ABSTRACT
Emerging evidence has revealed the novel role of gut microbiota in the development of cancer. The characteristics of function and composition in the gut microbiota of patients with breast cancer patients has been reported, however the detailed causation between gut microbiota and breast cancer remains uncertain. In the present study, 16S rRNA sequencing revealed that Prevotella, particularly the dominant species Prevotella copri, is significantly enriched and prevalent in gut microbiota of breast cancer patients. Prior-oral administration of P. copri could promote breast cancer growth in specific pathogen-free mice and germ-free mice, accompanied with sharp reduction of indole-3-pyruvic acid (IPyA). Mechanistically, the present of excessive P. copri consumed a large amount of tryptophan (Trp), thus hampering the physiological accumulation of IPyA in the host. Our results revealed that IPyA is an intrinsic anti-cancer reagent in the host at physiological level. Briefly, IPyA directly suppressed the transcription of UHRF1, following by the declined UHRF1 and PP2A C in nucleus, thus inhibiting the phosphorylation of AMPK, which is just opposite to the cancer promoting effect of P. copri. Therefore, the exhaustion of IPyA by excessive P. copri strengthens the UHRF1-mediated negative control to inactivated the energy-controlling AMPK signaling pathway to promote tumor growth, which was indicated by the alternation in pattern of protein expression and DNA methylation. Our findings, for the first time, highlighted P. copri as a risk factor for the progression of breast cancer.
1. Background
Breast cancer is the leading cause of cancer-related mortality in females with top new incidence in 2020.Citation1 The rise in the incidence of breast cancer among younger individuals, particularly those in regions with high human development index, is increasingly alarming.Citation1 In addition to the germline mutations in breast cancer susceptibility gene 1 (BRCA1) and BRCA2, the development of breast cancer could also be attributed to other non-hereditary factors, including pregnancy-associated factors, hormonal therapy, lifestyle factors.Citation2–4 Currently, surgery, radiotherapy and pharmacotherapy are the main options for the treatments of breast cancer. Drug regimens include chemotherapy, endocrine therapy targeted therapy, etc, depending on the multiple molecular classification. Nevertheless, some kinds of breast cancer, in particular, the triple-negative breast cancer (TNBC), remains a worrying public health problem due to metastasis, recrudescence, resistance and the unavailability of endocrine therapy or targeted therapy options. Therefore, strategies for the promotion of health lifestyle and early detection have been strongly initiated by the World Health Organization Global Breast Cancer Initiative, to reduce the incidence and malignant progression by prompt intervention.
Emerging evidence has shown that the commensal microbiome, especially gut microbiota and its versatile effects on the host, has unexpected association with cancer. It has been revealed that commensal microbiota dysbiosis is closely related to the progression of both local (e.g. colon cancer,Citation5 liver cancer,Citation6 and pancreatic cancer)Citation7 and non-local (e.g. breast cancerCitation8 and pancreatic cancerCitation9 cancers. It is a breakthrough finding with profound implications that gut microbiota plays a crucial role in the response to immunotherapy,Citation10 chemotherapy,Citation11 radiotherapy,Citation12 and targeted therapy.Citation13 In breast cancer, characteristic of function and composition has been found in the commensal microbiota that exists in breast tissueCitation14,Citation15 and gut.Citation8,Citation16 A latest study revealed that trimethylamine N-oxide (TMAO) derived from microbiota of the local breast tissue is capable of promoting antitumor immunity in TNBC.Citation17 Hence, commensal microbiota has been regarded as a promising target for the diagnosis and regime of cancer. Nonetheless, the underlying mechanism remains to be explored.
Prevotella is a diverse genus of Gram-negative anaerobe. De Filippo et al. and Yatsunenko et al. reported that, Prevotella spp. tend to dominate the gut microbiome in “non-westernized” populations who follow a more pre-industrial, “traditional” lifestyle and diet.Citation18,Citation19 Prevotella spp. inhabits multiple parts of the body in humans and animals, such as the gut,Citation20 airways,Citation21 and oral cavity.Citation22 Increasing studies revealed that enrichment of the Prevotella genus is related to various diseases, including cystic fibrosis,Citation21 gout,Citation23 obesity,Citation24 and rheumatoid arthritis.Citation25 Prevotella copri is the dominant species of the Prevotella genus in gut microbiota.Citation26 Tett et al. found four distinct clades (A, B, C, and D) that constitute the P. copri complex.Citation27 Indeed, clade A was ubiquitously found among both the Westernized and non-Westernized populations, while clades B, C, and D were predominantly found in non-Westernized populations. Hence despite the prevalence in gut microbiota, it remains unclear whether the controversial role of P. copri in human health would be due to the discrepancies of these four clades.
In the present study, we found that Prevotella in gut microbiota, especially the dominant species P. copri, was enriched in patients with breast cancer, which is accordant with the report by Zhu and colleagues.Citation8 The aim of the present study was to explore the causality and mechanism underlying the promotive effect of P. copri on the progression of breast cancer.
2. Results
2.1. Increase of Prevotella is closely related to the development of breast cancer
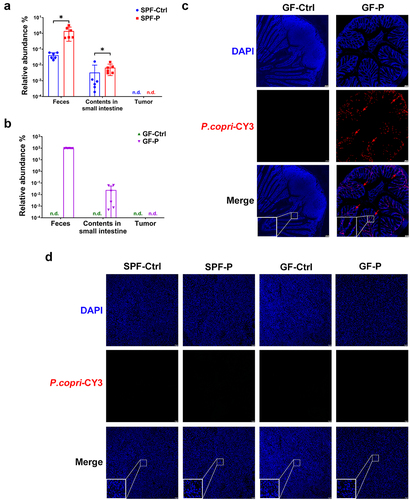
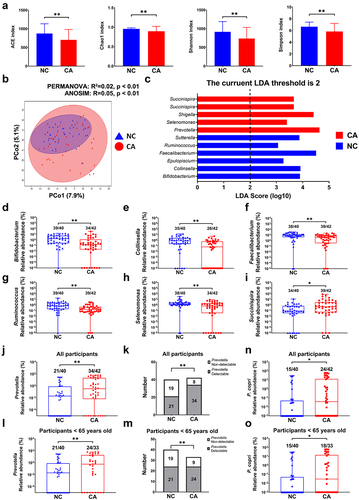
Several studies have revealed characteristics of the gut microbiota in patients with breast cancer (CA).Citation8,Citation28 In the present study, we found that alpha diversity of CA was evidently lower than that of the patients with benign breast disease (NC), as indicated by the decreased Chao1, Abundance-based coverage estimators (ACE), Shannon, and Simpson metrics (). Principal coordinates analysis (PCoA) showed that beta diversity of CA was remarkably different from that of NC, which was confirmed by permutational multivariate analysis of variance (PERMANOVA; R2 = 0.02, p < .01) and analysis of similarities (ANOSIM; R = 0.05, p < .01) (). Linear discriminant analysis with effect size (LEfSe) was applied to investigate the microorganisms contributing to this difference. It was found that several genera were enriched in CA, while some others were decreased (, Sheet S1). In brief, relative abundances of Bifidobacterium, Collinsella, Faecalibacterium, Ruminococcus, and Selenomonas were remarkably lower (), whereas those of Succinispira and Prevotella were significantly higher in CA (). In addition to the higher relative abundance, the prevalence of Prevotella was higher in CA versus NC (34/42 vs. 21/40, respectively, ). As showed in Table S1, the median age of CA (median: 53 years, lower quartile: 48 years, upper quartile: 63 years) was significantly higher than that of NC (median: 44 years, lower quartile: 38 years, upper quartile: 48 years), and all subjects in NC group was lower than 65 years. Even so, relative abundance and prevalence of P. copri in CA lower than 65 years (24/33) were higher than those of their NC counterparts (21/40) (), suggesting that age differences are not responsible for the higher prevalence and abundance of Prevotella in breast cancer patients. Moreover, P. copri was the dominant species of the Prevotella genus in all the participants (Sheet S1) in accordance with previous reports,Citation26,Citation29 and P. copri was enriched more in the gut microbiota of CA (). Interestingly, it was found that Prevotella was also enriched in breast cancer-bearing mice compared with their healthy counterparts in our previous animal studies.Citation30,Citation31 These findings suggest that the increase of Prevotella, particularly P. copri, was closely associated with the development of breast cancer.
Figure 1. Gut microbiota characteristics of breast cancer patients. (a) Alpha diversity metrics. (b) Beta diversity showed by PCoA, and the statistic difference was analyzed by PERMANOVA and ANOSIM. (c) Enriched genera analysis by LEfSe. (d~i) relative abundance of Bifidobacterium, Collinsella, Faecalibacterium, Ruminococcus, Selenomonas and Succinispira genera. (j, k) relative abundance and prevalence of prevotella genus in all participants. (l, m) relative abundance and prevalence of prevotella genus in participants lower than 65 years old. (n, o) relative abundance of P. copri in all participants and that in participants lower than 65 years old. Numbers on top of each column indicated the ratio of prevotella-detectable or P. copri-detectable participants to the total participants. CA: breast cancer patients, n = 42; NC: control participant, n = 40. *, represents p < .05; **, represents p < .01.
2.2. P. copri promotes the development of breast cancer in the 4T1 mice model
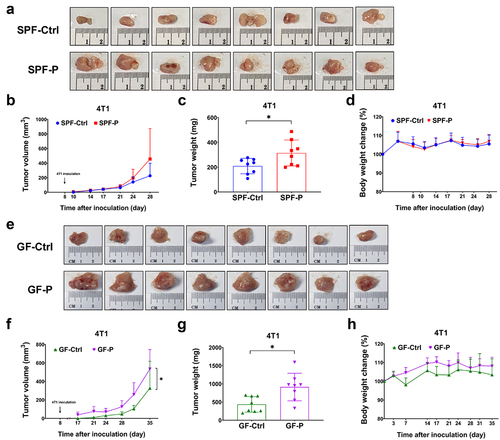
4T1 murine model of breast cancer was utilized to explore the potential role of P. copri in the progression of breast cancer. P. copri (2.5 × 109 cfu/mouse, once weekly) was orally administrated to SPF-P (for 4 weeks) and GF-P (for 5 weeks) groups prior to the inoculation of 4T1 cell. It was found that although the tumor volume of SPF-P group was not different from that of SPF-Ctrl group after the four or five-week treatment (), 4T1 tumors grew at a markedly higher rapid rate in GF mice treated with P. copri (GF-P group) than those in the non-treated GF mice (GF-Ctrl group, ). Finally, tumor was significantly heavier in SPF-P and GF-P groups than their counterparts (), but the administration of P. copri did not affect the body weight (). Moreover, relative abundance of P. copri in feces and content of small intestine of SPF-P group was evidently higher than that in SPF-Ctrl groups (). In GF mice, P. copri dominated in feces microbiome of GF-P group (97 ± 2%), while few P. copri was found in the contents of small intestine (). The colonization of P. copri in the gut of GF-P group was confirmed by fluorescent in situ hybridization (FISH, ). Nevertheless, P. copri could not be detected in tumor of any of the groups by RT-qPCR or FISH (). Taken together, these results suggest that enrichment of P. copri plays critical role in the progression of breast cancer.
Figure 2. P. copri promotes 4T1 tumor growth in SPF and GF mice. (a~d) tumor pictures, tumor volumes change, tumor weight, body weight change. (e~h) tumor pictures, tumor volumes change, tumor weight, body weight change. SPF-Ctrl: control group under SPF; SPF-P: P. copri-treated group under SPF; GF-Ctrl: control group under GF; GF-P: P. copri-treated group under GF. n = 8. *, represents p < .05; **, represents p < .01.
2.3. AMP-activated protein kinase (AMPK) signaling pathway is inactivated by ubiquitin like with PHD and ring finger domains 1 (UHRF1)-regulated DNA methylation in the promotion of breast cancer by P. copri
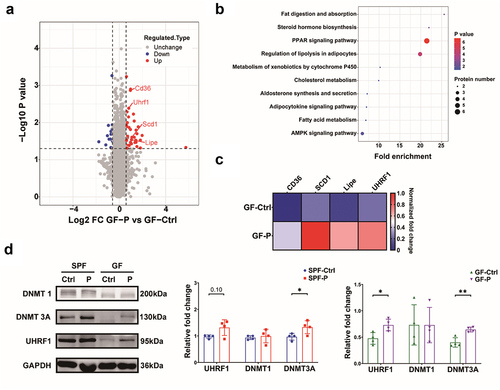
4D-label free protein omics was employed to explore the mechanism through which P. copri affects tumor growth (Sheet S2). A total of 51 differentially-expressed proteins (DEPs), including 38 significantly up-regulated proteins and 13 down-regulated proteins, were found in the GF-P group (). They are involved in several signaling pathways based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment. Among these DEPs, the upregulation of transcription protein UHRF1 is noteworthy because of its critical role in epigenetic control.Citation32 Verification by western blotting (WB) showed that the expression of UHRF1 in tumors was evidently higher in GF-P group versus GF-Ctrl groups; a tendency toward upregulation was observed in SPF-P group (). Moreover, the DNA methylation transferase 3A (DNMT 3A, a critical initiator of DNA methylation) was increased in tumors of P. copri-treated mice (SPF-P and GF-P groups), while the level of methylation maintainer DNMT1 was not affected ().
Figure 4. P. copri regulates protein profile of the 4T1 tumor. (a) Differential-expressed proteins (DEPs) showed by volcano plot. (b) Signaling pathway enrichment for DEPs based on KEGG. (c) DEPs involved in AMPK signaling pathway showed by heatmap. (d) Verification by WB for DNMT1, DNMT 3A, and UHRF1. SPF-Ctrl: control group under SPF; SPF-P: P. copri-treated group under SPF; GF-Ctrl: control group under GF; GF-P: P. copri-treated group under GF. For 4D-lable free protein omics, n = 3; for WB, n = 4. *, represents p < .05; **, represents p < .01.
Since UHRF1 plays a key role in epigenetics regulation, especially in DNA methylation,Citation32 we employed reduced representation bisulfite sequencing (RRBS) to explore the changes in DNA methylome. Results showed that although level of methylation in GF-P group was not evidently different from that in GF-Ctrl group (), the overall methylation profile of GF-P group was different from that of GF-Ctrl group (). Briefly, 2,675 and 2,651 differential methylation regions (DMRs) were hypermethylated and hypomethylated, respectively, in the GF-P group (, Sheet S3). Moreover, pathway enrichment based on KEGG showed that AMPK signaling pathway was one of the critical signaling pathways, in which the genes with promoters that existed differential-methylated loci was involved (). RT-qPCR verifications confirmed that the mRNA of CF transmembrane conductance regulator (CFTR), 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3), peroxisome proliferator activated receptor gamma (PPARG), stearoyl-CoA desaturase 1 (SCD1), and leptin receptor (LEPR) in AMPK signaling pathway were differentially expressed in tumor of GF-P group compared with those of GF-Ctrl group. Particularly, CFTR was up-regulated at mRNA level with a hypomethylated DMR in promoter region, while PFKFB3 was down-regulated with hypermethylated DMR. In addition, PPARG and LEPR were markedly affected by P. copri treatment in SPF-P group compared with those in SPF-Ctrl group, which was similar to those observed in GF mice ().
Figure 5. P. copri alters DNA methylation profile of the 4T1 tumor. (a) DNA methylation level in various genome function regions. (b) Numbers of the hypermethylated and hypomethylated DMRs. (c) Signaling pathway enrichment for genes with promoters that existed differential-methylated regions (DMRs) based on KEGG. (d) Genes with DMRs involved in AMPK signaling pathway showed by heatmap. (e) Verification by qPCR for the genes with differential methylated promoters. (f) Analysis for activity of AMPK, mTOR and ULK1. SPF-Ctrl: control group under SPF; SPF-P: P. copri-treated group under SPF; GF-Ctrl: control group under GF; GF-P: P. copri-treated group under GF. For RRBS, n = 3; for qPCR, n = 6 ~ 8; for WB, n = 4. *, represents p < .05; **, represents p < .01.
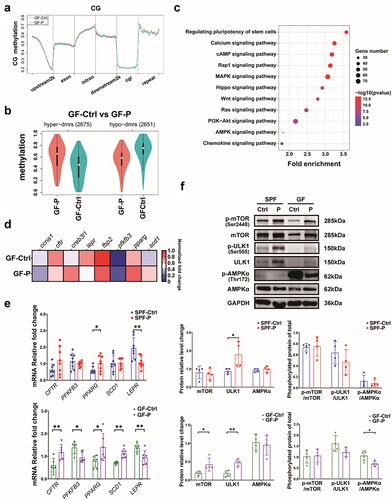
Interestingly, the metabolism and energy-controlling AMPK (AMP-activated protein kinase) signaling pathway was the only common pathway that was enriched both in proteome and DNA methylome (), suggesting that AMPK pathway plays an important role in the tumor promotion of P. copri. AMPK is a key sensor for metabolism and energy, and its impact on the downstream includes to dephosphorylate mammary target of rapamycin (mTOR),Citation33 to phosphorylate unc-51-like kinase 1 (ULK1),Citation34 etc. Therefore, we analyzed the impact of treatment with P. copri on AMPK, as well as those on its downstream mTOR and ULK1. Results by WB showed that the expression of total AMPK was not affected by P. copri, whereas the total ULK1 were significantly up-regulated in tumors of both the SPF-P group and GF-P group, although an evident upregulation of total mTOR were only found in GF mice. Nonetheless, AMPK phosphorylation was evidently suppressed upon treatment with P. copri under GF condition (). In contrast, P. copri did not affect the phosphorylation of mTOR or ULK1. Taken together, both proteome and DNA methylome indicated that the critical metabolism and energy-controlling AMPK signaling pathway was inactivated by UHRF1-regulated DNA methylation, which remarkably contributed to the promotion of cancer by P. copri.
2.4. P. copri exhausts host’s indole-3-pyruvic acid (IPyA) that suppresses tumor growth
It is of significance to explore the mechanism underlying the promotion of cancer by P. copri. Since it was found that secretion of P. copri did not exert an evident effect on cell proliferation (Figure S1), we hypothesized that P. copri influence cell proliferation by modulating the metabolism profile of the host. Approximately 200 metabolites were quantified in the serum by targeted metabonomic with ultra-high performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS analysis, Sheet S4). Results showed that the relative abundance of various metabolite classes in P. copri-treated groups (SPF-P and GF-P groups) were significantly different from those recorded in non-treated groups (SPF-Ctrl and GF-Ctrl groups) (Figure S2a, b). Univariate analysis revealed that 10 and 30 metabolites were increased and decreased, respectively, in SPF-P mice (univariate statistics p < .05, Figure S2c), while in GF-P mice, 27 and 20 metabolites were increased and decreased, respectively (Figure S2d). In multivariate analysis, 46 of the differential metabolites (univariate statistics p < .05 and Orthogonal Partial Least Squares-Discriminant Analysis [OPLS-DA] value > 1) were identified as potential biomarkers in the comparison between SPF-Ctrl and SPF-P groups, while there were 39 potential biomarkers in comparison between GF-Ctrl and GF-P groups (, Figure S2e, f). The intersection of univariate and multivariate analysis revealed that P. copri treatment decreased and increased 29 and 10 metabolites (a total of 39), respectively, in SPF tumor-bearing mice; while in the GF counterparts, it decreased and increased 18 and 26 metabolites (a total of 44), respectively (). Moreover, among the 13 common differential metabolites (Venn diagram in ), the level of fumaric acid, oxoglutaric acid, oxoadipic acid, and IPyA were significantly declined in both SPF-P and GF-P groups compared with their counterparts (Figure S2g-i, ). Notably, the content of IPyA was highest among the four metabolites (up to approximately 2 mM in SPF-Ctrl and GF-Ctrl groups), while it was dramatically reduced by P. copri treatment (). It was decreased from 1.7 ± 0.4 mM (SPF-Ctrl group) to 1.3 ± 0.2 mM (SPF-P group) in SPF mice, and, from 2.7 ± 0.4 mM (GF-Ctrl group) to 0.069 ± 0.066 mM (GF-P group) in GF mice. There are three more detected indoles, including indoleacetic acid (IAA), indole-3-propionic acid (also named indole-3-propionate, IPA), and indolelactic acid (ILA). IPA and ILA were significantly declined in SPF-P mice, but they were not affected in GF mice. However, IAA were increased in GF-P mice (). Moreover, the contents of tryptophan (Trp), from which IPyA is mainly derived, were also significantly decreased in P. copri-treated SPF mice, although no evident decrease was not found in GF mice ().
Figure 6. P. copri modulates host’s metabolites profile and exhausted the intrinsic anti-cancer IPyA. (A) Differential metabolites showed by heatmap in comparison between SPF-Ctrl and SPF-P, and that between GF-Ctrl and GF-P, n = 6. (B) Common differential metabolites showed by Venn diagram. (C) Contents of Indoleacetic acid (IAA), indolelactic acid (ILA), indole-3-propionic acid (IPA), and indole-3-pyruvic acid (IPyA), and tryptophan (Trp) in serum, n = 6. (D) Contents of Trp and IPyA in PYG medium after P. copri was cultured with Trp for 48 h, n = 3. (E) Tumor pictures, tumor volumes change, tumor weight, and body weight change in SPF mice treated by IPyA, n = 8. *, represents p < .05; **, represents p < .01.
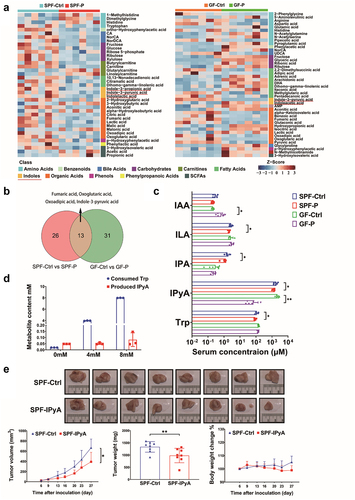
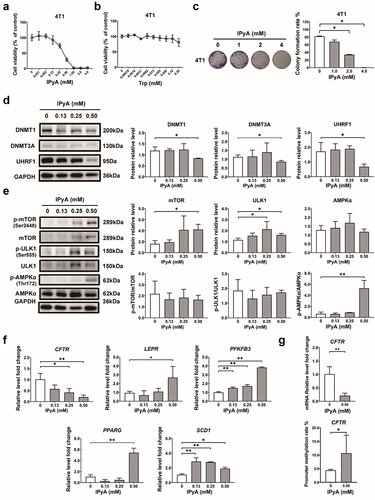
Since both Trp and IPyA were reduced upon P. copri administration, we sought to investigate whether P. copri intervene in the conversion of Trp to IPyA. Tintelnot et al reported that various Bacteroides (Bacteroides fragilis and Bacteroides thetaiotaomicron) metabolize Trp as indole-3-acetic acid based on genomic predictions, whereas P. copri lacks such ability.Citation35 Interestingly, our results showed that Trp was consumed by P. copri almost up to 3.9 ± 0.04 mM and 8.0 ± 0.02 mM, respectively, when it (4 mM and 8 mM) was co-cultured with P. copri for 48 h, while few IPyA (0.051 ± 0.008 mM and 0.082 ± 0.055 mM) was produced in this process (). This indicated that P. copri can consume the Trp in the host with limited production of IPyA. As the promotion of tumor growth by treatment with P. copri was accompanied by a sharp reduction in IPyA, IPyA may be an intrinsic cytotoxic metabolite in the host. As expected, oral administration of IPyA (120 mg/kg) evidently suppressed tumor growth (). Moreover, in vitro results showed that IPyA could inhibit the cell proliferation and colony formation of murine breast cancer cell line 4T1 (), and those of human breast cancer cell lines MCF-7 and MDA-MB-231 (Figure S3a, c, e). IPyA also significantly suppressed cell proliferation of normal colon epithelium cells (FHC, Figure S4a) and cervical cancer cell lines (SiHa and Hela, Figure S4c, e). Nevertheless, it is interesting that IPyA evidently promoted cell proliferation of murine splenocytes (Figure S4g). By contrast, Trp, the precursor of IPyA, did not affected cell viability of the above cells (, Figure S3b, d, and Figure S4b, d, f, h). Collectively, these results indicated that P. copri promote tumor growth mainly relying on the exhaustion of IPyA from Trp, and IPyA is an intrinsic reagent with versatile activity depending on cell types, which allows the other cells in the host to withstand its toxicity.
Figure 7. IPyA is cytotoxic to 4T1 cells. (A, B) cell viability of 4T1 cells treated by IPyA and Trp. (C) Colony formation of 4T1 cells treated by IPyA. (D) Expression of UHRF1, DMMT3A, and DNMT1 in 4T1 cells. (E) Total expression and phosphorylation of mTOR, ULK1, and AMPK in 4T1 cells. (F) mRNA level of CFTR, and PFKFB3, PPARG, SCD1, and LEPR by RT-qPCR. (G) Promoter methylation of CFTR by MS-qPCR. n = 3. *, represents p < .05; **, represents p < .01.
2.5. IPyA activates AMPK via suppressing UHRF1-mediated negative regulation to inhibit cell proliferation
Subsequently, we explore the mechanism underlying the cytotoxicity of IPyA on 4T1 cells. Results demonstrated that when exhibiting cytotoxicity, IPyA down-regulated the expression of UHRF1, DMMT3A, and DNMT1, and promoted the phosphorylation of AMPK (); meanwhile, the expressions of total mTOR and ULK1 were up-regulated without apparent changes in phosphorylation (). Additionally, the cytotoxicity of IPyA was associated with regulation on genes involved in AMPK signaling pathway, including the down-regulated CFTR, and up-regulated PFKFB3, PPARG, SCD1, and LEPR (). Furthermore, the promoter methylation of CFTR was increased by IPyA, which was in accordance with the suppressed mRNA level CFTR (). All these results were just opposite to the effect of P. copri on the alternations in protein profile and DNA methylation in tumor, demonstrating the critical role of IPyA exhaustion in P. copri’s encouragement of tumor.
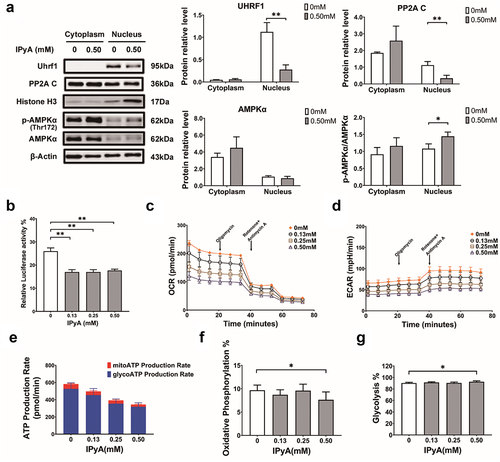
Xu et al found that the nucleus-residing UHRF1 suppresses nuclear AMPK activity by dephosphorylating AMPKα via protein phosphatase 2A (PP2A).Citation36 Due to the cycling between nucleus and cytoplasm via protein trafficking, the nuclear retention of AMPK modulated by UHRF1 contributes to a strong inactivation of AMPK. In the present work, it was revealed that IPyA inhibited the expressions of UHRF1 and PP2A C in nucleus, following by increased phosphorylation of AMPK (). Furthermore, IPyA suppressed the transcription of UHRF1 as indicated by the decreased luciferase activity in 4T1 cells transfected with UHRF1 reporter plasmid (), which is in accordant with the inhibition of UHRF1 protein expression. Additionally, results from adenosine triphosphate (ATP) production analysis were in agreement with the activation of AMPK by IPyA. Kinetic curves for oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) were showed in (. Based on results of OCR and ECAR, it could be calculated that both ATP produced from both mitochondrial oxidative phosphorylation (mitoATP) and glycolysis (glyATP) were substantially reduced by IPyA, resulting in an evident lower production of total ATP ( E). Moreover, mitoATP accounted for a significant lower proportion of the total ATP production, while glyATP took more percentage upon IPyA treatment (), which was a typical consequence of AMPK activation.
Figure 8. IPyA activates AMPK via suppressing the UHRF1-mediated negative regulation. (A) Expression of UHRF1, PP2A C, and AMPK phosphorylation in cytoplasm and nucleus, respectively. (B) The relative luciferase activity in 4T1 cells transfected with UHRF1 reporter plasmid. (C and D) kinetic curves for oxygen consumption rate (OCR) and extracellular acidification rate (ECAR). (E~G) ATP production analysis, including ATP produced from mitochondrial oxidative phosphorylation (mitoATP) and that from glycosis (glyATP). n = 3. *, represents p < .05; **, represents p < .01.
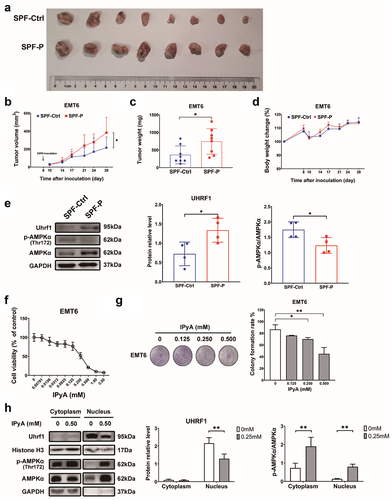
To further confirm the promotion of P. corpi on breast cancer progression, we explored the effect of P. corpi in a breast cancer model induced by another murine breast cancer cell line, EMT6, as well as the impact of IPyA on this cell line. As expected, oral administration of P. copri made the EMT6 tumor grew faster, and the tumor weight of P. copri-treated mice was significantly higher than that of the non-treated mice (). Similar with the findings in 4T1 cell lines, UHRF1 was upregulated in the P. copri-treated mice, while the phosphorylation of AMPK was inhibited (). By contrast, IPyA suppressed the proliferation and colony formation of EMT6 in vitro (). It downregulated the expression of UHRF1 but promoted the phosphorylation of AMPK (). All these results, again, suggested the promotion of P. copri in the development of breast cancer.
Figure 9. P. corpi promotes EMT6 tumor growth, while IPyA is cytotoxic to 4T1 cells. (A~D) tumor pictures, tumor volumes change, tumor weight, body weight change., n = 8. (E) Expression of UHRF1 and AMPK in tumor tissue by WB, n = 4. (F) Cell viability of EMT6 cells treated by IPyA, n = 3. (G) Colony formation of EMT6 cells treated by IPyA, n = 3. (H) Expression of UHRF1, total AMPK and phosphorylated AMPK in EMT6 cells, n = 3. *, represents p < . 05; **, represents p < . 01.
Taken together, the above results illustrated that IPyA activated AMPK by suppressing UHRF1-mediated negative regulation, reprogrammed cellular energy homeostasis, and finally inhibited cell proliferation. These results indicated that the substantially reduction of IPyA would be fundamentally important for the breast cancer promotion by P. copri.
3. Discussion
Mounting evidence has revealed the pivotal role of commensal microbiota, including the tissue-resident microbiota and gut microbiota, in cancer occurrence and progression. Thus far, characteristics of tumor-resident microbiota and gut microbiota has been reported in patients with breast cancer. In breast tumor tissue, the Staphylococcus genus is enriched,Citation14 and contributes to metastatic colonization.Citation15 In terms of gut microbiota, several microbes that had not been previously linked to the progression of cancer, such as Prevotella amnii and Proteus_mirabilis, were enriched in breast cancer patients.Citation8 Although both microbes residing in tumor tissue and gut were potential biomarkers or therapeutic targets, it is the better accessibility that makes gut microbiota attract much more attention for study and application.
In accordance with previous studies, we found that gut microbiota diversities of CA were significantly different from those of the counterparts. Most notably, the Prevotella genus was remarkably enriched and more prevalent in CA. Coincidentally, the Prevotella genus was also enriched in breast cancer-bearing mice.Citation30,Citation31 These findings suggest that the increase of Prevotella was closely related the progression of breast cancer. Prevotella genus is a Gram-negative anaerobe identified in 1990,Citation37 although the type strain Prevotella melaninogenica was firstly isolated in 1921.Citation38 Owing to the advance in cultivation-free high throughput profiling, it has been shown that Prevotella spp. are common and abundant microbial communities in humans, which inhabit in multiple body sites (e.g., gastrointestinal tract, oral cavity, and vagina) and body fluid (e.g., synovial fluid). Among the gut-resident Prevotella spp., P. copri is the most recognized and relevant memberCitation29 due to its high abundance and prevalence whereas controversial role in health. Numerous studies have reported the detrimental effects of P. copri in inflammatory conditions, such as rheumatoid arthritisCitation39,Citation40 and ankylosing spondylitis.Citation41 However, there are not yet any studies about the exact causation of Prevotella spp. in cancer progression. Strikingly, we found that P. copri is significantly enriched in gut microbiota of patients with breast cancer, and it evidently promote tumor growth in SPF mice. These findings were confirmed in GF tumor-bearing mice. This is the first evidence that reveals the unexpected contribution of P. copri to the progression of breast cancer.
Alterations in the protein expression profile were initially analyzed to understand the promotive effect of P. copri on cancer. Of the verified DEPs, UHRF1 was evidently up-regulated in tumors of P. copri-treated mice under SPF and GF conditions. This up-regulation is consistent with the reported evidence indicating that UHRF1 is highly expressed in breast cancer,Citation42 and is positively related to poorer prognosis.Citation43 UHRF1 (also referred as Np95 in mice and ICB90 in humans) is a multifunctional epigenetic regulator, particularly involved in the maintenance of DNA methylation. Physiologically, UHRF1 is tightly regulated by the cell cycle machinery,Citation44,Citation45 and expressed only in actively proliferating cells in the corresponding tissue (e. g., immune cells, erythroid cells, plasma cells, breast glandular cells, and distal enterocytes). In contrast, it is hardly detectable in cells of terminally differentiated tissues (e. g. neurocytes, hepatocytes, basal respiratory cells, and fibroblasts).Citation46 However, UHRF1 is overexpressed in almost all cancers due to the loss of precise regulation by the cell cycle machinery, thus promoting tumorigenesis and cancer progression. Maintenance of DNA methylation is the most studied function of UHRF1, which recruits or interacts with the DNMT family for de novo methylation (catalyzed by DNMT3A and DNMT3B)Citation47 or maintenance of methylation (by recruiting DNMT1 to hemi-methylated DNA sites).Citation48 Our results showed that in addition to UHRF1, the methylation-initiating DNMT3A was also upregulated following the administration of P. copri, indicating that P. copri influences the progression of breast cancer by altering the pattern of DNA methylation.
DNA methylation is a fundamental epigenetic modification that regulates gene activity and nuclear architecture. It has been demonstrated that DNA methylation in cancer cells displayed a specific pattern compared with normal cells. Generally, DNA regions with few CpG in cancer cells are hypomethylated, whereas the CpG-rich regions (termed CpG islands, CGIs) that localize near the gene promoters or enhancers are hypermethylated.Citation49 Due to the hypermethylation of CpG islands, the transcription of tumor-suppressor genes in cancer cells is inhibited, thereby greatly contributing to tumorigenesis.Citation50 Our analysis of the DNA methylome by RRBS demonstrated that, although did not affect the overall DNA methylation level, P. copri administration induced 2,675 and 2,651 hypermethylated and hypomethylated DMRs, respectively, during tumor growth. Coincidentally, both analysis for DNA methylome and proteome indicated that AMPK signaling pathway was one of the critical signaling pathways, in which several genes and proteins were evidently affected by P. copri administration. The subsequent verifications by WB and qPCR confirmed that AMPK signaling pathway was suppressed, as indicated by the repressed AMPK phosphorylation and upregulation of the downstream factors (mTOR and ULK1). As has been proved that UHRF1 would serve as a suppressor against AMPK activity,Citation36 our findings suggest that P. copri would promote the development of breast cancer via suppressing the metabolism and energy-controlling AMPK signaling pathway, in which the up-regulated UHRF1 by P. copri not only restrained AMPK activity directly, but also affected the pattern of DNA methylation pattern in genes that are involved in this pathway.
Thereafter, we explored what is the exact substance responsible for the cancer-promoting activity of P. copri. Unexpectedly, we discovered that the IPyA is a potent cytotoxic reagent that has never been discovered, suggesting that deficiency of intrinsic IPyA, rather than the secretion of P. copri, was critical for the cancer-promoting activity. IPyA is an indole product from the essential amino acid Trp during the interaction between host and microbiota, and it is usually accumulated in the host at physiological levels mainly relying on the conversion by tryptophan hydroxylases (Tph) in various microbes.Citation51 There have been several paradox reports concerning the relationship between Prevotella and Trp. For example, it has been found that the decrease in fecal Trp was associated with an increase of Prevotella/Bacteroides ratio in obesity.Citation24 Of note, diet with adequate amount of Trp was positively related to the maintenance of Prevotella in weaned piglets.Citation52,Citation53 In the present study, it was found that P. copri consumed a large amount of Trp without IPyA production, thus substantially decreasing the level of IPyA during tumor progression. This observation was consistent with results reported by Tintelnot et al.Citation35 These results indicated that the exhaustion of IPyA resulting from a negative relationship between P. copri and Trp contribute to the tumor promotion by P. copri.
Indeed, several studies have revealed the favorite role of indoles in the treatment of tumor. For example, Tintelnot et al. found that indole-3-acetic acid (IAA) is enriched in pancreatic ductal adenocarcinoma patients who respond to polychemotherapy in combine with folinic acid (FOLFIRINOX), in which show the efficacy of 3-IAA and chemotherapy is licensed by neutrophil-derived myeloperoxidase, thereby compromising the metabolic fitness and proliferation of cancer cells.Citation35 Bender et al. found that indole-3-aldehyde can promote immune checkpoint inhibitor efficacy melanoma patients, which relies on driving Tc1 effector function through the induction of aryl hydrocarbon receptor-dependent CREB activity.Citation54 We found that the cytotoxicity against breast cancer cells by IPyA is characterized by activation of AMPK. AMPK is a key sensor for metabolism and energy. By acting on various downstream factors (e.g., mTORCitation33 and ULK1,Citation34 AMPK promotes the oxidation of fatty acids, and inhibits the synthesis of proteins, fatty acids and cholesterol, thereby restricting cell proliferation.Citation55 Although several studies have revealed the paradoxical role of AMPK in carcinogenesis, it has been found that activated AMPK is negatively associated with the development of breast cancer.Citation56,Citation57 Similarly, we found that upon the cytotoxicity of IPyA, AMPK in breast cancer cells was evidently activated. This activation was accompanied with dramatic reduction of ATP production and suppression on the downstream (mTOR and ULK). Moreover, we found that IPyA directly suppressed the transcription of UHRF1, following by the declined UHRF1 and PP2A C in nucleus. These results imply that IPyA inhibits the activity of AMPK. via suppressing UHRF1-mediated negative regulation, which is just opposite to the cancer promoting effect of P. copri. All these results confirmed that the exhaustion of IPyA from Trp is a pivotal step in the promotion of breast cancer by P. copri, which is probably attributed to the inactivation of AMPK via UHRF1-mediated negative regulation.
It has been well recognized that several tryptophan-derived indoles, such as indole-3-acetaldehyde, indole-3-aldehyde, and indole-3-acetic acid, are AhR agonists. Numbers of papers suggested that the upregulation of CYP1A1, CYP1A2, CYP1B1, is a key indicator of AhR activation.Citation58-62 For example, Aoki et al Citation60 found that IPyA is an activator of aryl hydrocarbon receptor (AhR) in colon cells and colon tissue to suppress experimental colitis in mice, in which IPyA increased the transcription of CYP1A1 by AhR and the mRNA level. For example, Aoki et al Citation60 found that IPyA is an activator of aryl hydrocarbon receptor (AhR) in colon cells and colon tissue to suppress experimental colitis in mice, in which IPyA increased the transcription of CYP1A1 by AHR and the mRNA level. It was found that in the two cell lines, IPyA upregulated both protein and mRNA level of CYP1B1, another downstream of AhR activation, and the increase in mRNA level was more evident than that in protein level (Figure S5a, b and Figure S6a, b). In 4T1 cells, the protein and mRNA level of CYP1B1 IPyA treatment (0.250 mM) was up-regulated about 100% and 200%, respectively; in EMT6 cells, they were increased about 25% and 500%, respectively. And chromatin immunoprecipitation (ChIP) analysis showed that IPyA promoted the binding of AhR and the promoter of CYP1B1 as indicated by the increased fold enrichment (Figure S5c, and Figure S6c), implying that IPyA enhanced the transcription of CYP1B1. In addition, IPyA not only increased expression of AhR in nucleus (Figure S5d, and Figure S6d), but also promoted the nuclear location of AhR in the two cell lines (Figure S5e, and Figure S6e), indicating an enhanced nuclear translocation. The above results suggested an agonism activity of IPyA on AhR. Our findings and several previous studiesCitation63–65 showed that indoles and their metabolites are favorable in the inhibition on breast cancer; whereas, the anti-breast cancer activity of indoles is paradoxical to their agonism for AhR, which has been associated with the development of breast cancer.Citation66 The agonism of IPyA on AhR in breast cancer cell lines remains in doubt, and the exact role of IPyA in modulation of AhR is interesting and deserves further investigation. On the other hand, it was found that IPyA suppressed the proliferation and colony formation of the two breast cancer cell lines, and downregulated the expression of UHRF1 but promoted the phosphorylation of AMPK, which reversed the effect of P. copri on the development of breast cancer. These results indicated that activation of AMPK by suppressing UHRF1-mediated negative regulation would be the key event that drives the cytotoxicity of IPyA in breast cancer cells, rather than its effect on AhR.
A limitation of this study is the small size and retrospective nature. Moreover, there was significant difference in the age of CA and that of NC. The median age of CA was significantly higher than that of NC, and all participants in NC group were aged <65 years. Nevertheless, the relative abundance and prevalence of P. copri in all patients with breast cancer, and even those aged <65 years, were higher than their counterparts. It has been reported that the prevalence of P. copri was increased in an age-dependent manner regardless of Westernization, while the relative abundance drops slightly from adulthood to old age (more than 65 years old).Citation26 These studies suggest that age difference would unlikely affect the higher prevalence and abundance of Prevotella in breast cancer patients. In the future research, we would like to enroll more breast cancer patients with age-matched controls to verify the effect of P. copri.
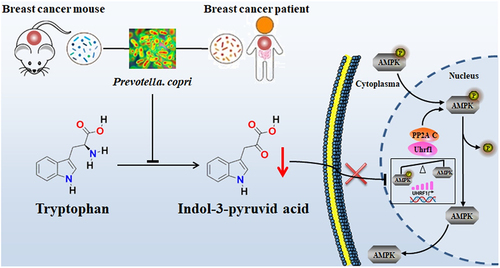
Overall, the present study revealed that P. copri is a significantly enriched and prevalent bacterium in gut microbiota of breast cancer patients, and may serve as a novel risk factor in the progression of breast cancer (). Mechanistically, the excess P. copri consumes large amount of Trp, thus hampering the physiological accumulation of IPyA in the host. As demonstrated in our results, IPyA is an intrinsic anti-cancer reagent in host at physiological level. IPyA directly suppressed the transcription of UHRF1, following by the declined UHRF1 and PP2A C in nucleus, thus inhibiting the phosphorylation of AMPK, which is just opposite to the cancer promoting effect of P. copri. Therefore, the exhaustion of IPyA by excessive P. copri strengthens the UHRF1-mediated negative control to inactivated the energy-controlling AMPK signaling pathway to promote tumor growth, which was indicated by the alternation in pattern of protein expression and DNA methylation. Our findings, for the first time, highlighted P. copri as a risk factor for the progression of breast cancer.
Figure 10. Working scheme. The excess P. copri consumes large amount of tryptophan (Trp), thus hampering the physiological accumulation of IPyA in host. IPyA is an intrinsic anti-cancer reagent in host at physiological level. Therefore, the deficiency of IPyA by P. copri inactivated the energy-controlling AMPK signaling pathway by the UHRF1-mediated negative control as indicated by the alternation in profiles of protein expression and DNA methylation.
4. Materials and methods
4.1. Clinical study design and fecal sample collection
This was a retrospective study approved by the Ethics Committee of Guangdong Hospital of Traditional Chinese Medicine (Guangzhou, China) (approval number: ZF2018–179). Fecal samples were collected from 42 patients with breast cancer (CA) and 40 controls with benign breast disease (NC), who were enrolled from May, 2018 to March, 2019. Demographic characteristics of all the participants was showed in Table S1. The status of all participants was confirmed by surgical and pathological examination at Guangdong Provincial Hospital of Traditional Chinese Medicine, and fecal samples were collected prior to surgery. All fecal samples were stored in sealed plastic containers with RNA later and frozen at −80°C within 30 min. Primary inclusion criteria were as follows: (1) female; (2) 18–80 years; (3) with breast disease and being scheduled for which surgery or core needle biopsy. Exclusions were: (1) undergoing neoadjuvant chemotherapy, endocrine therapy, immunotherapy, or radiotherapy; (2) complications of diabetes, digestive system disease (e.g., gastritis, diarrhea, ulcerative colitis, Crohn’s disease), or presence of other infectious diseases; (3) administration of antibiotics, steroid hormones, Chinese herbal medicine (oral, intramuscular, intravenous injection, etc.), or probiotics (e.g., yogurt) in the last 3 months.
4.2. DNA extraction and 16S ribosome RNA V3~V4 region sequencing
DNA extraction and 16S rRNA V3~V4 region sequencing were performed by Personal Biotechnology Co., Ltd. (Shanghai, China) as described by Guan et al.Citation67 Total genomic DNA from feces was extracted with OMEGA Soil DNA Kit (Omega Bio-Tek, Norcross, GA, USA). V3-V4 region of bacterial 16S rRNA gene was amplified by PCR with Q5® High-Fidelity DNA Polymerase ((New England Biolabs, Ipswich, MA, USA) with the forward primer (5′-ACTCCTACGGGAGGCAGCA-3′) and reward primer (5′-ACTCCTACGGGAGGCAGCA-3′). Sample-specific 7-bp barcodes were incorporated into the primers for multiplex sequencing. The resulting amplicons were purified with Vazyme VAHTSTM DNA Clean Beads (Vazyme Biotech Co., Ltd., Nanjing, China) and quantified by Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA). Amplicons were pooled in equal amounts, and pair-end 2 × 250 bp sequencing was conducted on the Illlumina MiSeq platform with MiSeq Reagent Kit v3.
Microbiome bioinformatics analysis was performed with Quantitative Insights Into Microbial Ecology (QIIME2 2019.4).Citation68 The high-quality sequencing data was trimmed, assembled, and aligned against the SILVA Release 132 Database.Citation69 Non-singleton amplicon operational taxonomic units (OTUs) were aligned with MAFFTCitation70 to construct a phylogeny with fasttree2. Alpha diversity was compared by ACE, Chao1, Simpson, and Shannon metrics. Beta diversity was assessed with PCoA, PERMANOVA and ANOSIM. LEfSe were applied to identify specific taxa microbes among groups using the default parameters.
4.3. Antibodies and chemicals
Details regarding to the primary and secondary antibodies were listed Table S2. IPyA and Trp were purchase from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China).
4.4. Animals
All animal experiments were approved by Guangdong Institute of Microbiology Laboratory Animal Ethics Committee (approval number: GT-IACUC201910121) according to the Guidelines for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication, revised 2011). All female Balb/c mice were purchased from Charles River Co., Ltd. (Beijing, China). In the experiment under SPF condition, mice were raised under controlled temperature (23 ± 2°C), humidity (50% ± 5%), and 12 h light/dark cycle, and they were free access to food and water. In the experiment under GF condition, mice were established and raised in sterile isolators with positive pressure differential and filter-top cages with irradiation-sterilization bedding, and ad libitum access to food and autoclaved water ad libitum. GF status was regularly verified by assessing fecal 16S rRNA via PCR.
4.5. P. copri culture
P. copri (DSM 18,205, DSMZ-German Collection of Microorganisms and Cell Cultures, GmbH, Braunschweig, Germany) was cultured on EG agar medium (Qingdao Hi-Tech Industrial Park Hope Bio-Technology Co., Ltd., Qingdao, China) supplemented with 5% sterilized defibrinated sheep blood (Hongquan Biotechnology Co., Ltd., Guangzhou, China), and maintained in a bio-bag at 37°C. After 72 h of culture, P. copri colonies were scrapped with normal saline and centrifuged at 5000×g for 5 min. The bacterial pellet was washed once and resuspended with normal saline, and subjected to dilution for oral administration and in vitro experiment.
4.6. Cell culture
Murine breast cancer cell lines 4T1 and EMT6 was provided by Cell bank of Chinese Academy of Sciences (Shanghai, China) and American Type Culture Collection (Manassas, VA, US). Both cells were maintained in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, NY, USA) and 100 IU/mL penicillin/streptomycin (Gibco), which were refereed as complete medium hereafter. Cell culture was maintained in incubator at 37°C with an atmosphere of 5% CO2.
4.7. Breast cancer model and treatments
For P. copri experiment, female Balb/c mice under SPF condition (6 to 8-weeks old) were randomly divided into SPF-Ctrl and SPF-P groups, and those under GF condition (6 to 8-weeks old) were divided into GF-Ctrl and GF-P groups (n = 8/group). P. copri (2.5 × 109 cfu/mouse, once weekly) was orally administrated to SPF-P (for 4 weeks) and GF-P (for 5 weeks) since 1st day. An equal volume vehicle was administrated to SPF-Ctrl and GF-Ctrl groups. On 8th day, 4T1 cells (8 × 104 cells/mouse) or EMT6 cells (4 × 105 cells/mouse) were subcutaneously inoculated into the right fourth breast fat pad of all the mice. Body weight and tumor growth were monitored twice weekly for 3 weeks (SPF) or 4 weeks (GF) since inoculation.
For IPyA experiment, female Balb/c mice under SPF condition (6 to 8-weeks old) were inoculated with 4T1 cells (8 × 104 cells/mouse) as described above, and body weight and tumor growth were recorded twice weekly until the end of IPyA treatment. The mice were randomly divided into SPF-Ctrl and SPF-IPyA groups when the tumor volume reached approximately 30 mm3. IPyA (120 mg/kg) was orally administrated to SPF-IPyA group for 3 weeks, while equal volume vehicle was administrated to SPF-Ctrl group.
Tumor volume was calculated as V = a×b2/2, where a and b indicated the longer and shorter diameter, respectively. On the last day, peripheral blood was collected from the orbital vein plexus prior to sacrifice by cervical dislocation, and then tumors were harvested, weighed, photographed, and segmented. Feces were collected for comparison of the relative abundance of P. copri.
4.8. P. copri detection by fluorescent in situ hybridization (FISH)
Colonization of P. copri in the colon or tumor was verified by FISH with DNA FISH kit (Future Biotech Co.,Ltd, Beijing, China). In brief, the paraffin-embedded colons were sliced into 3-μm thick sections. Then the sections were deparaffinized, dehydrated, denatured, and followed by hybridization with probe for P. copri (TTTCGCTTGGCCGCTGACCTGTTC, labeling with Cy3 at 5‘and 3’) for P. copri for 72 h at 37°C. After washed, the slides were stained with DAPI, and observed under a laser scanning confocal microscope (Leica, Wetzlar, Germany).
4.9. Protein profile analysis for tumors by 4D label-free quantitative proteomics
To compare the protein profile between GF-Ctrl and GF-P groups, 4D label-free quantitative proteomics analysis was performed by PGEM Co., Ltd. (Guangzhou, China) as described by Li et al.Citation71 The samples frozen at −80°C were homogenized four times with ice-cold lysis buffer (containing 8 M urea and a cocktail of proteinase inhibitors). After centrifugation, the supernatants (samples) were obtained as protein samples and quantified by Bicinchoninic acid (BCA) protein assay kit (Biosharp Co., Ltd., Beijing, China). Thereafter, proteins were enzymolyzed into peptides with trypsin. The peptides were dissolved with aqueous solution containing 0.1% formic acid and 2% acetonitrile, separated with NanoElute UPLC system (Bruker, Billerica, MA, USA), ionized by capillary ion source, and finally analyzed by trapped ion mobility spectrometry-time of flight (TIMS-TOF) Pro tandem mass spectrometry (Bruker). The obtained MS/MS data were processed using Maxquant search engine (v. 1.6.6.0), and the tandem mass spectra were searched against Mus musculus UniProt database concatenated with reverse decoy database. The main parameters for analysis were set as follows: mass tolerance for precursor ions in first search and in main search: 20 ppm; false discovery rate (FDR): <1%; minimum score for the modified peptides: >40. The verified proteins were annotated and those with significant expression difference (p < .05) were subjected to functional enrichment based on KEGG.
4.10. DNA methylation profile analysis for tumor by RRBS
RRBS was conducted by Shenzhen Acegen Technology Co., Ltd. (Shenzhen, China) to compare the DNA methylation profile between GF-Ctrl and GF-P groups. Genomic DNA from the tumor was extracted using DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) for RRBS library preparation as described previously.Citation72 Briefly, genomic DNA (1.5 mg) was digested with the MspI enzyme (NEB), following by end repair, A-base tailing and 5-methylcytosine-modified adapter ligation. DNA fractions from MspI-digested products in the range of 40–250 bp were subjected to bisulfite treatment with ZYMO EZ DNA Methylation-Gold Kit (Zymo Research, Irvine, CA, USA). DNA fragments were enriched by 12-cycle PCR and each library was integrated with the DNA index. The libraries were quantified, quality controlled, and subjected to paired-end 150 bp multiplex sequencing on the NovaSeq 6000 platform. Raw sequencing data were processed via the Illumina base-calling pipeline. High-quality reads that contained more than 70% ‘N’s and showed a high-quality value (>80) in over 90% of the sequence were used for analysis, and they were aligned to the Ensembl Mus musculus reference genome (Sscrofa10.2) with bisulfite sequence MAPping program (BSMAP). The methylation levels of individual cytosines were defined as the ratio of the sequenced depth of the ascertained methylated CpG cytosines to the total sequenced depth of individual CpG cytosines. Genes were annotated and those with significant DMRs (p < .05) were subjected to KEGG functional enrichment analyses.
4.11. Targeted metabolomics analysis and quantification by UPLC-MS/MS
The targeted metabolomics analysis was performed by Metabo-Profile (Shanghai, China) as described by Xie et al.Citation73 The serum and supernatant from P. copri culture medium were extracted separately with ice-cold methanol containing internal standards. The resulting supernatants were subjected to derivatization with 3-nitrophenylhydrazine (3-NPH) and N-(3-(dimethylamino)propyl)-N′-ethylcarbodiimide (EDC)·HCl (Sigma-Aldrich, St. Louis, MO, USA). Finally, all samples were analyzed by a UPLC-MS/MS system (ACQUITY UPLC-Xevo TQ-S, Waters Corp., Milford, MA, USA), equipped with a UPLC BEH C18 column (2.1 × 100 mm, 1.7 µM). The raw data files generated by UPLC-MS/MS were processed using the Targeted Metabolome Batch Quantification (TMBQ) software (v1.0, HMI, Shenzhen, Guangdong, China) to perform peak integration, calibration, and quantitation for each metabolite. The self-developed platform iMAP (v1.0, Metabo-Profile, Shanghai, China) was used for statistical analyses, including univariate analysis (Student’s t-test or Mann – Whitney U test, as shown by volcano plot), multi-dimensional analysis (OPLS-DA), and pathway analysis, et al. Metabolites with univariate statistics p < .05 and OPLS-DA value > 1 were considered as potential markers that are responsible for the difference between groups.
4.12. Quantification for conversion of Trp to IPyA
P. copri (1 × 109 cfu/mL, 100 μL/well) were co-cultured with Trp (0, 4, 8 mM) in Peptone Yeast Glucose (PYG) medium (Qingdao Hi-Tech Industrial Park Hope Bio-Technology Co., Ltd., Qingdao, China), which was freshly supplemented with 0.1% vitamin K and 0.5% hemin, for 48 h in bio-bag at 37°C. The supernatants were collected for quantitation of Trp and IPyA by UPLC-MS/MS as described above. The amounts of consumed Trp and produced IPyA were calculated as follows: consumed Trp (mM) = iTrp (mM) + rTrp (mM) -bTrp (mM), where iTrp indicates the input Trp in co-culture, rTrp indicates the residual Trp in the supernatant after the 48-hour co-culture, and bTrp indicates Trp in blank PYG medium after the 48-hour co-culture. Produced IPyA (mM) = rIPyA (mM) -bIPyA (mM), where rIPyA indicates the residual IPyA in the supernatant after the 48-hour co-culture, and bIPyA indicates IPyA in blank PYG medium after the 48-hour co-culture.
4.13. Cell viability assay
4T1 cells or EMT6 cells were seeded in 96-well plates at a density of 1 × 104 cells/mL (100 μL/well) with complete medium. After adherence overnight, cells were treated with IPyA (0 ~ 4 mM) for 48 h. Next, the medium was replaced with complete medium containing 10% CCK-8 (GlpBio Technology, Montclair, CA, USA), and the optical density at 490 nm was measured with a Multiscan MK3 microplate reader (Thermo Fisher, USA) 2 h later.
4.14. Colony formation assay
4T1 cells (1 × 103 cells/well. 1 mL/well) or EMT6 cells (2 × 102 cells/well. 1 mL/well) were seeded in 24-well plates with complete medium. After adherence overnight, cells were treated with IPyA (1, 2, 4 mM) for several days depending on the clone size, and the drug-containing complete medium was refreshed every three days. On the last day, the medium was discarded, and the cells were washed with PBS once. Then the cells were fixed with 4% paraformaldehyde for 30 min at room temperature, washed once with phosphate-buffered saline (PBS), and stained with crystal violet. The plates were photographed, and the number of colonies was counted to calculated the clone formation ratio as follows: colony formation (%) = number of colony/total number of seeded cells × 100%.
4.15. Western blot (WB) analysis
For total proteins extraction, tumor tissues were homogenized with radioimmunoprecipitation assay buffer (RIPA, Beyotime Biotechnology. Co., Ltd., Shanghai, China) supplemented with cocktail of proteinase and phosphatase inhibitors (Beyotime Biotechnology. Co., Ltd., Shanghai, China) at 4°C. Cells were lysed with RIPA directly. Proteins of cytoplasm and nucleus from cells were separated with NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific). All proteins were quantified by BCA protein assay kit.
Subsequently, the proteins were separated by sodium dodecyl sulfate-polyacrylamide gel-electrophoresis (SDS-PAGE) with 8% or 15% gels depending on the molecular weight of target protein, and transferred onto 0.45 μm polyvinylidene difluoride membrane (PVDF). After blocked with 5% skimmed milk in phosphate-buffered saline (TBST) for 2 h, the membranes were probed with primary antibodies overnight at 4°C, and with secondary antibodies for 1 hour on the next day. The membranes were developed with enhanced chemiluminescence (ECL) detection reagents (Biosharp Tech Co., Ltd., Beijing, China). After that, some membranes were stripped to detect another protein. Band intensity was quantified using Image J software (NIH Image, Bethesda, MD, USA).
4.16. Real-Time quantitative Polymerase Chain Reaction (RT-qPCR) analysis
For total RNAs from tumor tissues and cells, they were extracted with Animal total RNA isolation kit (Foregene Co., Ltd., Chengdu, China), and subjected to reverse transcription with ReverAid First Strand cDNA Synthesis Kit (Takara Bio, Shiga, Japan). RT-qPCR reactions were performed with TB Green® Premix Ex Taq™ II (Takara Bio, Shiga, Japan) using CFX ConnectTM Real-Time system (Bio-Rad, Hercules, CA, USA). The primer sequences for RT-qPCR are shown in Table S3. The levels of targets were normalized with those of β-actin (internal control), and analyzed by the 2−△△Ct method.
To analyze the relative abundance of P. copri, total DNAs from feces and contents in small intestine were extracted with OMEGA Soil DNA Kit, and DNAs from sections of paraffin-embedded tumor were extracted with FFPE DNA Kit (TIANGEN BIOTECH Co.,Ltd, Beijing, China). Then they were subject to RT-qPCR. Relative abundance of P. copri was calculated as follows: P. copri relative abundance (%) = 1/(2 Ctp-Ct16s) × 100%, where Ctp represents the cycle threshold achieved with P. copri primers, and Ct16s represents the cycle threshold achieved with 16S primers. Primers were listed in Table S3.
4.17. Methylation specific quantitative PCR (MS qPCR) assays
Genomic DNA from cells was extracted by FastPure® Cell/Tissue DNA Isolation Mini Kit (Vazyme Biotech Co., Ltd.), and subjected to bisulfite conversion with ZYMO EZ DNA Methylation-Gold Kit. The modified DNA was amplified and subjected to qPCR as described above, to determine the methylation rate of the promoter region of target genes to the unmethylated ones. Primers sequences for MS qPCR were listed in Table S4. The rate of promoter methylation rate was calculated as follows: promoter methylation rate (%) = 1/(2 Ctme-Ctunm) × 100%, where Ctme represents the cycle threshold achieved with methylation primers in bisulfite-conversed DNA samples, and Ctunme represents the cycle threshold achieved with unmethylation primers in unmodified DNA samples.
4.18. Cytoplasm and nucleus protein separation
After 48 h of IPyA treatment, the cells were collected and proteins from cytoplasm and nucleus were extracted by Nuclear Protein Extraction Kit (Solarbio, Beijing China) for western blot analysis or Co-immunoprecipitation assay.
4.19. Dual luciferase reporter assay
The promoter sequence of UHRF1 were subcloned into the pGL3 vector (Promega, Madison, Wisconsin, USA) to build the reporter plasmid. 4T1 cells were simultaneously transfected with the UHRF1 reporter plasmid and Renilla pRL-TK internal control vector using Lipo8000™ Transfection Reagent (Beyotime Biotech. Inc., Shanghai, China) in 96-well plates. After 6 h of transfection, the medium was replaced with IPyA (0, 0.125, 0.25, 0.5 mM)-containing completed RPMI1640 without penicillin or streptomycin for another 48-h incubation. The cells were lysed and reporter gene expression was assessed using a Dual Luciferase Reporter Gene Assay Kit (Beyotime Biotech. Inc., Shanghai, China).
4.20. ATP production analysis
ATP production in cells treated with IPyA was analyzed with Seahorse XF Real-Time ATP Rate Assay Kit (Agilent, Santa Clara, CA, USA). In brief, cells treated with IPyA were harvested, and seeded in a Seahorse XF 96-well microplate (20000 cells/well). After adherence overnight, the medium was replaced with the assay medium. Oligomycin and Rotenone/antimycin A provided in the kit were loaded into the corresponding ports of a hydrated sensor cartridge. Finally, the assay was performed in the Seahorse XF96 Analyzer (Agilent).
4.21. Statistical analysis
Data were presented as mean ± standard deviation (SD). Statistical analysis was performed with Statistical Package for the Social Sciences (SPSS version 22.0; IBM Corp., Armonk, NY, USA). Normal distribution test was conducted prior to the following analysis. For datasets in complying with normal distribution, they were analyzed by one-way analysis of variance (ANOVA) or Student’s t-test; for those of non-normal distribution, they were compared by Mann-Whitney U test or Kruskal–Wallis test. Prevalence was compared using the Chi-squared test, and datasets of body weight and tumor volume were compared with repeated measurement data analysis of variance.
Author contributions
Conceptualization: JYS, QJC, CLX. Methodology: JYS, XJL, YZX, QJC, CLX. Investigation: JYS, XJL, DL, CMY, SML, XHC, XJY, BTP, RX, LPR, YFZ. Funding acquisition: JYS, QJC, CLX. Project administration: YZX, QJC, CLX. Supervision: YZX, QJC, CLX. Writing – original draft: JYS, XJL, DL, YZX. Writing – review & editing: JYS, XJL, DL, CLX.
Availability of data and materials
All data generated during the current study are included in this article and its supplementary information files, and they are available from the corresponding authors upon reasonable request. The sequences generated and analyzed in this study were uploaded to the NCBI Sequence Read Archive (SRA) data repository, with project numbers PRJNA887717 (microbiota raw sequencing data) and PRJNA890318 (DNA methylation raw sequencing data).
Ethics approval and consent to participate
The enrollment of participants and sample collection was approved by the Ethics Committee of the Guangdong Provincial Hospital of Chinese Medicine (Guangzhou, China, Ethics Approval number: ZF2018–179). Consent for participation was obtained when the patients were enrolled.
All animal experiments were approved by Guangdong Institute of Microbiology Laboratory Animal Ethics Committee (permission number: GT-IACUC201910121) according to the Guidelines for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication, revised 2011).
Supplemental Material
Download Zip (20.7 MB)Acknowledgments
We would like to acknowledge and thank Prof. Liwu Fu (Sun Yat-sen University Cancer Center) for the help in manuscript drafting and editing, and thank Prof. Wang Sheng (Beijing Institute of Technology) for the help in cell and bacterial culture.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2024.2347757
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clinicians. 2021;71(3):209–27. doi:10.3322/caac.21660.
- Derakhshan F, Reis-Filho JS. Pathogenesis of triple-negative breast cancer. Annu Rev Pathol. 2022;17(1):181–204. doi:10.1146/annurev-pathol-042420-093238.
- Houghton SC, Hankinson SE. Cancer progress and priorities: breast cancer. Cancer Epidemiol Biomarkers Prev. 2021;30(5):822–844. doi:10.1158/1055-9965.EPI-20-1193.
- Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. Lancet. 2021;397(10286):1750–1769. doi:10.1016/S0140-6736(20)32381-3.
- Lau HCH, Sung JJ, Yu J. Gut microbiota: impacts on gastrointestinal cancer immunotherapy. Gut Microbes. 2021;13(1):1–21. doi:10.1080/19490976.2020.1869504.
- Schwabe RF, Greten TF. Gut microbiome in HCC – mechanisms, diagnosis and therapy. J Hepatol. 2020;72(2):230–238. doi:10.1016/j.jhep.2019.08.016.
- Pan LL, Li BB, Pan XH, Sun J. Gut microbiota in pancreatic diseases: possible new therapeutic strategies. Acta Pharmacol Sin. 2020;42(7):1027–1039. doi:10.1038/s41401-020-00532-0.
- Zhu J, Liao M, Yao Z, Liang W, Li Q, Liu J, Yang H, Ji Y, Wei W, Tan A. et al. Breast cancer in postmenopausal women is associated with an altered gut metagenome. Microbiome. 2018;6(1):136. doi:10.1186/s40168-018-0515-3.
- Yu Q, Newsome RC, Beveridge M, Hernandez MC, Gharaibeh RZ, Jobin C, Thomas RM. Intestinal microbiota modulates pancreatic carcinogenesis through intratumoral natural killer cells. Gut Microbes. 2022;14(1):2112881. doi:10.1080/19490976.2022.2112881.
- Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, Adler K, Dick-Necula D, Raskin S, Bloch N. et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2020;6529(2021):602–609.
- Daillère R, Vétizou M, Waldschmitt N, Yamazaki T, Isnard C, Poirier-Colame V, Duong CM, Flament C, Lepage P, Roberti M. et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity. 2016;45(4):931–943. doi:10.1016/j.immuni.2016.09.009.
- Guo H, Chou WC, Lai Y, Liang K, Tam JW, Brickey WJ, Chen L, Montgomery ND, Li X, Bohannon LM. et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science. 2020;370(6516):370. doi:10.1126/science.aay9097.
- Di Modica M, Gargari G, Regondi V, Bonizzi A, Arioli S, Belmonte B, De Cecco L, Fasano E, Bianchi F, Bertolotti A. et al. Gut microbiota condition the therapeutic efficacy of trastuzumab in HER2-positive breast cancer. Cancer Res. 2021;81(8):2195–2206. doi:10.1158/0008-5472.CAN-20-1659.
- Urbaniak C, Gloor GB, Brackstone M, Scott L, Tangney M, Reid G, Goodrich-Blair H. The microbiota of Breast Tissue and its association with Breast cancer. Appl Environ Microb. 2016;82(16):5039–5048. doi:10.1128/AEM.01235-16.
- Fu A, Yao B, Dong T, Chen Y, Yao J, Liu Y, Li H, Bai H, Liu X, Zhang Y. et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell. 2022;185(8):1356–1372.e26. doi:10.1016/j.cell.2022.02.027.
- Ma J, Sun L, Liu Y, Ren H, Shen Y, Bi F, Zhang T, Wang X. Alter between gut bacteria and blood metabolites and the anti-tumor effects of faecalibacterium prausnitzii in breast cancer. BMC Microbiol. 2020;20(1):82. doi:10.1186/s12866-020-01739-1.
- Wang H, Rong X, Zhao G, Zhou Y, Xiao Y, Ma D, Jin X, Wu Y, Yan Y, Yang H. et al. The microbial metabolite trimethylamine N-oxide promotes antitumor immunity in triple-negative breast cancer. Cell Metab. 2022;34(4):581–594.e8. doi:10.1016/j.cmet.2022.02.010.
- De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107(33):14691–14696. doi:10.1073/pnas.1005963107.
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP. et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–227. doi:10.1038/nature11053.
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto J-M. et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi:10.1038/nature09944.
- O’Connor JB, Mottlowitz M, Kruk ME, Mickelson A, Wagner BD, Harris JK, Wendt CH, Laguna TA. Network analysis to identify multi-omic correlations in the Lower Airways of children with cystic fibrosis. Front Cell Infect Microbiol. 2022;12:805170. doi:10.3389/fcimb.2022.805170.
- Eriksson K, Lundmark A, Delgado LF, Hu YOO, Fei G, Lee L, Fei C, Catrina AI, Jansson L, Andersson AF. et al. Salivary microbiota and host-inflammatory responses in periodontitis affected individuals with and without rheumatoid arthritis. Front Cell Infect Microbiol. 2022;12:841139. doi:10.3389/fcimb.2022.841139.
- Chu Y, Sun S, Huang Y, Gao Q, Xie X, Wang P, Li J, Liang L, He X, Jiang Y. et al. Metagenomic analysis revealed the potential role of gut microbiome in gout. npj Biofilms Microbiomes. 2021;7(1):66–. doi:10.1038/s41522-021-00235-2.
- Dong TS, Guan M, Mayer EA, Stains J, Liu C, Vora P, Jacobs JP, Lagishetty V, Chang L, Barry RL. et al. Obesity is associated with a distinct brain-gut microbiome signature that connects Prevotella and Bacteroides to the brain’s reward center. Gut Microbes. 2022;14(1):2051999. doi:10.1080/19490976.2022.2051999.
- Jiang L, Shang M, Yu S, Liu Y, Zhang H, Zhou Y, Wang M, Wang T, Li H, Liu Z. et al. A high-fiber diet synergizes with Prevotella copri and exacerbates rheumatoid arthritis. Cell Mol Immunol. 2022;19(12):1414–1424. doi:10.1038/s41423-022-00934-6.
- Tett A, Pasolli E, Masetti G, Ercolini D, Segata N. Prevotella diversity, niches and interactions with the human host. Nat Rev Microbiol. 2021;19(9):585–599. doi:10.1038/s41579-021-00559-y.
- Tett A, Huang KD, Asnicar F, Fehlner-Peach H, Pasolli E, Karcher N, Armanini F, Manghi P, Bonham K, Zolfo M. et al. The prevotella copri complex comprises four distinct clades underrepresented in westernized populations. Cell Host Microbe. 2019;26(5):666–79.e7. doi:10.1016/j.chom.2019.08.018.
- Mao AW, Barck H, Young J, Paley A, Mao J, Chang H. Identification of a novel cancer microbiome signature for predicting prognosis of human breast cancer patients. Clin Transl Oncol. 2021;24(3):597–604. doi:10.1007/s12094-021-02725-3.
- Pasolli E, Asnicar F, Manara S, Zolfo M, Karcher N, Armanini F, Beghini F, Manghi P, Tett A, Ghensi P. et al. Extensive unexplored human microbiome diversity revealed by over 150,000 genomes from metagenomes spanning age, geography, and lifestyle. Cell. 2019;176(3):649–62.e20. doi:10.1016/j.cell.2019.01.001.
- Su J, Su L, Li D, Shuai O, Zhang Y, Liang H, Jiao C, Xu Z, Lai Y, Xie Y. et al. Antitumor activity of extract from the sporoderm-breaking spore of ganoderma lucidum: restoration on exhausted cytotoxic T cell with gut microbiota remodeling. Front Immunol. 2018;9:1765. doi:10.3389/fimmu.2018.01765.
- Su J, Li D, Chen Q, Li M, Su L, Luo T, Liang D, Lai G, Shuai O, Jiao C. et al. Anti-breast cancer enhancement of a polysaccharide from spore of ganoderma lucidum with paclitaxel: suppression on tumor metabolism with gut microbiota reshaping. Front Microbiol. 2018;9:3099. doi:10.3389/fmicb.2018.03099.
- Sidhu H, Capalash N. UHRF1: the key regulator of epigenetics and molecular target for cancer therapeutics. Tumour Biol. 2017;39(2):1010428317692205. doi:10.1177/1010428317692205.
- Inoki K, Kim J, Guan KL. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol. 2012;52(1):381–400. doi:10.1146/annurev-pharmtox-010611-134537.
- Hu CA, Wu Z, Wang J. Amino acids and autophagy: their crosstalk, interplay and interlock. Amino Acids. 2015;47(10):2035–2036. doi:10.1007/s00726-015-2098-7.
- Tintelnot J, Xu Y, Lesker TR, Schonlein M, Konczalla L, Giannou AD, Pelczar P, Kylies D, Puelles VG, Bielecka AA. et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature. 2023;615(7950):168–174. doi:10.1038/s41586-023-05728-y.
- Xu X, Ding G, Liu C, Ding Y, Chen X, Huang X, Zhang C-S, Lu S, Zhang Y, Huang Y. et al. Nuclear UHRF1 is a gate-keeper of cellular AMPK activity and function. Cell Res. 2022;32(1):54–71. doi:10.1038/s41422-021-00565-y.
- Shah HN, Collins DM. Prevotella, a new genus to include bacteroides melaninogenicus and related species formerly classified in the genus bacteroides. Int J Syst Bacteriol. 1990;40(2):205–208. doi:10.1099/00207713-40-2-205.
- Oliver WW, Wherry WB. Notes on some bacterial parasites of the human mucous membranes. J Infect Dis. 1921;34(4):341–344. doi:10.1093/infdis/28.4.341.
- Alpizar-Rodriguez D, Lesker TR, Gronow A, Gilbert B, Raemy E, Lamacchia C, Gabay C, Finckh A, Strowig T. Prevotella copri in individuals at risk for rheumatoid arthritis. Ann Rheum Dis. 2019;78(5):590–593. doi:10.1136/annrheumdis-2018-214514.
- Pianta A, Arvikar S, Strle K, Drouin EE, Wang Q, Costello CE, Steere AC. Evidence of the immune relevance of Prevotella copri, a gut microbe, in patients with rheumatoid arthritis. Arthritis & Rheumatol (Hoboken, NJ). 2017;69(5):964–975. doi:10.1002/art.40003.
- Wen C, Zheng Z, Shao T, Liu L, Xie Z, Le Chatelier E, He Z, Zhong W, Fan Y, Zhang L. et al. Quantitative metagenomics reveals unique gut microbiome biomarkers in ankylosing spondylitis. Genome Biol. 2017;18(1):142. doi:10.1186/s13059-017-1271-6.
- Chandrashekar DS, Bashel B, Balasubramanya SAH, Creighton CJ, Ponce-Rodriguez I, Chakravarthi B, Varambally S. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia (New York, NY). 2017;19(8):649–658. doi:10.1016/j.neo.2017.05.002.
- Gao SP, Sun HF, Li LD, Fu WY, Jin W. UHRF1 promotes breast cancer progression by suppressing KLF17 expression by hypermethylating its promoter. Am J Cancer Res. 2017;7:1554–1565.
- Chen H, Ma H, Inuzuka H, Diao J, Lan F, Shi YG, Wei W, Shi Y. DNA damage regulates UHRF1 stability via the SCF β-TrCP E3 ligase. Mol Cell biol. 2013;33(6):1139–1148. doi:10.1128/MCB.01191-12.
- Vaughan RM, Dickson BM, Whelihan MF, Johnstone AL, Cornett EM, Cheek MA, Ausherman CA, Cowles MW, Sun Z-W, Rothbart SB. Chromatin structure and its chemical modifications regulate the ubiquitin ligase substrate selectivity of UHRF1. Proc Natl Acad Sci USA. 2018;115(35):8775–8780. doi:10.1073/pnas.1806373115.
- Karlsson M, Zhang C, Méar L, Zhong W, Digre A, Katona B, Sjöstedt E, Butler L, Odeberg J, Dusart P. et al. A single–cell type transcriptomics map of human tissues. Sci Adv. 2021;7(31):eabh2169. doi:10.1126/sciadv.abh2169.
- Meilinger D, Fellinger K, Bultmann S, Rothbauer U, Bonapace IM, Klinkert WE, Spada F, Leonhardt H. Np95 interacts with de novo DNA methyltransferases, Dnmt3a and Dnmt3b, and mediates epigenetic silencing of the viral CMV promoter in embryonic stem cells. EMBO Rep. 2009;10(11):1259–1264. doi:10.1038/embor.2009.201.
- Bostick M, Kim JK, Estève PO, Clark A, Pradhan S, Jacobsen SE. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science. 2007;317(5845):1760–1764. doi:10.1126/science.1147939.
- Nishiyama A, Nakanishi M. Navigating the DNA methylation landscape of cancer. Trends Genet. 2021;37(11):1012–1027. doi:10.1016/j.tig.2021.05.002.
- Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet. 2007;8(4):286–298. doi:10.1038/nrg2005.
- Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23(6):716–724. doi:10.1016/j.chom.2018.05.003.
- Rao Z, Li J, Shi B, Zeng Y, Liu Y, Sun Z, Wu L, Sun W, Tang Z. Dietary tryptophan levels impact growth performance and intestinal microbial ecology in weaned piglets via tryptophan metabolites and intestinal antimicrobial peptides. Anim: An Open Access J MDPI. 2021;11(3):11. doi:10.3390/ani11030817.
- Liang H, Dai Z, Liu N, Ji Y, Chen J, Zhang Y, Yang Y, Li J, Wu Z, Wu G. et al. Dietary L-Tryptophan modulates the structural and functional composition of the intestinal microbiome in weaned piglets. Front Microbiol. 2018;9:1736. doi:10.3389/fmicb.2018.01736.
- Bender MJ, McPherson AC, Phelps CM, Pandey SP, Laughlin CR, Shapira JH, Medina Sanchez L, Rana M, Richie TG, Mims TS. et al. Dietary tryptophan metabolite released by intratumoral lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell. 2023;186(9):1846–1862.e26. doi:10.1016/j.cell.2023.03.011.
- Yuan J, Dong X, Yap J, Hu J. The MAPK and AMPK signalings: interplay and implication in targeted cancer therapy. J Hematol Oncol. 2020;13(1):113. doi:10.1186/s13045-020-00949-4.
- El-Houjeiri L, Biondini M, Paquette M, Kuasne H, Pacis A, Park M, Siegel PM, Pause A. Folliculin impairs breast tumor growth by repressing TFE3-dependent induction of the Warburg effect and angiogenesis. J Clin Invest. 2021;131(22):131. doi:10.1172/JCI144871.
- Chen LM, Yang PP, Al Haq AT, Hwang PA, Lai YC, Weng YS, Chen MA, Hsu H-L. Oligo-Fucoidan supplementation enhances the effect of olaparib on preventing metastasis and recurrence of triple-negative breast cancer in mice. J Biomed Sci. 2022;29(1):70. doi:10.1186/s12929-022-00855-6.
- Hezaveh K, Shinde RS, Klotgen A, Halaby MJ, Lamorte S, Ciudad MT, Quevedo R, Neufeld L, Liu ZQ, Jin R. et al. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity. 2022;55(2):324–340.e8. doi:10.1016/j.immuni.2022.01.006.
- Iizuka T, Yin P, Zuberi A, Kujawa S, Coon J, Bjorvang RD, Damdimopoulou P, Pacyga DC, Strakovsky RS, Flaws JA. et al. Mono-(2-ethyl-5-hydroxyhexyl) phthalate promotes uterine leiomyoma cell survival through tryptophan-kynurenine-AHR pathway activation. Proc Natl Acad Sci USA. 2022;119(47):e2208886119. doi:10.1073/pnas.2208886119.
- Aoki R, Aoki-Yoshida A, Suzuki C, Takayama Y. Indole-3-Pyruvic Acid, an Aryl Hydrocarbon Receptor Activator, Suppresses Experimental Colitis in Mice. J Immunol. 2018;201(12):3683–3693. doi:10.4049/jimmunol.1701734.
- Chen W, Wen L, Bao Y, Tang Z, Zhao J, Zhang X, Wei T, Zhang J, Ma T, Zhang Q. et al. Gut flora disequilibrium promotes the initiation of liver cancer by modulating tryptophan metabolism and up-regulating SREBP2. Proc Natl Acad Sci USA. 2022;119(52):e2203894119. doi:10.1073/pnas.2203894119.
- Renga G, Nunzi E, Pariano M, Puccetti M, Bellet MM, Pieraccini G, D’Onofrio F, Santarelli I, Stincardini C, Aversa F. et al. Optimizing therapeutic outcomes of immune checkpoint blockade by a microbial tryptophan metabolite. J Immunother Cancer. 2022;10(3):10. doi:10.1136/jitc-2021-003725.
- Meng Q, Qi M, Chen DZ, Yuan R, Goldberg ID, Rosen EM, Auborn K, Fan S. Suppression of breast cancer invasion and migration by indole-3-carbinol: associated with up-regulation of BRCA1 and E-cadherin/catenin complexes. J Mol Med (Berl). 2000;78(3):155–165. doi:10.1007/s001090000088.
- Okino ST, Pookot D, Basak S, Dahiya R. Toxic and chemopreventive ligands preferentially activate distinct aryl hydrocarbon receptor pathways: implications for cancer prevention. Cancer Prev Res (Philadelphia, Pa). 2009;2(3):251–256. doi:10.1158/1940-6207.CAPR-08-0146.
- Wattenberg LW, Loub WD. Inhibition of polycyclic aromatic hydrocarbon-induced neoplasia by naturally occurring indoles. Cancer Res. 1978;38:1410–1413.
- Donovan MG, Selmin OI, Romagnolo DF. Aryl hydrocarbon receptor diet and breast cancer risk. Yale J Biol Med. 2018;91:105–127.
- Guan X, Ma F, Sun X, Li C, Li L, Liang F, Li S, Yi Z, Liu B, Xu B. et al. Gut microbiota profiling in patients with HER2-negative metastatic breast cancer receiving metronomic chemotherapy of capecitabine compared to those under conventional dosage. Front Oncol. 2020;10:10. doi:10.3389/fonc.2020.00902.
- Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–857. doi:10.1038/s41587-019-0209-9.
- Kõljalg U, Nilsson RH, Abarenkov K, Tedersoo L, Taylor AF, Bahram M, Bates ST, Bruns TD, Bengtsson‐Palme J, Callaghan TM. et al. Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol. 2013;22(21):5271–5277. doi:10.1111/mec.12481.
- Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30(14):3059–3066. doi:10.1093/nar/gkf436.
- Li Q, Yuan Q, Jiang N, Zhang Y, Su Z, Lv L, Sang X, Chen R, Feng Y, Chen Q. Dihydroartemisinin regulates immune cell heterogeneity by triggering a cascade reaction of CDK and MAPK phosphorylation. Sig Transduct Target Ther. 2022;7(1):222. doi:10.1038/s41392-022-01028-5.
- Gao F, Zhang J, Jiang P, Gong D, Wang JW, Xia Y, Østergaard MV, Wang J, Sangild PT. Marked methylation changes in intestinal genes during the perinatal period of preterm neonates. BMC Genomics. 2014;15(1):716. doi:10.1186/1471-2164-15-716.
- Zheng X, Chen T, Zhao A, Ning Z, Kuang J, Wang S, You Y, Bao Y, Ma X, Yu H. et al. Hyocholic acid species as novel biomarkers for metabolic disorders. Nat Commun. 2021;12(1):1487. doi:10.1038/s41467-021-21744-w.