ABSTRACT
Nuclear pore proteins interact dynamically with chromatin to regulate gene activities. A key question is how nucleoporin interactions mechanistically alter a gene's intranuclear position and transcriptional output. We reported recently on a direct interaction between the nuclear pore-associated TREX-2 complex and promoter-bound Mediator. This highlights how nuclear-pore associated adaptors gain regulatory access to the core transcription machinery. In this Extra View, we discuss an additional implication that arises from our work and the recent literature: how promoter elements may regulate mRNA metabolism beyond transcription initiation.
Introduction
Nuclear pore complexes (NPCs) are gatekeepers at the nuclear envelope mediating traffic between the nucleus and cytoplasm. Beyond transport, NPCs dynamically interact with chromatin to regulate its architecture and activity. Genome-wide studies in yeast, flies and humans have shown that nuclear pore proteins (nucleoporins) interact with numerous active genes, but also with heterochromatin boundaries and repressed genes.Citation1 The TREX-2 (Transcription-coupled Export) complex is conserved from yeast to humans and associates with the NPC via the NPC basket structure.Citation2,3 The S.cerevisiae TREX-2 complex was found to regulate a surprisingly diverse number of chromatin-associated processes including transcriptionCitation4,5 and mRNA export,Citation2,6 targeting of activated genes to NPCs,Citation7 DNA replication,Citation8 and genome stability.Citation9 Yeast TREX-2 is composed of Sac3, Thp1, Sem1, Sus1 and Cdc31 and can be divided into a PCI domain part (a protein scaffold also found in the Proteasome lid, CSN, and eIF3 complexes) and an NPC-basket anchor element (). This general domain architecture is conserved between yeast and human TREX-2.Citation10,11 Like in yeast, metazoan TREX-2 plays a role in mRNA export,Citation12 although its role in transcription remains to be fully explored. The molecular mechanism by which TREX-2 impacts on gene expression was a major question in the field, because of its implications for understanding how nucleoporins regulate chromatin architecture and function.
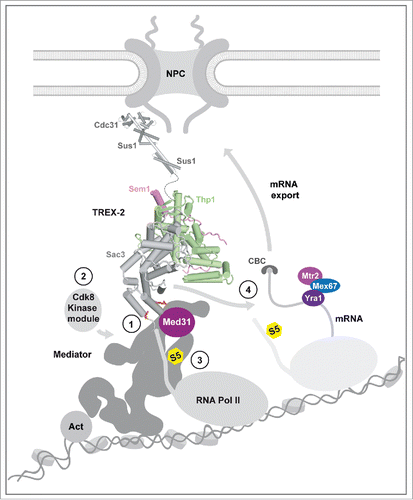
Figure 1. Mechanism for a relay between TREX-2, Mediator, and Pol II. Model depicts the putative overall topology of the NPC-associated TREX-2 complex and its interaction with Mediator. Mediator cartoon follows the outline of the yeast Mediator cryo-EM structure. TREX-2 is subdivided into an NPC-anchor domain (upper part) and a PCI domain part (lower part). (1) Docking to Mediator involves a conserved pair of basic Sac3 residues (red sticks) and the Med31 submodule. (2) TREX-2 regulates Cdk8 kinase module association. (3) TREX-2 impacts on RNA Pol II CTD Ser5 phosphorylation (S5; yellow). (4) TREX-2 also influences mRNA export via the same PCI surface used for interacting with Mediator. Other mRNA adaptor/export proteins are indicated. Transition between Pol II initiation and early elongation is shown. Act : transcription activator; CBC: Cap Binding Complex.
Mediator, on the other hand, is the central scaffold for transcription initiation in eukaryotes. Transcription initiation at protein-coding genes requires RNA Pol II, general transcription factors, and Mediator. Assembly of these factors on promoter DNA results in a core initiation complex, which recruits the TFIIH complex to unwind DNA and to phosphorylate the RNA Polymerase (Pol) II C-terminal domain (CTD) at Ser5. Mediator is recruited by transcription activators, stabilizes the initiation complex and stimulates TFIIH kinase activity.Citation13,14
We recently reported that TREX-2 (1) interacts directly with Mediator via a specific interface of its PCI domain, (2) regulates Mediator association with the Cdk8 kinase, a central activity switch on Mediator and (3) influences RNA Pol II phosphorylation at Ser5, the defining mark of transcription initiation ().Citation15 This has been a major advance in elucidating the biological function of TREX-2, as it uncovered a direct role of TREX-2 in modulating the core transcription machinery. TREX-2 and Mediator were shown to co-regulate a distinct subset of constitutive (e.g. the superpathway of sulfur amino acid biogenesis) and highly inducible (e.g., GAL1 and HXK1) genes in yeast. Moreover, the interaction between the 2 complexes is required for the dynamic targeting of activated genes to NPCs. In essence, Mediator is a relay for communication between TREX-2 and Pol II. Here, we discuss the emerging role of promoters in regulating different aspects of mRNA metabolism and how promoter-bound Mediator and TREX-2 could impact on these transactions.
RNA Pol II-dependent coupling events during early transcription
The biogenesis of eukaryotic mRNAs involves not only pre-mRNA synthesis by RNA Pol II, but also cotranscriptional RNA processing comprising 5′ capping, intron splicing, 3′ RNA cleavage and polyadenylation (3′ processing). The mature mRNA is packaged with RNA-binding proteins into messenger ribonucleoprotein particles (mRNPs), which is essential for its export to the cytoplasm and protein synthesis.Citation16,17 The prevailing view is that, in contrast to a simple linear assembly line, gene expression machines are extensively coupled to ensure that each step in gene expression is timely, accurate and efficient. Coupling is a term that generally refers to the enhancement of one step in gene expression by another and can reflect physical and/or functional interactions between factors. This topic has been the subject of several excellent reviews.Citation18-20 Here, we will focus on Pol II-dependent coupling events that accompany the earliest steps of transcription.
A prime example for sequential coupling reactions is the RNA Pol II C-terminal domain (CTD), which undergoes dynamic phosphorylation as the Pol II progresses through initiation, elongation, and termination.Citation21 CTD phosphorylation creates sequential “landing pads” for numerous mRNP components.Citation18 The first mark to appear on the Pol II CTD is the phosphorylation of Ser5, which is catalyzed by yeast Kin28 (human CDK7), a component of TFIIH. Mediator stimulates Ser5 CTD phosphorylation,Citation22 which is thought to promote the eviction of Pol II from the promoter-bound preinitiation complex and transition to elongation.Citation23-25 Beyond promoting Pol II promoter escape, Ser5 phosphorylation is linked to the capping of the pre-mRNA transcript, the very first event in co-transcriptional RNA processing.Citation26 Recruitment of the capping enzyme (yeast Cet1/Ceg1) involves binding of Ceg1 to the Ser5 phosphorylated CTD of Pol II, which occurs during transcription initiation. Notably, the capping enzyme docks onto the Pol II wall in immediate proximity of the mRNA exit tunnel of Pol II. mRNA has to be at least 15 nucleotides in length to reach the Pol II surface where capping can occur. Thus, the first steps in pre-mRNA capping take place when the nascent RNA reaches the polymerase surface. The physical coupling of capping enzymes with RNA Pol II nicely illustrates how cells achieve seamless protection of RNA from degradation by 5′-exonucleases right from the start of transcription.
A common model is that acquisition of export competence begins with the recruitment of the conserved TREX (Transcription-coupled Export) complex.Citation16,17 TREX also functions in various aspects of co-transcriptional mRNP formation, yet, exhibits no protein homology with TREX-2. In yeast, TREX is continuously loaded onto emerging transcripts during transcription elongation, which facilitates folding of nascent transcripts into mRNPs and helps to recruit additional RNA-binding proteins.Citation27-29 When exactly the first mRNA export adaptor binds to the nascent mRNA is not well established in yeast, but intriguing insights came from studies in Xenopus already in 1990, which showed that the 5′ cap and the interacting cap binding complex (CBC20/CBC80 in metazoa) are required for mRNA export.Citation30 Furthermore, the human TREX complex is recruited in a cap- and splicing-dependent manner to the 5′ end of the mRNA. This recruitment requires the cap binding subunit CBP80, which interacts directly with human TREX.Citation31 These observations highlight the fact that the earliest steps of transcription are characterized by several interconnected events with the potential to imprint the destination of the transcript.
Our study revealed an unexpected link between TREX-2 and Mediator and the phosphorylation status of RNA Pol II. Specifically, we found that a loss of TREX-2 function leads to an increase of Ser5 phosphorylation of the Pol II CTD, which indicated that TREX-2 may have a regulatory impact when bound to Mediator. In support of this notion, we found that TREX-2 binds directly to the Med31/Med7 submodule, which is positioned in proximity to the Pol II CTD binding site on Mediator. Whether and how the impact of TREX-2 on the Pol II “phospho-code” plays a role in choreographing subsequent mRNA processing events will require further analysis.
Role of promoters in mRNA metabolism
Since TREX-2 is necessary for mRNA export, its interaction with promoter-bound Mediator raises the interesting question whether the mRNA export function of TREX-2 is linked to Mediator's role in initiating transcription. This would imply that cells connect the earliest and latest steps of nuclear gene expression. Notably, a growing number of studies has reported promoter-dependent effects on downstream mRNA metabolism, which altogether raise the question whether transcription initiation is indeed a promoter's only task. In 2011, 2 studies showed that transcription factors and DNA promoters can directly influence the stability of the transcripts that they produce, independent of the transcript sequence. One paper described destabilization of SWI5 and CLB2 mRNAs at the onset of metaphase in S.cerevisiae.Citation32 This stability switch requires promoter-dependent deposition of the mitotic exit kinase Dbf2 on both mRNAs during transcription. The second paper described a similar decay-enhancing effect mediated by the transcription factor Rap1.Citation33 Rap1 stimulates both the synthesis and the decay of a specific population of endogenous mRNAs suggesting that Rap1 association with the promoter affects the composition of the exported mRNP, which in turn regulates mRNA decay in the cytoplasm. More recently, yeast promoter sequences were shown to direct both the localization of mRNAs and their translation during starvation.Citation34 S.cerevisiae responds to glucose starvation by translating a subset of transcriptionally activated mRNAs while decreasing translation of others. The information specifying the differential localization and protein production of these 2 classes of mRNAs was found to depend on specific promoters targeted by heat-shock factor 1 (Hsf1). Last but not least, promoter-proximal pausing was shown to be involved in regulating alternative cleavage and polyadenylation of mRNAs in Drosophila neurons.Citation35 In flies, the RNA-binding protein ELAV (Embryonic Lethal Abnormal Visual System) inhibits RNA processing at proximal polyadenylation sites, thereby promoting the formation of exceptionally long 3′UTRs. Paused Pol II promotes recruitment of ELAV and leads to extended genes, and this effect is regulated by the promoter. In sum, promoters can influence gene expression by mechanisms other than transcriptional control, perhaps through mediating the loading of proteins onto mRNAs.
The emerging theme of all these studies is an impact of promoters on gene expression that clearly goes beyond transcription initiation. Promoters appear to reach out to influence later steps of mRNA processing and mRNP formation. A central assumption underlying promoter-coupled mRNA processing events is that promoter elements are somehow brought into physical proximity with the nascent mRNA to allow several types of molecular cross-talk. However, the molecular details of these transactions are largely unclear.
Mediator as an emerging regulator of mRNA processing
Given the influence of promoter-bound Mediator on Pol II Ser5 phosphorylation and the role of the CTD in scaffolding various mRNA processing and packaging events, it is conceivable that Mediator impacts on the mRNA life cycle through setting the proper RNA Pol II “phospho code.” But is there any evidence for a direct role of Mediator in dictating mRNA fate? Surprising insights came from a study, which reported direct physical interactions between the Mediator subunit MED23 and the hnRNP L protein, a regulator of alternative splicing in metazoans.Citation36 Functionally, MED23 regulates a subset of alternative splicing and alternative cleavage and polyadenylation events. These results suggested a crosstalk between Mediator and the splicing machinery and advanced the idea that Mediator could be involved in coupling transcription initiation with mRNA processing possibly by serving as a stepping-stone for splicing-related factors. Although yeast has no MED23 ortholog, the role of Mediator in scaffolding diverse promoter-functions could be a conserved property. We propose that Mediator's interaction with TREX-2 could similarly mediate the coupling of transcription initiation and export of specific mRNAs through the nuclear pore.
The role of TREX-2 in transcription and mRNA export
A surprising finding of our study was that TREX-2 employs identical Sac3 PCI domain residues to promote both transcription and mRNA export. TREX-2 directly interacts with the Mediator Med31/Med7 submodule via conserved polar residues on the Sac3 PCI domain surface. Notably, TREX-2 uses the same polar residues to contact Mediator and to regulate mRNA export. This could indicate that TREX-2 is involved in a sequential coupling reaction, in which binding to Med31/Med7 is displaced by an interaction with a yet unknown mRNP factor. TREX-2 could therefore either promote the loading of factors onto the nascent mRNA or become itself loaded onto RNA. These possibilities remain to be explored, but are conceivable given that mutually exclusive protein-protein and protein-RNA interactions are a common feature of many co-transcriptional events. Such handover reactions are thought to impose directionality and a sequential order to mRNP formation.Citation20
TREX-2 also binds to the general mRNA exporter Mex67/Mtr2 Citation2 as well as DNA or RNA in vitro.Citation11 Moreover, TREX-2 was suggested to undergo large-scale conformational changes from extended to more compact forms in vitro.Citation37 The functional relevance of this structural plasticity and the spatiotemporal order of TREX-2 interactions with Mediator, Mex67/Mtr2 and the NPC basket during gene expression will have to be explored in detail. Such studies will be essential to understand the precise mechanism by which TREX-2 promotes the coupling of transcription and mRNA export in cells.
Conclusion and perspective
We have recently described a direct link between the TREX-2 complex and Mediator using structural biology, in vitro biochemical reconstitution and various in vivo functional analyses. These findings are key to understanding how NPC-associated adaptors modulate transcription, mRNA export and nuclear gene localization. This has revealed a molecular mechanism of TREX-2 function and opens new avenues for exploring how promoters can regulate multiple aspects of mRNA biology beyond transcription initiation.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Jakub Cibulka for comments on the manuscript.
Funding
A. Köhler is funded by an ERC grant (281354; NPC GENEXPRESS) and a START grant from the Austrian Science Fund (FWF; Y557-B11).
References
- Ibarra A, Hetzer MW. Nuclear pore proteins and the control of genome functions. Gen Dev 2015; 29:337-49; PMID:25691464; http://dx.doi.org/10.1101/gad.256495.114
- Fischer T, Strasser K, Racz A, Rodriguez-Navarro S, Oppizzi M, Ihrig P, Lechner J, Hurt E. The mRNA export machinery requires the novel Sac3p-Thp1p complex to dock at the nucleoplasmic entrance of the nuclear pores. EMBO J 2002; 21:5843-52; PMID:12411502; http://dx.doi.org/10.1093/emboj/cdf590
- Jani D, Valkov E, Stewart M. Structural basis for binding the TREX2 complex to nuclear pores, GAL1 localisation and mRNA export. Nucleic Acids Res 2014; 42(10):6686-97; PMID:24705649
- Gallardo M, Luna R, Erdjument-Bromage H, Tempst P, Aguilera A. Nab2p and the Thp1p-Sac3p complex functionally interact at the interface between transcription and mRNA metabolism. J Biol Chem 2003; 278:24225-32; PMID:12702719; http://dx.doi.org/10.1074/jbc.M302900200
- Santos-Pereira JM, Garcia-Rubio ML, Gonzalez-Aguilera C, Luna R, Aguilera A. A genome-wide function of THSC/TREX-2 at active genes prevents transcription-replication collisions. Nucleic acids Res 2014; 42(19):12000-14; PMID:25294824
- Lei EP, Stern CA, Fahrenkrog B, Krebber H, Moy TI, Aebi U, Silver PA. Sac3 is an mRNA export factor that localizes to cytoplasmic fibrils of nuclear pore complex. Mol Biol Cell 2003; 14:836-47; PMID:12631707; http://dx.doi.org/10.1091/mbc.E02-08-0520
- Cabal GG, Genovesio A, Rodriguez-Navarro S, Zimmer C, Gadal O, Lesne A, Buc H, Feuerbach-Fournier F, Olivo-Marin JC, Hurt EC, et al. SAGA interacting factors confine sub-diffusion of transcribed genes to the nuclear envelope. Nature 2006; 441:770-3; PMID:16760982; http://dx.doi.org/10.1038/nature04752
- Bermejo R, Capra T, Jossen R, Colosio A, Frattini C, Carotenuto W, Cocito A, Doksani Y, Klein H, Gomez-Gonzalez B, et al. The replication checkpoint protects fork stability by releasing transcribed genes from nuclear pores. Cell 2011; 146:233-46; PMID:21784245; http://dx.doi.org/10.1016/j.cell.2011.06.033
- Gonzalez-Aguilera C, Tous C, Gomez-Gonzalez B, Huertas P, Luna R, Aguilera A. The THP1-SAC3-SUS1-CDC31 complex works in transcription elongation-mRNA export preventing RNA-mediated genome instability. Mol Biol Cell 2008; 19:4310-8; PMID:18667528; http://dx.doi.org/10.1091/mbc.E08-04-0355
- Jani D, Lutz S, Hurt E, Laskey RA, Stewart M, Wickramasinghe VO. Functional and structural characterization of the mammalian TREX-2 complex that links transcription with nuclear messenger RNA export. Nucleic acids Res 2012; 40:4562-73; PMID:22307388; http://dx.doi.org/10.1093/nar/gks059
- Ellisdon AM, Dimitrova L, Hurt E, Stewart M. Structural basis for the assembly and nucleic acid binding of the TREX-2 transcription-export complex. Nat Struct Mol Biol 2012; 19:328-36; PMID:22343721; http://dx.doi.org/10.1038/nsmb.2235
- Umlauf D, Bonnet J, Waharte F, Fournier M, Stierle M, Fischer B, Brino L, Devys D, Tora L. The human TREX-2 complex is stably associated with the nuclear pore basket. J Cell Sci 2013; 126(Pt 12):2656-67; PMID:23591820
- Allen BL, Taatjes DJ. The Mediator complex: a central integrator of transcription. Nat Rev Mol Cell Biol 2015; 16:155-66; PMID:25693131; http://dx.doi.org/10.1038/nrm3951
- Tsai KL, Tomomori-Sato C, Sato S, Conaway RC, Conaway JW, Asturias FJ. Subunit Architecture and Functional Modular Rearrangements of the Transcriptional Mediator Complex. Cell 2014; 157(6):1430-44
- Schneider M, Hellerschmied D, Schubert T, Amlacher S, Vinayachandran V, Reja R, Pugh BF, Clausen T, Kohler A. The Nuclear Pore-Associated TREX-2 Complex Employs Mediator to Regulate Gene Expression. Cell 2015; 162:1016-28; PMID:26317468; http://dx.doi.org/10.1016/j.cell.2015.07.059
- Luna R, Rondon AG, Aguilera A. New clues to understand the role of THO and other functionally related factors in mRNP biogenesis. Biochimica et biophysica acta 2012; 1819:514-20; PMID:22207203; http://dx.doi.org/10.1016/j.bbagrm.2011.11.012
- Nino CA, Herissant L, Babour A, Dargemont C. mRNA nuclear export in yeast. Chem Rev 2013; 113:8523-45; PMID:23731471; http://dx.doi.org/10.1021/cr400002g
- Hsin JP, Manley JL. The RNA polymerase II CTD coordinates transcription and RNA processing. Gen Dev 2012; 26:2119-37; PMID:23028141; http://dx.doi.org/10.1101/gad.200303.112
- Maniatis T, Reed R. An extensive network of coupling among gene expression machines. Nature 2002; 416:499-506; PMID:11932736; http://dx.doi.org/10.1038/416499a
- Bentley DL. Coupling mRNA processing with transcription in time and space. Nat Rev Genet 2014; 15:163-75; PMID:24514444; http://dx.doi.org/10.1038/nrg3662
- Heidemann M, Hintermair C, Voss K, Eick D. Dynamic phosphorylation patterns of RNA polymerase II CTD during transcription. Biochimica et biophysica acta 2013; 1829:55-62; PMID:22982363; http://dx.doi.org/10.1016/j.bbagrm.2012.08.013
- Plaschka C, Lariviere L, Wenzeck L, Seizl M, Hemann M, Tegunov D, Petrotchenko EV, Borchers CH, Baumeister W, Herzog F, et al. Architecture of the RNA polymerase II-Mediator core initiation complex. Nature 2015; 518:376-80; PMID:25652824; http://dx.doi.org/10.1038/nature14229
- Wong KH, Jin Y, Struhl K. TFIIH Phosphorylation of the Pol II CTD Stimulates Mediator Dissociation from the Preinitiation Complex and Promoter Escape. Mol Cell 2014; 54(4):601-12; http://dx.doi.org/10.1016/j.molcel.2014.03.024
- Sogaard TM, Svejstrup JQ. Hyperphosphorylation of the C-terminal repeat domain of RNA polymerase II facilitates dissociation of its complex with mediator. J Biol Chem 2007; 282:14113-20; PMID:17376774; http://dx.doi.org/10.1074/jbc.M701345200
- Jeronimo C, Robert F. Kin28 regulates the transient association of Mediator with core promoters. Nat Struct Mol Biol 2014; 21(5):449-55; PMID:24704787
- Martinez-Rucobo FW, Kohler R, van de Waterbeemd M, Heck AJ, Hemann M, Herzog F, Stark H, Cramer P. Molecular Basis of Transcription-Coupled Pre-mRNA Capping. Mol Cell 2015; 58:1079-89; PMID:25959396; http://dx.doi.org/10.1016/j.molcel.2015.04.004
- Strasser K, Masuda S, Mason P, Pfannstiel J, Oppizzi M, Rodriguez-Navarro S, Rondon AG, Aguilera A, Struhl K, Reed R, et al. TREX is a conserved complex coupling transcription with messenger RNA export. Nature 2002; 417:304-8; PMID:11979277; http://dx.doi.org/10.1038/nature746
- Huertas P, Aguilera A. Cotranscriptionally formed DNA:RNA hybrids mediate transcription elongation impairment and transcription-associated recombination. Mol Cell 2003; 12:711-21; PMID:14527416; http://dx.doi.org/10.1016/j.molcel.2003.08.010
- Zenklusen D, Vinciguerra P, Wyss JC, Stutz F. Stable mRNP formation and export require cotranscriptional recruitment of the mRNA export factors Yra1p and Sub2p by Hpr1p. Mol Cell Biol 2002; 22:8241-53; PMID:12417727; http://dx.doi.org/10.1128/MCB.22.23.8241-8253.2002
- Hamm J, Mattaj IW. Monomethylated cap structures facilitate RNA export from the nucleus. Cell 1990; 63:109-18; PMID:2208274; http://dx.doi.org/10.1016/0092-8674(90)90292-M
- Cheng H, Dufu K, Lee CS, Hsu JL, Dias A, Reed R. Human mRNA export machinery recruited to the 5′ end of mRNA. Cell 2006; 127:1389-400; PMID:17190602; http://dx.doi.org/10.1016/j.cell.2006.10.044
- Trcek T, Larson DR, Moldon A, Query CC, Singer RH. Single-molecule mRNA decay measurements reveal promoter- regulated mRNA stability in yeast. Cell 2011; 147:1484-97; PMID:22196726; http://dx.doi.org/10.1016/j.cell.2011.11.051
- Bregman A, Avraham-Kelbert M, Barkai O, Duek L, Guterman A, Choder M. Promoter elements regulate cytoplasmic mRNA decay. Cell 2011; 147:1473-83; PMID:22196725; http://dx.doi.org/10.1016/j.cell.2011.12.005
- Zid BM, O'Shea EK. Promoter sequences direct cytoplasmic localization and translation of mRNAs during starvation in yeast. Nature 2014; 514:117-21; PMID:25119046; http://dx.doi.org/10.1038/nature13578
- Oktaba K, Zhang W, Lotz TS, Jun DJ, Lemke SB, Ng SP, Esposito E, Levine M, Hilgers V. ELAV links paused Pol II to alternative polyadenylation in the Drosophila nervous system. Mol Cell 2015; 57:341-8; PMID:25544561; http://dx.doi.org/10.1016/j.molcel.2014.11.024
- Huang Y, Li W, Yao X, Lin QJ, Yin JW, Liang Y, Heiner M, Tian B, Hui J, Wang G. Mediator complex regulates alternative mRNA processing via the MED23 subunit. Mol Cell 2012; 45:459-69; PMID:22264826; http://dx.doi.org/10.1016/j.molcel.2011.12.022
- Dimitrova L, Valkov E, Aibara S, Flemming D, McLaughlin SH, Hurt E, Stewart M. Structural Characterization of the Chaetomium thermophilum TREX-2 Complex and its Interaction with the mRNA Nuclear Export Factor Mex67:Mtr2. Structure 2015; 23:1246-57; PMID:26051714; http://dx.doi.org/10.1016/j.str.2015.05.002
