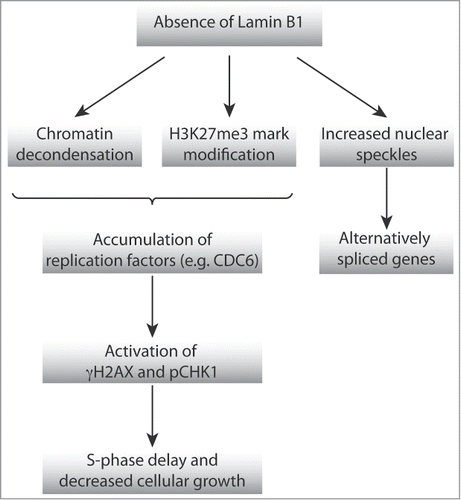
Abstract
Lamins constitute an integral structural component of the nuclear lamina. However, their impact on the structure and stability of chromosome territories, and on the regulation of gene expression is explored to a lesser extent. By 3D-FISH, Camps and colleagues showed that lamin B1 (LMNB1) is required for proper chromosome condensation in interphase nuclei, and deficiency of LMNB1 triggers the relocation of the epigenetic mark of facultative heterochromatin, H3K27me3, toward the interior of the nucleus. Additionally, LMNB1 repression slowed cellular growth due to S-phase delays and increased genomic instability. Finally, silencing of LMNB1 resulted in enlarged nuclear speckles and in extensive changes in alternative splicing of multiple genes. Altogether, the data suggest a central role of LMNB1 for the condensation of chromosome territories, for the distribution of heterochromatin, and for the regulation of gene expression and splicing.
Introduction
The nucleus is a compartmentalized, yet dynamic structure. While chromosomes are organized in territories (CT), the nuclear interchromatin compartment (IC) is a DNA free space expanding between chromatin domain clusters in which central cellular processes, including DNA replication, transcription and splicing occur.Citation1 The nuclear lamina (NL) is a meshwork of proteins located just inside the inner layer of the nuclear envelope involved in the maintenance of the physiological equilibrium of cells. The major constituents of the NL are the type-V intermediate filament proteins known as lamins, which include lamins A/C, lamin B1 and lamin B2. Lamin A and lamin C are the products of alternative splicing of LMNA, whereas LMNB1 and LMNB2 encode for the proteins lamin B1 and lamin B2, respectively. The NL is involved in many nuclear activities, including DNA replication and transcription, nuclear and chromatin organization, cell cycle regulation, cell development and differentiation, nuclear migration, and apoptosis.Citation2 Furthermore, the NL provides structure and support for the nucleus, controls the position of nuclear pore complexes, interacts with pericentrometric heterochromatin, and initiates mitosis.Citation3 It has also become evident that the NL, as well as several lamina-associated proteins, play a significant role in organizing the post-mitotic nucleus and in repositioning chromosomes after cell division. In addition, mutations in the genes encoding the lamins result in laminopathies, usually leading to premature aging conditions, such as the Hutchinson-Gilford progeria syndrome.Citation4
The goal of our study was to determine the extent to which the cross-talk between the NL and the chromatin affects global chromosome organization and gene expression.
Functional Compensation of The Nuclear Lamins
As in many cell types the NL might be composed of four different proteins, one cannot exclude the possibility that their respective functions are to a certain extent redundant. We therefore tested a potential compensation effect using immunoblot analysis. Using LMNB1-depleted DLD-1 cells, we could detect higher levels of LMNB2 and/or LMNA/C only in one out of three stable lines, indicating that the function of LMNB1 is not always compensated by increased expression of either one of the other three members of this family (). This interpretation is supported by a recent report showing that LMNB1 and LMNB2 have unique functions in the developing brain and that increased production of one B-type lamin cannot compensate for the loss of the other.Citation5
Figure 1. Functional consequences of silencing LMNB1. (A) Immunoblot showing decreased levels of LMNB1 in three single cell subclones (shown in duplicates). Note that in two out of three clones no major disruption of the NL was observed when assessed the expression of lamin B2 and lamin A/C. GAPDH was used as loading control. (B) Cell viability analysis of LMNB1-depleted cells after silencing LMNB2 and LMNA by siRNA assessed at 96 hours after transfection. AllStar Death was used as positive control.
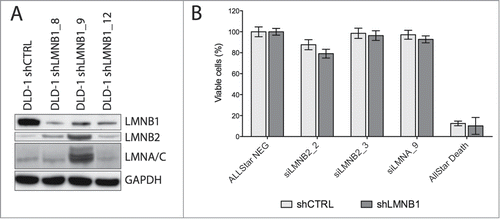
Nuclear lamins are essential proteins for the survival of mammalian somatic cells.Citation6 However, in our model, not only did LMNB1 depletion not induce activation of apoptosis or senescence, but co-transfection of LMNB1-depleted DLD-1 cells with short interference oligonucleotides against LMNB2 or LMNA barely decreases cellular viability, suggesting that cancer cells are less dependent on the physiological stoichiometry of this network (). This is in agreement with previous reports where it has been shown that B-type lamins are dispensable in differentiation of keratinocytes as well as in maintaining the nuclear structure of both keratinocytes and hepatocytes.Citation7,8 These authors suggested that lamin A/C might be complementing the biological roles of B-type lamins, at least in some cell types. On the other hand, while loss of LMNB1 is a senescence-associated biomarker in primary human and murine cultured normal fibroblasts induced by DNA damage, replicative exhaustion, or oncogene expression,Citation9,10 increased levels of LMNB1 in response to reactive oxygen species (ROS)-induced senescence through p38 MAPK activation have also been observed in ataxia telangiectasia cells.Citation11 This phenotype is not observed in LMNB1-depleted DLD-1 cells. Several hypotheses are possible to explain why these cells are so resistant to structural dysfunction of lamins: (i) nuclear membrane assembly after karyokinesis in these cancer cells is not subjected to strict cellular checkpoints, (ii) only little protein is enough to assemble the lamina mesh, (iii) proteins other than nuclear lamins are also involved in the maintenance of the nuclear structure.
Nuclear Distribution and Compaction of Chromosome Territories
Chromosomes are non-randomly distributed in the interphase nuclei of mammalian cells.Citation12 In fact, gene-rich chromosomes are positioned more toward the interior of the nucleus while gene-poor chromosomes locate toward the periphery. The extent to which the non-random positioning of chromosome territories is regulated by components of the NL remains largely elusive; however, it is reasonable to speculate that the positioning of chromosome territories, in particular those that are localized more peripherally, is altered upon disruption of the NL. This is supported by the close association between LMNB1 and heterochromatin, as well as the association of gene-rich chromosome regions in LMNA/C rich nuclear blebs formed in LMNB1 knockout HeLa cells.Citation13 In addition, genes that are transcriptionally deregulated in Lmnb1−/- mouse primary fibroblasts clustered in chromosomes whose location shifted in comparison to normal primary fibroblasts, suggesting a correlation between transcriptional activity and nuclear positioning.Citation14 In contrast to these findings, 3D-FISH analysis with whole chromosome painting probes for human chromosomes 18 (gene-poor) and 19 (gene-rich) in LMNB1-depleted DLD-1 cells showed that the chromosome distribution is maintained. This suggests, that at least in DLD-1 cells, nuclear positioning of chromosome territories is not strictly dependent on the presence of LMNB1. The question that naturally arises, but still remains unresolved, is whether chromosome territories are tethered to the NL, thus defining the nuclear structure or whether there are self-organizing principles that act as a driving force. And also, what factors other than the NL are implicated in organizing chromosome territories in the 3D space and whether these factors show functional cell type specificity.
We next wanted to explore whether depletion of LMNB1 had effects on chromatin structure. In order to ensure that only a few cells were in S-phase, so close to none of the cells had relaxed chromatin conformation due to DNA replication, we first treated the cultures with nocodazole to block cells at G2/M. Five hours after the removal of nocodazole we proceeded with fixation and 3D-FISH experiments. We observed that chromosome territories were considerably less compacted in the LMNB1-depleted cells ().Citation15 In order to quantify this observation, we applied 3D image reconstruction and measured volume and surface for chromosome 18 and 19 territories (). For both chromosomes, volume and surface were approximately 2-fold higher in LMNB1-depleted cells (P < 0 .0001). Analyses of the width, height and depth distances after 3D-FISH reconstruction further confirmed such enlargement of chromosome territories. Thus, these results suggest that LMNB1 plays a role in maintaining the chromatin compaction in the nucleus rather than preserving the radial positioning of chromosome territories.
Figure 2. Role of LMNB1 in the regulation of the chromosome architecture and DNA transcriptional activity. (A) 3D-FISH showing chromosome 18 labeled in Spectrum Orange and chromosome 19 in FITC in control and LMNB1-depleted DLD-1 cells. Bar, 5 μm. (B-C) Plots indicating the increase of both volume and surface of chromosome territories for HSA18 and HSA19 in the LMNB1-depleted cells. Measurements of the digitalized chromosome territories were taken to determine changes in volume and surface. (D) Scattered distribution of the histone mark H3K27me3 in LMNB1-depleted cells compare to the peripheral localization in control cells. Bar, 2 μm. (E) Quantification of the signal distribution of the epigenetic histone mark H3K27me3 determined by using Image J software (n = 600). (F) Functional GO terms significantly deregulated associated with transcriptionally altered spliced genes as a consequence of LMNB1 silencing.
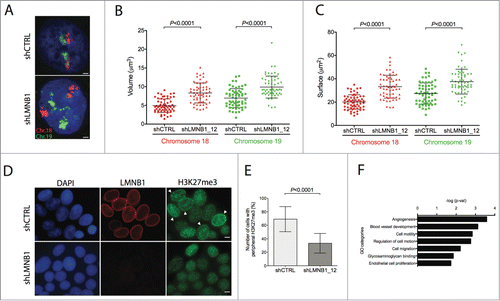
In order to explore the stoichiometry between chromosome condensation and the total nuclear area when LMNB1 is disrupted, we performed measurements of the nuclear size and compared to wild type DLD-1 cells. The fact that chromosome territories are larger in cells with depleted LMNB1 translates to a 65% increase in the nuclear size, most likely to accommodate the increase volume of the chromosome territories. It remains to be explored in further detail how enlargement of chromosome territories affects the ratio of the space of the chromatin territories compared to the interchromatin compartment, and to which extent the interaction between chromatin and the NL is disturbed. In fact, Camps and colleagues have shown a very drastic redistribution of the epigenetic mark H3K27me3, which is a surrogate for facultative heterochromatin and usually marks peripheral, repressed DNA. Under physiological conditions, H3K27me3 is present as a rim at the nuclear periphery and in the nucleoplasmatic compartment, surrounding polycomb repressive complexes involved in development and cellular differentiation. LMNB1-depleted cells showed a significantly reduced intensity of the peripheral distribution of H3K27me3, and the overall intensity of this epigenetic mark decreased as well (). Intriguingly, a similar phenotype has been identified in LMNA mutated cells, which showed a decrease in the localization of heterochromatin at the nuclear periphery. This loss of peripheral heterochromatin was accompanied with a reduction of the levels of H3K27m3 as well as H3K9m3 epigenetic marks.Citation16-18 In our model, though, the distribution and intensity of H3K9me3 remained stable in LMNB1-depleted cells, indicating that constitutive heterochromatin was not affected. Additionally, in LMNB1-depleted cells generated by replicative and oncogene-induced senescence, Shah and colleagues identified chromatin reorganization of the H3K27me3 and H3K4me3 distribution compared with proliferating cells by ChIP-seq.Citation19 Their analysis revealed large domain losses of H3K27me3 genome-wide, while H3K27me3-enriched genome span showed a striking overlap with H3K4me3.
Effect of The Nuclear Lamina on Regulating Gene Expression
DNA-lamin interactions at the nuclear membrane are determinants of chromatin organization and function. The nuclear periphery, and per its extension, the NL associated chromatin is perceived as a repressive environment for gene expression. DNA at the nuclear periphery is strongly associated with heterochromatin and gene silencing.Citation20-23 By ChIP-DamID experiments, it has also been shown that LMNB1-associated domains are characterized by low gene expression levels, and preferentially correlate well with the radial positioning of chromosomes.Citation24
Despite the fact that the majority of genes associated with the NL are repressed, it is also interesting to note the extent as to which chromatin dynamics of lamina-associated domains determines the expression of certain genes involved in processes such as differentiation.Citation25 Changes in chromatin-lamina interactions may reflect cell identity, as release from the repressed lamina meshwork may result in the activation of some genes. Thus, it seems that the NL can be selectively repressive of gene expression, and this selectivity might depend on several factors, e.g., epigenetic marks, proximity to the nuclear pore complex (NPC), etc. Recent reports have shown that nucleoporin-chromatin interactions at the NPC or within the nucleoplasm have an impact on gene expression in higher eukaryotes that was previously underappreciated.Citation26 These authors could demonstrate that a set of non-active genes interacted with the NPC, while nucleoporins within the nucleoplasm predominantly interacted with transcriptionally active genes, in particular those involved in developmental and cell cycle regulation.
By depleting the expression of LMNB1, thus inducing changes in the epigenetic mark H3K27me3, we hypothesized that genes located at the nuclear periphery would have a major impact on transcription, because they would be more profoundly affected by changes of facultative heterochromatin. In order to assess this hypothesis, we interrogated the levels of gene expression by whole genome microarray analysis. To our surprise, we observed only minor changes. In fact, unsupervised hierarchical clustering and principal component analysis were unable to differentiate LMNB1-depleted clones from empty vector transfected clones and wild-type cells. Using a ≥ 2-fold change threshold and a p-value ≤ 0.05, a total of 405 oligonucletotide features were deregulated, 129 showed overexpression and 276 were expressed at lower levels. As a proof of principle, one of the top 10 downregulated genes was LMNB1. These transcriptional changes were less prominent than results previously published in the comparison of Lmnb1−/- with wild-type mouse embryonic fibroblasts.Citation14 Indeed, there is very poor concordance in the top deregulated genes, most likely because of difference in the models and conditions of the studies. In addition, deregulated genes did not cluster to any particular region of the genome (e.g., enrichment for gene poor areas located toward the nuclear periphery, data not shown).
In order to further explore the transcriptional effects after depleting LMNB1, we focused our attention on the results from two previous reports. First, Tang and colleagues showed that depletion of LMNB1 resulted in structural changes in the morphology of nuclear speckles, which mimic those seen when pre-mRNA synthesis is inhibited.Citation27 Second, in cells of patients with progeria syndrome, progressive telomere damage led to extensive changes in alternative splicing in multiple genes other than LMNA.Citation28 When we looked at the structure of nuclear speckles of LMNB1-depleted DLD-1 cells by visualizing the splicing factor SC-35, we identified a significant increase in the amount of total nuclear speckles and an enlarged size of SC-35 foci compared to control cells. Thereafter, we proceeded to investigate whether genome-wide transcriptional changes could affect alternative splicing genes. We used the human Exon 1.0 ST array, a splicing-sensitive microarray with probes targeting individual exons or exon junctions. This design allows for the detection of RNA isoforms expressed while simultaneously profiling gene expression. Our results demonstrate that changes in alternative splicing were much more prominent in LMNB1-depleted cells. In order to identify an association of the 262 significantly deregulated alñternative spliced genes with putative functional roles, gene ontology enrichment analysis using the DAVID database was performed. The top affected pathways included angiogenesis, cell motility, and regulation of cell motion (), indicating the potential linkage between the NL and cellular structures related to cell mobility.
The mechanisms how the integrity of the NL might affect pre-mRNA processing machinery are not completely clear. Cao and colleagues suggested that telomere shortening in normal human fibroblasts during senescence played a causative role in the activation, not only of the cryptic splice site in LMNA, but also of many other splice sites.Citation28 In contrast to their findings, our cells did not show cellular senescence; however, depletion of LMNB1 also resulted in telomere shortening, which could lead to an increase of alternative splicing in multiple genes. It has been demonstrated that interaction between telomeres and the NL is not mediated by TTAGGG repeat binding factor, TRF.Citation29 Telomeres are localized in condensed sites distributed throughout the NL. Destabilization of heterochromatin by cryptic splicing of LMNA or depletion of LMNB1 could affect the repressive chromatin environment creating the telomere position effect (TPE), thereby exposing telomeres affecting telomeres and stability. However, the mechanism for increased splicing events due to telomere damage has yet to be elucidated.
LMNB1 Depletion Affects DNA Replication
Despite the absence of apoptotic or senescent cells, we observed decreased proliferative potential when LMNB1 was stably silenced. We thus performed an EdU assay to confirm decreased cellular proliferation. However, similar amounts of EdU positive cells were observed when comparing LMNB1-depleted with control cells, therefore we considered the possibility of changes of cells at certain stages of the cell cycle (). As EdU is incorporated in S-phase, higher amounts of DNA replicating cells would explain an equal amount of EdU cells, and a simultaneously reduced growth potential. Actually, time-lapse experiments showed that LMNB1-depleted cells spent extended time to go through S-phase (). As DNA replication takes place in S-phase, it is reasonable to argue that LMNB1 might affect DNA synthesis. In fact, lamins are known to play an important role in DNA replication. In the mid-nineties, intranuclear LMNB1 foci have been shown to bind to proliferating cell nuclear antigen (PCNA) during late S-phase in mouse 3T3 cells.Citation30 It was also shown that DNA replication is inhibited when Lamin B3 is depleted in Xenopus nuclei.Citation31,32 Furthermore, when dominant negative lamin mutants are introduced into interphase extracts containing Xenopus egg nuclei they result in a redistribution of the endogenous lamin network into intranuclear foci, which results in an inhibition of replication.Citation33,34 Transferring such nuclei into fresh extracts leads to normal lamin distribution and to the re-initiation of the elongation phase of replication. More recently, work from Tang and colleagues suggested that LMNB1 depletion resulted in a 50% decline of cells within S-phase, but increased the proportion of mid/late S-phase cells.Citation27 These same authors also stated that reduced LMNB1 expression resulted in an extended duration of S-phase and in the abortion of DNA synthesis before S-phase is complete. Taken together, these results suggest an important role of LMNB1 in DNA replication, mediated through the organization of intranuclear replication scaffolds. In accordance to our data, LMNB1-depleted DLD-1 cells showed a prolongation of S-phase, rather than a complete blockage of the cell cycle.
Figure 3. LMNB1 cooperates with the formation of DNA pre-replication complexes. (A) Cells were incubated with EdU for 30 or 60 minutes, respectively. After EdU incorporation cells were fixed and stained. EdU positive cells were counted (n > 400 ). (B) FACS analysis showing differences in cell cycle stages in shCTRL and shLMNB1 cells. (C) Cellular viability after LMNB1-depleted cells were transfected with siRNA against CDC6 and MCM3. (D) Immunoblot for CDC6 and the phosphorylated form of CHK1 showing an increase amount of these proteins when LMNB1 is silenced.
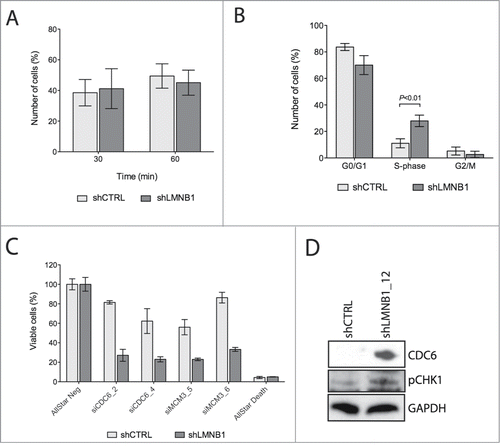
Intriguingly, when LMNB1 was depleted, DLD-1 cells became much more sensitive to transient silencing of key factors involved in licensing the initiation of the DNA replication process, such as CDC6 and MCM3 by siRNA (). Several reports investigated to which extent lamin-associated proteins actually participate in the regulation of processes such as DNA replication. Along with this, Martins and colleagues showed that the interaction between LAP2B and the chromatin-associated factor HA95 is required to bind CDC6 to the pre-replication complex of the DNA during late G1 phase.Citation35 Data derived from follow-up studies in our laboratory provide evidence that LMNB1-depleted cells show an increase of the DNA associated protein CDC6, which would suggest that the arrest in S-phase happens during the formation of the pre-replication complex.Citation36 Granted that CDC6 physically interacts with ATR aiding the activation of replication checkpoints,Citation37 it seems reasonable that the increased amount of CDC6, together with activation of the phosphorylated form of H2AX, result in the activation of the replication stress-response factor CHK1 ().
Summary and Perspective
We have observed that the absence of LMNB1 induced changes of the higher-order chromatin organization, including decondensation of chromosome territories, but not chromosome positioning, and modification of histone marks associated with heterochromatin (). In addition, we have observed telomere attrition, potentially leading to increased alternative splicing changes in multiple genes. Finally, LMNB1-depleted cells showed a prolonged S-phase, most likely due to activation of replication checkpoints. Altogether, these data indicate that the absence of LMNB1 causes a phenotype that recapitulates the one observed as a consequence of dysregulation of LMNA, suggesting that a delicate balance of NL components is needed to maintain the nuclear structure and function.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are grateful to Dr. Thomas Cremer for critical review and helpful discussion. The authors would also like to thank Dr. Abdel Elkalhoun, director of the Microarray Core of the National Human Genome Research Institute, Dr. Stephen M Wincovitch and Dr. Amalia Dutra from the Cytogenetics and Microscopy Core of the National Human Genome Research Institute, and Barbara J Taylor, Facility Head of the FACS Core Laboratory of the National Cancer Institute for technical support. The authors would also like to thank Buddy Chen for editorial assistance.
Funding
This project was supported by the Intramural Research Program of the NIH, National Cancer Institute. JC is supported by Instituto de Salud Carlos III (CP13/00160).
References
- Cremer T, Cremer M. Chromosome territories. Cold Spring Harb Perspect Biol 2010; 2:a003889; PMID:20300217; http://dx.doi.org/10.1101/cshperspect.a003889
- Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev 2008; 22:832-53; PMID:18381888; http://dx.doi.org/10.1101/gad.1652708
- Goldman RD, Gruenbaum Y, Moir RD, Shumaker DK, Spann TP. Nuclear lamins: building blocks of nuclear architecture. Genes Dev 2002; 16:533-47; PMID:11877373; http://dx.doi.org/10.1101/gad.960502
- Broers JL, Ramaekers FC, Bonne G, Yaou RB, Hutchison CJ. Nuclear lamins: laminopathies and their role in premature ageing. Physiol Rev 2006; 86:967-1008; PMID:16816143; http://dx.doi.org/10.1152/physrev.00047.2005
- Lee JM, Tu Y, Tatar A, Wu D, Nobumori C, Jung HJ, Yoshinaga Y, Coffinier C, de Jong PJ, Fong LG, et al. Reciprocal knock-in mice to investigate the functional redundancy of lamin B1 and lamin B2. Mol Biol Cell 2014; 25:1666-75; PMID:24672053; http://dx.doi.org/10.1091/mbc.E14-01-0683
- Harborth J, Elbashir SM, Bechert K, Tuschl T, Weber K. Identification of essential genes in cultured mammalian cells using small interfering RNAs. J Cell Sci 2001; 114:4557-65; PMID:11792820
- Yang SH, Chang SY, Yin L, Tu Y, Hu Y, Yoshinaga Y, de Jong PJ, Fong LG, Young SG. An absence of both lamin B1 and lamin B2 in keratinocytes has no effect on cell proliferation or the development of skin and hair. Hum Mol Genet 2011; 20:3537-44; PMID:21659336; http://dx.doi.org/10.1093/hmg/ddr266
- Yang SH, Jung HJ, Coffinier C, Fong LG, Young SG. Are B-type lamins essential in all mammalian cells? Nucleus 2011; 2:562-9; PMID:22127257; http://dx.doi.org/10.4161/nucl.2.6.18085
- Freund A, Laberge RM, Demaria M, Campisi J. Lamin B1 loss is a senescence-associated biomarker. Mol Biol Cell 2012; 23:2066-75; PMID:22496421; http://dx.doi.org/10.1091/mbc.E11-10-0884
- Shimi T, Butin-Israeli V, Adam SA, Hamanaka RB, Goldman AE, Lucas CA, Shumaker DK, Kosak ST, Chandel NS, Goldman RD. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev 2011; 25:2579-93; PMID:22155925; http://dx.doi.org/10.1101/gad.179515.111
- Barascu A, Le Chalony C, Pennarun G, Genet D, Imam N, Lopez B, Bertrand P. Oxidative stress induces an ATM-independent senescence pathway through p38 MAPK-mediated lamin B1 accumulation. EMBO J 2012; 31:1080-94; PMID:22246186; http://dx.doi.org/10.1038/emboj.2011.492
- Cremer T, Cremer C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet 2001; 2:292-301; PMID:11283701; http://dx.doi.org/10.1038/35066075
- Shimi T, Pfleghaar K, Kojima S, Pack CG, Solovei I, Goldman AE, Adam SA, Shumaker DK, Kinjo M, Cremer T, et al. The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Genes Dev 2008; 22:3409-21; PMID:19141474; http://dx.doi.org/10.1101/gad.1735208
- Malhas A, Lee CF, Sanders R, Saunders NJ, Vaux DJ. Defects in lamin B1 expression or processing affect interphase chromosome position and gene expression. J Cell Biol 2007; 176:593-603; PMID:17312019; http://dx.doi.org/10.1083/jcb.200607054
- Camps J, Wangsa D, Falke M, Brown M, Case CM, Erdos MR, Ried T. Loss of lamin B1 results in prolongation of S phase and decondensation of chromosome territories. FASEB J 2014; 28:3423-34; PMID:24732130; http://dx.doi.org/10.1096/fj.14-250456
- Columbaro M, Capanni C, Mattioli E, Novelli G, Parnaik VK, Squarzoni S, Maraldi NM, Lattanzi G. Rescue of heterochromatin organization in Hutchinson-Gilford progeria by drug treatment. Cell Mol Life Sci 2005; 62:2669-78; PMID:16261260; http://dx.doi.org/10.1007/s00018-005-5318-6
- Goldman RD, Shumaker DK, Erdos MR, Eriksson M, Goldman AE, Gordon LB, Gruenbaum Y, Khuon S, Mendez M, Varga R, et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA 2004; 101:8963-8; PMID:15184648; http://dx.doi.org/10.1073/pnas.0402943101
- Shumaker DK, Dechat T, Kohlmaier A, Adam SA, Bozovsky MR, Erdos MR, Eriksson M, Goldman AE, Khuon S, Collins FS, et al. Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc Natl Acad Sci U S A 2006; 103:8703-8; PMID:16738054; http://dx.doi.org/10.1073/pnas.0602569103
- Shah PP, Donahue G, Otte GL, Capell BC, Nelson DM, Cao K, Aggarwala V, Cruickshanks HA, Rai TS, McBryan T, et al. Lamin B1 depletion in senescent cells triggers large-scale changes in gene expression and the chromatin landscape. Genes Dev 2013; 27:1787-99; PMID:23934658; http://dx.doi.org/10.1101/gad.223834.113
- Finlan LE, Sproul D, Thomson I, Boyle S, Kerr E, Perry P, Ylstra B, Chubb JR, Bickmore WA. Recruitment to the nuclear periphery can alter expression of genes in human cells. PLoS Genet 2008; 4:e1000039; PMID:18369458; http://dx.doi.org/10.1371/journal.pgen.1000039
- Kourmouli N, Theodoropoulos PA, Dialynas G, Bakou A, Politou AS, Cowell IG, Singh PB, Georgatos SD. Dynamic associations of heterochromatin protein 1 with the nuclear envelope. EMBO J 2000; 19:6558-68; PMID:11101528; http://dx.doi.org/10.1093/emboj/19.23.6558
- Reddy KL, Zullo JM, Bertolino E, Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature 2008; 452:243-7; PMID:18272965; http://dx.doi.org/10.1038/nature06727
- Shaklai S, Amariglio N, Rechavi G, Simon AJ. Gene silencing at the nuclear periphery. FEBS J 2007; 274:1383-92; PMID:17489096; http://dx.doi.org/10.1111/j.1742-4658.2007.05697.x
- Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, Eussen BH, de Klein A, Wessels L, de Laat W, et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature 2008; 453:948-51; PMID:18463634; http://dx.doi.org/10.1038/nature06947
- Peric-Hupkes D, Meuleman W, Pagie L, Bruggeman SW, Solovei I, Brugman W, Gräf S, Flicek P, Kerkhoven RM, van Lohuizen M, et al. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol Cell 2010; 38:603-13; PMID:20513434; http://dx.doi.org/10.1016/j.molcel.2010.03.016
- Kalverda B, Pickersgill H, Shloma VV, Fornerod M. Nucleoporins directly stimulate expression of developmental and cell-cycle genes inside the nucleoplasm. Cell 2010; 140:360-71; PMID:20144760; http://dx.doi.org/10.1016/j.cell.2010.01.011
- Tang CW, Maya-Mendoza A, Martin C, Zeng K, Chen S, Feret D, Wilson SA, Jackson DA. The integrity of a lamin-B1-dependent nucleoskeleton is a fundamental determinant of RNA synthesis in human cells. J Cell Sci 2008; 121:1014-24; PMID:18334554; http://dx.doi.org/10.1242/jcs.020982
- Cao K, Blair CD, Faddah DA, Kieckhaefer JE, Olive M, Erdos MR, Nabel EG, Collins FS. Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J Clin Invest 2011; 121:2833-44; PMID:21670498; http://dx.doi.org/10.1172/JCI43578
- Luderus ME, van Steensel B, Chong L, Sibon OC, Cremers FF, de Lange T. Structure, subnuclear distribution, and nuclear matrix association of the mammalian telomeric complex. J Cell Biol 1996; 135:867-81; PMID:8922373; http://dx.doi.org/10.1083/jcb.135.4.867
- Moir RD, Montag-Lowy M, Goldman RD. Dynamic properties of nuclear lamins: lamin B is associated with sites of DNA replication. J Cell Biol 1994; 125:1201-12; PMID:7911470; http://dx.doi.org/10.1083/jcb.125.6.1201
- Lopez-Soler RI, Moir RD, Spann TP, Stick R, Goldman RD. A role for nuclear lamins in nuclear envelope assembly. J Cell Biol 2001; 154:61-70; PMID:11448990; http://dx.doi.org/10.1083/jcb.200101025
- Shumaker DK, Solimando L, Sengupta K, Shimi T, Adam SA, Grunwald A, Strelkov SV, Aebi U, Cardoso MC, Goldman RD. The highly conserved nuclear lamin Ig-fold binds to PCNA: its role in DNA replication. J Cell Biol 2008; 181:269-80; PMID:18426975; http://dx.doi.org/10.1083/jcb.200708155
- Ellis DJ, Jenkins H, Whitfield WG, Hutchison CJ. GST-lamin fusion proteins act as dominant negative mutants in Xenopus egg extract and reveal the function of the lamina in DNA replication. J Cell Sci 1997; 110 (Pt 20):2507-18; PMID:9372440
- Spann TP, Moir RD, Goldman AE, Stick R, Goldman RD. Disruption of nuclear lamin organization alters the distribution of replication factors and inhibits DNA synthesis. J Cell Biol 1997; 136:1201-12; PMID:9087437; http://dx.doi.org/10.1083/jcb.136.6.1201
- Martins S, Eikvar S, Furukawa K, Collas P. HA95 and LAP2 beta mediate a novel chromatin-nuclear envelope interaction implicated in initiation of DNA replication. J Cell Biol 2003; 160:177-88; PMID:12538639; http://dx.doi.org/10.1083/jcb.200210026
- Speck C, Stillman B. Cdc6 ATPase activity regulates ORC x Cdc6 stability and the selection of specific DNA sequences as origins of DNA replication. J Biol Chem 2007; 282:11705-14; PMID:17314092; http://dx.doi.org/10.1074/jbc.M700399200
- Yoshida K, Sugimoto N, Iwahori S, Yugawa T, Narisawa-Saito M, Kiyono T, Fujita M. CDC6 interaction with ATR regulates activation of a replication checkpoint in higher eukaryotic cells. J Cell Sci 2010; 123:225-35; PMID:20048340; http://dx.doi.org/10.1242/jcs.058693