4316.e21. doi:10.1016/j.cell.2022.10.001.
- Yin Y, Guo Q, Zhou X, Duan Y, Yang Y, Gong S, Han M, Liu Y, Yang Z, Chen Q, et al. Role of brain-gut-muscle axis in human health and energy homeostasis. Front Nutr. 2022;9:947033. doi:10.3389/fnut.2022.947033.
- Tu Y, Yang R, Xu X, Zhou X. The microbiota-gut-bone axis and bone health. J Leukoc Biol. 2021;110(3):525–20. doi:10.1002/JLB.3MR0321-755R.
- Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. 2020;72(3):558–577. doi:10.1016/j.jhep.2019.10.003.
- Weersma RK, Zhernakova A, Fu J. Interaction between drugs and the gut microbiome. Gut. 2020;69(8):1510–1519. doi:10.1136/gutjnl-2019-320204.
- Ting NLN, Lau HCH, Yu J. Cancer pharmacomicrobiomics: targeting microbiota to optimise cancer therapy outcomes. Gut. 2022;71(7):1412–1425. doi:10.1136/gutjnl-2021-326264.
- Conti G, D’Amico F, Fabbrini M, Brigidi P, Barone M, Turroni S. Pharmacomicrobiomics in anticancer therapies: why the gut microbiota should be pointed out. Genes (Basel). 2022;14(1):55. doi:10.3390/genes14010055.
- Kaźmierczak-Siedlecka K, Roviello G, Catalano M, Polom K. Gut microbiota modulation in the context of immune-related aspects of lactobacillus spp and bifidobacterium spp. in gastrointestinal cancers. Nutrients. 2021;13(8):2674. doi:10.3390/nu13082674.
- Kaźmierczak-Siedlecka K, Skonieczna-Żydecka K, Hupp T, Duchnowska R, Marek-Trzonkowska N, Połom K. Next-generation probiotics - do they open new therapeutic strategies for cancer patients? Gut Microbes. 2022;14(1):2035659. doi:10.1080/19490976.2022.2035659.
- Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Sci. 2018;359(6371):104–108. doi:10.1126/science.aao3290.
- Shi N, Li N, Duan X, Niu H. Interaction between the gut microbiome and mucosal immune system. Mil Med Res. 2017;4(1):14. doi:10.1186/s40779-017-0122-9.
- Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018;33(4):570–580. doi:10.1016/j.ccell.2018.03.015.
- Vitorino M, Baptista de Almeida S, Alpuim Costa D, Faria A, Calhau C, Azambuja Braga S. Human microbiota and immunotherapy in breast cancer - a review of recent developments. Front Oncol. 2022;11:815772. doi:10.3389/fonc.2021.815772.
- Doestzada M, Vila AV, Zhernakova A, Koonen DPY, Weersma RK, Touw DJ, Kuipers F, Wijmenga C, Fu J. Pharmacomicrobiomics: a novel route towards personalized medicine? Protein Cell. 2018;9(5):432–445. doi:10.1007/s13238-018-0547-2.
- Leigh SJ, Lynch CMK, Bird BRH, Griffin BT, Cryan JF, Clarke G. Gut microbiota-drug interactions in cancer pharmacotherapies: implications for efficacy and adverse effects. Expert Opin Drug Metab Toxicol. 2022;18(1):5–26. doi:10.1080/17425255.2022.2043849.
- Skonieczna-Żydecka K, Łoniewski I, Misera A, Stachowska E, Maciejewska D, Marlicz W, Galling B. Second-generation antipsychotics and metabolism alterations: a systematic review of the role of the gut microbiome. Psychopharmacol (Berl). 2019;236(5):1491–1512. doi:10.1007/s00213-018-5102-6.
- Misera A, Łoniewski I, Palma J, Kulaszyńska M, Czarnecka W, Kaczmarczyk M, Liśkiewicz P, Samochowiec J, Skonieczna-Żydecka K. Clinical significance of microbiota changes under the influence of psychotropic drugs. An updated narrative review. Front Microbiol. 2023;14:1125022. doi:10.3389/fmicb.2023.1125022.
- Lehouritis P, Cummins J, Stanton M, Murphy CT, McCarthy FO, Reid G, Urbaniak C, Byrne WL, Tangney M. Local bacteria affect the efficacy of chemotherapeutic drugs. Sci Rep. 2015;5(1):14554. doi:10.1038/srep14554.
- Demeckova V, Mudronova D, Gancarcikova S, Kubatka P, Kajo K, Kassayova M, Bojkova B, Adamkov M, Solár P. 5-fluorouracil treatment of CT26 colon cancer is compromised by combined therapy with IMMODIN. Int J Mol Sci. 2022;23(12):6374. doi:10.3390/ijms23126374.
- Shen SR, Chen WJ, Chu HF, Wu SH, Wang YR, Shen TL, Jung YH. Amelioration of 5-fluorouracil-induced intestinal mucositis by Streptococcus thermophilus ST4 in a mouse model. PloS One. 2021;16(7):e0253540. doi:10.1371/journal.pone.0253540.
- Valencia-Lazcano AA, Hassan D, Pourmadadi M, Shamsabadipour A, Behzadmehr R, Rahdar A, Medina DI, Díez-Pascual AM, Shamsabadipour A. 5-fluorouracil nano-delivery systems as a cutting-edge for cancer therapy. Eur J Med Chem. 2023;246:114995. doi:10.1016/j.ejmech.2022.114995.
- Liao AH, Lee YA, Lin DL, Chuang HC, Wang JK, Chang CE, Li HT, Wu TY, Shih CP, Wang CH. Treatment efficacy of low-dose 5-fluorouracil with ultrasound in mediating 5-fluorouracil-loaded microbubble cavitation in head and neck cancer. Drug Deliv. 2023;30(1):1–13. doi:10.1080/10717544.2022.2154410.
- Spanogiannopoulos P, Kyaw TS, Guthrie BGH, Bradley PH, Lee JV, Melamed J, Melamed J, Malig YNA, Lam KN, Gempis D, et al. Host and gut bacteria share metabolic pathways for anti-cancer drug metabolism. Nature Microbiology. 2022;7(10):1605–1620. doi:10.1038/s41564-022-01226-5.
- Wan L, Li H, Sun G, Zhang L, Xu H, Su F, He S, Xiao F. Mutational pattern induced by 5-fluorouracil and oxaliplatin in the gut microbiome. Front Microbiol. 2022;13:841458. doi:10.3389/fmicb.2022.841458.
- Gori S, Inno A, Belluomini L, Bocus P, Bisoffi Z, Russo A, Arcaro G. Gut microbiota and cancer: How gut microbiota modulates activity, efficacy and toxicity of antitumoral therapy. Crit Rev Oncol Hematol. 2019;143:139–147. doi:10.1016/j.critrevonc.2019.09.003.
- Vitorino M, Costa DA, Vicente R, Caleça T, Santos C. Local breast microbiota: a “new” player on the block. Cancers Basel. 2022;14(15):3811. doi:10.3390/cancers14153811.
- Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, Rotter-Maskowitz A, Weiser R, Mallel G, Gigi E, et al. The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Sci. 2020;368(6494):973–980. doi:10.1126/science.aay9189.
- Banerjee S, Wei Z, Tan F, Peck KN, Shih N, Feldman M, Rebbeck TR, Alwine JC, Robertson ES. Distinct microbiological signatures associated with triple negative breast cancer. Sci Rep. 2015;5(1):15162. doi:10.1038/srep15162.
- Banerjee S, Tian T, Wei Z, Shih N, Feldman MD, Peck KN, DeMichele AM, Alwine JC, Robertson ES. Distinct microbial signatures associated with different breast cancer types. Front Microbiol. 2018;9:951. doi:10.3389/fmicb.2018.00951.
- LaCourse KD, Zepeda-Rivera M, Kempchinsky AG, Baryiames A, Minot SS, Johnston CD, Bullman S. The cancer chemotherapeutic 5-fluorouracil is a potent Fusobacterium nucleatum inhibitor and its activity is modified by intratumoral microbiota. Cell Rep. 2022;41(7):111625. doi:10.1016/j.celrep.2022.111625.
- Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, Qian Y, Kryczek I, Sun D, Nagarsheth N, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170(3):548–563.e16. doi:10.1016/j.cell.2017.07.008.
- Zhang S, Yang Y, Weng W, Guo B, Cai G, Ma Y, Cai S. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J Exp Clin Cancer Res. 2019;38(1):14. doi:10.1186/s13046-018-0985-y.
- An J, Ha EM. Combination therapy of Lactobacillus plantarum supernatant and 5-fluouracil increases chemosensitivity in colorectal cancer cells. J Microbiol Biotechnol. 2016;26(8):1490–1503. doi:10.4014/jmb.1605.05024.
- Kim HJ, An J, Ha EM. Lactobacillus plantarum-derived metabolites sensitize the tumor-suppressive effects of butyrate by regulating the functional expression of SMCT1 in 5-FU-resistant colorectal cancer cells. J Microbiol. 2022;60(1):100–117. doi:10.1007/s12275-022-1533-1.
- Hong BY, Sobue T, Choquette L, Dupuy AK, Thompson A, Burleson JA, Salner AL, Schauer PK, Joshi P, Fox E, et al. Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome. 2019;7(1):66. doi:10.1186/s40168-019-0679-5.
- Sato T, Nakazawa F. Coaggregation between prevotella oris and porphyromonas gingivalis. J Microbiol Immunol Infect. 2014;47(3):182–186. doi:10.1016/j.jmii.2012.09.005.
- de Andrade KQ, Almeida-da-Silva CLC, Coutinho-Silva R. Immunological pathways triggered by porphyromonas gingivalis and Fusobacterium nucleatum: therapeutic possibilities? Mediators Inflamm. 2019;2019:1–20. doi:10.1155/2019/7241312.
- Denk D, Petrocelli V, Conche C, Drachsler M, Ziegler PK, Braun A, Kress A, Nicolas AM, Mohs K, Becker C, et al. Expansion of T memory stem cells with superior anti-tumor immunity by urolithin a-induced mitophagy. Immunity. 2022;55(11):2059–2073.e8. doi:10.1016/j.immuni.2022.09.014.
- Lee HJ, Jung YH, Choi GE, Kim JS, Chae CW, Lim JR, Kim SY, Yoon JH, Cho JH, Lee SJ, et al. Urolithin A suppresses high glucose-induced neuronal amyloidogenesis by modulating TGM2-dependent ER-mitochondria contacts and calcium homeostasis. Cell Death Differ. 2021;28(1):184–202. doi:10.1038/s41418-020-0593-1.
- García-Villalba R, Tomás-Barberán FA, Iglesias-Aguirre CE, Giménez-Bastida JA, González-Sarrías A, Selma MV, Espín JC. Ellagitannins, urolithins, and neuroprotection: human evidence and the possible link to the gut microbiota. Mol Aspects Med. 2023;89:101109. doi:10.1016/j.mam.2022.101109.
- Tao H, Li W, Zhang W, Yang C, Zhang C, Liang X, Yin J, Bai J, Ge G, Zhang H, et al. Urolithin a suppresses RANKL-induced osteoclastogenesis and postmenopausal osteoporosis by, suppresses inflammation and downstream NF-κB activated pyroptosis pathways. Pharmacol Res. 2021;174:105967. doi:10.1016/j.phrs.2021.105967.
- Ghosh S, Singh R, Vanwinkle ZM, Guo H, Vemula PK, Goel A, Haribabu B, Jala VR. Microbial metabolite restricts 5-fluorouracil-resistant colonic tumor progression by sensitizing drug transporters via regulation of FOXO3-FOXM1 axis. Theranostics. 2022;12(12):5574–5595. doi:10.7150/thno.70754.
- Chang L, Wei Y, Hashimoto K. Brain-gut-microbiota axis in depression: a historical overview and future directions. Brain Res Bull. 2022;182:44–56. doi:10.1016/j.brainresbull.2022.02.004.
- Zhang F, Chen H, Zhang R, Liu Y, Kong N, Guo Y, Xu M. 5-fluorouracil induced dysregulation of the microbiome-gut-brain axis manifesting as depressive like behaviors in rats. Biochim Biophys Acta Mol Basis Dis. 2020;1866(10):165884. doi:10.1016/j.bbadis.2020.165884.
- Miao H, Chen X, Luan Y. Small molecular gemcitabine prodrugs for cancer therapy. Curr Med Chem. 2020;27(33):5562–5582. doi:10.2174/0929867326666190816230650.
- Beutel AK, Halbrook CJ. Barriers and opportunities for gemcitabine in pancreatic cancer therapy. Am J Physiol Cell Physiol. 2023;324(2):C540–52. doi:10.1152/ajpcell.00331.2022.
- Gebregiworgis T, Bhinderwala F, Purohit V, Chaika NV, Singh PK, Powers R. Insights into gemcitabine resistance and the potential for therapeutic monitoring. Metabolomics. 2018;14(12):156. doi:10.1007/s11306-018-1452-7.
- Vande Voorde J, Sabuncuoğlu S, Noppen S, Hofer A, Ranjbarian F, Fieuws S, Balzarini J, Liekens S. Nucleoside-catabolizing enzymes in mycoplasma-infected tumor cell cultures compromise the cytostatic activity of the anticancer drug gemcitabine. J Biol Chem. 2014;289(19):13054–13065. doi:10.1074/jbc.M114.558924.
- Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, Gavert N, Zwang Y, Cooper ZA, Shee K, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Sci. 2017 wrzesień 15;357(6356):1156–1160. doi:10.1126/science.aah5043.
- Geller LT, Straussman R. Intratumoral bacteria may elicit chemoresistance by metabolizing anticancer agents. Mol Cell Oncol. 2018;5(1):e1405139. doi:10.1080/23723556.2017.1405139.
- Kang X, Bu F, Feng W, Liu F, Yang X, Li H, Yu Y, Li G, Xiao H, Wang X. Dual-cascade responsive nanoparticles enhance pancreatic cancer therapy by eliminating tumor-resident intracellular bacteria. Adv Mater. 2022;34(49):e2206765. doi:10.1002/adma.202206765.
- Panebianco C, Adamberg K, Jaagura M, Copetti M, Fontana A, Adamberg S, Kolk K, Vilu R, Andriulli A, Pazienza V. Influence of gemcitabine chemotherapy on the microbiota of pancreatic cancer xenografted mice. Cancer Chemother Pharmacol. 2018;81(4):773–782. doi:10.1007/s00280-018-3549-0.
- Kaźmierczak-Siedlecka K, Marano L, Merola E, Roviello F, Połom K. Sodium butyrate in both prevention and supportive treatment of colorectal cancer. Front Cell Infect Microbiol. 2022;12:1023806. doi:10.3389/fcimb.2022.1023806.
- Panebianco C, Villani A, Pisati F, Orsenigo F, Ulaszewska M, Latiano TP, Potenza A, Andolfo A, Terracciano F, Tripodo C, et al. Butyrate, a postbiotic of intestinal bacteria, affects pancreatic cancer and gemcitabine response in in vitro and in vivo models. Biomed Pharmacother. 2022;151:113163. doi:10.1016/j.biopha.2022.113163.
- Maldonado RF, Sá-Correia I, Valvano MA, Whitfield C. Lipopolysaccharide modification in gram-negative bacteria during chronic infection. FEMS Microbiol Rev. 2016;40(4):480–493. doi:10.1093/femsre/fuw007.
- Guenther M, Gil L, Surendran SA, Palm MA, Heinemann V, von Bergwelt-Baildon M, Mayerle J, Engel J, Werner J, Boeck S, et al. Bacterial Lipopolysaccharide as a negative predictor of adjuvant gemcitabine efficacy in pancreatic cancer. JNCI Cancer Spectr. 2022;6(3):kac039. doi:10.1093/jncics/pkac039.
- Wilkie T, Verma AK, Zhao H, Charan M, Ahirwar DK, Kant S, Pancholi V, Mishra S, Ganju RK. Lipopolysaccharide from the commensal microbiota of the breast enhances cancer growth: role of S100A7 and TLR4. Mol Oncol. 2022;16(7):1508–1522. doi:10.1002/1878-0261.12975.
- Urbaniak C, Gloor GB, Brackstone M, Scott L, Tangney M, Reid G, Goodrich-Blair H. The microbiota of breast tissue and its association with breast cancer. Appl Environ Microbiol. 2016;82(16):5039–5048. doi:10.1128/AEM.01235-16.
- Parida S, Sharma D. The power of small changes: comprehensive analyses of microbial dysbiosis in breast cancer. Biochim Biophys Acta Rev Cancer. 2019;1871(2):392–405. doi:10.1016/j.bbcan.2019.04.001.
- Costa DA, Nobre JG, Vaz Batista M, Ribeiro C, Calle C, Cortes A, Marhold M, Negreiros I, Borralho P, Brito M, et al. Human microbiota and breast cancer—is there any relevant link?—a literature review and new horizons toward personalised medicine. Front Microbiol. 2021;12:584332. doi:10.3389/fmicb.2021.584332.
- Parhi L, Alon-Maimon T, Sol A, Nejman D, Shhadeh A, Fainsod-Levi T, Yajuk O, Isaacson B, Abed J, Maalouf N, et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat Commun. 2020;11(1):3259. doi:10.1038/s41467-020-16967-2.
- Barroso-Sousa R, Krop IE, Trippa L, Tan-Wasielewski Z, Li T, Osmani W, Andrews C, Dillon D, Richardson ET, Pastorello RG, et al. A phase II study of pembrolizumab in combination with palliative radiotherapy for hormone receptor-positive metastatic breast cancer. Clin Breast Cancer. 2020;20(3):238–245. doi:10.1016/j.clbc.2020.01.012.
- Aarnoutse R, Ziemons J, de Vos-Geelen J, Valkenburg-van Iersel L, Wildeboer ACL, Creemers VAGJM, Baars A, Vestjens HJHMJ, Le GN. The role of intestinal microbiota in metastatic colorectal cancer patients treated with capecitabine. Clin Colorectal Cancer. 2022;21(2):e87–97. doi:10.1016/j.clcc.2021.10.004.
- Guan X, Ma F, Sun X, Li C, Li L, Liang F, Li S, Yi Z, Liu B, Xu B. Gut microbiota profiling in patients with HER2-negative metastatic breast cancer receiving metronomic chemotherapy of capecitabine compared to those under conventional dosage. Front Oncol. 2020;10:902. doi:10.3389/fonc.2020.00902.
- Kong C, Gao R, Yan X, Huang L, He J, Li H, You J, Qin H. Alterations in intestinal microbiota of colorectal cancer patients receiving radical surgery combined with adjuvant CapeOx therapy. Sci China Life Sci. 2019;62(9):1178–1193. doi:10.1007/s11427-018-9456-x.
- Kaźmierczak-Siedlecka K, Skonieczna-Żydecka K, Palma J, Sobocki BK, Świerblewski M, Siedlecka-Kroplewska K, Kalinowski L, Połom K. Microbiota-derived metabolites in colorectal cancer patients in preoperative period. Eur Rev Med Pharmacol Sci. 2023;27(4):1443–1449. doi:10.26355/eurrev_202302_31384.
- Ziemons J, Aarnoutse R, Heuft A, Hillege L, Waelen J, de Vos-Geelen J, van Iersel LV, van Hellemond IEG, Creemers GJM, Baars A, et al. Fecal levels of SCFA and BCFA during capecitabine in patients with metastatic or unresectable colorectal cancer. Clin Exp Med. 2023;23(7):3919–3933. Online ahead of print. doi:10.1007/s10238-023-01048-7.
- Rahimpour M, Ashabi G, Rahimi AM, Halimi S, Panahi M, Alemrajabi M, Nabavizadeh F. Lactobacillus rhamnosus R0011 treatment enhanced efficacy of capecitabine against colon cancer in male balb/c mice. Nutr Cancer. 2022;74(7):2622–2631. doi:10.1080/01635581.2021.2014901.
- Chen Y, Liao X, Li Y, Cao H, Zhang F, Fei B, Bao C, Cao H, Mao Y, Chen X, et al. Effects of prebiotic supplement on gut microbiota, drug bioavailability, and adverse effects in patients with colorectal cancer at different primary tumor locations receiving chemotherapy: study protocol for a randomized clinical trial. Trials. 2023;24(1):268. doi:10.1186/s13063-023-07137-y.
- Aarnoutse R, de Vos-Geelen JMPGM, Penders J, Boerma EG, Warmerdam FARM, Goorts B, Olde Damink SWM, Soons Z, Rensen SSM, Smidt ML. Study protocol on the role of intestinal microbiota in colorectal cancer treatment: a pathway to personalized medicine 2.0. Int J Colorectal Dis. 2017;32(7):1077–1084. doi:10.1007/s00384-017-2819-3.
- Tang D, Zeng T, Wang Y, Cui H, Wu J, Zou B, Tao Z, Zhang L, Garside GB, Tao S. Dietary restriction increases protective gut bacteria to rescue lethal methotrexate-induced intestinal toxicity. Gut Microbes. 2020;12(1):1714401. doi:10.1080/19490976.2020.1714401.
- Letertre MPM, Munjoma N, Wolfer K, Pechlivanis A, McDonald JAK, Hardwick RN, Cherrington NJ, Coen M, Nicholson JK, Hoyles L, et al. A two-way interaction between methotrexate and the gut microbiota of male Sprague–Dawley rats. J Proteome Res. 2020;19(8):3326–3339. doi:10.1021/acs.jproteome.0c00230.
- Wang W, Zhou H, Liu L. Side effects of methotrexate therapy for rheumatoid arthritis: a systematic review. Eur J Med Chem. 2018;158:502–516. doi:10.1016/j.ejmech.2018.09.027.
- Huang X, Fang Q, Rao T, Zhou L, Zeng X, Tan Z, Chen L, Ouyang D. Leucovorin ameliorated methotrexate induced intestinal toxicity via modulation of the gut microbiota. Toxicol Appl Pharmacol. 2020;391:114900. doi:10.1016/j.taap.2020.114900.
- Scher JU, Nayak RR, Ubeda C, Turnbaugh PJ, Abramson SB. Pharmacomicrobiomics in inflammatory arthritis: gut microbiome as modulator of therapeutic response. Nat Rev Rheumatol. 2020;16(5):282–292. doi:10.1038/s41584-020-0395-3.
- Yan H, Su R, Xue H, Gao C, Li X, Wang C. Pharmacomicrobiology of methotrexate in rheumatoid arthritis: gut microbiome as predictor of therapeutic response. Front Immunol. 2021;12:789334. doi:10.3389/fimmu.2021.789334.
- Wang C, Zhao S, Xu Y, Sun W, Feng Y, Liang D, Guan Y. Integrated microbiome and metabolome analysis reveals correlations between gut microbiota components and metabolic profiles in mice with methotrexate-induced hepatoxicity. Drug Des Devel Ther. 2022;16:3877–3891. doi:10.2147/DDDT.S381667.
- Frank M, Hennenberg EM, Eyking A, Rünzi M, Gerken G, Scott P, Parkhill J, Walker AW, Cario E. TLR signaling modulates side effects of anticancer therapy in the small intestine. J Immunol. 2015;194(4):1983–1995. doi:10.4049/jimmunol.1402481.
- Cario E. Toll-like receptors in the pathogenesis of chemotherapy-induced gastrointestinal toxicity. Curr Opin Support Palliat Care. 2016;10(2):157–164. doi:10.1097/SPC.0000000000000202.
- da Silva Ferreira AR, Wardill HR, Havinga R, Tissing WJE, Harmsen HJM. Prophylactic treatment with vitamins C and B2 for methotrexate-induced gastrointestinal mucositis. Biomolecules. 2020;11(1):34. doi:10.3390/biom11010034.
- Habibi S, Rashidi A. Fecal microbiota transplantation in hematopoietic cell transplant and cellular therapy recipients: lessons learned and the path forward. Gut Microbes. 2023;15(1):2229567. doi:10.1080/19490976.2023.2229567.
- Kaźmierczak-Siedlecka K, Daca A, Fic M, Van de Wetering T, Folwarski M, Makarewicz W. Therapeutic methods of gut microbiota modification in colorectal cancer management - fecal microbiota transplantation, prebiotics, probiotics, and synbiotics. Gut Microbes. 2020;11(6):1518–1530. doi:10.1080/19490976.2020.1764309.
- Wardill HR, van der Aa SAR, da Silva Ferreira AR, Havinga R, Tissing WJE, Harmsen HJM. Antibiotic-induced disruption of the microbiome exacerbates chemotherapy-induced diarrhoea and can be mitigated with autologous faecal microbiota transplantation. Eur J Cancer. 2021;153:27–39. doi:10.1016/j.ejca.2021.05.015.
- Ergin AD, Oltulu Ç, Koç B. Enhanced cytotoxic activity of 6-mercaptopurine-loaded solid lipid nanoparticles in hepatic cancer treatment. Assay Drug Dev Technol. 2023;21(5):212–221. doi:10.1089/adt.2023.007.
- Huynh T, Murray J, Flemming CL, Kamili A, Hofmann U, Cheung L, Roundhill EA, Yu DMT, Webber HT, Schwab M, et al. CCI52 sensitizes tumors to 6-mercaptopurine and inhibits MYCN-amplified tumor growth. Biochemical Pharmacology. 2020;172:113770. doi:10.1016/j.bcp.2019.113770.
- Wei X, Zhuang J, Li N, Zheng B, Sun H, Cai J, Huang X, Zhang G, Zhuang J. NUDT15 genetic testing-guided 6-mercaptopurine dosing in children with ALL likely to be cost-saving in China. Int J Hematol. 2022;115(2):278–286. doi:10.1007/s12185-021-03237-0.
- Timmer A, Patton PH, Chande N, McDonald JWD, MacDonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2016;2016(5):CD000478. doi:10.1002/14651858.CD000478.pub4.
- Bradford K, Shih DQ. Optimizing 6-mercaptopurine and azathioprine therapy in the management of inflammatory bowel disease. World J Gastroenterol. 2011;17(37):4166–4173. doi:10.3748/wjg.v17.i37.4166.
- Oancea I, Movva R, Das I, Aguirre de Cárcer D, Schreiber V, Yang Y, Purdon A, Harrington B, Proctor M, Wang R, et al. Colonic microbiota can promote rapid local improvement of murine colitis by thioguanine independently of T lymphocytes and host metabolism. Gut. 2017;66(1):59–69. doi:10.1136/gutjnl-2015-310874.
- Wang S, Qin Y, Wen Q, Xia Q, Gu R, Wang S, Chen GJ, Tan C, Shen C, Song S. Intestinal microbiota-mediated biotransformations alter the pharmacokinetics of the major metabolites of azathioprine in rats after oral administration. Drug Metab Pharmacokinet. 2022;45:100458. doi:10.1016/j.dmpk.2022.100458.
- Effenberger M, Reider S, Waschina S, Bronowski C, Enrich B, Adolph TE, Koch R, Moschen AR, Rosenstiel P, Aden K. Microbial butyrate synthesis indicates therapeutic efficacy of azathioprine in IBD patients. J Crohns Colitis. 2021;15(1):88–98. doi:10.1093/ecco-jcc/jjaa152.
- Karpiński TM, Adamczak A. Anticancer activity of bacterial proteins and peptides. Pharmaceutics. 2018;10(2):54. doi:10.3390/pharmaceutics10020054.
- Kong J, Yi L, Xiong Y, Huang Y, Yang D, Yan X, Shen B, Duan Y, Zhu X. The discovery and development of microbial bleomycin analogues. Appl Microbiol Biotechnol. 2018;102(16):6791–6798. doi:10.1007/s00253-018-9129-8.
- Quan Y, Yin Z, Chen S, Lang J, Han L, Yi J, Zhang L, Yue Q, Tian W, Chen P, et al. The gut-lung axis: gut microbiota changes associated with pulmonary fibrosis in mouse models induced by bleomycin. Front Pharmacol. 2022;13:985223. doi:10.3389/fphar.2022.985223.
- Fu Y, Gao H, Hou X, Chen Y, Xu K. Pretreatment with IPA ameliorates colitis in mice: colon transcriptome and fecal 16S amplicon profiling. Front Immunol. 2022;13:1014881. doi:10.3389/fimmu.2022.1014881.
- Kaźmierczak-Siedlecka K, Folwarski M, Ruszkowski J, Skonieczna-Żydecka K, Szafrański W, Makarewicz W. Effects of 4 weeks of lactobacillus plantarum 299v supplementation on nutritional status, enteral nutrition tolerance, and quality of life in cancer patients receiving home enteral nutrition - a double-blind, randomized, and placebo-controlled trial. Eur Rev Med Pharmacol Sci. 2020;24(18):9684–9694. doi:10.26355/eurrev_202009_23059.
- Yoon YM, Hrusch CL, Fei N, Barrón GM, Mills KAM, Hollinger MK, Velez TE, Leone VA, Chang EB, Sperling AI. Gut microbiota modulates bleomycin-induced acute lung injury response in mice. Respir Res 10 grudzień. 2022;23(1):337. doi:10.1186/s12931-022-02264-7.
- Xie Y, Li W, Lu C, Zhu L, Qin S, Du Z. The effects of phycocyanin on bleomycin-induced pulmonary fibrosis and the intestinal microbiota in C57BL/6 mice. Appl Microbiol Biotechnol. 2019;103(20):8559–8569. doi:10.1007/s00253-019-10018-7.
- Jiang L, Wang Y, Yin Q, Liu G, Liu H, Huang Y, Li B. Phycocyanin: a potential drug for cancer treatment. J Cancer. 2017;8(17):3416–3429. doi:10.7150/jca.21058.
- Huang R, Liao G, Gao M, Ou Y. Phycocyanin inhibits pancreatic cancer metastasis via suppressing epithelial-mesenchymal transition and targeting Akt/β-catenin pathway. Neoplasma. 2021;210609N773. doi:10.4149/neo_2021_210609N773.
- van Der Heijden R, Jacobs DI, Snoeijer W, Hallard D, Verpoorte R. The Catharanthus alkaloids: pharmacognosy and biotechnology. Curr Med Chem. 2004;11(5):607–628. doi:10.2174/0929867043455846.
- Zhang J, Hansen LG, Gudich O, Viehrig K, Lassen LMM, Schrübbers L, Adhikari KB, Rubaszka P, Carrasquer-Alvarez E, Chen L, et al. A microbial supply chain for production of the anti-cancer drug vinblastine. Nat wrzesień. 2022;609(7926):341–347. doi:10.1038/s41586-022-05157-3.
- Vinblastine. 2023. https://go.drugbank.com/drugs/DB00570
- Below J, Das M, Vincristine J. W: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. http://www.ncbi.nlm.nih.gov/books/NBK537122/.
- Caron JM, Herwood M. Vinblastine, a chemotherapeutic drug, inhibits palmitoylation of tubulin in human leukemic lymphocytes. Chemotherapy. 2007;53(1):51–58. doi:10.1159/000098419.
- Rtibi K, Grami D, Selmi S, Amri M, Sebai H, Marzouki L. Vinblastine, an anticancer drug, causes constipation and oxidative stress as well as others disruptions in intestinal tract in rat. Toxicol Rep. 2017;4:221–225. doi:10.1016/j.toxrep.2017.04.006.
- Waclawiková B, Codutti A, Alim K, El Aidy S. Gut microbiota-motility interregulation: insights from in vivo, ex vivo and in silico studies. Gut Microbes. 2022;14(1):1997296. doi:10.1080/19490976.2021.1997296.
- Zheng Z, Tang J, Hu Y, Zhang W. Role of gut microbiota-derived signals in the regulation of gastrointestinal motility. Front Med. 2022;9:961703. doi:10.3389/fmed.2022.961703.
- Kamiński M, Skonieczna-Żydecka K, Łoniewski I, Koulaouzidis A, Marlicz W. Are probiotics useful in the treatment of chronic idiopathic constipation in adults? A review of existing systematic reviews, meta-analyses, and recommendations. Prz Gastroenterol. 2020;15(2):103–118. doi:10.5114/pg.2019.86747.
- Singh V, Ahlawat S, Mohan H, Singh Gill S, Kant Sharma K. Balancing reactive oxygen species generation by rebooting gut microbiota. J Appl Microbiol. 2022;132(6):4112–4129. doi:10.1111/jam.15504.
- Sternberg CN, de Mulder PHM, Schornagel JH, Théodore C, Fossa SD, van Oosterom AT, Witjes F, Spina M, van Groeningen CJ, de Balincourt C, et al. Randomized phase III trial of high–dose-Intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European organization for research and treatment of cancer protocol no. 30924. J Clin Oncol. 2001;19(10):2638–2646. doi:10.1200/JCO.2001.19.10.2638.
- Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016;S0016-5085(16):00223–7. doi:10.1053/j.gastro.2016.02.032.
- Spruss T, Bernhardt G, Schönenberger H, Schiess W. Hyaluronidase significantly enhances the efficacy of regional vinblastine chemotherapy of malignant melanoma. J Cancer Res Clin Oncol. 1995;121(4):193–202. doi:10.1007/BF01366962.
- Boulanger J, Ducharme A, Dufour A, Fortier S, Almanric K. Management of extravasation of antineoplastic agents. Support Care Cancer. 2015;23(5):1459–1471. doi:10.1007/s00520-015-2635-7.
- Hynes WL, Walton SL. Hyaluronidases of gram-positive bacteria. FEMS Microbiol Lett. 2000;183(2):201–207. doi:10.1111/j.1574-6968.2000.tb08958.x.
- Peiris V, Oppenheim BA. Antimicrobial activity of cytotoxic drugs may influence isolation of bacteria and fungi from blood cultures. J Clin Pathol. 1993;46(12):1124–1125. doi:10.1136/jcp.46.12.1124.
- López-Gómez L, Díaz-Ruano S, Girón R, López-Pérez AE, Vera G, Herradón Pliego E, López-Miranda V, Nurgali K, Martín-Fontelles MI, Uranga JA, et al. Preclinical evaluation of the effects on the gastrointestinal tract of the antineoplastic drug vincristine repeatedly administered to rats. Neurogastroenterol Motil. 2018;30(11):e13399. doi:10.1111/nmo.13399.
- Gao Y, Tang Y, Zhang H, Chu X, Yan B, Li J, Liu C. Vincristine leads to colonic myenteric neurons injury via pro-inflammatory macrophages activation. Biochemical Pharmacol. 2021;186:114479. doi:10.1016/j.bcp.2021.114479.
- Vera G, López-Pérez A, Uranga J, Girón R, Martín-Fontelles MI, Abalo R. Involvement of cannabinoid signaling in vincristine-induced gastrointestinal dysmotility in the rat. Front Pharmacol. 2017;8:37. doi:10.3389/fphar.2017.00037.
- Suh SH, Choe K, Hong SP, Jeong SH, Mäkinen T, Kim KS, Alitalo K, Surh CD, Koh GY, Song JH. Gut microbiota regulates lacteal integrity by inducing VEGF-C in intestinal villus macrophages. EMBO Rep. 2019;20(4):e46927. doi:10.15252/embr.201846927.
- Zeng MY, Inohara N, Nuñez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10(1):18–26. doi:10.1038/mi.2016.75.
- Taper HS, Roberfroid MB. Possible adjuvant cancer therapy by two prebiotics-inulin or oligofructose. Vivo. 2005;19:201–204.
- Zhang C, Xu C, Gao X, Yao Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics. 2022;12(5):2115–2132. doi:10.7150/thno.69424.eCollection2022.
- Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi:10.1016/j.ejphar.2014.07.025.
- Gui QF, Lu HF, Zhang CX, Xu ZR, Yang YH. Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genet Mol Res. 2015;14(2):5642–5651. doi:10.4238/2015.May.25.16.
- Miyake M, Oda Y, Owari T, Iida K, Ohnishi S, Fujii T, Nishimura N, Miyamoto T, Shimizu T, Ohnishi K, et al. Probiotics enhances anti-tumor immune response induced by gemcitabine plus cisplatin chemotherapy for urothelial cancer. Cancer Sci. 2023;114(3):1118–1130. doi:10.1111/cas.15666.
- Liang M, Liu Y, Zhang Z, Yang H, Dai N, Zhang N, Sun W, Guo Y, Kong J, Wang X, et al. Fusobacterium nucleatum induces MDSCs enrichment via activation the NLRP3 inflammosome in ESCC cells, leading to cisplatin resistance. Ann Med. 2022;54(1):989–1003. doi:10.1080/07853890.2022.2061045.
- Samaan TMA, Samec M, Liskova A, Kubatka P, Büsselberg D. Paclitaxel’s mechanistic and clinical effects on breast cancer. Biomolecules. 2019;9(12):789. doi:10.3390/biom9120789.
- Cristiano C, Cuozzo M, Coretti L, Liguori FM, Cimmino F, Turco L, Avagliano C, Aviello G, Mollica MP, Lembo F, et al. Oral sodium butyrate supplementation ameliorates paclitaxel-induced behavioral and intestinal dysfunction. Biomed Pharmacother. 2022;153:113528. doi:10.1016/j.biopha.2022.113528.
- Kesh K, Mendez R, Abdelrahman L, Banerjee S, Banerjee S. Type 2 diabetes induced microbiome dysbiosis is associated with therapy resistance in pancreatic adenocarcinoma. Microb Cell Fact. 2020;19(1):75. doi:10.1186/s12934-020-01330-3.
- Zhao H, Yu J, Zhang R, Chen P, Jiang H, Yu W. Doxorubicin prodrug-based nanomedicines for the treatment of cancer. Eur J Med Chem. 2023;258:115612. doi:10.1016/j.ejmech.2023.115612.
- Guilherme Gonçalves-Nobre J, Gaspar I, Alpuim Costa D. Anthracyclines and trastuzumab associated cardiotoxicity: is the gut microbiota a friend or foe? – a mini-review. Front Microbiomes. 2023;2. doi:10.3389/frmbi.2023.1217820.
- Bawaneh A, Wilson AS, Levi N, Howard-McNatt MM, Chiba A, Soto-Pantoja DR, Cook KL. Intestinal microbiota influence doxorubicin responsiveness in triple-negative breast cancer. Cancers Basel. 2022;14(19):4849. doi:10.3390/cancers14194849.
- Chiba A, Bawaneh A, Velazquez C, Clear KYJ, Wilson AS, Howard-McNatt M, Levine EA, Levi-Polyachenko N, Yates-Alston SA, Diggle SP, et al. Neoadjuvant chemotherapy shifts breast tumor microbiota populations to regulate drug responsiveness and the development of metastasis. Mol Cancer Res. 2020;18(1):130–139. doi:10.1158/1541-7786.MCR-19-0451.
- Yan A, Culp E, Perry J, Lau JT, MacNeil LT, Surette MG, Wright GD. Transformation of the anticancer drug doxorubicin in the human gut microbiome. ACS Infect Dis. 2018;4(1):68–76. doi:10.1021/acsinfecdis.7b00166.
- Katsila T, Balasopoulou A, Tsagaraki I, Patrinos GP. Pharmacomicrobiomics informs clinical pharmacogenomics. Pharmacogenom. 2019;20(10):731–739. doi:10.2217/pgs-2019-0027.
- Javdan B, Lopez JG, Chankhamjon P, Lee YCJ, Hull R, Wu Q, Wang X, Chatterjee S, Donia MS. Personalized mapping of drug metabolism by the human gut microbiome. Cell. 2020;181(7):1661–1679.e22. doi:10.1016/j.cell.2020.05.001.
- Merenstein D, Pot B, Leyer G, Ouwehand AC, Preidis GA, Elkins CA, Hill C, Lewis ZT, Shane AL, Zmora N, et al. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes. 2023;15(1):2185034. doi:10.1080/19490976.2023.2185034.
- Özdemir V, Arga KY, Aziz RK, Bayram M, Conley SN, Dandara C, Endrenyi L, Fisher E, Garvey CK, Hekim N, et al. Digging deeper into precision/personalized medicine: cracking the sugar code, the third alphabet of life, and sociomateriality of the cell. OMICS. 2020;24(2):62–80. doi:10.1089/omi.2019.0220.
- Klünemann M, Andrejev S, Blasche S, Mateus A, Phapale P, Devendran S, Vappiani J, Simon B, Scott TA, Kafkia E, et al. Bioaccumulation of therapeutic drugs by human gut bacteria. Nature. 2021;597(7877):533–538. doi:10.1038/s41586-021-03891-8.
- Sousa T, Paterson R, Moore V, Carlsson A, Abrahamsson B, Basit AW. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int J Pharm. 2008;363(1–2):1–25. doi:10.1016/j.ijpharm.2008.07.009.
- Wang Z, Chow MS. Overview and appraisal of the current concept and technologies for improvement of sublingual drug delivery. Ther Deliv. 2014;5(7):807–816. doi:10.4155/tde.14.50.
- Takx RAP, Suchá D, Park J, Leiner T, Hoffmann U. Sublingual nitroglycerin administration in coronary computed tomography angiography: a systematic review. Eur Radiol. 2015;25(12):3536–3542. doi:10.1007/s00330-015-3791-3.
- Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, Goodman AL. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. 2019;570(7762):462–467. doi:10.1038/s41586-019-1291-3.
- Rosen LS, Jacobs IA, Burkes RL. Bevacizumab in colorectal cancer: current role in treatment and the potential of biosimilars. Target Oncol. 2017;12(5):599–610. doi:10.1007/s11523-017-0518-1.
- Jaswal K, Todd OA, Behnsen J. Neglected gut microbiome: interactions of the non-bacterial gut microbiota with enteric pathogens. Gut Microbes. 2023;15(1):2226916. doi:10.1080/19490976.2023.2226916.
- Kaźmierczak-Siedlecka K, Ruszkowski J, Fic M, Folwarski M, Makarewicz W. Saccharomyces boulardii CNCM I-745: a non-bacterial microorganism used as probiotic agent in supporting treatment of selected diseases. Curr Microbiol. 2020;77(9):1987–1996. doi:10.1007/s00284-020-02053-9.
- Oar A, Lee M, Le H, Wilson K, Aiken C, Chantrill L, Simes J, Nguyen N, Barbour A, Samra J, et al. Agitg masterplan: a randomised phase II study of modified FOLFIRINOX alone or in combination with stereotactic body radiotherapy for patients with high-risk and locally advanced pancreatic cancer. BMC Cancer. 2021;21(1):936. doi:10.1186/s12885-021-08666-y.