ABSTRACT
Human aging is characterized by gut microbiome alteration and differential loss of gut commensal species associated with the onset of frailty. The administration of cultured commensal strains to replenish lost taxa could potentially promote healthy aging. To investigate the interaction of whole microbiomes and administered strains, we transplanted gut microbiota from a frail or healthy elderly subject into germ-free mice. We supplemented the frail-donor recipient group with a defined consortium of taxa (the “S7”) that we identified by analyzing healthy aging subjects in our previous studies and whose abundance correlated with health-promoting dietary intervention. Inoculation with a frail or a healthy donor microbiome resulted in differential microbiota compositions in murine recipients 5 weeks post-transplantation. Fecal acetate levels were significantly higher in healthy donor recipient mice than in frail donor recipient mice after 4 weeks. However, the frailty-related phenotype was not replicated in recipient mice with single-dose microbiota transplantation from a healthy and a frail donor. Five S7 species colonized successfully in germ-free mice, with a relatively high abundance of Barnesiella intestinihominis and Eubacterium rectale. The engraftment of five S7 species in germ-free mice increased fecal acetate levels and reduced colon permeability and plasma TNF-ɑ concentration. Supplementation with the S7 in frail-microbiota recipient mice did not increase alpha-diversity but significantly increased the abundance of Barnesiella intestinihominis. S7 supplementation showed the potential for improving spatial reference memory in frail-microbiota recipient mice. Collectively, these data highlight the challenge of elderly microbiota engraftment in the germ-free mouse model but show promise for modulating the gut microbiome of frail elderly subjects by administering an artificial gut microbe consortium associated with healthy aging.
Introduction
Aging is defined as the time-dependent functional decline that increases vulnerability to death,Citation1 and maintaining functional ability is considered “healthy” aging. However, older people (typically 65 y old and above) experience reduced function of multiple systems, including energy harvest, physical ability, and cognition, collectively contributing to different extents of frailty. When frailty becomes established, functional decline is accelerated and homeostatic mechanisms start failing.Citation2 Research and practice seeking to promote healthy aging aims to delay the onset of frailty or even avoid developing chronic diseases.
Aging is linked to gut microbiota alteration associated with chronic inflammation, gut permeability changes, and physical and cognitive dysfunction.Citation3 The microbiota of older people is characterized by lower alpha diversity, reduced abundance of subdominant taxa, and the loss of bifidobacterial and fiber-responsive taxa compared to that of younger adults.Citation4,Citation5 An increase in the proportional abundance of pathobionts has also been reported in people above 100 y old.Citation6–10 Aging-related microbiota changes can potentially make the host more susceptible to certain diseases, accelerating functional decline in the frail elderly. We previously identified a group of multiple disease-associated taxa in elderly subjects that differ from those involved in microbiome alterations in non-communicable diseases in younger people.Citation11 In general, the gut microbiota of the elderly with higher frailty is characterized by loss of commensals associated with health at all ages and gain of disease-associated pathobionts that also relate to medication, reduced mobility, and consumption of a less diverse diet.Citation3
Diet-based and microbe-based interventions are thus the two main strategies for microbiome modulation in the elderly. A previous study from our laboratory has shown that fiber-associated taxa were difficult to restore by administration of a mix of five prebiotics for 6 months in the elderly.Citation12 Microbe-based strategies include fecal microbiota transplantation (FMT) and live biotherapeutics. FMT is a valid clinical option for treating Clostridioides difficile infections (CDI), but there are operational challenges, such as occasional adverse events and the requirement for strict donor screening.Citation13–15 Administering a defined consortia of cultured bacteria is an alternative therapeutic strategy to fecal transplants. It has been shown that a synthetic bacterial suspension consisting of 13 microbial species is an efficacious therapeutic for CDI.Citation16 Current probiotic supplement studies are based on recognized probiotics, usually Lactobacillus or Bifidobacterium species.Citation17–20 Despite the reported high efficacy of those probiotics in infants, children, and young adults,Citation20,Citation21 the associations of these species with healthy aging have not been clearly demonstrated in the elderly. In contrast to younger people who gradually gain disease-associated gut microbes, people older than 50 tend to lose gut commensals.Citation22 Thus, microbiome-based therapeutic strategies targeting the elderly could attempt to restore selected commensals in a consortium format to prevent or delay the onset of frailty and age-related inflammation
The importance of some specific species in the aging-subject microbiome and the potential for therapeutic use of defined bacterial consortia has gathered momentum as the role of microbiota in healthy aging is elucidated. Characteristic changes in the microbiota composition of older people are correlated with frailty, co-morbidity, and inflammation markers despite the great inter-individual variation of the microbiota of older people (aged 60 and above).Citation11,Citation23 Previous diet intervention studies have revealed that specific taxa whose abundances are increased by a Mediterranean diet are positively associated with lower frailty and improved cognitive function in older people over 65.Citation24 A previous study reported significantly different abundances of 17 gut microbes between “highly frail” and “low frail” individuals aged over 70.Citation25 A repertoire of frailty-related genus-level taxa was identified by us in higher abundance in Long-stay subjects compared to Community-dwelling subjects of the ELDERMET cohort (aged above 60).Citation3 A distinctive microbiota configuration associated with long-term care and frailty has also been identified.Citation26 In a recent study, we extensively cultured taxa above 1% relative abundance in healthy community-dwelling subjects and used the ensuing Microbiome Culture Collection 100 (MCC100) strains to modulate the gut microbiota of elderly donors in an in vitro human colon model.Citation27 Supplementation of the artificial colon with the MCC100 increased microbiome alpha-diversity and the levels of health-associated amino acids, motivating us to develop and refine this approach.
Compositional or functional changes in gut microbiota have been associated with various diseases, including inflammatory bowel disease,Citation28 colorectal cancer,Citation29,Citation30 and metabolic syndrome.Citation31 However, establishing causal relationships between microbes and disease conditions is complicated by highly individualized microbiomes and regulatory issues for administering live biotherapeutics in human intervention studies. Human microbiota-associated (HMA) mice are a powerful model for studying the involvement of gut microbiota in human health. The “Humanization” process involves the transplantation of human gut microbiota into germ-free (GF) mice to establish human gut microbial communities in mouse models. Many human fecal microbiota transplantation studies have successfully recapitulated human donor phenotypes such as obesity,Citation32 allergies,Citation33 inflammatory bowel disease,Citation34 and autism.Citation35 HMA mice hold promise for studying the association of gut microbiota with frailty in the elderly. However, the extent to which the recipient microbiota represents that of the donor following transplant is not always assessed. It is unknown whether the transplantation of elderly human microbiota to GF mice could recapitulate the human donor phenotype, in this case, frailty.
To establish a preclinical humanized mouse model for aging studies, we first identified a healthy aging-associated bacterial consortium – the S7 species. We then conducted transplantation of gut microbiota from a frail and a healthy elderly subject to GF mice to replicate human frailty phenotypes. We also investigated the potential of the S7 consortium for counteracting gut permeability changes, physical and cognitive decline, and inflammation.
Results
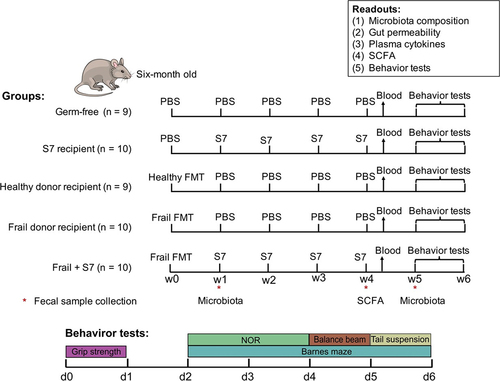
Despite the highly individualized microbiota of elderly subjects, many taxa have been reported to be associated with frailty. Thus, this study aimed to investigate whether the engraftment of the microbiota of donors with different levels of frailty, namely a “frail” donor and a “healthy” donor, could replicate human frailty-related phenotypes using a germ-free mouse model. Furthermore, the effect of administering a seven-species consortium (the S7) linked to healthy aging was examined ().
Figure 1. Overview of the experimental design and study timeline.
Engraftment and temporal abundance trajectory of elderly human gut microbiota in germ-free mice
Representative “healthy” donor and “frail” donor (n = 1) was selected based upon their clinical and health measurement and microbiome relatedness to a subset of the well-studied ELDERMET cohort (Supplementary Figure S1a, Supplementary Table S1). Our sequencing data showed that microbial compositions of frozen donor samples are similar to corresponding fresh fecal samples with a 0.03 Jensen-Shannon Divergence (JSD)Citation36 between fresh and frozen healthy donor samples and a 0.04 JSD between fresh and frozen frail donor samples. Furthermore, all taxa in fresh fecal samples can be detected in frozen fecal samples at family level (Supplementary Figure S1 a and b). The plating results showed that frail and healthy donor samples remained 109 CFU/ml after storage at −80°C, suggesting that the sample storage did not affect the microbiota of selected donors. Most importantly, the 16S rRNA sequencing results confirmed that the typical community or long-stay microbiota profiles were present in the donors.
The efficacy of taxon transfer from frozen elderly donor fecal microbiota was evaluated in germ-free (GF) mice using a single healthy or frail donor. GF mice were humanized with single oral gavage of healthy or frail microbiota at week 0 (as shown schematically in ). The total bacterial numbers in healthy and frail donor feces were measured by qPCR as 5.0 × 109 and 3.4 × 109 genome copies/g, respectively. The gut microbiota of the healthy donor showed higher alpha-diversity indices (numbers of observed species and Shannon Index) than the frail donor microbiota (). As expected, the frail and healthy donor stools harbored different microbiota compositions, with a higher relative abundance of Firmicutes and Proteobacteria and a lower relative abundance of Bacteriodota, Actinobacteriota, and Desulfobacterota in the frail donor compared with the healthy donor (Supplementary Figure S2a).
Figure 2. Engraftment of human donor microbiota in murine recipients.
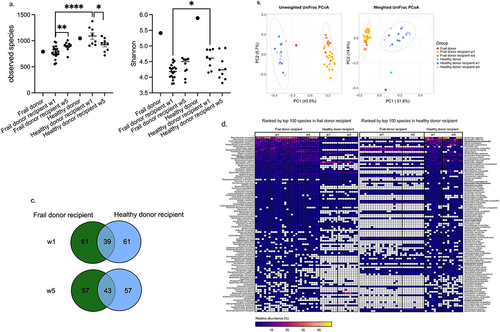
Fecal samples were collected from recipient mice for microbiome profiling by 16S rRNA gene amplicon sequencing at weeks 1 and 5 post-transplantation. Measurement of the alpha-diversity revealed significantly lower numbers of observed species (p <.0001, One-way ANOVA post hoc Holm-Sidak) and Shannon index (p =.016, One-way ANOVA post hoc Holm-Sidak) values in frail donor recipient mice compared with healthy donor recipient mice at 1-week post-transplantation (). Unweighted and weighted UniFrac distances analysis revealed distinct frail and healthy donor microbiota in the germ-free mouse model 1 week after the transplantation. The differences remained up to 5 weeks post-transplantation (), whereas no differences were observed in alpha diversity between healthy and frail donor recipient mice at week 5. Pairwise similarity analysis revealed significant differences in Unweighted (p =.001) and Weighted (p =.001) UniFrac distances between the microbiota of frail donor recipient mice at weeks 1 and 5. Notably, there was no significant difference in weighted UniFrac distances between healthy donor recipient mice at weeks 1 and 5 (p =.13), suggesting more stable colonization of healthy elderly microbiota in germ-free mice than frail elderly microbiota (Supplementary Table S2).
16S rRNA profiling of fecal samples obtained from frail donor recipient mice showed partial engraftment of the donor gut microbiota: 7/9 phyla colonized at both weeks 1 and 5, while only 51% (47/93) at week 1 and 54% at week 5 (50/93) of genus-level taxa of the frail donor were detected among the recipient mice (Supplementary Table S3 and S4). In contrast, 7/8 phyla from healthy donor stool colonized at both week 1 and week 5, 66% (76/115) and 65% (75/115) of healthy donor genus-level taxa were detected in the recipient mice at weeks 1 and 5, respectively.
Evaluation of family-level microbial community composition in recipient mice revealed a greater relative abundance of the families Bacteroidaceae and Akkermansiaceae in mouse communities compared to humans, indicating the adaptation of those human bacteria in the murine gut environment. Additionally, Rikenellaceae, Tannerellaceae, Sutterellaceae, and Erysipelatoclostridiaceae exhibited higher relative abundance in the frail donor recipient mouse gut than that of the frail human donor itself, which was not observed in healthy donor recipients (Supplementary Figure S2b). We then performed a more refined analysis of the engraftment capacity of donor microbiota. For this purpose, we computed pairwise Spearman correlations between recipient mice and their donor using the relative abundances in each fecal sample at the genus/family level. Correlation analysis revealed higher similarity (p <.0001, One-way ANOVA post hoc Holm-Sidak) between healthy donor recipient mice and healthy donor (genus 0.46 ± 0.03, family 0.65 ± 0.01) compared to the similarity between frail donor recipient and frail donor (genus 0.27 ± 0.01, family 0.53 ± 0.01) at week 1. Similarly, the engraftment efficacy of healthy donor microbiota was greater than that of frail donor microbiota at week 5 (Supplementary Table S5, Supplementary Figure S3).
We next compared the top 100 species from the frail donor and healthy donor with their mouse recipients to assess species engraftment. The presence of the species in >50% of the recipient samples was considered successful engraftment, and <10% was considered failed engraftment. Notably, more species from the healthy donor were detected in the respective recipient murine gut compared with the frail donor species (Supplementary Figure S2c). In the case of frail donor microbiota, 38% of the top 100 species engrafted successfully in mouse recipients, while 33% of the top 100 species could not be recovered from the murine gut. In contrast, 60% of the healthy donor microbiota species engrafted successfully, and only 25% of the top 100 species failed to colonize the mouse gut. The engraftment efficacy reviewed at different taxonomic levels revealed that the healthy donor microbiota engrafted more effectively in the GF mouse model than the frail donor microbiota.
To compare the gut microbiota composition of frail and healthy donor recipient mice, we identified the top 100 species from the recipient animal microbiota. Frail and healthy donor recipient mice shared only 39 and 43 of the top 100 species at weeks 1 and 5, respectively (). Interestingly, some of the most abundant species in frail donor recipient mice were present with low abundance in healthy donor recipient mice, such as Bacteroides cellulosilyticus (24% vs. 0.04%), Akkermansia muciniphila (7% vs. 1.7%), and Lachnospiraceae unclassified (9% vs. 2.6%). Likewise, highly abundant species in healthy donor recipients, including Bacteroides eggerthii (24% vs. 0.01%), Bacteroides uniformis (12% vs. 1.1%), and Faecalibacterium prausnitzii (7.6% vs. 0.005%), are present with low abundance in frail donor recipient mice ().
Collectively, these data revealed a greater engraftment efficacy of healthy elderly microbiota taxa than those of frail elderly microbiota. The engraftment of healthy or frail human microbiota resulted in distinct microbial composition in recipient mice, which were retained for up to 5 weeks post-transplantation.
The relative abundances of the S7 consortium are negatively associated with frailty in the elderly
By analyzing disease- and diet-associated taxa in elderly subjects, a candidate group of species (that we refer to here as the S7), consisting of Alistipes putredinis, Barnesiella intestinihominis, Coprococcus catus, Dorea longicatena, Eubacterium rectale, Faecalibacterium prausnitzii, and Roseburia hominis, was identified as frailty-associated taxa (see Methods). To verify the correlation between these selected taxa and frailty, we re-analyzed the shotgun fecal microbiome data of 188 elderly Irish individuals (64–102 y old) from the ELDERMET cohort (Supplementary Table S6). Our results showed that the abundances of A. putredinis, B. intestinihominis, C. catus, D. longicatena, E. rectale, F. prausnitzii, and R. hominis were differentially negatively associated with frailty (). In the ELDERMET cohort, A. putredinis is the most abundant species of the S7 species across all frailty categories, followed by B. intestinihominis, E. rectale, and F. prausnitzii. Relative abundances of C. catus, D. longicatena, and R. hominis are relatively low (<0.1%) in the selected Irish population (Supplementary Table S7). We previously isolated the Microbiome Culture Collection 100 (MCC100) strains from fecal samples of healthy donors.Citation27 The genomes of the MCC100 strains had been sequenced (Supplementary Table S8), and their antimicrobial susceptibility was assessed.Citation27 The availability of cultured isolates of the healthy aging-associated S7 species in our MCC100 collection facilitated their usage in the current study.
Figure 3. Prevalence and abundance of S7 taxa in human and mouse gut microbiome datasets.
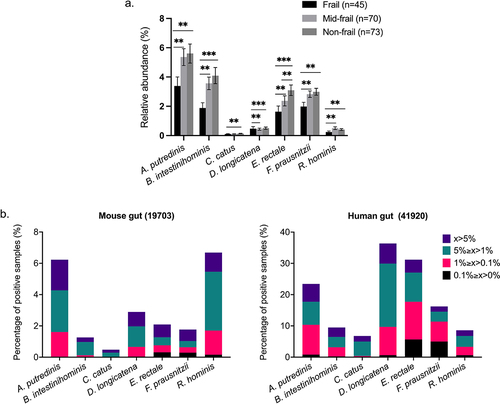
There are very few published studies of fecal microbiota transplantation (FMT) from elderly human donors to GF mice, so we performed a meta-analysis to measure the presence and relative abundance of the S7-related sequences in the gut of human and mice using published 16S rRNA gene amplicon datasets contained in IMNGS.Citation37 Although the prevalence of S7-related sequences (99% similarity) is lower in the mouse gut compared to the human gut samples, the presence of all seven species was detected in the mouse gut, suggesting the possibility of S7 colonization in the mouse gut. The observed patterns of higher prevalence of S7-related taxa in humans compared with mice support their being regarded as human gut bacteria ().
Engraftment of the S7 species in germ-free and frail donor recipient mice
We first assessed the engraftment of the S7 species originating from the human donor microbiota. We applied a threshold of presence in at least 50% of the recipient mice as successful engraftment. Seven and five S7 species were detected in healthy and frail donor microbiota, respectively. Six of seven S7 species originating from healthy donor microbiota were detected at week 1, and five were retained at week 5. Only two frail donor-originated S7 species were detected at weeks 1 and 5 (), suggesting different engraftment efficacies of S7 species in distinct microbial communities.
Figure 4. Effect of S7 supplementation on the microbiome of frail donor recipient mice.
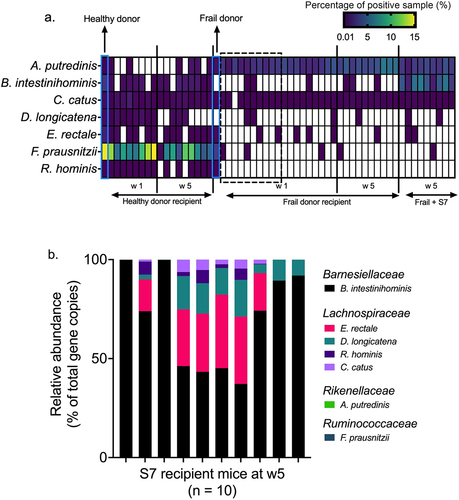
To investigate the engraftment of the defined S7 consortium, we supplemented GF (and no other microbial inoculum) and frail-donor recipient mice with the S7 consortium, as shown in . Engraftment efficacy of S7 in GF mice was assessed using S7 strain-specific 16S rRNA primers. Five S7 species successfully colonized the S7-mono-associated GF mice, with the highest proportional abundances achieved by B. intestinihominis, followed by E. rectale, D. longicatena, R. hominis, C. catus (). Colonization of A. putredinis and F. prausnitzii was not detected in GF mice. Next, we examined the engraftment of the S7 in frail donor recipient mice to test if the treatment with the S7 consortium might attenuate the difference between the gut microbiota of frail- and healthy-donor recipient mice. While the supplementation with S7 did not change the microbiota alpha-diversity and weighted UniFrac beta-diversity of frail-donor recipient mice, unweighted UniFrac beta-diversity was affected by S7 treatment (R2 = .09, p =.007) (Supplementary Figure S4 a and b). At the species level, the S7 treatment primarily increased the relative abundance of B. intestinihominis in frail donor recipient mice (). DESeq2 analysisCitation38 further revealed an increased abundance of Bacteroides and Lachnospiraceae, and a lower abundance of Clostridium saccharogumia in frail-donor recipient mice receiving the S7 supplementation (Supplementary Figure S4c). However, all taxa mentioned above, except B. intestinihominis, were present with low relative abundances (<0.2%).
Effect of S7 consortium on fecal SCFA levels
Fecal samples were collected at week 4 for short-chain fatty acid (SCFA) measurement (as shown schematically in ). Healthy donor recipient mice displayed higher fecal acetate (p =.036, one-way ANOVA post hoc Holm-Sidak) levels compared to frail donor recipient mice 4 weeks post-transplantation (Supplementary Figure S5). We observed no differences in microbial metabolites between frail donor-recipient mice with or without S7 supplementation. However, S7 supplementation in germ-free mice increased acetate levels and decreased glucose concentrations (p <.001, one-way ANOVA post hoc Holm-Sidak) compared with the germ-free controls. Lactose, succinic acid, and butyric acid were not detected in all samples, and there was no difference in lactose and formic acid between groups.
Inflammatory cytokine alteration before and after restraint stress
Transplantation of gut microbiota from old mice (17 months) to young mice (7–10 weeks) has been shown to affect inflammaging.Citation39 Proinflammatory cytokines, such as TNF-ɑ, IL-6, and MCP-1, are considered relevant mediators during the onset of frailty.Citation40–42 Thus, we examined aging-related inflammatory cytokines in plasma, specifically TNF-ɑ, IL-6, and MCP-1. We tested the plasma cytokine concentrations before and after the stress test because blood cytokine profile variations have been reported under stress conditions.Citation43–45 At baseline, we observed reduced plasma TNF- ɑ in the S7 recipient group compared to GF control (p =.09, One-way ANOVA, uncorrected Fisher’s LSD). Furthermore, transferring human microbiota from a frail donor to GF mice could also reduce the TNF-ɑ concentration in plasma (p =.06, One-way ANOVA, uncorrected Fisher’s LSD). Notably, TNF-ɑ concentrations in healthy donor recipient and Frail+S7 mice were significantly lower compared to germ-free control mice at baseline. Restraint stress caused a drop in plasma TNF-ɑ (p =.07, t-test) in germ-free mice, but this trend was not observed in all the other groups (Supplementary Figure S6a). There was no difference in MCP-1 concentration at baseline and after restraint stress between microbiome treatment groups (Supplementary Figure S6b). IL-6 levels were not significantly different between the groups at baseline, but restraint stress-induced IL-6 was observed in all groups, and stress-induced IL-6 was highest in frail donor recipient mice and was lowered by S7 treatment (Supplementary Figure S6c).
Colonization by donor microbiota and the S7 species did not change the overall gut permeability, but reduced the colon epithelial permeability
We next investigated whether S7 administration modulated intestinal permeability in the humanized murine model in vivo at baseline and following acute stress by measuring plasma levels of orally administered FITC-dextran. There was no significant difference in gut permeability between groups at baseline (Kruskal-Wallis, p =.7958) or after 1 hour of restraint stress (Kruskal-Wallis, p =.6349) (Supplementary Figure S7a). The association of GF mice with either healthy or frail donor microbiota did not alter gut permeability compared with the GF control. Furthermore, S7 administration did not significantly alter the gut permeability of S7 recipient or Frail+S7 recipient mice compared with GF and frail donor recipient controls, despite a non-significant trend for all S7 recipient mice to show higher levels of basal (pre-stress) permeability. Notably, restraint stress significantly increased the gut permeability of humanized or S7-inoculated mice but not that of GF mice.
To characterize specifically colon epithelial permeability, we measured the efficacy of FITC diffusion across the epithelium in colon samples ex vivo (FITC flux). We observed reduced colon permeability in S7 recipient mice compared to their GF controls, which was also observed in other treatment groups (Supplementary Figure S7b). However, the S7 supplement in frail donor recipient mice did not affect colon permeability compared to frail donor recipient mice.
Altered behavior in S7-treated frail donor recipients
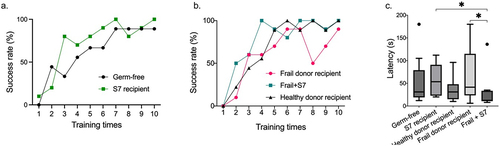
To test if S7 supplementation affected the gut-brain axis in frail donor recipient mice, we performed a series of behavior tests, including (1) grip strength test, (2) tail suspension test for depression-like behavior, (3) novel objective recognition test for cognitive function, (4) balance beam test for sensorineural balance and coordination, (5) Barnes maze test for spatial reference memory and learning. There was no significant difference between the treatment groups for grip strength, depression-like behavior, cognitive function, sensorineural balance, or coordination (Supplementary Figure S8a-d). However, S7 treatment of frail donor recipient mice improved the performance of female mice for grip strength and in the Barnes maze, which was not observed in male mice (Supplementary Figure S9). We observed improvement over time in all groups in the Barnes maze test, reflected in the increased number of individuals who were able to finish the test within 3 min (success rate) from training day 1 to the test day (). Notably, GF and frail donor recipients treated with S7 showed higher success rates than their GF and frail donor recipient controls (). On the test day, frail donor recipient mice with S7 treatment demonstrated improved spatial memory and learning ability (p =.03, one-way ANOVA post hoc Holm-Sidak) compared to frail donor recipient mice without S7 treatment (). These data suggest that S7 supplementation, which predominantly increased B. intestinihominis abundance, can potentially improve spatial memory and learning ability in frail donor-recipient mice.
Figure 5. Supplementation with the S7 consortium improves behavior in frail donor recipient mice.
Discussion
An accumulation of evidence indicates that the gut microbiota is an environmental modulator of aging-related health loss, and microbiota modulation or restoration has been proposed as an effective strategy for promoting healthy aging. However, precision microbiome manipulation in humans is technically challenging, expensive, and requires regulatory hurdles to be overcome, collectively making it highly desirable to be able to pre-screen for promising strains. It is, therefore, imperative to establish a valid animal model to generate mechanistic understanding of host–microbe interactions in the aging process. In this study, we used a human microbiota-associated (HMA) model to evaluate whether engraftment of a healthy or frail human microbiota could recapitulate host frailty-related phenotypes, and this model was used to investigate the effect of a seven-species consortium (the “S7”) as a microbiome-based therapeutic strategy for healthy aging. Metabolomic analysis revealed higher acetate levels in the feces of healthy donor recipient mice compared with that of frail donor recipient mice (Supplementary Figure S4). Consistent with the metabolic data, acetate-producing bacteria,Citation46 including Prevotella spp. and Bifidobacterium spp., were present in the healthy donor recipient mice while absent in the frail donor recipient mice (Supplementary Figure S2).
While a previous study reported that fecal microbiota transplantation of frozen samples into GF mice resulted in the successful transfer of 85% of genera after 1 week,Citation47 only 51% and 66% of frail and healthy donor genus-level taxa were transferred into mouse recipients 1 week after transplantation in our current study. This difference in transplantation efficacy could be due to the lower microbial diversity of the elderly healthy – and frail – donor we recruited. Lower engraftment efficacy of frail donor microbiota could be due to lower alpha-diversity and a low Prevotella to Bacteroides ratio compared to healthy donor microbiota.Citation48 Furthermore, the germ-free mouse gut is characterized by increased mucin production, higher pH value, urea and oxygen levels, and low or absent short-chain fatty acids, substantially affecting gut microbiota colonization.Citation49–51 Even though previous studies have shown a single dose of FMT is enough to replicate human microbiota in germ-free mice,Citation12,Citation47 some studies have highlighted that multiple gavage instead of a single dose increases the number of detected taxa,Citation52,Citation53 which should be considered in future studies to improve the engraftment rate of the elderly microbiota. It may alternatively be necessary to trial other preclinical models for human microbiome–frailty interaction if engraftment efficiency or readouts in a murine germ-free model prove limiting.
Using the selected readouts for several physiological systems, we also found that the human frailty phenotype could not be transmitted to GF mice by microbiota transfer, even though the engraftment of healthy or frail donor microbiota resulted in distinct microbiota in recipient mice. It has been reported that taxa that colonize in recipient animals may not engage in the host–microbe interactions that drive “native” disease-associated alterations.Citation34,Citation35,Citation54 The engraftment in HMA mice with healthy or frail-associated microbiota may simply reflect the adaptation of very different microbial communities to a new gut environment rather than the human donors’ pathological states, thereby masking disease signatures. The transplantation of “healthy” and “frail” donor microbiota resulted in distinct microbiota in recipient mice at week 5 despite no difference in alpha diversity (). To replicate the frailty-related phenotype in recipient mice, the engraftment of frailty-related taxa holds greater importance than alpha diversity which is useful in describing the microbiome states.Citation23 The successful engraftment of two frailty-positive-related taxa, Coprobacillus and Parabacteroides, in frail donor recipients but not in healthy donor recipients may play a role in replicating frailty-related phenotype (Supplementary Information). However, the inefficient engraftment of two frailty-positive-related taxa, Escherichia-Shigella and Streptococcus, could be one of the reasons why there were no measurable differences in frailty-related phenotypes (Supplementary Table S11), including inflammation, grip strength, and cognitive function, between frail and healthy donor microbiota recipients. Furthermore, the lack of changes in gut permeability in this study could hinder the crosstalk within the microbiota-gut-brain axis.
With a view to modeling the modulation of the gut microbiome of older people, we identified seven healthy aging-related species and supplemented GF and frail donor recipient mice with those frailty-negative-associated S7 species (). Consistent with previous probiotic studiesCitation55–57, S7 supplementation as a live biotherapeutic consortium in frail donor recipient mice did not change the alpha-diversity. Five S7 species were detected in GF mice at week 5 using 16S rRNA-specific primers. However, S7 supplementation in frail donor recipient mice increased only the abundance of Barnesiella intestinihominis, suggesting that S7 species encounter a higher degree of colonization resistance in the humanized mice than in the GF mice. We also observed the loss of donor-originated S7 species in the murine recipients (). Alistipes putredinis was detected in the healthy donor but failed to colonize in the murine gut at weeks 1 and 5. In another case, Dorea longicatena from a healthy donor was detected in the mouse gut microbiota at week 1 but was absent at week 5. Similarly, frail donor-originated Eubacterium rectale, Faecalibacterium prausnitzii, and Roseburia hominis failed to colonize in the mouse gut. The distinct engraftment efficacies of the same species in different microbial communities suggest that the interaction of whole microbiomes and administered strains is crucial for successful engraftment. The loss of donor-originated S7 species also indicates the challenges of recovering administered strains.
Changes in the gut microbiota might influence the bidirectional signaling between the gastrointestinal tract and the brain, thus modulating behavior.Citation58,Citation59 B. intestinihominis has been reported to be more abundant in high-functioning older adults (aged above 70) compared with low-functioning adults, and the transfer of high-functioning donor microbiota into GF mice improved their grip strengthCitation60. Our data reveal improved spatial memory and learning ability in frail donor recipient mice receiving S7 treatment, mainly induced by increased B. intestinihominis, which may provide a tractable reductionist model for future studies seeking to identify microbial effectors and host mechanisms.
Supplementation of S7 in GF mice significantly increased fecal acetate levels (Supplementary Figure S5), confirming in vivo the reported ability of B. intestinihominisCitation61 and D. longicatenaCitation62 to produce acetate. Furthermore, S7 supplementation reduced the plasma TNF-ɑ concentration compared to the GF controls, which is consistent with reduced colon permeability in S7 recipient mice, suggesting the beneficial effects of the S7 consortium. Although it was previously reported that the transfer of gut microbiome from old mice into young mice affects inflammaging, the reduction in TNF-ɑ in S7-supplemented animals we observed may have been related to the stress test response. The S7 consortium did not change the short-chain fatty acids (SCFA) level of frail donor recipients. The lack of observed effect of the S7 consortium on SCFA in frail donor recipients could be attributed to one or more of the following: i) SCFA is the product of fermentation of gut microbiota, which is regulated by microbe–microbe and microbe–host interactions, and frail microbiota is a complex community, ii) SCFA can be directly used as energy and carbon sources by the murine host,Citation63 and iii) difficulties of SCFA measurement in the feces due to their volatility and hydrophilicity. The S7 supplementation in frail donor recipient mice did not change the overall gut permeability and colon permeability compared to frail donor recipients. However, the stress-reduced IL-6 was higher (p = .08) in frail donor recipients compared to GF mice, which was not observed in frail donor recipients with S7 supplementation (Frail + S7). The inconsistent change of stress-reduced IL-6 between Frail + S7 and frail donor recipients could be explained by the permeability changes in other intestinal locations. It has also been reported that humanized GF mice display reduced immune stimulation compared to mouse microbiota-transplanted mice.Citation64 Moreover, GF mice are known to have an underdeveloped immune system due to the lack of gut microbiota,Citation65,Citation66 further highlighting the challenge of adapting this model for mechanistic studies.
Because of the usage of single donors in the preclinical model, we could not test the effect of different donors/microbiome composition within healthy and frail groups and compare them at higher taxonomic resolution (i.e., strain level) for colonization efficiency and phenotype transmission. Although some studies seeking to transfer human phenotypes to experimental animals via the microbiome have used pooled samples from multiple donors, we chose not to do this because it produces artificial trophic networks and functional redundancies. Although we carefully selected donors that were representative of clinical phenotypes and the corresponding microbiome type, using single donors for the two groups is a limitation we acknowledge. We also recognize that the current study does not prove the causality of improved spatial reference memory and learning ability. Further studies will be needed to understand the role of B. intestinihominis in improving these readouts, supported by a wider battery of tests targeting this aspect of cognitive function. Overall, this study demonstrates the difficulty of replicating the frailty phenotype in the humanized GF mouse model and provides essential information for design considerations in elderly microbiota transplantation studies.
Methods
Identification of healthy aging-related taxa for constructing a synthetic consortium
Diet is a significant modulator of the gut microbiota at all life stages and one that particularly drives alteration of the gut microbiota in aging. Our previous study investigated the effect of a 1-y Mediterranean diet intervention (NU-AGE diet) in a large cohort of more than 1200 elderly individuals aged 65–79 y, distributed across five countries (UK, France, Netherlands, Italy, and Poland). This study revealed that pre-frail elderly subjects receiving a 1-y NU-AGE diet experienced delayed onset of further frailty by modulating the gut microbiota.Citation24 Taxa enriched by adherence to the NU-AGE diet were referred to as “diet-positive” taxa. The NU-AGE study thus provided a short list of “diet-positive” taxa that have the potential to promote healthy aging, including Faecalibacterium prausnitzii, Eubacterium rectale, Roseburia hominis, Prevotella copri, Blautia spp., and Clostridium spp.
Aging is associated with multiple diseases, and a previous study in our lab also identified aging-associated microbiome disease markers across five major diseases in a multi-cohort dataset, including more than 2,500 individuals from different countries of North America, Europe, and Asia.Citation11 In Ghosh et al., we reported reduced abundance of Alistipes putredinis, Barnesiella intestinihominis, Coprococcus catus, Dorea longicatena, and Eubacterium rectale in at least two diseases.Citation11 Furthermore, F. prausnitzii has been negatively associated with frailty in a twin study.Citation26 Combining the overlapping results of disease- and diet-associated taxa, a candidate group of species (that we refer to here as the S7), namely Alistipes putredinis, Barnesiella intestinihominis, Coprococcus catus, Dorea longicatena, Eubacterium rectale, Faecalibacterium prausnitzii, and Roseburia hominis, was selected for further analysis.
Relative abundance of the S7 taxa in the ELDERMET cohort and IMNGS database
The ELDERMET shotgun data were retrieved from the European Nucleotide Archive (ENA) with the project accession number PRJEB37017. Samples with available Functional Independence Measure (FIM)Citation67 and Barthel ScoresCitation68 were selected for the S7 profile analysis (Supplementary Table S6). Selected samples comprise 188 elderly Irish individuals (aged between 64 and 102 y of age), with 135 subjects living in the community and 55 subjects living in Residential care (Long-stay). FIM values equal to or less than 90 are classified as frail,Citation67 values between 90 and 126 are classified as mid-frail, and values equal to 126 are defined as non-frail/healthy.
The occurrence and prevalence of 16S rRNA gene sequences related to the S7 taxon were analyzed with the Integrated Microbial Next-Generation Sequencing (IMNGS) platform.Citation37 All Sequence Read Archive (SRA) amplicon sequence datasets with the Origin from the human and mouse gut were selected for further analyses. A 99% sequence similarity cutoff was used to identify S7-related 16S rRNA gene sequences in the selected dataset, which includes 41,920 human and 19,703 mouse samples.
Donor fecal sample and inoculum preparation
The “healthy” and “frail” donors (n = 1) were selected from a subset of the well-phenotyped ELDERMET subjectsCitation69 who live in community or long-stay residential care. Claesson et al. reported that gut microbiota of the ELDERMET cohort formed groups, correlating with residence location and diets. Furthermore, the clustering of subjects by diet separated them by the same residence locations and microbiota groupings.Citation3 The average age of ELDERMET is 78 ± 8 y, with a range of 64 to 102. Regardless of age variance, the gut microbiota of ELDERMET cohort exhibited distinct clustering patterns correlated to frailty.Citation3 The following exclusion criteria were used for donor selection: usage of antibiotics there months before the sampling; usage of immunosuppressive or anti-inflammatory drugs or any drug affecting intestinal mobility; participants on warfarin; participation in another clinic or intervention study involving investigational drugs, including investigational prebiotic, prebiotic or symbiotic preparations in the preceding 6 months.
The “healthy” and “frail” donor samples were selected based on consideration of their clinical measurements in ELDERMET project, as well as microbiome configuration. The diagnosis of frailty was provided by their consultant gerontologist based on combined evaluation of the body mass index, calf circumference, systolic blood pressure, Barthel index,Citation68 functional independence measurement (FIM), mini-mental state exam, mini-nutritional assessment, geriatric depression test, and Charlson index of comorbidity.Citation3 Barthel Index, Charlson co-morbidity index, and handgrip strength at sampling were also used as confirmatory references for donor selection (Suplementary Table S1). The microbiome composition of candidate donors was determined by 16S rRNA gene sequencing. We also compared the microbiota of candidate donors (from clinical evaluation) to the microbiomes previously analyzed ELDERMET samples to ensure the typical community or long-stay microbiota profiles were present in the donors (Supplementary Figure S1). Eventually, one “healthy” and one “frail” donors were selected for fecal microbiota transplantation (FMT). Upon collection, fecal samples were transferred to an anaerobic cabinet immediately after collection and homogenized in reduced sterile phosphate-buffered saline (PBS) with 20% glycerol, then stored at −80°C for transplantation. Additionally, the survival rate of donor taxa was checked by plating before and after the storage to ensure no taxa loss caused by the preservation method.
The S7 culture is constituted of a total of seven bacterial species from the previously isolated and characterized the Microbiome Culture Collection 100 (MCC100)Citation27 and S7 species were cultivated in a modified YCFA medium as described previouslyCitation27 at 37°C in an anaerobic chamber under an anoxic atmosphere (90% N2, 5% CO2, 5% H2). S7 pure cultures were harvested in the late exponential growth phase and centrifuged to reach the maximum cell count. The cell counts of S7 species were measured by plating on YCFA agar. The final S7 culture mix consisted of A. putredinis (1.83 × 108 CFU/ml), B. intestinihominis (5.0 × 108 CFU/ml), C. catus (2.14 × 108 CFU/ml), D. longicatena (3.28 × 108 CFU/ml), E. rectale (1.15× 108 CFU/ml), F. prausnitzii (4.0 × 105 CFU/ml), R. hominis (4.5 × 10Citation7 CFU/ml). Bacterial cell mixtures were kept at −80°C in 20% (v/v) glycerol/PBS until further use.
Pre-clinical animal model
All animal protocols were approved by the Animal Experimentation Ethics Committee at University College Cork and by the Health Products Regulatory Authority (HPRA) of Ireland in accordance with EU Directive 2010/63/EU (HPRA Project authorization number AE19130/P147).
Six-month-old C57BL/6 mice from the gnotobiotic line bred at the APC Microbiome Institute’s Germ-Free Platform were used for the experiment as fecal microbiota transplantation (FMT) from aged mice to young mice has been proven to be sufficient to change microbial metabolites in the host and to cause a reduction in cognitive function.Citation39,Citation70 We ensured gender balance within the experimental groups by incorporating an equal number of male and female mice (Supplementary Table S9). All the mice were group housed 3–4 per cage in the same temperature and humidity-controlled animal room with a 12 h light: dark cycle and were maintained on ad libitum sterile standard chow and water unless otherwise noted. Mice were colonized by pipette dosing with 200 µl of FMT (109 CFU/ml) from either a single healthy or frail elderly donor (or sterile PBS as vehicle control). The “healthy” donor sample was used to humanize all the mice in the group “Healthy donor recipient,” and the “frail” donor sample was used to humanize all mice in groups ‘Frail donor recipient” and “Frail + S7”. Following 1 week of colonization, mice were gavaged once a week (for 4 weeks) with 200 µl of either the S7 treatment or vehicle control. After transplantation, mice that received the same treatment were co-housed in individually ventilated cages for the duration of the experiment. The cages, food, bedding, and water were sterilized by autoclave to maintain germ-free condition. Fecal samples were collected 1 week after FMT inoculation and 4 weeks after S7 supplementation to assess the effects of FMT on recipient fecal microbiota and the influence of S7 treatment. Following 4 weeks of S7 supplementation, blood was collected for gut permeability and inflammation assessment (see sections on FITC measurement and cytokine assessment). Mice then underwent behavioral tests to assess physical and cognitive frailty, including grip strength, tail suspension, balance beam, and Barnes maze. Finally, mice were sacrificed to collect blood and tissues for further analysis.
DNA extraction for 16S rRNA amplicon microbiota analysis
Fecal DNA was extracted using the DNeasy Blood & Tissue Kit (QIAGEN) according to the manufacturer’s instructions. The V3-V4 region of the 16S rRNA gene was amplified, and the amplicons were quantified with a Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific). Sequencing was performed on an Illumina MiSeq Platform (2 × 300 bp reads) by the Eurofins Genomics Next-Generation Sequencing service (London, UK).
High-throughput 16S rRNA gene amplicon analysis
Raw reads were used for quality filtering and trimming using DADA2 (version 1.24.0)Citation71 with parameters trimLeft = 12, truncLen = 250, and maxEE = 2 in R (version 4.2.1). Only forward reads were further processed and used for downstream analysis due to decreased quality of the reverse reads, which can negatively affect sample inference in the DADA2 pipeline. Taxonomy assignment was conducted using the SILVA SSURef database release v. 138.Citation72 Species were assigned using spingo v1.3.Citation73 Raw read counts were used as input data for differential analysis using DESeq2.Citation38
Quantitative PCR (qPCR) of S7 16S rRNA genes and total 16S rRNA gene copies in donor feces
To investigate the engraftment of S7 in germ-free mice, the fecal DNAs from S7 recipient mice were subjected to quantitative PCR using primers specifically targeting the individual S7 16S rRNA gene (Supplementary Table S10). 16S rRNA-specific primers were designed using Primer3Plus.Citation74 Standard curves were generated from genomic DNA extracted from S7 pure cultures. In order to quantify the total bacteria, DNA was extracted from human donor feces using the QIAamp DNA Stool Mini kit following the manufacturer’s instructions. The 515F/806 R primers were chosen to quantify total bacteria, and standard curves were constructed using the PCR product of the 16S rRNA gene of E. coli. The PCR product was purified using the QIAquick PCR Purification Kit (QIAGEN, Germany) and quantified with a Qubit dsDNA HS Assay kit (Thermo Fisher Scientific, Ireland). One PCR reaction (total volume of 15 μl) contains 0.75 μl of primers (10 μM), 7.5 μl SYBR Green I, 5 μl nuclease-free water, and 100 ng DNA template. PCR conditions were 95°C for 10 min, followed by 40 cycles of 95°C for 10 s, 55°C for 15 s, and 72°C for 15 s. Fluorescence signals were obtained at the end of each cycle. Amplification and detection were performed using the LightCycler 480 system (Roche, US). All qPCR assays were performed in duplicate, and the amplification efficiencies of all qPCR assays are >98%.
SCFA and organic acid analysis
Mouse fecal pellets were diluted 1:5 (w/v) with water, vortex-mixed for 5 min and sonicated for 5 min. Homogenized samples were centrifuged for 10 min at 13,000 g. The supernatant was collected and filtered through a membrane filter (0.45 μm), and a volume of 20 μl sample was used for further measurement. Short-chain fatty acids (SCFAs) were determined with an Agilent 1200 HPLC system (Agilent Technologies, CA, USA) with a REZEX 8 µL 8% H + organic acid column (300 × 7.8 mm) (Phenomenex, CA, USA) operating at 65°C using 0.01 N H2SO4 as a solvent at a flow rate of 0.6 mL/min. Organic compounds were detected with a refractive index detector. Reference standards of SCFAs and organic acids, including glucose (10 mM), lactose (10 mM), lactic acid (10 mM), formic acid (10 mM), succinic acid (10 mM), propionic acid (10 mM), acetic acid (10 mM), butyric acid (10 mM), were used for data analysis.
Cytokine measurement
Inflammation-related cytokines IL-6, CCL2 (MCP-1), and TNF-ɑ were assessed using the LEGENDplex Mouse Inflammation Panel (Mix and Match Subpanel) assay (BioLegend) according to the manufacturer’s instructions. Four tail blood samples from each group before (baseline) and after restraint stress were collected using a heparinized capillary tube for cytokine measurement. Samples were run in duplicate. Data analyses were performed using BioLegend’s LEGENDplex™ data analysis software.
Intestinal permeability assay by FITC-dextran
In vivo FITC permeability
Intestinal barrier function was assessed using fluorescein isothiocyanate-labeled dextran (FITC-D) (FD4, Sigma Aldrich, Ireland) as previously described.Citation75 Briefly, the mice were fasted overnight and orally gavaged with FITC-D (600 mg/kg, 80 mg/ml) the next morning. One hour following gavage, mice were restrained, and tail blood samples were collected using a heparinized capillary tube for baseline permeability. Blood samples for stress-induced permeability were collected following 1 hour of restraint stress. Blood samples were kept on ice and centrifuged to collect plasma. Plasma samples were stored at −20°C for FITC measurement. FITC in plasma was measured in Nunc™ 384-well Optical Bottom plate (Thermo Fisher Scientific) at 485 nm excitation and 535 nm emission wavelengths using a multi-mode plate reader (Victor 3, Perkin Elmer). Serial dilutions of FITC in PBS were used for the standard curve.
Ex-vivo FITC colonic permeability
Fresh colon segments (1–2 cm) were collected at the end of the experiment to examine the colonic permeability. Colon tissues were washed with PBS and were mounted into vertical NaviCyte diffusion chambers with 4 mm round aperture (0.126 cm2 exposed tissue area). No seromuscular stripping was performed. Then, 4.0 mL of Krebs buffer (1.2 mM NaH2PO4, 116 mM NaCl, 4.8 mM KCl, 1.2 mM MgCl2, 25 mM NaHCO3, 2.5 mM CaCl2, and 10 mM D-glucose) was added to both the mucosal and serosal chambers. Chambers were kept at 37°C and continuously supplied with carbogen (95% O2 and 5% CO2). To assess the transepithelial permeability, 4 KDa FITC-dextran (FD4, Sigma-Aldrich) was added to the luminal chamber (final concentration of 2.5 mg/ml). Samples (200 μl) from the serosal chamber were collected at 0 h, 1 h, 1.5 h, and 2 h.Citation76 FITC absorbance was measured in 96 well plates as mentioned above. FITC mucosal-to-serosal flux was presented in µg/h/cm2.
Behavioral tests
All behavior tests were conducted as the last tests () and scored blinded. Both the grip strength and tail suspension tests were conducted under sterile conditions in a biosafety cabinet. Due to the size of the Barnes Maze, novel object recognition, and balance beam tests, these tests were conducted outside of a sterile environment.
Novel object recognition (NOR)
To assess short-term recognition memory, mice were subjected to the NOR task as previously described.Citation77 Animals were habituated to the room 60 min before the test. On the pre-trial day, mice were habituated to the empty, open arena (40 × 45 × 45 cm, L × W × H) in two habituation phases (10 min each, with 3 h time in between). The following day, mice were exposed for 10 min to two identical objects placed in the arena’s corners (acquisition phase). Three hours later, one of the familiar objects was substituted with a novel object, and the animal was allowed to explore the objects for 10 min (retention phase). The test was conducted under dimmed lighting (15 lux). Animal behavior was video recorded, and the exploration time of the objects was recorded. The discrimination index was calculated according to the formula: (t(novel)-t(familiar))/(t(novel)+t(familiar)).
Balance beam
Mice were placed onto a wooden balance beam (12 mm wide, 125 cm long, elevated 60 cm above the floor), and the time to reach the escape box (20 × 20 × 20 cm) at the end of the beam was recorded. If animals did not readily walk across the beam, they were gently encouraged at 2, 3, 3:30, 4, and 4:30 min. The test was ended if the mice failed to cross the beam in 5 min. A net was placed below the beam in case of falls, and if an animal fell, it was placed back on the starting end of the beam, and the number of falls was recorded.
Grip strength
Forelimb grip strength was measured using a grip strength meter (Ugo Basile, Italy), which measures the peak pull force (g) exerted by the mice on the grasping apparatus. Mice were held with their forelimbs on the grip bar and were gently pulled backward along a horizontal plane while they grasped the bar. The forces from five consecutive trials were recorded and used for analysis.
Barnes Maze
The Barnes maze is used to assess spatial reference memory and learning and was conducted as previously described.Citation78 Briefly, mice were trained over 3 d, with three trials per day, each lasting 3 min to allocate the submerged platform spatially. The latency to find the correct hole was analyzed by comparing the average of three trials across each training day to evaluate learning performance.
Tail suspension
The tail suspension test is used for screening potential antidepressant therapeutics.Citation79 Mice were suspended by their tails from a horizontal bar for 6 min, which resulted in initial escape-oriented behaviors and immobile posture after several minutes. The time to the first immobility and the total immobility time were recorded for further analysis.
Statistical analyses
Statistical analyses were conducted using R and GraphPad Prism v.9.4.1 (GraphPad Software). Outliers were excluded using the ROUT method with q = 1%.Citation80 Figures with outliers were presented in Supplementary Figure S10. Normality analyses were conducted using the Shapiro–Wilk test. Unless otherwise indicated, nonparametric data were analyzed by Kruskal–Wallis post-hoc Dunn’s and parametric data by one-way ANOVA post-hoc Holm-Sidak. The similarity between the samples was calculated with Spearman’s correlation (ρ) using cor() in R (version 4.2.1).Citation81 All tests were two-sided, and statistical significance was set at P <.05.
Author contributions
PWOT has contributed to the conception and experimental design. HY and PWOT wrote the manuscript. TSG, CMH, KV, AVG, and NPH revised the manuscript. HY, CMH, and KV performed the experiments and acquired data. HY, CMH, and TSG analyzed the data. All authors read and approved the final manuscript.
Supplemental Material
Download Zip (3.3 MB)Acknowledgments
We thank the APC Microbiome Ireland Flow Cytometry Platform (led by Ken Nally) for the cytokine measurement. We also thank Daniel Walsh for technical assistance with the HPLC analysis. The authors are grateful for the invaluable help with sampling from Dara Meehan, Cian Hurley, Dalimil Bujdos, Peter Cronin, and Junhui Li.
Disclosure statement
The seven species described in this study are the subject of patent EP3808358A1, coauthored by MP, AN, HT, and PWOT.
Data availability statement
All 16S rRNA gene sequence data are deposited to the Sequence Read Archive (SRA) under BioProject accession PRJNA943425.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2023.2282796
Additional information
Funding
References
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–20. doi:10.1016/j.cell.2013.05.039.
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi:10.1016/S0140-6736(12)62167-9.
- Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HMB, Coakley M, Lakshminarayanan B, O’Sullivan O, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi:10.1038/nature11319.
- Salazar N, Valdés-Varela L, González S, Gueimonde M, de Los Reyes-Gavilán CG. Nutrition and the gut microbiome in the elderly. Gut Microbes. 2017;8:82–97. doi:10.1080/19490976.2016.1256525.
- Ghosh TS, Shanahan F, O’Toole PW. The gut microbiome as a modulator of healthy ageing. Nat Rev Gastroenterol Hepatol. 2022;19:565–584. doi:10.1038/s41575-022-00605-x.
- Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkïla J, Monti D, Satokari R, Franceschi C, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE. 2010;5:e10667. doi:10.1371/journal.pone.0010667.
- Xu C, Zhu H, Qiu P. Aging progression of human gut microbiota. BMC Microbiol. 2019;19:236. doi:10.1186/s12866-019-1616-2.
- Odamaki T, Kato K, Sugahara H, Hashikura N, Takahashi S, Xiao J-Z, Abe F, Osawa R. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 2016;16:90. doi:10.1186/s12866-016-0708-5.
- Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S, Consolandi C, Quercia S, Scurti M, Monti D, et al. Gut Microbiota and extreme longevity. Curr Biol. 2016;26:1480–1485. doi:10.1016/j.cub.2016.04.016.
- Wu L, Zeng T, Zinellu A, Rubino S, Kelvin DJ, Carru C. A cross-sectional study of compositional and functional profiles of gut microbiota in Sardinian centenarians. mSystems. 2019;4. doi:10.1128/mSystems.00325-19.
- Ghosh TS, Das M, Jeffery IB, O’Toole PW. Adjusting for age improves identification of gut microbiome alterations in multiple diseases. Elife. 2020;9. doi:10.7554/eLife.50240.
- Tran TTT, Cousin FJ, Lynch DB, Menon R, Brulc J, Brown J-M, O’Herlihy E, Butto LF, Power K, Jeffery IB, et al. Prebiotic supplementation in frail older people affects specific gut microbiota taxa but not global diversity. Microbiome. 2019;7:39. doi:10.1186/s40168-019-0654-1.
- Cheng Y-W, Alhaffar D, Saha S, Khanna S, Bohm M, Phelps E, Ghabril M, Orman E, Sashidhar S, Rogers N, et al. Fecal Microbiota transplantation is safe and effective in patients with Clostridioides difficile infection and cirrhosis. Clin Gastroenterol Hepatol. 2021;19:1627–1634. doi:10.1016/j.cgh.2020.06.051.
- Baunwall SMD, Lee MM, Eriksen MK, Mullish BH, Marchesi JR, Dahlerup JF, Hvas CL. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: an updated systematic review and meta-analysis. EClinicalMedicine. 2020;29–30:100642. doi:10.1016/j.eclinm.2020.100642.
- Saha S, Mara K, Pardi DS, Khanna S. Long-term safety of fecal Microbiota transplantation for recurrent Clostridioides difficile infection. Gastroenterology. 2021;160:1961–1969.e3. doi:10.1053/j.gastro.2021.01.010.
- Quaranta G, Ianiro G, De Maio F, Guarnaccia A, Fancello G, Agrillo C, Iannarelli F, Bibbo S, Amedei A, Sanguinetti M, et al. “Bacterial Consortium”: A potential evolution of fecal Microbiota transplantation for the treatment of Clostridioides difficile infection. Biomed Res Int. 2022;2022:5787373. doi:10.1155/2022/5787373.
- Tamtaji OR, Heidari-Soureshjani R, Mirhosseini N, Kouchaki E, Bahmani F, Aghadavod E, Tajabadi-Ebrahimi M, Asemi Z. Probiotic and selenium co-supplementation, and the effects on clinical, metabolic and genetic status in Alzheimer’s disease: a randomized, double-blind, controlled trial. Clin Nutr. 2019;38:2569–2575. doi:10.1016/j.clnu.2018.11.034.
- Inoue T, Kobayashi Y, Mori N, Sakagawa M, Xiao J-Z, Moritani T, Sakane N, Nagai N. Effect of combined bifidobacteria supplementation and resistance training on cognitive function, body composition and bowel habits of healthy elderly subjects. Benef Microbes. 2018;9:843–853. doi:10.3920/BM2017.0193.
- Di Gioia D, Aloisio I, Mazzola G, Biavati B. Bifidobacteria: their impact on gut microbiota composition and their applications as probiotics in infants. Appl Microbiol Biotechnol. 2014;98:563–577. doi:10.1007/s00253-013-5405-9.
- Dempsey E, Corr SC. Lactobacillus spp. For gastrointestinal health: Current and future perspectives. Front Immunol. 2022;13:840245. doi:10.3389/fimmu.2022.840245.
- Saturio S, Nogacka AM, Alvarado-Jasso GM, Salazar N, de Los Reyes-Gavilán CG, Gueimonde M, Arboleya S. Role of bifidobacteria on infant health. Microorganisms. 2021;9:2415. doi:10.3390/microorganisms9122415.
- Shanahan F, Ghosh TS, O’Toole PW. The healthy microbiome-what is the definition of a healthy gut microbiome? Gastroenterology. 2021;160:483–494. doi:10.1053/j.gastro.2020.09.057.
- Ghosh TS, Shanahan F, O’Toole PW. Toward an improved definition of a healthy microbiome for healthy aging. Nat Aging. 2022;2:1054–1069. doi:10.1038/s43587-022-00306-9.
- Ghosh TS, Rampelli S, Jeffery IB, Santoro A, Neto M, Capri M, Giampieri E, Jennings A, Candela M, Turroni S, et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut. 2020;69:1218–1228. doi:10.1136/gutjnl-2019-319654.
- van Tongeren SP, Slaets JPJ, Harmsen HJM, Welling GW. Fecal microbiota composition and frailty. Appl Environ Microbiol. 2005;71:6438–6442. doi:10.1128/AEM.71.10.6438-6442.2005.
- Jackson MA, Jeffery IB, Beaumont M, Bell JT, Clark AG, Ley RE, O’Toole PW, Spector TD, Steves CJ. Signatures of early frailty in the gut microbiota. Genome Med. 2016;8:8. doi:10.1186/s13073-016-0262-7.
- Perez M, Ntemiri A, Tan H, Harris HMB, Roager HM, Ribière C, O’Toole PW. A synthetic consortium of 100 gut commensals modulates the composition and function in a colon model of the microbiome of elderly subjects. Gut Microbes. 2021;13:1–19. doi:10.1080/19490976.2021.1919464.
- Damman CJ, Miller SI, Surawicz CM, Zisman TL. The microbiome and inflammatory bowel disease: is there a therapeutic role for fecal microbiota transplantation? Am J Gastroenterol. 2012;107:1452–1459. doi:10.1038/ajg.2012.93.
- Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800–812. doi:10.1038/nrc3610.
- Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–298. doi:10.1101/gr.126573.111.
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi:10.1038/4441022a.
- Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Sci. 2013;341:1241214. doi:10.1126/science.1241214.
- Feehley T, Plunkett CH, Bao R, Choi Hong SM, Culleen E, Belda-Ferre P, Campbell E, Aitoro R, Nocerino R, Paparo L, et al. Healthy infants harbor intestinal bacteria that protect against food allergy. Nat Med. 2019;25:448–453. doi:10.1038/s41591-018-0324-z.
- Britton GJ, Contijoch EJ, Mogno I, Vennaro OH, Llewellyn SR, Ng R, Li Z, Mortha A, Merad M, Das A, et al. Microbiotas from humans with inflammatory bowel disease alter the balance of gut Th17 and RORγt+ regulatory T cells and exacerbate colitis in mice. Immunity. 2019;50:212–224.e4. doi:10.1016/j.immuni.2018.12.015.
- Sharon G, Cruz NJ, Kang D-W, Gandal MJ, Wang B, Kim Y-M, Zink EM, Casey CP, Taylor BC, Lane CJ, et al. Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell. 2019;177:1600–1618.e17. doi:10.1016/j.cell.2019.05.004.
- Antwis RE, Lea JMD, Unwin B, Shultz S. Gut microbiome composition is associated with spatial structuring and social interactions in semi-feral Welsh mountain ponies. Microbiome. 2018;6:207. doi:10.1186/s40168-018-0593-2.
- Lagkouvardos I, Joseph D, Kapfhammer M, Giritli S, Horn M, Haller D, Clavel T. IMNGS: a comprehensive open resource of processed 16S rRNA microbial profiles for ecology and diversity studies. Science Reports. 2016;6:33721. doi:10.1038/srep33721.
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15. doi:10.1186/s13059-014-0550-8.
- Fransen F, van Beek AA, Borghuis T, Aidy SE, Hugenholtz F, van der Gaast-de Jongh C, Savelkoul HFJ, De Jonge MI, Boekschoten MV, Smidt H, et al. Aged gut microbiota contributes to systemical inflammaging after transfer to germ-free mice. Front Immunol. 2017;8:1385. doi:10.3389/fimmu.2017.01385.
- Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;8(3):131–136. doi:10.1097/00062752-200105000-00001.
- Conley MN, Wong CP, Duyck KM, Hord N, Ho E, Sharpton TJ. Aging and serum MCP-1 are associated with gut microbiome composition in a murine model. PeerJ. 2016;4:e1854. doi:10.7717/peerj.1854.
- Soysal P, Arik F, Smith L, Jackson SE, Isik AT. Inflammation, frailty and cardiovascular disease. Adv Exp Med Biol. 2020;1216:55–64. doi:10.1007/978-3-030-33330-0_7.
- Qing H, Desrouleaux R, Israni-Winger K, Mineur YS, Fogelman N, Zhang C, Rashed S, Palm NW, Sinha R, Picciotto MR, et al. Origin and function of stress-induced IL-6 in murine models. Cell. 2020;182(6):1660. doi:10.1016/j.cell.2020.08.044.
- Voorhees JL, Tarr AJ, Wohleb ES, Godbout JP, Mo X, Sheridan JF, Eubank TD, Marsh CB. Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PLoS ONE. 2013;8(3):e58488. doi:10.1371/journal.pone.0058488.
- Tang L, Cai N, Zhou Y, Liu Y, Hu J, Li Y, Yi S, Song W, Kang L, He H. Acute stress induces an inflammation dominated by innate immunity represented by neutrophils in mice. Front Immunol. 2022;13:1014296. doi:10.3389/fimmu.2022.1014296.
- Rey FE, Faith JJ, Bain J, Muehlbauer MJ, Stevens RD, Newgard CB, Gordon JI. Dissecting the in vivo metabolic potential of two human gut acetogens. J Biol Chem. 2010;285(29):22082–22090. doi:10.1074/jbc.m110.117713.
- Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6ra14. doi:10.1126/scitranslmed.3000322.
- Wilson BC, Vatanen T, Jayasinghe TN, Leong KSW, Derraik JGB, Albert BB, Chiavaroli V, Svirskis DM, Beck KL, Conlon CA, et al. Strain engraftment competition and functional augmentation in a multi-donor fecal microbiota transplantation trial for obesity. Microbiome. 2021;9(1):107. doi:10.1186/s40168-021-01060-7.
- Al-Asmakh M, Zadjali F. Use of germ-free animal models in microbiota-related research. J Microbiol Biotechnol. 2015;25(10):1583–1588. doi:10.4014/jmb.1501.01039.
- Gillilland MG 3rd, Erb-Downward JR, Bassis CM, Shen MC, Toews GB, Young VB, Huffnagle GB. Ecological succession of bacterial communities during conventionalization of germ-free mice. Appl Environ Microbiol. 2012;78(7):2359–2366. doi:10.1128/AEM.05239-11.
- Tomas J, Wrzosek L, Bouznad N, Bouet S, Mayeur C, Noordine M-L, Honvo-Houeto E, Langella P, Thomas M, Cherbuy C. Primocolonization is associated with colonic epithelial maturation during conventionalization. FASEB J. 2013;27(2):645–655. doi:10.1096/fj.12-216861.
- Choo JM, Rogers GB. Establishment of murine gut microbiota in gnotobiotic mice. iScience. 2021;24(2):102049. doi:10.1016/j.isci.2021.102049.
- Wrzosek L, Ciocan D, Borentain P, Spatz M, Puchois V, Hugot C, Ferrere G, Mayeur C, Perlemuter G, Cassard A-M. Transplantation of human microbiota into conventional mice durably reshapes the gut microbiota. Sci Rep. 2018;8(1): doi:10.1038/s41598-018-25300-3.
- Walter J, Armet AM, Finlay BB, Shanahan F. Establishing or exaggerating causality for the gut microbiome: lessons from human microbiota-associated rodents. Cell. 2020;180(2):221–232. doi:10.1016/j.cell.2019.12.025.
- Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. 2016;8(1): doi:10.1186/s13073-016-0300-5.
- Laursen MF, Laursen RP, Larnkjær A, Michaelsen KF, Bahl MI, Licht TR. Administration of two probiotic strains during early childhood does not affect the endogenous gut microbiota composition despite probiotic proliferation. BMC Microbiol. 2017;17(1): doi:10.1186/s12866-017-1090-7.
- Bazanella M, Maier TV, Clavel T, Lagkouvardos I, Lucio M, Maldonado-Gòmez MX, Autran C, Walter J, Bode L, Schmitt-Kopplin P, et al. Randomized controlled trial on the impact of early-life intervention with bifidobacteria on the healthy infant fecal microbiota and metabolome. Am J Clin Nutr. 2017;106(5):1274–1286. doi:10.3945/ajcn.117.157529.
- Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The neuroendocrinology of the microbiota-gut-brain axis: a behavioural perspective. Front Neuroendocrinol. 2018;51:80–101. doi:10.1016/j.yfrne.2018.04.002.
- Boehme M, Guzzetta KE, Bastiaanssen TFS, van de Wouw M, Moloney GM, Gual-Grau A, Spichak S, Olavarría-Ramírez L, Fitzgerald P, Morillas E, et al. Microbiota from young mice counteracts selective age-associated behavioral deficits. Nat Aging. 2021;1(8):666–676. doi:10.1038/s43587-021-00093-9.
- Fielding RA, Reeves AR, Jasuja R, Liu C, Barrett BB, Lustgarten MS. Muscle strength is increased in mice that are colonized with microbiota from high-functioning older adults. Exp Gerontol. 2019;127:110722. doi:10.1016/j.exger.2019.110722.
- Morotomi M, Nagai F, Sakon H, Tanaka R. Dialister succinatiphilus sp. nov. and Barnesiella intestinihominis sp. nov., isolated from human faeces. Int J Syst Evol Microbiol. 2008;58(12):2716–2720. doi:10.1099/ijs.0.2008/000810-0.
- Taras D, Simmering R, Collins MD, Lawson PA, Blaut M. Reclassification of Eubacterium formicigenerans Holdeman and Moore 1974 as Dorea formicigenerans gen. nov., comb. nov., and description of Dorea longicatena sp. nov., isolated from human faeces. Int J Syst Evol Microbiol. 2002;52(2):423–428. doi:10.1099/00207713-52-2-423.
- Hoces D, Lan J, Sun W, Geiser T, Stäubli ML, Cappio Barazzone E, Arnoldini M, Challa TD, Klug M, Kellenberger A, et al. Metabolic reconstitution of germ-free mice by a gnotobiotic microbiota varies over the circadian cycle. PLoS Biol. 2022;20(9):e3001743. doi:10.1371/journal.pbio.3001743.
- Lundberg R, Toft MF, Metzdorff SB, Hansen CHF, Licht TR, Bahl MI, Hansen AK. Human microbiota-transplanted C57BL/6 mice and offspring display reduced establishment of key bacteria and reduced immune stimulation compared to mouse microbiota-transplantation. Sci Rep. 2020;10(1):7805. doi:10.1038/s41598-020-64703-z.
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–323. doi:10.1038/nri2515.
- Fiebiger U, Bereswill S, Heimesaat MM. Dissecting the interplay between intestinal microbiota and host immunity in health and disease: lessons learned from germfree and gnotobiotic animal models. Eur J Microbiol Immunol (Bp). 2016;6(4):253–271. doi:10.1556/1886.2016.00036.
- Wang C-Y, Graham JE, Karmarkar AM, Reistetter TA, Protas EJ, Ottenbacher KJ. FIM motor scores for classifying community discharge after inpatient rehabilitation for hip fracture. PM R. 2014;6(6):493–497. doi:10.1016/j.pmrj.2013.12.008.
- Collin C, Wade DT, Davies S, Horne V. The Barthel ADL index: a reliability study. Int Disabil Stud. 1988;10(2):61–63. doi:10.3109/09638288809164103.
- Jeffery IB, Lynch DB, O’Toole PW. Composition and temporal stability of the gut microbiota in older persons. ISME J. 2016;10(1):170–182. doi:10.1038/ismej.2015.88.
- Lee J, Venna VR, Durgan DJ, Shi H, Hudobenko J, Putluri N, Petrosino J, McCullough LD, Bryan RM. Young versus aged microbiota transplants to germ-free mice: increased short-chain fatty acids and improved cognitive performance. Gut Microbes. 2020;12(1):1814107–1814114. doi:10.1080/19490976.2020.1814107.
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581–583. doi:10.1038/nmeth.3869.
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41(D1):D590–6. doi:10.1093/nar/gks1219.
- Allard G, Ryan FJ, Jeffery IB, Claesson MJ. SPINGO: a rapid species-classifier for microbial amplicon sequences. BMC Bioinform. 2015;16(1):324. doi:10.1186/s12859-015-0747-1.
- Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. Primer3—new capabilities and interfaces. Nucleic Acids Res. 2012;40(15):e115. doi:10.1093/nar/gks596.
- Scott KA, Ida M, Peterson VL, Prenderville JA, Moloney GM, Izumo T, Murphy K, Murphy A, Ross RP, Stanton C, et al. Revisiting Metchnikoff: age-related alterations in microbiota-gut-brain axis in the mouse. Brain Behav Immun. 2017;65:20–32. doi:10.1016/j.bbi.2017.02.004.
- Boscaini S, Cabrera-Rubio R, Golubeva A, Nychyk O, Fülling C, Speakman JR, Cotter PD, Cryan JF, Nilaweera KN. Depletion of the gut microbiota differentially affects the impact of whey protein on high-fat diet-induced obesity and intestinal permeability. Physiol Rep. 2021;9(11):e14867. doi:10.14814/phy2.14867.
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study “recognition memory. Nat Protoc. 2006;1(3):1306–1311. doi:10.1038/nprot.2006.205.
- Sunyer B, Patil S, Höger H, Luber G. Barnes maze, a useful task to assess spatial reference memory in the mice. Protoc Exch. 2007. doi:10.1038/nprot.2007.390.
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29(4–5):571–625. doi:10.1016/j.neubiorev.2005.03.009.
- Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression – a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 2006;7(1):123. doi:10.1186/1471-2105-7-123.
- Ripley BD. The R project in statistical computing. MSOR Connect. 2001;1(1):23–25. doi:10.11120/MSOR.2001.01010023.
