ABSTRACT
Classically, Axin1 is considered a regulator of Wnt/β-catenin signaling. However, Axin1’s roles in host-microbial interactions have been unknown. Our recent study has demonstrated that deletion of intestinal epithelial Axin1 in epithelial cells and Paneth cells protects the host against colitis by enhancing Akkermansia muciniphila. Loss of intestinal epithelial or Paneth cell Axin1 results in increased Wnt/β-catenin signaling, proliferation, and cell migration. This is associated with morphologically altered goblet and Paneth cells, including increased Muc2 and decreased lysozyme. Axin1 deletion specifically enriched Akkermansia muciniphila. Akkermansia muciniphila in Axin1 knockout mice is the driver of protection against DSS-induced inflammation. Here, we feature several significant conceptual changes, such as differences between Axin1 and Axin2, Axin1 in innate immunity and microbial homeostasis, and Axin1 reduction of Akkermansia muciniphila. We discuss an important trend in the field related to Paneth cells and tissue-specific Axin1 manipulation of microbiome in health and inflammation.
Introduction
Axin1 is known as a negative regulator of the canonical Wnt/β-catenin pathway. It forms a complex with adenomatous polyposis coli (APC), glycogen synthase kinase-3 (GSK3), and casein kinase-1 (CK1). The complex phosphorylates β-catenin, triggering ubiquitination and ultimately leads to the degradation of β-catenin. The Wnt/β-catenin pathway contributes to a wide range of cellular activities, including embryonic development, cell proliferation, tissue regeneration, stem cell maintenance, and cell fate determination. Dysregulation of the Wnt/β-catenin pathway due to Axin1 mutations is associated with various cancers, especially gastrointestinal (GI) cancers, such as hepatocellular carcinoma (HCC), gastric cancer, and colorectal cancer. For example, Axin1 alteration is found in 8–10% of HCCs.Citation1,Citation2 However, little is known regarding its specific function in host-microbial interactions. In a recent study published in EngineeringCitation3, Garrett et al. have demonstrated a new role for intestinal epithelial Axin1 in colitis by reducing Akkermansia muciniphila (A. muciniphila), thus making the host susceptible to colitis. Conditional deletion of Axin1 in epithelial cells and Paneth cells protects against colitis with enriched A. muciniphila in the microbiome community. In the current commentary, we will highlight several significant conceptual changes related to the role of Axin1 in innate immunity and microbial homeostasis.
Axin1 contributes to intestinal inflammation by regulating the microbiome, not just through the Wnt/β-catenin pathway
When the Wnt/β-catenin pathway is activated, the Axin1-centered complex will be sequestered (note that it’s not destroyed). As a result, free β-catenin in the cytosol can translocate into the nucleus and initiate transcription by interacting with transcription factors, TCF/LEF; as a result, one of the endogenous Wnt target genes, as well as an Axin1 homolog, Axin2, is induced following pathway activation. Sanson et al.Citation4 suggest that the function of Axin1 in the Wnt/β-catenin pathway can be entirely compensated for by Axin2, and vice versa. It remains a subject of debate as other studies describe otherwise. Thorvaldsen et al. have observed that in tankyrase (TNKS) inhibitor-induced degradation of β-catenin, Axin2 plays a more significant part in forming degradosome in CRC cell lines, indicating that Axin2 might have a greater role in turning the pathway off while Axin1 is more dominant in the turn-on process.Citation5 Roo et al. also emphasize that Axin2 is crucial and non-redundant for precise hematopoiesis and T lymphopoiesis, as demonstrated in a mTurquoise2-reporter mouse model, suggesting its fine-tuning function is irreplaceable by Axin1.Citation6 Overall, Axin1 may appear genetically redundant with Axin2 in the Wnt/β-catenin pathway, as they can act rather dissimilarly on proteostatic and transcriptional levels, and their effects can also be highly tissue-specific.Citation6 For instance, Axin2 knockout mice show dental and cranial agenesis, whereas Axin1 knockout has proved to be lethal.Citation7–9 Previous studies in vitro have demonstrated that Axin2 is not involved in inhibiting intestinal cell bacterial invasion and inflammationCitation10. It is unknown how Axin2 may contribute to intestinal homeostasis, microbiome, and inflammation in vivo.
Axin1 might be more than a mere gatekeeper in the Wnt/β-catenin pathway.Citation11 A study utilizing RNA-seq has unveiled that Axin1 is linked to IFN-γ/STAT1/Th1 immune program through an unidentified mechanism; moreover, CRC patients exhibiting an Axin1-dependent gene expression signature tend to have a more favorable prognosis.Citation4 Guo et al. propose that Axin1 enhances the antiviral response to influenza by activating the JNK/c-Jun and Smad3 pathways, reducing virus replication, and increasing interferon (IFN) production. Overexpressing Axin1 or using the Axin1 stabilizer XAV939 has been shown to decrease influenza virus replication in lung epithelial cells.Citation12 Our previous study has demonstrated that pathogenic Salmonella manipulates Axin1 signaling for its infection and invasion in vitroCitation13. In our current paperCitation3, we found that Axin1 knockout alleviates DSS-induced colitis via alteration of intestinal environment and microbiota. These findings imply that Axin1 could play multiple roles in different signaling pathways, such as pro-inflammation and anti-tumor responses.
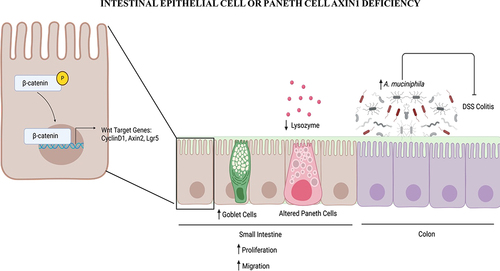
In the Axin1 intestinal epithelial cell (IEC) knockout Axin1ΔIEC mice, we found increased intestinal epithelial Axin2, the Wnt target gene, at the mRNA level. The absence of intestinal epithelial Axin1 altered Wnt/β-catenin signaling and enhanced proliferation, which may contribute to protection from DSS injury as well. This cellular protection is likely to be microbiome-dependent because co-housing Axin1ΔIEC and Axin1LoxP mice reduced A. muciniphila in Axin1ΔIEC mice and made them more susceptible to colitis. Moreover, A. muciniphila treatment protected against DSS-induced colitis in Axin1LoxP mice. Thus, intestinal epithelial Axin1 contributes to inflammation by regulating the microbiome, not just through the Wnt/β-catenin pathway.
New findings of Axin1 in inflammatory bowel disease (IBD)
There is limited study on Axin1 links to human IBD. Our paper reported increased Axin1 expression at both the mRNA and protein levels in human IBD, including ulcerative colitis (UC), and Crohn’s disease (CD). Specifically, we identified increased Axin1 expression positively correlated with pro-inflammatory cytokines IL-6 and TNF-α in human CD. Dysfunction of Wnt/β-catenin was reported in human IBD and Axin1 serum level was elevated in patients with UCCitation14. However, the focus of previous studies is on the host impacts mediated through the Wnt/β-catenin pathway. For the first time, our study identifies links between the gut microbiota and intestinal Axin1 in intestinal inflammation through tissue-specific deletion of Axin1 in intestinal epithelium and Paneth cells. Loss of intestinal Axin1 plays a novel role in intestinal inflammation by altering Paneth cells and microbiome (e.g., enriched A. muciniphila). Investigation of the interactions between microbiota and Axin1 will provide novel mechanistic strategies for therapeutic approaches for human IBD.
Axin1 deletion leads to altered Paneth cells
Loss of intestinal epithelial or Paneth cell (PC) Axin1 results in increased Wnt/β-catenin signaling, proliferation, and cell migration. This is associated with altered goblet and Paneth cell morphology with increased MUC2 and decreased lysozyme, respectively. PCs can regulate innate immunity by sensing the microbiota and adjusting their production of antimicrobial peptides, like lysozymeCitation15,Citation16. Axin1 is a known regulator of Wnt/β-catenin signaling which promotes the differentiation and maturation of PCs, thereby regulating antimicrobial peptide production. Specifically, mice lacking the Wnt/β-catenin signaling regulator TCF-1 demonstrated a decrease in lysozymeCitation17. In addition, mice lacking PC lysozyme have increased goblet cells and Muc2 expressionCitation18. Axin1 may regulate lysozyme expression in a Wnt/β-catenin dependent fashion. Perturbations of PC lysozyme function in the lyz1−/− mice increase mucolytic bacteria, e.g., Ruminococcus gnavus, alter the gut community landscape, and protect against DSS-induced colitisCitation18. In addition, increased lysozyme has been observed in patients with UCCitation19,Citation20. We have demonstrated that PC Axin1 maintains intestinal and microbial homeostasis, which may be the driving factor in protection against colitis. Axin1 deficiency leads to alterations in the gut microbiome (e.g., enriched A. muciniphila) due to decreased lysozyme processing of the gut microbiota and a potential anti-colitogenic type 2 immune response as seen in Lyz1−/− miceCitation18. Further ex vivo and in vitro studies may narrow down the cellular role of Axin1 in PC function.
The tissue-specific role of Axin1 in immunity is still unknown. A recent study reported that Axin1 stimulates intestinal inflammation via an interferon-gamma/Th1 program that prevents tumor growthCitation4. Our paper has demonstrated that Axin1 and the microbiome are promising targets for colitis. Axin1 status in the epithelium is critical for PC function, but how Axin1 regulates the immune system in IBD is not fully understood. It has been shown that mice with deficiency in LRP5/6 in CD11c+ cells, that have a stabilized form of β-catenin, decreased DSS-induced colitis severity, and reduced inflammatory cytokinesCitation21. Many other studies support the anti-inflammatory role of β-catenin in other mouse models. Specifically, Wnt signaling in dendritic cells can regulate intestinal immunity by promoting T-cell responses and secretion of cytokines to a tolerogenic stateCitation22. It is unknown whether Axin1 plays a similar role in CD11 myeloid cells in the context of colitis. Besides Paneth and intestinal epithelial cells, further study is required to determine whether myeloid Axin1 plays a role in intestinal homeostasis and inflammation. Additional in vivo studies would also determine an overall immune profile in Axin1-deficient mice. Furthermore, we have developed a myeloid cell Axin1 knockout mouse model. Explorations in myeloid Axin1 may elucidate its mechanistic role in modulating- gut microbiota.
Axin1 regulation of mucus barrier and Akkermansia muciniphila
Axin1-specific alterations in intestinal mucosa and thinning of the mucus barrier lead to enriched A. muciniphila and goblet cells. The presence of A. muciniphila in Axin1 knockout mice is the driver of protection against DSS-induced inflammation. In addition, the loss of Muribaculaceae during co-housing is another contributing factor to DSS colitis susceptibility.
We found that Axin1ΔIEC mice are protected from DSS-induced inflammation with increased MUC2. We found increased MUC2 and goblet cells in Axin1ΔIEC mice. Axin1 knockout in IECs did not significantly change intestinal permeability (data not shown). Moreover, A. muciniphila was enhanced in Axin1ΔIEC mice. MUC2 likely provides a favorable environment to stimulate the abundance of A. muciniphila. Thus, increased MUC2 and A. muciniphila collectively may have granted the Axin1ΔIEC mice increased colitis protection compared with controls (). It has been widely reported that A. muciniphila and Muribaculaceae are underrepresented in metabolic and neurodegenerative disorders and intestinal inflammationCitation23–26. There are trends to explore these strains as new probiotics for disease prevention and treatment.
Future directions
Garrett et al. demonstrate a novel and critical role of Axin1 in regulating intestinal epithelial development and microbial homeostasisCitation3. Our study has provided insights into the molecular mechanism that might contribute to IBD. There are no human studies assessing the role of intestinal epithelial and Paneth cell Axin1 in inflammation and microbiome. It is unknown how Axin1 may directly regulate PC function to protect against DSS injury. Increased Wnt/β-catenin signaling and intestinal proliferation may be a contributing factor, and further investigations in the colon may elucidate this mechanism. Moreover, dissecting the colonic mechanisms of Axin1 may elucidate its role in intestinal homeostasis and colorectal cancerCitation4,Citation27,Citation28. Microbiome and spatially resolved metabolomics analysis reveal the anticancer role of intestinal A. muciniphila by crosstalk with intratumoral microbiota and reprogramming tumoral metabolism in a lung cancer modelCitation29. Explorations in the role of myeloid Axin1 can potentially expound the tissue-specific function of Axin1 in auto-immune diseases. Overall, further investigation of the microbiota-Axin1 interactions will provide novel insights into the development of chronic diseases, e.g., IBD and colon cancer, and mechanistic strategies for therapeutic approaches.
Loss of intestinal epithelial or Paneth cell Axin1 results in increased Wnt/β-catenin signaling, proliferation, and cell migration. This is associated with altered goblet and Paneth cell morphology with increased MUC2 and decreased lysozyme, respectively. Axin1-specific deletion in the intestinal mucosa leads to an altered gut microbiome (e.g., enriched A. muciniphila) and thinning of the mucus barrier. The presence of A. muciniphila in Axin1 knockout mice is the driver of protection against inflammation.
Author contributions
Shari Garrett and Jun Sun prepared the figures and the draft text; Monica Asada contributed to the section on Axin1 and Axin2 and the draft text; and Jun Sun obtained funds, designed the study, and directed the project. All authors contributed to the writing of the manuscript.
Compliance with ethics guidelines
Shari Garrett, Monica C. Asada, and Jun Sun declare that they have no conflict of interest.
Acknowledgments
We would like to acknowledge the VA Merit Award (1 I01BX004824-01), Crohn’s & Colitis Foundation Senior Research Award (Award Number: 902766) and the National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health grants (R01 DK134343, DK105118, DK114126) to Jun Sun. Working model was created using BioRender. R01DK114126 supplement is to promote Diversity in Health-Related Research for PHD student Shari Garrett. The study sponsors play no role in the study design, data collection, analysis, and interpretation of data. The contents do not represent the views of the United States Department of Veterans Affairs or the United States Government.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Li Vivian SW, Ng Ser S, Boersema Paul J, Low Teck Y, Karthaus Wouter R, Gerlach Jan P, Mohammed S, Heck AR, Maurice M, Mahmoudi T, et al. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245–1773. doi:10.1016/j.cell.2012.05.002.
- Qiao Y, Wang J, Karagoz E, Liang B, Song X, Shang R, Evert K, Xu M, Che L, Evert M, et al. Axis inhibition protein 1 (Axin1) deletion–induced hepatocarcinogenesis requires intact β‐catenin but not notch cascade in mice. Hepatology. 2019;70(6):2003–2017. doi:10.1002/hep.30556.
- Garrett S, Zhang Y, Xia Y, Sun J. Intestinal epithelial Axin1 deficiency protects against colitis via altered gut microbiota. Engineering. 2023. doi:10.1016/j.eng.2023.06.007.
- Sanson R, Lazzara SL, Cune D, Pitasi CL, Trentesaux C, Fraudeau M, Letourneur F, Saintpierre B, Le Gall M, Bossard P, et al. Axin1 protects colon carcinogenesis by an immune-mediated effect. Cell Mol Gastroenterol Hepatol. 2023;15:689–715. doi:10.1016/j.jcmgh.2022.10.017.
- Thorvaldsen TE, Pedersen NM, Wenzel EM, Stenmark H, Katoh M. Differential roles of AXIN1 and AXIN2 in tankyrase inhibitor-induced formation of degradasomes and β-catenin degradation. PLoS One. 2017;12(1):e0170508. doi:10.1371/journal.pone.0170508.
- JJDd R, Chhatta A, Garcia-Perez L, Naber BAE, Vloemans SA, Salvatori DCF, Pike-Overzet K, Mikkers H, Staal FJT. Axin2/Conductin is required for normal haematopoiesis and T Lymphopoiesis. Cells. 2022;11:2679. doi:10.3390/cells11172679.
- Moshkovsky AR, Kirschner MW. The nonredundant nature of the Axin2 regulatory network in the canonical Wnt signaling pathway. Proc Natl Acad Sci. 2022;119:e2108408119. doi:10.1073/pnas.2108408119.
- Wang W, Liu P, Lavrijsen M, Li S, Zhang R, Li S, van de Geer WS, van de Werken HJG, Peppelenbosch MP, Smits R, et al. Evaluation of AXIN1 and AXIN2 as targets of tankyrase inhibition in hepatocellular carcinoma cell lines. Sci Rep. 2021;11(1):7470. doi:10.1038/s41598-021-87091-4.
- Chia IV, Costantini F. Mouse axin and Axin2/Conductin proteins are functionally equivalent in vivo. Mol Cell Biol. 2005;25:4371–4376. doi:10.1128/MCB.25.11.4371-4376.2005.
- Zhang Y-G, Wu S, Xia Y, Chen D, Petrof EO, Claud EC, Hsu W, Sun J. Axin1 Prevents Salmonella Invasiveness and Inflammatory Response in Intestinal Epithelial Cells. PLoS One. 2012;7:e34942. doi:10.1371/journal.pone.0034942.
- Mazzoni SM, Fearon ER. AXIN1 and AXIN2 variants in gastrointestinal cancers. Cancer Lett. 2014;355(1):1–8. doi:10.1016/j.canlet.2014.09.018.
- Guo Y, Bamunuarachchi G, Vaddadi K, Zhu Z, Gandikota C, Ahmed K, Pushparaj S, More S, Xiao X, Yang X, et al. Axin1: a novel scaffold protein joins the antiviral network of interferon. Mol Microbiol. 2022;118(6):731–743. doi:10.1111/mmi.14995.
- Zhang YG, Wu S, Xia Y, Chen D, Petrof EO, Claud EC, Hsu W, Sun J. Axin1 prevents Salmonella invasiveness and inflammatory response in intestinal epithelial cells. PLoS One. 2012;7:e34942. doi:10.1371/journal.pone.0034942.
- Moraes L, Magnusson MK, Mavroudis G, Polster A, Jonefjäll B, Törnblom H, Sundin J, Simrén M, Strid H, Öhman L, et al. Systemic inflammatory protein profiles distinguish irritable bowel syndrome (IBS) and ulcerative colitis, irrespective of inflammation or IBS-Like symptoms. Inflamm Bowel Dis. 2020;26(6):874–884. doi:10.1093/ibd/izz322.
- Riba A, Olier M, Lacroix-Lamandé S, Lencina C, Bacquié V, Harkat C, Gillet M, Baron M, Sommer C, Mallet V, et al. Paneth cell defects induce microbiota dysbiosis in mice and promote visceral hypersensitivity. Gastroenterology. 2017;153(6):1594–606.e2. doi:10.1053/j.gastro.2017.08.044.
- Salzman NH. Paneth cell defensins and the regulation of the microbiome: détente at mucosal surfaces. Gut Microbes. 2010;1(6):401–406. doi:10.4161/gmic.1.6.14076.
- Beisner J, Teltschik Z, Ostaff MJ, Tiemessen MM, Staal FJT, Wang G, Gersemann, M, Perminow, G, Vatn, MH, Schwab, M, et al. TCF-1-mediated Wnt signaling regulates Paneth cell innate immune defense effectors HD-5 and -6: implications for Crohn’s disease. Ame J Physiol-Gastrointest and Liver Physiol. 2014;307(5):G487–G498. doi:10.1152/ajpgi.00347.2013.
- Yu S, Balasubramanian I, Laubitz D, Tong K, Bandyopadhyay S, Lin X, Flores J, Singh R, Liu Y, Macazana C, et al. Paneth cell-derived lysozyme defines the composition of mucolytic microbiota and the inflammatory tone of the intestine. Immunity. 2020;53(2):398–416.e8. doi:10.1016/j.immuni.2020.07.010.
- Meyer K, Gellhorn A, Prudden JF, Lehman WL, Steinberg A. Lysozyme in chronic ulcerative colitis. Proceedings of the Society for Experimental Biology and Medicine. 1947;65(2):221–222.
- Di Ruscio M, Vernia F, Ciccone A, Frieri G, Latella G. Surrogate fecal biomarkers in inflammatory bowel disease: rivals or complementary tools of fecal calprotectin? Inflamm Bowel Dis. 2018;24(1):78–92. doi:10.1093/ibd/izx011.
- Swafford D, Shanmugam A, Ranganathan P, Hussein MS, Koni PA, Prasad PD, Thangaraju M, Manicassamy S. Canonical Wnt signaling in CD11c+ APCs regulates microbiota-induced inflammation and immune cell homeostasis in the colon. J Immunol. 2018;200:3259–3268. doi:10.4049/jimmunol.1701086.
- Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Y-C W, Pulendran B. Activation of β-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Sci. 2010;329:849–853. doi:10.1126/science.1188510.
- Crovesy L, Masterson D, Rosado EL. Profile of the gut microbiota of adults with obesity: a systematic review. Eur J Clin Nutr. 2020;74(9):1251–1262. doi:10.1038/s41430-020-0607-6.
- Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, Chen Y, Ji L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One. 2013;8:e71108. doi:10.1371/journal.pone.0071108.
- Chang C-S, Liao Y-C, Huang C-T, Lin C-M, Cheung CHY, Ruan J-W, Yu W-H, Tsai Y-T, Lin I-J, Huang C-H, et al. Identification of a gut microbiota member that ameliorates DSS-induced colitis in intestinal barrier enhanced Dusp6-deficient mice. Cell Rep. 2021;37(8):110016. doi:10.1016/j.celrep.2021.110016.
- Imhann F, Vich Vila A, Bonder MJ, Fu J, Gevers D, Visschedijk MC, Spekhorst LM, Alberts R, Franke L, van Dullemen HM, et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut. 2018;67(1):108–119. doi:10.1136/gutjnl-2016-312135.
- Jackson DN, Alula KM, Delgado-Deida Y, Tabti R, Turner K, Wang X, Venuprasad K, Souza RF, Désaubry L, Theiss AL, et al. The synthetic small molecule FL3 combats intestinal tumorigenesis via Axin1-mediated inhibition of Wnt/β-catenin signaling. Cancer Res. 2020;80:3519–3529. doi:10.1158/0008-5472.CAN-20-0216.
- Alula KM, Delgado-Deida Y, Jackson DN, Venuprasad K, Theiss AL. Nuclear partitioning of prohibitin 1 inhibits Wnt/beta-catenin-dependent intestinal tumorigenesis. Oncogene. 2021;40:369–383. doi:10.1038/s41388-020-01538-y.
- Zhu Z, Cai J, Hou W, Xu K, Wu X, Song Y, Bai C, Mo Y-Y, Zhang Z. Microbiome and spatially resolved metabolomics analysis reveal the anticancer role of gut Akkermansia muciniphila by crosstalk with intratumoral microbiota and reprogramming tumoral metabolism in mice. Gut Microbes. 2023;15:2166700. doi:10.1080/19490976.2023.2166700.