Abstract
Cell phenotype and fate are driven by the mechanical properties of their surrounding environment. Changes in matrix rigidity or application of force have been shown to impact profoundly cell behavior and phenotype, demonstrating that the molecular mechanisms which “sense” and transduce these signals into biochemical pathways are central in cell biology. In this commentary, we discuss recent evidence showing that mechanotransduction mechanisms occur in the nucleus, allowing dynamic regulation of the nucleoskeleton in response to mechanical stress. We will review this nucleoskeletal response and its impact on both nuclear structure and function.
Introduction
Over the last 15 years, studies have revealed that mechanical tension, whether it is externally applied or generated by the cell in response to ECM rigidity, is a major determinant of cell phenotype, sufficient to direct stem cell differentiationCitation1 or promote malignant behavior.Citation2 Although it is clear that mechanical tension can regulate gene expression,Citation3 very little is known about the molecular mechanisms by which tension is sensed by the transcriptional machinery.
Mechanotransduction mechanisms are mediated by load-bearing subcellular structures whose conformations change in response to mechanical stress,Citation4 resulting in activation of biochemical signaling pathways. Mechanotransduction pathways have been identified in adhesion and cytoskeletal structuresCitation4,5 and have been shown to signal to the nucleus via the passage of cytoplasmic components such as transcriptional co-activators into the nucleus.Citation6,7 However, it has long been speculated that nuclear structures might respond directly to mechanical tension after its propagation though the cytoskeleton and that this response to tension may control gene transcription.Citation8,9 Recent evidence supports this hypothesis and demonstrates that the nucleoskeleton can respond to force, indicating that molecular mechanotransduction mechanisms exist within the nucleus and participate in the cellular response to mechanical stress.Citation10,11
I-The Nucleus is Connected to the Cytoskeleton
Nuclear movements have been studied in a large variety of eukaryotic cellsCitation12,13 and the position of the nucleus has been observed to vary significantly during cellular processes such as cell division, migration and differentiation.Citation12,13 Early observations of these nuclear movements rapidly led to the conclusion that active mechanisms move and maintain the nucleus in the proper cellular location, suggesting a physical connection between the cytoskeleton and the nucleus.
More recently, the proteins responsible for this connection have been identifiedCitation14,15 and constitute the LINC (Linker of Nucleoskeleton and Cytoskeleton) complex. This complex is composed of SUN (Sad1p, UNC-84) and KASH (Klarsicht/ANC-1/Syne Homology) family members, which are membrane proteins of the inner nuclear membrane and the outer nuclear membrane respectively. KASH proteins interact with cytoskeletal elements through their C-terminal extremity, including intermediate filaments, actin filaments and microtubules, whereas SUN proteins are connected to lamins by their nucleoplasmic tails. SUN and KASH proteins interact within the perinuclear space, forming a bridge that connects the cytoskeleton with the nucleoskeleton (). This connection has been shown to play a central role in many processes which hinge on correct nuclear positioning. Disruption of the LINC complex affects actin cytoskeletal organization and cell mechanics.Citation16-18 For example, the LINC complex is necessary during migration, allowing nuclear movements through both microtubule and actin-dependent movements.Citation13,19 Interestingly, recent advances demonstrate the LINC complex plays a central role when cells migrate in 3D, as it participates in the formation of a pressure gradient within the cell contributing to drive extension.Citation20 SUN and KASH proteins also play a role during cell division, and SUN proteins have been recently reported to participate in chromatin separation from the nuclear envelope and organization of the mitotic spindle.Citation21
Figure 1. The nucleoskeleton responds to mechanical tension. Application of tension on the LINC complex triggers SFK-dependent emerin phosphorylation (1). This reinforces the connection between the LINC complex and lamin A-C (2). Lamin A dephoshorylation may participate in this response. Emerin phosphorylation affects SRF-dependent gene expression (3).
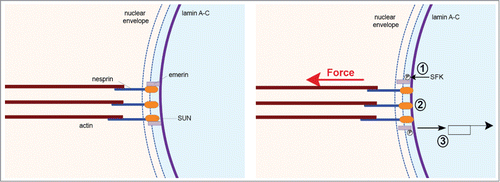
Creating a physical continuum between the cytoskeleton and the nucleoskeleton, the LINC complex can transmit not only tension generated by the cytoskeleton, but also mechanical stress applied to the cell surface. In a seminal paper, the Ingber group observed that application of tensional forces on cell surface adhesion receptors resulted in nuclear envelope distortion, demonstrating for the first time that mechanical stress can be transmitted from the extracellular matrix to the nucleus.Citation22 Since that early work other studies have shown that various mechanical stimuli, such as stretch or compression, can affect nuclear shapeCitation23–25 or can impact the organization of nucleoplasmic structures.Citation26 Although it has been demonstrated that nuclei experience force in various physiological and pathological situations,Citation27 the direct effect of force on the nucleus had not been examined until recently.
II-The Nucleus Can Respond to Mechanical Stress
When a nucleus is isolated from a cell or when the actin cytoskeleton is disrupted, nuclear size and shape change drastically, suggesting that the nucleus is mechanically constrained in intact cells. But does mechanical stress regulate nuclear structure and function? To tackle this question, we recently developed a method to stimulate isolated nuclei with tensional forces.Citation10 In order to mimic transmission of mechanical stress from the cytoskeleton to the nucleus, we used magnetic tweezers to apply pico newton pulses of force on the LINC complex component nesprin 1. Surprisingly, we observed decreasing nuclear strain in response to pulses of force, indicating local nuclear stiffening. We found that neither chromatin, nor nuclear actin were involved in this response to force. Interestingly, both lamin A-C and its binding partner emerin were necessary for nuclear stiffening, although they seem to play opposite roles in nuclear strain.Citation10 Whereas lamin A-C depletion caused an increase in nuclear deformation, nuclei isolated from emerin knockdown cells displayed decreased deformation in response to force on nesprin. Investigating further the molecular mechanism of the nuclear response to force, we found that emerin becomes tyrosine phosphorylated in response to tension on the LINC complex and this phosphorylation mediates the nuclear mechanical response to force by reinforcing the connection between lamin A-C and the LINC complex ().Citation10 One could envision various outcomes of this nuclear response to force. This strengthening response may limit the magnitude of nuclear deformation when the cytoskeleton is pulling on the LINC complex, conferring robust attachment to the cytoskeleton and thereby permitting efficient nuclear movement and positioning. Interestingly, we observed fewer bundles of actin filaments in cells expressing phosphoresistant emerin.Citation10 Consistent with this finding, other studies have reported defects in actin cytoskeletal organization in response to lamin A-C depletion or LINC complex disruption.Citation18,28,29 This nuclear stiffening response may also protect chromatin from excessive strain, in order to preserve chromatin organization and nuclear functions. Other work has reported similar nuclear stiffening in response to flow in intact cellsCitation30,31 Intriguingly, flow-induced nuclear hardening was associated with lamin A-C recruitment at the nuclear periphery.Citation31 Further investigations should reveal if lamin A-C recruitment in response to flow is regulated by emerin-dependent mechanisms and increased association to the LINC complex.
Additionally, we analyzed the consequences of this nuclear response to tension on gene expression. We found that expression of an emerin phosphoresistant mutant altered serum response factor (SRF) dependent transcription, suggesting that the nuclear response to force may impact gene expression.Citation10 This is consistent with the recent finding from the Lammerding group who showed that lamin A-C and emerin regulate megakaryoblastic leukemia 1 (MKL1, also known as MRTF) nuclear localization and SRF-dependent transcription.Citation32 Other work reported that lamin A-C mutation can impact YAP-dependent mechanosensing in myoblasts.Citation33 Together, these results indicate that the nucleoskeletal response to tension may play a central role in regulating mechanosensitive gene expression. Interestingly, Swift and colleagues reported that lamin A-C levels are regulated in response to matrix rigidity.Citation11 Remarkably, they observed that lamin A level scales with tissue elasticity in mice and human cells, partly due to lamin dephosphorylation and stabilization. This mechanism controlling gene expression seems to participate in matrix–directed stem cell differentiation. Applying shear stress directly to isolated nuclei, the authors observed that lamin A undergoes conformational changes in response to stress. However these conformational changes were not observed in stem cells cultured on matrix with different rigidities.Citation11 This could be the consequence of stress normalization due to the recruitment of lamin at the nuclear periphery in response to increased tension. Decreased phosphorylation of lamin A could result from tension-dependent regulation of a nuclear kinase or phosphatase specific for lamin A. We observed that emerin phosphorylation strengthens the connection between lamin A-C and the LINC complex in response to tension leading us to wonder whether emerin phosphorylation affects lamin A phosphorylation and triggers its recruitment to the LINC complex. Further work will be necessary to determine if tension-dependent emerin phosphorylation and lamin dephosphoryation are interlinked.
Conclusion
The idea that the nucleus may directly respond to mechanical stress was envisioned long ago.Citation8,9 Our results and those from the Discher group support this hypothesis and show that the nucleoskeleton is regulated by mechanical tension. Application of mechanical stress to isolated nuclei reveal that emerin and lamin A-C undergo post translational modifications, which impact nuclear structure and function. In intact cells, this nucleoskeletal response hinges on mechanical stress propagation mediated by the cytoskeleton and LINC complex. Adhesion maturation, nesprin isoform expression and lamin expression may result in substantial differences in stress transmission between the extracellular matrix and the nucleus, leading to different subsets of “mechanosensitive” nuclear structures and different responses depending on the cell type. Development of nuclear tension sensors, such as those developed for adhesion structures,Citation34,35 will be crucial to determine the amplitude and frequency of the stress experienced by the nucleus in physiological and pathological contexts. Interestingly we found that emerin phosphorylation can affect gene expression. Lamin and emerin have been shown to interact with chromatin,Citation36 indicating that the nucleoskeletal response to mechanical stress may also impact chromatin structure directly. This may explain observations of structural changes in Cajal bodies in response to force.Citation37 Observation of nucleoskeletal responses to tension in isolated nuclei suggests that nuclear proteins detect and transduce mechanical signals into biochemical signaling pathways which regulate nucleoskeletal proteins. But what are these nuclear mechanosensors? Tension on the LINC complex may result in emerin extension and conformational changes, resulting in its phosphorylation with no apparent changes in kinase activity. Similar mechanotransduction mechanism have been described for p130-Cas at focal adhesions.Citation38
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
CG is supported by grants from the Agence National de la Recherche (ANR-13-JSV1-0008), from the European Union Seventh Framework Programme (Marie Curie Career Integration n˚304162) and from the Institut National de la Santé et de la Recherche Médicale (INSERM). KB is supported by National Institutes of Health (GM029860).
References
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006; 126:677-89; PMID:16923388; http://dx.doi.org/10.1016/j.cell.2006.06.044
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005; 8:241-54; PMID:16169468; http://dx.doi.org/10.1016/j.ccr.2005.08.010
- Discher DE, Janmey P, Wang Y-L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005; 310:1139-43; PMID:16293750; http://dx.doi.org/10.1126/science.1116995
- Hoffman BD, Grashoff C, Schwartz MA. Dynamic molecular processes mediate cellular mechanotransduction. Nature 2011; 475:316-23; PMID:21776077; http://dx.doi.org/10.1038/nature10316
- Smutny M, Yap AS. Neighborly relations: cadherins and mechanotransduction. J. Cell Biol 2010; 189:1075-7; PMID:20584914; http://dx.doi.org/10.1083/jcb.201005151
- Hervy M, Hoffman L, Beckerle MC. From the membrane to the nucleus and back again: bifunctional focal adhesion proteins. Curr Opin Cell Biol 2006; 18:524-32; PMID:16908128; http://dx.doi.org/10.1016/j.ceb.2006.08.006
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature 2011; 474:179-83; PMID:21654799; http://dx.doi.org/10.1038/nature10137
- Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol 2009; 10:75-82; PMID:19197334; http://dx.doi.org/10.1038/nrm2594
- Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol 1982; 99:31-68; PMID:6892044; http://dx.doi.org/10.1016/0022-5193(82)90388-5
- Guilluy C, Osborne LD, Van Landeghem L, Sharek L, Superfine R, Garcia-Mata R, Burridge K. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat Cell Biol 2014; 16:376-81; PMID:24609268; http://dx.doi.org/10.1038/ncb2927
- Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 2013; 341:1240104; PMID:23990565; http://dx.doi.org/10.1126/science.1240104
- Morris NR. Nuclear positioning: the means is at the ends. Curr Opin Cell Biol 2003; 15:54-9; PMID:12517704; http://dx.doi.org/10.1016/S0955-0674(02)00004-2
- Gundersen GG, Worman HJ. Nuclear positioning. Cell 2013; 152:1376-89; PMID:23498944; http://dx.doi.org/10.1016/j.cell.2013.02.031
- Starr DA, Han M. Role of ANC-1 in tethering nuclei to the actin cytoskeleton. Science 2002; 298:406-9; PMID:12169658; http://dx.doi.org/10.1126/science.1075119
- Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol 2006; 172:41-53; PMID:16380439; http://dx.doi.org/10.1083/jcb.200509124
- Khatau, S. B., Hale CM, Stewart-Hutchinson PJ, Patel MS, Stewart CL, Searson PC, Hodzic D, Wirtz D. A perinuclear actin cap regulates nuclear shape. Proc Natl Acad Sci U S A 2009; 106:19017-22; PMID:19850871; http://dx.doi.org/10.1073/pnas.0908686106
- Luxton GWG, Starr DA. KASHing up with the nucleus: novel functional roles of KASH proteins at the cytoplasmic surface of the nucleus. Curr Opin Cell Biol 2014; 28:69-75; PMID:24704701; http://dx.doi.org/10.1016/j.ceb.2014.03.002
- Dahl KN, Kalinowski A. Nucleoskeleton mechanics at a glance. J Cell Sci 2011; 124:675-8; PMID:21321324; http://dx.doi.org/10.1242/jcs.069096
- Zhang X, Lei K, Yuan X, Wu X, Zhuang Y, Xu T, Xu R, Han M. SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron 2009; 64:173-87; PMID:19874786; http://dx.doi.org/10.1016/j.neuron.2009.08.018
- Petrie RJ, Koo H, Yamada KM. Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science 2014; 345:1062-5; PMID:25170155; http://dx.doi.org/10.1126/science.1256965
- Turgay Y, Champion L, Balazs C, Held M, Toso A, Gerlich DW, Meraldi P, Kutay U. SUN proteins facilitate the removal of membranes from chromatin during nuclear envelope breakdown. J. Cell Biol 2014; 204:1099-109; PMID:24662567; http://dx.doi.org/10.1083/jcb.201310116
- Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A 1997; 94:849-54; PMID:9023345; http://dx.doi.org/10.1073/pnas.94.3.849
- Lombardi,ML, Jaalouk DE, Shanahan CM, Burke B, Roux KJ, Lammerding J. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J Biol Chem 2011; 286:26743-53; PMID:21652697; http://dx.doi.org/10.1074/jbc.M111.233700
- Guilak F. Compression-induced changes in the shape and volume of the chondrocyte nucleus. J Biomech 1995; 28:1529-41; PMID:8666592; http://dx.doi.org/10.1016/0021-9290(95)00100-X
- Anno T, Sakamoto N, Sato M. Role of nesprin-1 in nuclear deformation in endothelial cells under static and uniaxial stretching conditions. Biochem Biophys Res Commun 2012; 424:94-9; PMID:22728879; http://dx.doi.org/10.1016/j.bbrc.2012.06.073
- Booth-Gauthier EA, Alcoser TA, Yang G, Dahl KN. Force-induced changes in subnuclear movement and rheology. Biophys J 2012; 103:2423-31; PMID:23260044; http://dx.doi.org/10.1016/j.bpj.2012.10.039
- Isermann P, Lammerding J. Nuclear mechanics and mechanotransduction in health and disease. Curr Biol CB 2013; 23:R1113-21; PMID:24355792; http://dx.doi.org/10.1016/j.cub.2013.11.009
- Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest 2004; 113:370-8; PMID:14755334; http://dx.doi.org/10.1172/JCI200419670
- Luxton GWG, Gomes ER, Folker ES, Vintinner E, Gundersen GG. Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science 2010; 329:956-9; PMID:20724637; http://dx.doi.org/10.1126/science.1189072
- Deguchi S, Maeda K, Ohashi T, Sato M. Flow-induced hardening of endothelial nucleus as an intracellular stress-bearing organelle. J Biomech 2005; 38:1751-9; PMID:16005465; http://dx.doi.org/10.1016/j.jbiomech.2005.06.003
- Philip JT, Dahl KN. Nuclear mechanotransduction:response of the lamina to extracellular stress with implications in aging. J Biomech 2008; 41:3164-70; PMID:18945430; http://dx.doi.org/10.1016/j.jbiomech.2008.08.024
- Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature 2013; 497:507-11; PMID:23644458; http://dx.doi.org/10.1038/nature12105
- Bertrand AT, Ziaei S, Ehret C, Duchemin H, Mamchaoui K, Bigot A, Mayer M, Quijano-Roy S, Desguerre I, Lainé J, et al. Cellular microenvironments reveal defective mechanosensing responses and elevated YAP signaling in LMNA-mutated muscle precursors. J Cell Sci 2014; 127:2873-84; PMID:24806962; http://dx.doi.org/10.1242/jcs.144907
- Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 2010; 466:263-6; PMID:20613844; http://dx.doi.org/10.1038/nature09198
- Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol CB 2013; 23:1024-30; PMID:23684974; http://dx.doi.org/10.1016/j.cub.2013.04.049
- Simon DN, Wilson KL. The nucleoskeleton as a genome-associated dynamic ‘network of networks’. Nat Rev Mol Cell Biol 2011; 12:695-708; PMID:21971041; http://dx.doi.org/10.1038/nrm3207
- Poh Y-C, Shevtsov SP, Chowdhury F, Wu DC, Na S, Dundr M, Wang N. Dynamic force-induced direct dissociation of protein complexes in a nuclear body in living cells. Nat Commun 2012; 3:866; PMID:22643893; http://dx.doi.org/10.1038/ncomms1873
- Sawada, Y., Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 2006; 127:1015-26; PMID:17129785; http://dx.doi.org/10.1016/j.cell.2006.09.044
