ABSTRACT
The nuclear lamina (NL) is a structural component of the nuclear envelope and makes extensive contacts with integral nuclear membrane proteins and chromatin. These interactions are critical for many cellular processes, such as nuclear positioning, perception of mechanical stimuli from the cell surface, nuclear stability, 3-dimensional organization of chromatin and regulation of chromatin-binding proteins, including transcription factors. The NL is present in all nucleated metazoan cells but its composition and interactome differ between tissues. Most likely, this contributes to the broad spectrum of disease manifestations in humans with mutations in NL-related genes, ranging from muscle dystrophies to neurological disorders, lipodystrophies and progeria syndromes. We review here exciting novel insight into NL function at the cellular level, in particular in chromatin organization and mechanosensation. We also present recent observations on the relation between the NL and metabolism and the special relevance of the NL in muscle tissues. Finally, we discuss new therapeutic approaches to treat NL-related diseases.
Introduction
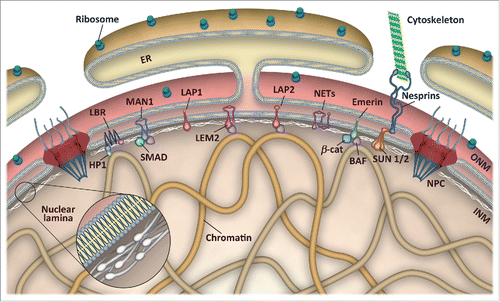
The nuclear lamina (NL), a filamentous protein network underlying the nuclear membranes (), has emerged as a critical player from basic cell biology to human health.Citation1 It serves as the anchoring point for chromatin and transcription factors at the nuclear periphery and plays a key role in signal transduction between the cytoskeleton and the nucleus. In addition, the NL provides mechanical stability to the nucleus, which is of particular relevance in migrating cells and cells undergoing physical tension. Reflecting its implication in fundamental cellular processes, the NL is causatively linked to various severe human diseases and understanding NL dynamics has become an important biomedical challenge. For these reasons, the International University of Andalusia organized together with Vicente Andrés, Tom Misteli and Peter Askjaer the workshop “The Nuclear Lamina in Health and Disease” in the historical Spanish town of Baeza in November 2015. The workshop brought together scientists working on different aspects of the NL and highlighted recent advances and remaining challenges. The exciting presentations and stimulating discussions inspired us to write an overview of the current situation of the field. Following a brief introduction to the NL we focus on recent discoveries and we encourage readers to consult more comprehensive reviews for a discussion and overview of earlier studies.Citation1,2
Figure 1. Schematic representation of the nuclear envelope. The nuclear envelope consists of an outer (ONM) and an inner nuclear membrane (INM), which are fused at nuclear pore complexes (NPC). The ONM is continuous with the endoplasmic reticulum (ER) and associated with ribosomes as well as nesprins and other proteins linking the nucleus to the cytoskeleton. The nuclear lamina is a proteinaceous network of intermediate filaments underneath the INM that forms extensive contacts with chromatin and INM proteins. The INM harbors multiple nuclear envelope transmembrane (NET) proteins, of which only a few are depicted. LEM domain proteins interact with the chromatin-binding protein BAF and transcription factors, such as SMADs, β-catenin and others, whereas lamin B receptor (LBR) associates with heterochromatin protein 1 (HP1) and directly with modified histones.
The NL is present in all animals and is composed of type V intermediate filaments called lamins plus lamin associated proteins. Mammalian genomes contain 3 lamin genes: LMNA, which encodes lamin A and lamin C (A-type lamins), as well as LMNB1 and LMNB2 that encode lamin B1 and B2 (B-type lamins), respectively. In addition to these 4 major isoforms, the lamin genes also produce 3 minor isoforms: AΔ10, C2 and B3. LMNA is mainly expressed in differentiated cells whereas all cells express at least one B-type lamin. B-type lamins are membrane-anchored via farnesylation of a C-terminal CaaX motif. Pre-lamin A, the lamin A precursor also contains a CaaX motif and is initially farnesylated however lamin C is not. Proteolytic cleavage by the ZMPSTE24 protease removes the last 15 amino acid residues and the hydrophobic farnesyl group to produce mature lamin A, which is found both at the NL and in the nuclear interior. Interestingly, specific LMNA mutations that cause an internal deletion of amino acids 607-656, including the sequence recognized by ZMPSTE24, lead to accumulation of aberrant lamin A, termed progerin, and development of a dramatic premature aging disease, known as Hutchinson-Gilford Progeria Syndrome (HGPS).Citation3 Moreover, the recent identification of a single amino acid substitution that disrupts the ZMPSTE24 cleavage site in prelamin A in a progeria patient has provided compelling evidence of the importance of correct lamin A processing.Citation4 In addition to the connection between the NL and HGPS a stunning number of disease-causing mutations have been identified in LMNA (http://www.umd.be/LMNA/), as well as in genes encoding NL-interacting proteins in the inner and outer nuclear membranes. These diseases span a remarkable spectrum of clinical manifestations, including neuropathies, muscle dystrophies, and lipodystrophies, collectively termed laminopathies (). More recently, B-type lamins have also been linked with human diseases.Citation1,2 The fact that lamins are broadly expressed but most laminopathies affect only a single or a few tissue types is surprising and has triggered several hypotheses. According to the “mechanical model,” alterations in the NL that affect its mechanoproperties (nuclear flexibility and stiffness, transmission of force from the cytoplasm to the nucleus, etc.) will mainly impact on tissues exposed to strong mechanical tension, such as muscles. Recent observations compatible with this model are discussed in the section “Mechanics of the nuclear lamina and cell migration” below. The “gene expression model” is based on the extensive contacts that exist between the NL and chromatin as well as between the NL and transcription factors and chromatin modifiers. As discussed in the next section, tissue specificity in this model arises from different 3-dimensional (3D) genome organization and expression of distinct subsets of NL-interacting nuclear envelope proteins.Citation5
Table 1. Examples of nuclear lamina-related diseases.
The nuclear lamina as chromatin organizer
Early electron microscopy observations of eukaryotic nuclei revealed that dark-staining heterochromatin is enriched at the nuclear periphery (and around nucleoli) whereas light-staining euchromatin occupies the nuclear interior. Fluorescence in situ hybridization experiments later determined that individual chromosomes are confined to “chromosome territories” where chromosomes with high or low density of active genes are more frequently positioned toward the nuclear center or the nuclear envelope, respectively.Citation6 Moreover, for several genes, a correlation has been demonstrated between locus position within the nucleus and expression: when these genes are inactive they are predominantly found at the nuclear periphery but upon stimuli or cell differentiation they accumulate in the nuclear interior. It should be noted that there exist also examples of genes for which such correlation is absent.Citation6 Experiments to establish cause-consequence relations through genome manipulations have yielded conflicting results (reviewed inCitation1,6), which in part might be because of effects of the particular genomic environment.Citation7 Indeed, recent work by Wendy Bickmore and colleagues has suggested that changes in chromatin condensation state, rather than transcription, regulates gene position.Citation8
To overcome the limitation intrinsic to single-locus studies, several methods have been developed that analyze interactions between the NL and chromatin more systematically. The laboratory of Bas van Steensel has pioneered the DNA adenine methyltransferase identification (DamID) technique to obtain global chromatin association profiles of NL components in a series of cell types. These studies have revealed that 35-40% of the human genome is organized into ∼1300 lamin-associated domains (LADs), which range from less than 10 kb to more than 10 Mb (median size of ∼0.5 Mb).Citation6 LADs have lower gene density, less transcriptional activity and are enriched for repressive chromatin marks (H3K27me3 and H3K9me2). Most LADs are found in several cell types (“constitutive LADs”) although there are also hundreds of regions of the genome that change association during differentiation (“facultative LADs”). Interestingly, the genomic positions of constitutive LADs are conserved between human and mouse.Citation9 It is still not understood what signatures drive association with the NL. Globally, constitutive LADs are A/T-rich,Citation9 whereas an analysis of a smaller subset of LADs identified elements that target DNA to the NL via the transcriptional regulators cKrox and YY1, the histone deacetylase HDAC3 and the inner nuclear membrane protein lamina-associated polypeptide 2β (LAP2β).Citation10,11 Similarly, examining the dynamics of LADs through the cell cycle using an ingenious imaging method led recently to the discovery that LADs are located in a ∼1 µm zone underneath the NL, implying that LADs have a restricted but detectable mobility in interphase.Citation12 Surprisingly, most LADs do not return to the nuclear periphery after mitosis, but localize instead to the nuclear interior and the surface of nucleoli. The mechanism controlling this dynamic behavior of LADs is still unknown, but it is interesting to note that LADs that re-associate with the NL after mitosis are enriched for H3K9me2, suggesting a possible memory function of this modification.Citation12 Methylation of H3K9 was previously identified to be critical for anchoring at the nuclear periphery and knockdown of H3K9 methyltransferases impedes interaction between LADs and the NL.Citation1,12-14 To further understand the variability in behavior of LADs in individual cells van Steensel and colleagues recently generated hundreds of single-cell DamID profiles for lamin B1.Citation15 This revealed a spectrum of interaction frequencies where some LADs were detected in almost all cells whereas other LADs were detected only occasionally. The previously defined constitutive LADs showed frequent interactions, suggesting that strong NL association is conserved both within and between populations of cells. Interestingly, behavior of neighboring LADs was found to correlate and have frequent inter-LAD contacts, indicative of long-range co-operativity.Citation15
Although A- and B-type lamins differ in their subcellular distribution with the former being more abundant in the nuclear interior, lamin A and lamin B1 DamID profiles are highly similar.Citation9,16 This suggests that interaction with intra-nuclear lamin A might be involved in the “reshuffling” of LADs after mitosis described above. In support of this hypothesis, knockdown of lamin A leads to increased interaction of LADs with lamin B.Citation16 Moreover, chromatin immunoprecipitation (ChIP) studies in adipose tissue stem cells identified lamin A-associated promoters both at the nuclear periphery and in the nuclear interior.Citation17 Upon differentiation into adipocytes many lamin A-promoter associations are lost and others are gained, but only ∼17% of the changes coincide with altered transcription.Citation17 In contrast to the similar DamID profiles, Roland Foisner and colleagues recently reported that ChIP of lamin A and lamin B1 identifies different chromatin domains when genomic DNA is fragmented by relatively mild sonication.Citation18 Under these conditions, lamin B1 interacts with gene poor and non-transcribed regions that overlap with DamID LADs. In contrast, lamin A peaks are enriched for transcribed genes and active chromatin marks, such as histone H3K4me3 and H3K9ac, and coincide with the ChIP profile of LAP2α. LAP2α, which in distinction to LAP2β is not membrane-associated, presumably plays an important role in these contacts, because lamin A interactions with euchromatin are reduced in LAP2α−/− cells.Citation18 Interestingly, stronger sonication to produce shorter chromatin fragments shifts the population of lamin A-interacting domains toward inactive chromatin, eventually generating a high overlap with lamin B1 ChIP and DamID profiles.Citation18,19 This demonstrates that lamins probably interact with several classes of chromatin and illustrates the potential bias that experimental procedures might impose on the results. The interaction between lamin A and LAP2α is also relevant in cells from HGPS patients, in which expression of LAP2α negatively correlates with progerin levels.Citation20 HGPS cells proliferate slower, but ectopic expression of LAP2α counteracts this phenotype, partly by stimulating the production of extracellular matrix components.Citation20
A crucial question in understanding NL-chromatin interactions is which factors are responsible for recruiting and anchoring chromatin at the nuclear periphery? Several proteins and chromatin modifications have been implicated (seeCitation1 for original references), but recent studies have identified novel players and new mechanisms. Surprisingly, lamins are dispensable for organization of the genome in LADs and inter-LAD regions, at least in murine embryonic stem cells, which indicates that other nuclear envelope proteins can independently tether chromatin to the nuclear periphery.Citation21 One interesting candidate is the inner nuclear membrane lamin B receptor (LBR) protein, which recognizes heterochromatic histone H4K20me2 via its Tudor domain.Citation22 In an elegant study of 39 different mammals, Irina Solovei and colleagues compared the distribution of heterochromatin in retinal cells versus endothelial cells: in nocturnal species the distribution is inverted in retinal cells with heterochromatin accumulating in the nuclear center and this correlates with absence of both lamin A/C and LBR in these cells.Citation23 In contrast, heterochromatin is at the nuclear periphery of retinal cells in diurnal animals which all express either lamin A/C or LBR. Further analysis of lamin A/C and LBR in mice revealed that many cell types express only one of them, often with LBR preceding lamin A/C. Interestingly, inversion of heterochromatin distribution was observed in Lbr−/− mice in tissues that do not express lamin A/C, and widespread inversion was also observed in mice lacking both lamin A/C and LBR.Citation23 Although lamin A/C and LBR are partially redundant in perinuclear tethering of heterochromatin, they have opposite effects on transcription in myoblasts: muscle-related genes have reduced and increased expression in Lmna−/− and Lbr−/− myoblasts, respectively.Citation23 Whereas LBR may interact directly with heterochromatin via its Tudor domain, Lamin A/C requires co-factors, such as the inner nuclear membrane protein emerin, to efficiently anchor chromatin to the NL.Citation23 Expression of emerin varies between tissues and is relatively abundant in contractile tissues both in mice and Caenorhabditis elegans,Citation23,24 suggesting that emerin might be particularly relevant for nuclear organization in those tissues. Indeed, although global DamID profiles of lamins and emerin are very similar,Citation21 experiments in C. elegans identified local differences, including specific enrichment of emerin at many muscle and neuronal genes.Citation25 Interestingly, loss of emerin leads to increased lamin association to these chromatin regions, and this was associated with altered neuromuscular junction activity indicating that the 2 proteins compete for binding.Citation25 Eric Schirmer and colleagues have systematically screened a series of less characterized inner nuclear membrane proteins and found that several of these (NET5, NET29, NET39, NET45 and NET47) are able to induce relocation of particular loci and entire chromosomes to the nuclear periphery.Citation26 Because these proteins are expressed in tissue-specific manners, they are attractive candidates for further analysis of cell type-specific nuclear organization. The Polycomb group (PcG) protein Ezh2 was recently also found to act together with lamin A/C to regulate muscle-related genes.Citation27 Chiara Lanzuolo and colleagues demonstrated that lamin A/C interacts with PcG proteins and affects their intranuclear dynamics. In addition, loss of lamin A/C expression impairs the repressive action of PcG and accelerates differentiation of myoblasts to myotubes.Citation27 Another remarkable example of the dynamics of interactions between the NL and chromatin was reported in a study of circadian genes.Citation28 Anita Göndör and colleagues identified an inter-chromosomal network of circadian genes whose relative position within the nucleus oscillates with a periodicity of ∼24 h. The recruitment to the nuclear periphery coincides with the appearance of H3K9me2 and reduced expression. Interestingly, knockdown of the chromatin regulator CTCF and its cofactor PARP1 or inhibition of the H3K9 methyltransferase G9a suppresses oscillation of gene positioning and transcription.Citation28
Gene position relative to the NL (or other subnuclear structures) can be precisely determined by fluorescence in situ hybridization (FISH), but the laborious nature of the protocol has prevented application of this method beyond studies on relatively few loci and/or gene positioning factors. To overcome this limitation, Tom Misteli and co-workers developed a high-throughput automated FISH workflow and demonstrated its usefulness by screening an siRNA library targeting 669 selected nuclear proteins.Citation29 The effects on positioning of 4 endogenous loci were analyzed in cells in 384-well plates and led to the identification of 50 validated hits. Twenty-six factors affected the position of a single locus, whereas 18 factors affected 2 loci. The factors that affected multiple loci did so in a variable manner: 11 of them caused peripheral and central loci to move toward and away from the nuclear center, respectively, whereas the remaining 13 factors induced a unidirectional shift in intranuclear position.Citation29 Together, this demonstrates that gene positioning within the nucleus is a combinatorial process that involves multiple factors acting at specific loci. As observed in other studies, repositioning of genes does not necessarily alter their expression level, but, interestingly, requires progression through S phase.Citation29
The NL also plays important roles during viral infection. The lamin-binding protein Barrier to autointegration factor (BAF) was identified almost 2 decades ago based on its role in integration of retroviral DNA into the host cell genome (see references inCitation30). More recently, Marina Lusic and colleagues discovered that human immunodeficiency virus 1 (HIV-1) preferentially integrates into perinuclear chromatin in the vicinity of nuclear pores and outside LADs.Citation31 ChIP analysis of several nucleoporins demonstrated an interaction with integration hot spots and viral DNA, whereas knockdown of the same nucleoporins impairs proviral transcription. Whether the frequent integration into nuclear pore-associated open chromatin reflects an active role of nucleoporins and/or rather a passive exclusion from closed LAD chromatin is an interesting question for future studies.Citation31
Mechanics of the nuclear lamina and cell migration
Cells in multicellular organisms are constantly exposed to various forms of mechanical stress and their response is fundamental for proper cellular functioning. These responses depend on the NL as well as linker of nucleoskeleton and cytoskeleton complex (LINC) proteins, such as SUN and KASH domain proteins, connecting the nuclear envelope to cytoplasmic filamentous actin, intermediate filaments and microtubules ().
Figure 2. Nuclear envelope protein networks controlling nuclear positioning and sensing tension. Trimeric SUN-domain proteins in the INM associate with KASH-domains of nesprins embedded in the ONM and the nuclear lamina to form linker of nucleoskeleton and cytoskeleton (LINC) complexes. In the cytoplasm, nesprins bind a variety of cytoskeletal structures, including filamentous actin, intermediate filaments and microtubules. In addition, LEM-domain proteins are also involved in anchoring of centrosomes to the nuclear envelope via unknown cytoplasmic or ONM proteins [24, 86]. Together, these contacts serve as sensors and transducers of mechanical stimuli from the cytoskeleton to regulate gene transcription as well as to position the nucleus within the cell. The ratio of A- and B-type lamins in the nuclear lamina and phosphorylation of lamins controls nuclear envelope flexibility, which is important during cell migration and tissue deformation.
![Figure 2. Nuclear envelope protein networks controlling nuclear positioning and sensing tension. Trimeric SUN-domain proteins in the INM associate with KASH-domains of nesprins embedded in the ONM and the nuclear lamina to form linker of nucleoskeleton and cytoskeleton (LINC) complexes. In the cytoplasm, nesprins bind a variety of cytoskeletal structures, including filamentous actin, intermediate filaments and microtubules. In addition, LEM-domain proteins are also involved in anchoring of centrosomes to the nuclear envelope via unknown cytoplasmic or ONM proteins [24, 86]. Together, these contacts serve as sensors and transducers of mechanical stimuli from the cytoskeleton to regulate gene transcription as well as to position the nucleus within the cell. The ratio of A- and B-type lamins in the nuclear lamina and phosphorylation of lamins controls nuclear envelope flexibility, which is important during cell migration and tissue deformation.](/cms/asset/c6d1b432-ffbd-44cc-aba2-c840e33e7925/kncl_a_1183848_f0002_oc.gif)
Tissues exhibit a spectrum of microelasticity diverging from soft tissues like brain or adipose tissue, which are exposed to low physical stress, to stiffer tissues like bone or muscle that have to deal with high stress. Recent proteomics analyses of tissue samples and cells by Dennis Discher and colleagues have shown that proper behavior of stiff tissues depends on the ratio between lamin A, which is ∼30-fold more abundant in stiff vs. soft tissues, and lamin B.Citation32 On the other hand, the expression of B-type lamins varies minimally between tissues and consequently they dominate in softer tissues like brain.Citation32 Moreover, collagens in the extracellular matrix (ECM) are a key determinant of tissue stiffness and scale with tissue stiffness whereas lamin A levels respond to changes in tissue elasticity.Citation32 Cellular stress is transmitted to the nucleus and cytoskeletal tension increases with the stiffness of the matrix.Citation33 Cells growing on a soft matrix (i.e. low stress) are more rounded with a highly wrinkled nuclear envelope, while on stiff matrix (i.e., high stress) the nucleus is flattened with a smoother NL, thus matrix stiffness alters the NL structure.Citation32 However, changes in the NL also affect the cytoskeleton. Lamin A interacts with actin and actin-binding proteins through components of the LINC () and lamin A knockdown in mesenchymal stem cells leads to repression of actomyosin cytoskeleton-related genes, particularly components of the serum response factor pathway.Citation34 It has been demonstrated that lamin A/C is phosphorylated both during interphase and, more prominently, during mitosis where phosphorylation promotes disassembly of the NL.Citation1,35 Interestingly, lamin A phosphorylation is stress-dependent, with low stress favoring lamin A phosphorylation and possibly lamin A solubilization.Citation32,34 Moreover, decreased expression of lamin A causes reduction of myosin-IIA protein and mRNA levels, whereas expression of an non-phosphorylatable lamin A in lamin A depleted cells caused an increase of myosin-IIA quantity.Citation34 In addition, the NL has been shown to act in tumor metastasis, more specifically to increase the resistance of tumor cells to fluid shear stress (FSS) in the bloodstream.Citation36 During metastasis, cancer cells detach from the primary tumor and enter the bloodstream where they have to cope with hemodynamic shear forces while circulating to other organs. Metastatic breast cancer cells are more resistant than non-malignant epithelial cells to elevated FSS in vitro and lamin A depletion reduces their resistance to FSS-induced cell death by increasing tumor cell apoptosis further highlighting the protective role of lamin A when cells are exposed to stress.Citation36
Cell migration is a fundamental part of many biological processes, including tissue formation, wound healing, cancer invasion and metastasis. During migration, the nucleus, which is the largest and the stiffest organelle of the cell, typically has to undergo dramatic deformations (). Cell migration requires actin polymerization, integrin-mediated adhesion to the ECM as well as ECM cleavage by cell surface metalloproteinases (MMPs) and finally contraction of the cell body followed by cell translocation mediated by the actomyosin cytoskeleton.Citation37 Cell migration was previously observed on flat, 2D substrates, but most physiological processes take place in 3D environments. This has given rise to emerging research of migration processes based on 3D devices.Citation37,38 These studies have demonstrated that cell migration is a linear function of pore size with the collagenase MT1-MMP being a limiting factor in 3D collagen matrices and that both integrin-based traction force and actomyosin contractility are necessary for cell migration in confined space. In the absence of MMPs, cell passage in a confined space induces further deformation of the nucleus, reaching a maximal compression when the nuclear diameter is reduced to 10% of its original size.Citation37,38 Cells present variability of nuclear shape, hence migrating cells present different forms of nuclear deformation; mononuclear cells display elongated hourglass-like or cigar-shaped deformations whereas polymorphonuclear cells produce a pearl chain-like configuration.
It has been shown that 3D migration through narrow pores is sensitive to lamin A levels, since overexpression or partial knockdown of lamin A limits or promotes cell passage, respectively.Citation38,39 However, in accordance with the protective role of the NL against stress, partial lamin A knockdown also causes an increase in apoptosis after cell migration through narrow pores, whereas further suppression of lamin A knockdown impairs resistance to migration stress and causes pronounced apoptosis.Citation39 Furthermore, the stoichiometry between A and B-type lamins influences the physical plasticity during cell migration with high lamin A:B ratios leading to persistent nuclear shape changes after cell migration.Citation39 Depletion of lamins also correlates with the frequency of local nuclear rupture when tumor cells migrate in confining 3D-environments.Citation40 Local nuclear envelope rupture is characterized mixing of nuclear and cytoplasmic factors, protrusions of chromatin through the nuclear envelope and increased DNA damage, whereas the endosomal sorting complexes required for transport-III (ESCRT-III) machinery is crucial for restoring the integrity of the nuclear envelope.Citation40-42
Cells migrating across 2D surfaces use lamellipodial protrusions at their leading edge, however Kenneth Yamada and coworkers have shown that cells moving through a physiological 3D matrix can generate cylindrical, blunt protrusions called lobopodia and migrate in a lamellipodia-independent manner.Citation43 Furthermore, they propose that during cell migration through 3D environments, the nucleus functions as a piston to generate differences in hydrostatic pressure in front of and behind the nucleus. This involves the outer nuclear membrane protein nesprin-3 as a linker between the nucleus and cytoskeleton, which pulls the nucleus forward by actomyosin contraction.
The nuclear lamina and metabolism
Several recent studies investigate the link between laminopathies and metabolic pathways, including post-translational sugar modification of serine or threonine residues by monosaccharide β-N-acetylglucosamine (O-GlcNAc).Citation44 O-GlcNAc is added and removed by O-GlcNac transferase (OGT) and O-GlcNacase (OGA) enzymes, respectively, and can act as a rival mechanism to protein phosphorylation.Citation45 O-GlcNAc modification is implicated in the regulation of transcription and translation, regulates protein trafficking and turnover, modulates insulin signaling and finally is implicated as an early response to cellular stress.Citation45,46 Moreover, overexpression of OGT and OGA affects mitosis and cytokinesis and has been implicated in diabetes, aging, cancer and heart diseases.Citation45
Proteins of the nuclear pore complexes are heavily modified by O-GlcNAc,Citation46 but it was recently found that emerin, apart from being a highly phosphorylated protein both during mitosis and interphase is also O-GlcNAcylated on at least 8 residues.Citation44 Mutation of Ser53 or Ser54 located close to the LEM-domain in emerin severely disrupts O-GlcNAcylation both in vitro and in vivo. Interestingly, substitution of Ser54 for phenylalanine (S54F) does not affect emerin expression but is sufficient to cause Emery-Dreifuss muscular dystrophy. In cell extracts, the interaction of emerin with BAF is enriched in histone and lamin B-containing fractions, and O-GlcNAcylation of emerin on Ser173, which is outside of the LEM domain, promotes association of emerin with BAF.Citation44 Post-translational sugar modification by OGT and OGA enzymes also influences chromatin organization, since all core histones are GlcNAcylated.Citation46 Moreover, recent studies have proposed that histone H2B GlcNAcylation on Ser112 functions as an epigenetic mark involved in remodeling of LADs after adipogenic induction.Citation47
While A- and B-type lamins form immobile protein networks, BAF is highly mobile both in human cell lines and in C. elegans embryos. Interestingly, C. elegans BAF mobility is regulated by environmental stress, since GFP-BAF-1 is immobilized by dietary restriction or heat shock, linking cell metabolism to NL dynamics.Citation30 Whether the changes in mobility are due to modifications of BAF or its interaction partners, such as emerin, is currently unknown, but it would be relevant to investigate O-GlcNAcylation levels.
Specific LMNA mutations affect adipose tissues causing lipodystrophies, characterized by severe metabolic alterations, fat atrophy and pathological accumulation of prelamin A at the nuclear periphery of affected cells (). Interestingly, HGPS patients also develop lipodystrophy and prelamin A accumulation affects human mesenchymal stem cell-derived adipocytes, leading to smaller lipid droplets, increased lipolysis, alteration in the structure of the endoplasmic reticulum, partial depolarization of mitochondria membranes and alteration of the lipid profile.Citation48,49
In conclusion, these observations illustrate the high complexity of protein interactions at the NL and point to extensive cross-talk between the NL and cell metabolism.
The nuclear lamina and muscles
All three major muscle groups, skeletal, cardiac and smooth muscle, are affected in various laminopathies. In progeria, vascular smooth muscle cell (VSMC) dysfunction and loss is the cause of premature death due to accelerated atherosclerosis, leading to heart attack and stroke. It appears that VSMCs are highly susceptible to the accumulation of mutant forms of prelamin A (progerin) in HGPS. This notion is further supported by findings in the general population showing that prelamin A species specifically accumulate in aged and diseased VSMCs and can act as a biomarker for vascular aging and disease.Citation3,50-52
Mouse models either expressing progerin or lacking ZMPSTE24 and thus accumulating prelamin A develop cardiovascular defects. These include VSMC loss and extracellular matrix changes which culminate in calcification of the vessel wall. Vascular calcification is a key feature of vascular aging and is also prevalent in patients with HGPS. Recent studies by the Andrés group have shown that calcification is exacerbated in ‘knock-in’ mice carrying the Lmna G609G mutation that leads to progerin production. These mice develop calcification due to defective extracellular metabolism of the key calcification inhibitor pyrophosphate (PPi) in VSMCs.Citation53 This deficiency in progerin-expressing VSMCs was associated with increased tissue expression and activity of tissue-nonspecific alkaline phosphatase (TNAP), which hydrolyzes PPi to inorganic phosphate, and mitochondrial dysfunction leading to reduced synthesis of the PPi substrate ATP through mechanisms that remain unclear. The absence of atherosclerosis in these mouse models is not unexpected, as mice do not naturally develop the disease. Therefore we await further studies investigating how prelamin A can impact on atherosclerotic disease in appropriate models. Interestingly these studies may not be confined to studies of VSMCs. Recent data suggests that the NL is important for T-cell function.Citation54 These cells are also a component of the chronic inflammation in atherosclerotic plaques suggesting that dysfunction in additional cell types may contribute to the accelerated atherosclerosis in HGPS.
Studies of normal human VSMCs in vitro have begun to dissect further how prelamin A can impact on cardiovascular pathologies. Using an in vitro model of VSMC calcification it was found that expression of prelamin A accelerated calcification. The mechanism involves increased DNA damage accumulation and the onset of premature senescence, leading to the induction of the senescence associated secretory phenotype (SASP). Many of the growth factors and inflammatory cytokines associated with the SASP, including BMP2 and IL6, have been shown to accelerate VSMC calcification.Citation51 Importantly, the greatest risk factor for cardiovascular disease is age, and prelamin A has been shown to interfere with DNA damage repair in a number of ways leading to premature senescence of cells.Citation55 In VSMCs, this interference may also impact on specific pathways. For instance, lamin A and its binding partner nesprin-2 have been discovered to play a role in regulating VSMC proliferation and DNA repair by stabilizing intranuclear active ERK complexes at PML bodies.Citation52 Clearly, further work is required to fully define the mechanisms whereby prelamin A drives premature vascular aging.
Mutations in LMNA and/or EMD (encoding emerin) cause Emery Dreifuss Muscular Dystrophy, which affects both skeletal and cardiac muscle leading to conduction defects. Other LMNA mutations affect only the heart, manifesting as dilated cardiomyopathy (DCM) or mainly skeletal muscle as occurs in various limb girdle muscular dystrophies (see ). There are no clear genotype-phenotype correlations for these diseases making it difficult to pinpoint an overarching mechanism for each disease state. However, observations by Giselle Bonne, who has performed seminal work in unraveling the role of the NL in muscle syndromes, suggest that cardiac defects might be the major cause of death in these diverse laminopathy syndromes.Citation56
One of the limitations has been that animal models do not reiterate all aspects of human disease. While most human LMNA mutations exert a dominant effect and show a phenotype in heterozygotes, homozygosity of mutations is often required in animal models. Indeed, deletion of genes such as EMD, which causes classic EDMD in man apparently show no phenotype even when homozygous in mice. However, the Bonne laboratory has recently developed a model where for the first time a heterozygous animal develops a cardiac specific phenotype. This model is a ‘knock-in’ of the LMNAΔK32 human mutation.Citation57 Mice develop DCM and die between 35 and 70 weeks of age. Interestingly, the disease appeared to have 2 phases. The first was haploinsufficiency of lamin A, which is a known cause of DCM, most likely associated with defects in mechanical function/signaling. This was followed by a later phase where lamin A levels were increased and this was associated with defects in the ubiquitin-proteosomal system; a key indicator of later stage heart failure. The pathogenesis of disease in this model highlights the complexities of defining molecular mechanisms in muscle laminopathies. The heart continuously undergoes adaptive remodeling in response to changes in mechanical signaling inducing a continuum of changes making cause and effect difficult to dissect apart. One way forward will be to compare various models of NL disruption. If disruption of mechanosignaling is the main driver of early disease, then models where other LINC components, such as SUN or nesprin proteins, are disrupted might be informative (). So far it has been shown that deletion of the C-terminal region of both nesprin-1 and −2 is required to induce cardiac symptoms in miceCitation58 and we now await further studies of LINC complex and NL binding partner knockouts, particularly those that are muscle specific, to further define disease processes.
Muscle, due to its structure and accessibility provides ideal material for gene therapy based techniques to treat laminopathies. Skeletal muscle is particularly amenable to localized delivery of nucleic acids and a recent technology known as exon-skipping which can be used to delete mutant exons while leaving the nucleic acid coding sequence in frame has been successfully applied to other skeletal muscle dystrophies such as Duchenne Muscular Dystrophy. Studies are emerging that have begun to explore the use of this technology in laminopathies and this and other innovative technologies aimed at normalizing LMNA mutations are likely to be implemented in the coming years.Citation59
Development of therapeutic strategies for lamin-related diseases
In the last decade, the identification of molecular mechanisms contributing to cellular decline in lamin-related diseases and the generation of mouse models has expedited the search for therapies to ameliorate the pathophysiology of these diseases. Much emphasis has been placed on the treatment of HGPS. HGPS patient-derived cells and mouse models of progeria have been valuable tools to design therapies for treatment. Initial preclinical studies identified farnesyltransferase inhibitors (FTIs) and prenylation inhibitors (statins and aminobisphosphonates) as potential beneficial treatments for HGPS. The encouraging results in mice led to the first clinical drug trial for children with progeria utilizing the FTI lonafarnib. However, the improvements were minor and FTIs alone are far from curing the disease.Citation3,60 A second trial with the triple combination lonafarnib, a bisphosphonate (zoledronate) and a statin (pravastatin) is ongoing.
In addition to prenylation inhibitors, therapeutic strategies are targeting other post-translational modifications of progerin. In particular, reduced expression of ICMT, the enzyme responsible for carboxymethylation of the farnesylcysteine of lamin A/progerin, has a dramatic effect ameliorating the phenotype of progeria mice, significantly increasing lifespan.Citation61 This study also demonstrated that activation of the AKT/mTOR pathway is responsible for the improvement of the phenotype of progeria cells. Consistent with this notion, depletion or inhibition of ICMT increases AKT-mTOR signaling and delays senescence in HGPS cells, while the mTOR inhibitor rapamycin reduces the proliferation of HGPS cells. These data suggest that ICMT and the AKT/mTOR pathway could be considered as targets for the treatment of HGPS patients. However, other reports have shown a beneficial effect of rapamycin on the phenotype of HGPS patient cells, primarily by increasing progerin clearance by autophagy.Citation62,63 Rapamycin is an FDA-approved drug used as an immunosuppressant, and thus the use of this drug in HGPS patients has been gaining momentum. Along the same line, sulforaphane, an antioxidant that stimulates proteasome activity and autophagy, enhances progerin clearance in HGPS cells. Sulforaphane has been suggested as a promising therapeutic avenue for children with HGPS.Citation64 Moreover, resveratrol, an enhancer of SIRT1 deacetylase activity, alleviates progeroid features and extends lifespan in progeria mice, although the effect on survival is relatively modest.Citation65
New evidence has also linked lamin dysfunction with reduced levels of antioxidant enzymes and increased generation of reactive oxygen species (ROS) and mitochondrial dysfunction.Citation53,66-68 Persistent accumulation of ROS can oxidize cysteines in lamins, causing NL structural alterations,Citation66 which could contribute to the increased levels of DNA damage and the genomic instability that characterize lamin-deficient cells. Support for this notion is the fact that treatment of progeroid cells with the ROS scavenger N-acetyl cysteine reduces the amount of unrepairable DNA damage.Citation69 Therefore, compounds that reduce the ROS levels could represent yet another strategy to reduce genomic instability in lamin-related diseases.Citation70
All these studies have generated controversy about the best treatment regimen for HGPS patients, especially because prenylation inhibitors can induce a variety of noxious side effects. Moreover, studies on mesodermal stem cells derived from HGPS pluripotent stem cells (iPS) with all 3 treatments (FTI, ZoPra, and/or rapamycin) showed tremendous variability in the phenotypes improved by the different treatments, as well as evidence for cytostatic effects with different combinations.Citation71 The current view is that we need to be cautious when designing clinical trials for HGPS patients, because the toxicity associated with some of these treatments on the long run might undermine the benefits. Thus, the main challenge in the field of progeria in the years to come is to find strategies to improve such dramatic phenotypes while lowering toxicity.
In the workshop “The Nuclear Lamina in Health and Disease,” researchers discussed new compounds showing beneficial effects in progeria models. The small molecule “remodelin,” an inhibitor of N-acetyltransferase-10 (NAT10) has been recently shown to improve a variety of phenotypes in both lamin A/C-depleted cells and HGPS patient-derived cells. Remodelin treatment restores nuclear shape, improves chromatin organization, and decreases DNA damage in these cells.Citation72 The effect of remodelin rescuing nuclear shape defects seems to be connected to NAT10 organizing the microtubule network. In addition, remodelin treatment improves DNA replication, enhances proliferation and decreases senescence. Current studies are investigating the effect of remodelin on gene expression and testing a potential benefit of remodelin treatment in mouse models of laminopathies. Small molecules such as remodelin might provide alternative strategies for treating laminopathies and premature aging diseases. In addition, retinoids are emerging as potential compounds with a benefit in progeria models. In vitro studies have shown that these compounds regulate directly expression from the LMNA gene, leading to decreased expression of progerin in HGPS patient-derived fibroblasts. Retinoic acid responsive elements (L-RARE) are present in the LMNA gene promoter,Citation32 which upon treatment with all trans retinoic acid (ATRA) downregulate progerin expression. Importantly, ATRA synergizes with rapamycin in reducing progerin levels, restoring heterochromatin organization and nuclear shape, reducing DNA damage markers, and improving cell viability.Citation73 Preclinical studies with retinoic acids alone or in combination with rapamycin are needed to determine a potential benefit in vivo. Other studies have revealed an unprecedented role for the active vitamin D metabolite 1,25 α-dihydroxyvitamin D3 (1,25D) rescuing DNA repair defects in lamin A/C-deficient cells.Citation74-76 Unpublished data from Susana Gonzalo and colleagues also indicates that vitamin D/vitamin D receptor (VDR) signaling ameliorates the phenotypes of HGPS patient-derived cells. Future studies need to determine if 1,25D, ATRA, or the combination, can reduce progerin levels in vivo without causing toxicity, resulting in a general amelioration of the premature aging phenotype.
In addition to the above approaches to target known HGPS-affected pathways with therapeutic purposes, unbiased global-scale screenings are being implemented to identify new molecular mechanisms behind disease pathology, new effectors, and new compounds to prevent progerin-induced effects. Tom Misteli and colleagues recently performed a high-throughput, high-content imaging based screening method to identify small molecules that ameliorate progeria-induced cytotoxicity. The screening of a library of FDA approved drugs identified retinoids as a class of compounds reversing the phenotypes of HGPS patient cells.Citation77 These results reinforce the need to test in vivo the efficacy of retinoids ameliorating the pathophysiology of HGPS. It is possible that the best strategy is the utilization of combination therapy to obtain synergy among compounds, while reducing the doses of each single compound in order to reduce toxicity. Other approaches utilized by the Misteli team include mapping on a global scale the interactome of lamin A, and how disease-associated mutations modify the map. The yeast 2-hybrid (Y2H) screening of an ORFeome library comprising over 12,000 genes for lamin A/C-binding proteins identified new proteins whose interaction is disrupted by disease-linked lamin A mutations.Citation78 The screen identified 225 progerin-specific interactions, 51 proteins that interacted with both lamin A and progerin, and 61 interactions specific from lamin A. The concomitant analysis of localization pattern revealed that although the majority of progerin-specific proteins (89%) are at the nuclear periphery, only half of lamin A-binding proteins accumulate at the nuclear envelope. Gene Ontology (GO) analysis indicates that progerin-specific and lamin A-specific interactors are functionally distinct. Lamin A interactors were enriched for nuclear envelope and lamina components, while progerin interactors comprised mostly integral and intrinsic membrane proteins with channel and transport function. These included components of the ER and proteins that participate in vesicle-mediated transport, membrane organization, and overall protein localization. Altogether, these data suggest that the tissue specificity of laminopathies might be driven by gain or loss of tissue-specific lamin A/C interactions. These interactions can sequester proteins either at the nuclear periphery or in the nuclear interior, altering their function.
The sequestration of proteins in the nucleus seems to be a recurrent theme for progeria. The association of chromatin remodeling factors such as NURD, and transcription factors such as Fos, Sp1, or SKIP with the NL regulate their function.Citation1,49 For example, the sequestration of transcription factors by progerin is responsible for the loss of NURD complex function observed in HGPS and in physiological aging. The association of NURD complex with the NL stabilizes the complex, which in the absence of lamins is targeted to degradation.Citation79
Another subject of active research in the field of progeria is the role of telomerase counteracting progerin toxicity. Accelerated telomere shortening is a hallmark of HGPS patient cells, which contributes to accumulation of DNA damage, genomic instability and proliferation defects. As such, expression of telomerase in HGPS cells prevents both proliferation defects and DNA damage, and suppresses changes in gene expression induced by progerin.Citation80,81 Unpublished studies performed by Colin Stewart and colleagues in embryonic stem cells (ESCs), which express B-type lamins but not lamin A, showed high expression of telomerase. Upon differentiation, lamin A expression is induced, concomitant with a reduction in telomerase levels. Expression of lamin A or progerin in ESCs did not show an overt effect, with cells still retaining their stemness and pluripotent characteristics, as well as a normal growth rate. However, the viability and proliferation of differentiated stem cell derivatives expressing progerin is significantly compromised, and differentiated cells accumulate DNA damage. Thus, one of the questions raised by these findings is whether the decrease in telomerase during differentiation of ESCs is responsible for the DNA damage. In fact, the Stewart's team discovered that telomerase-deficient ESCs are severely compromised in their tolerance to progerin, supporting the notion that telomerase protects from the detrimental effects of progerin.
Moreover, analysis of protein-protein interactions by the Stewart's team revealed that LAP2α interacts more weakly with progerin than with lamin ACitation82. Super-resolution microscopy showed that LAP2α association with telomeres is impaired in HGPS cells. Their studies suggest that the impaired interaction of progerin with LAP2α contributes to HGPS phenotypes. As such, LAP2α depletion in HGPS cells enhances their proliferation defects, while increasing LAP2α levels restores normal growth. The loss of LAP2α in progerin-expressing cells also contributes to the characteristic loss of heterochromatic marks such as H3K27me3, a hallmark of HGPS cells. These findings provide novel insights into the pathophysiology underlying HGPS, and how the NL regulates proliferation and chromatin organization through functional interactions with telomeres and LAP2α.
Concluding remarks
We are just beginning to unravel the diversity and complexity of functions of the NL and its associated binding partners. Progress has been made in understanding disease processes and more research is now focusing on the basic cell biological mechanisms involved in NL homeostasis, ranging from behavior of stem cellsCitation83 to regulation of phospholipidsCitation84 and protein turnover in aging.Citation85 The workshop in Baeza brought together leading scientists from interrelated topics in the NL field and fostered stimulating discussions to pave the way for exciting future discoveries that will be key to develop more efficient therapeutic strategies against laminopathies.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to the International University of Andalusia, in particular Joaquín Torreblanca López for making the exciting “The Nuclear Lamina in Health and Disease” workshop possible, as well as Vicente Andrés and Tom Misteli who co-organized the workshop together with PA. We also wish to thank Victor Carranco for help with figures. We apologize to our colleagues whose work has not been discussed here due to space limitations.
Funding
Work in the SG lab was supported by NIH RO1 GM094513-01. Work in the PA lab is funded by the Spanish Ministry of Economy and Competitiveness [BFU2013-42709P] and the European Regional Development Fund.
References
- Gruenbaum Y, Foisner R. Lamins: nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. Annu Rev Biochem 2015; 84:131-64; PMID:25747401; http://dx.doi.org/10.1146/annurev-biochem-060614-034115
- Burke B, Stewart CL. Functional architecture of the cell's nucleus in development, aging, and disease. Curr Top Dev Biol 2014; 109:1-52; PMID:24947235; http://dx.doi.org/10.1016/B978-0-12-397920-9.00006-8
- Gordon LB, Rothman FG, Lopez-Otin C, Misteli T. Progeria: a paradigm for translational medicine. Cell 2014; 156:400-7; PMID:24485450; http://dx.doi.org/10.1016/j.cell.2013.12.028
- Wang Y, Lichter-Konecki U, Anyane-Yeboa K, Shaw JE, Lu JT, Ostlund C, Shin JY, Clark LN, Gundersen GG, Nagy PL, Worman HJ. Mutation abolishing the ZMPSTE24 cleavage site in prelamin A causes a progeroid disorder. J Cell Sci. 2016 May 15; 129(10):1975-80. PMID: 27034136; http://dx.doi.org/10.1242/jcs.187302
- Worman HJ, Schirmer EC. Nuclear membrane diversity: underlying tissue-specific pathologies in disease? Curr Opin Cell Biol 2015; 34:101-12; PMID:26115475; http://dx.doi.org/10.1016/j.ceb.2015.06.003
- Bickmore WA, van Steensel B. Genome architecture: domain organization of interphase chromosomes. Cell 2013; 152:1270-84; PMID:23498936; http://dx.doi.org/10.1016/j.cell.2013.02.001
- Akhtar W, de Jong J, Pindyurin AV, Pagie L, Meuleman W, de Ridder J, Berns A, Wessels LF, van Lohuizen M, van Steensel B. Chromatin position effects assayed by thousands of reporters integrated in parallel. Cell 2013; 154:914-27; PMID:23953119; http://dx.doi.org/10.1016/j.cell.2013.07.018
- Therizols P, Illingworth RS, Courilleau C, Boyle S, Wood AJ, Bickmore WA. Chromatin decondensation is sufficient to alter nuclear organization in embryonic stem cells. Science 2014; 346:1238-42; PMID:25477464; http://dx.doi.org/10.1126/science.1259587
- Meuleman W, Peric-Hupkes D, Kind J, Beaudry JB, Pagie L, Kellis M, Reinders M, Wessels L, van Steensel B. Constitutive nuclear lamina-genome interactions are highly conserved and associated with A/T-rich sequence. Genome Res 2013; 23:270-80; PMID:23124521; http://dx.doi.org/10.1101/gr.141028.112
- Harr JC, Luperchio TR, Wong X, Cohen E, Wheelan SJ, Reddy KL. Directed targeting of chromatin to the nuclear lamina is mediated by chromatin state and A-type lamins. J Cell Biol 2015; 208:33-52; PMID:25559185; http://dx.doi.org/10.1083/jcb.201405110
- Zullo JM, Demarco IA, Pique-Regi R, Gaffney DJ, Epstein CB, Spooner CJ, Luperchio TR, Bernstein BE, Pritchard JK, Reddy KL, Singh H. DNA sequence-dependent compartmentalization and silencing of chromatin at the nuclear lamina. Cell 2012; 149:1474-87; PMID:22726435; http://dx.doi.org/10.1016/j.cell.2012.04.035
- Kind J, Pagie L, Ortabozkoyun H, Boyle S, de Vries SS, Janssen H, Amendola M, Nolen LD, Bickmore WA, van Steensel B. Single-cell dynamics of genome-nuclear lamina interactions. Cell 2013; 153:178-92; PMID:23523135; http://dx.doi.org/10.1016/j.cell.2013.02.028
- Towbin BD, Gonzalez-Aguilera C, Sack R, Gaidatzis D, Kalck V, Meister P, Askjaer P, Gasser SM. Step-Wise Methylation of Histone H3K9 Positions Heterochromatin at the Nuclear Periphery. Cell 2012; 150:934-47; PMID:22939621; http://dx.doi.org/10.1016/j.cell.2012.06.051
- Gonzalez-Sandoval A, Towbin BD, Kalck V, Cabianca DS, Gaidatzis D, Hauer MH, Geng L, Wang L, Yang T, Wang X, et al. Perinuclear Anchoring of H3K9-Methylated Chromatin Stabilizes Induced Cell Fate in C. elegans Embryos. Cell 2015; 163:1333-47; PMID:26607792; http://dx.doi.org/10.1016/j.cell.2015.10.066
- Kind J, Pagie L, de Vries SS, Nahidiazar L, Dey SS, Bienko M, Zhan Y, Lajoie B, de Graaf CA, Amendola M, et al. Genome-wide Maps of Nuclear Lamina Interactions in Single Human Cells. Cell 2015; 163:134-47; PMID:26365489; http://dx.doi.org/10.1016/j.cell.2015.08.040
- Kind J, van Steensel B. Stochastic genome-nuclear lamina interactions: modulating roles of Lamin A and BAF. Nucleus 2014; 5:124-30; PMID:24717229; http://dx.doi.org/10.4161/nucl.28825
- Lund E, Oldenburg AR, Delbarre E, Freberg CT, Duband-Goulet I, Eskeland R, Buendia B, Collas P. Lamin A/C-promoter interactions specify chromatin state-dependent transcription outcomes. Genome Res 2013; 23:1580-9; PMID:23861385; http://dx.doi.org/10.1101/gr.159400.113
- Gesson K, Rescheneder P, Skoruppa MP, von Haeseler A, Dechat T, Foisner R. A-type lamins bind both hetero- and euchromatin, the latter being regulated by lamina-associated polypeptide 2alpha. Genome Res. 2016 Apr; 26(4):462-73. PMID: 26798136; http://dx.doi.org/10.1101/gr.196220.115.
- Lund E, Oldenburg AR, Collas P. Enriched domain detector: a program for detection of wide genomic enrichment domains robust against local variations. Nucleic Acids Res 2014; 42:e92; PMID:24782521; http://dx.doi.org/10.1093/nar/gku324
- Vidak S, Kubben N, Dechat T, Foisner R. Proliferation of progeria cells is enhanced by lamina-associated polypeptide 2alpha (LAP2alpha) through expression of extracellular matrix proteins. Genes Dev 2015; 29:2022-36; PMID:26443848; http://dx.doi.org/10.1101/gad.263939.115
- Amendola M, van Steensel B. Nuclear lamins are not required for lamina-associated domain organization in mouse embryonic stem cells. EMBO Rep 2015; 16:610-7; PMID:25784758; http://dx.doi.org/10.15252/embr.201439789
- Hirano Y, Hizume K, Kimura H, Takeyasu K, Haraguchi T, Hiraoka Y. Lamin B receptor recognizes specific modifications of histone H4 in heterochromatin formation. J Biol Chem 2012; 287:42654-63; PMID:23100253; http://dx.doi.org/10.1074/jbc.M112.397950
- Solovei I, Wang AS, Thanisch K, Schmidt CS, Krebs S, Zwerger M, Cohen TV, Devys D, Foisner R, Peichl L, et al. LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell 2013; 152:584-98; PMID:23374351; http://dx.doi.org/10.1016/j.cell.2013.01.009
- Morales-Martinez A, Dobrzynska A, Askjaer P. Inner nuclear membrane protein LEM-2 is required for correct nuclear separation and morphology in C. elegans. J Cell Sci 2015; 128:1090-6; PMID:25653391; http://dx.doi.org/10.1242/jcs.164202
- Gonzalez-Aguilera C, Ikegami K, Ayuso C, de Luis A, Iniguez M, Cabello J, Lieb JD, Askjaer P. Genome-wide analysis links emerin to neuromuscular junction activity in Caenorhabditis elegans. Genome Biol 2014; 15:R21; PMID:24490688; http://dx.doi.org/10.1186/gb-2014-15-2-r21
- Zuleger N, Boyle S, Kelly DA, de las Heras JI, Lazou V, Korfali N, Batrakou DG, Randles KN, Morris GE, Harrison DJ, et al. Specific nuclear envelope transmembrane proteins can promote the location of chromosomes to and from the nuclear periphery. Genome Biol 2013; 14:R14; PMID:23414781; http://dx.doi.org/10.1186/gb-2013-14-2-r14
- Cesarini E, Mozzetta C, Marullo F, Gregoretti F, Gargiulo A, Columbaro M, Cortesi A, Antonelli L, Di Pelino S, Squarzoni S, et al. Lamin A/C sustains PcG protein architecture, maintaining transcriptional repression at target genes. J Cell Biol 2015; 211:533-51; PMID:26553927; http://dx.doi.org/10.1083/jcb.201504035
- Zhao H, Sifakis EG, Sumida N, Millan-Arino L, Scholz BA, Svensson JP, Chen X, Ronnegren AL, Mallet de Lima CD, Varnoosfaderani FS, et al. PARP1- and CTCF-Mediated Interactions between Active and Repressed Chromatin at the Lamina Promote Oscillating Transcription. Mol Cell 2015; 59:984-97; PMID:26321255; http://dx.doi.org/10.1016/j.molcel.2015.07.019
- Shachar S, Voss TC, Pegoraro G, Sciascia N, Misteli T. Identification of Gene Positioning Factors Using High-Throughput Imaging Mapping. Cell 2015; 162:911-23; PMID:26276637; http://dx.doi.org/10.1016/j.cell.2015.07.035
- Bar DZ, Davidovich M, Lamm AT, Zer H, Wilson KL, Gruenbaum Y. BAF-1 mobility is regulated by environmental stresses. Mol Biol Cell 2014; 25:1127-36; PMID:24501420; http://dx.doi.org/10.1091/mbc.E13-08-0477
- Marini B, Kertesz-Farkas A, Ali H, Lucic B, Lisek K, Manganaro L, Pongor S, Luzzati R, Recchia A, Mavilio F, et al. Nuclear architecture dictates HIV-1 integration site selection. Nature 2015; 521:227-31; PMID:25731161; http://dx.doi.org/10.1038/nature14226
- Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 2013; 341:1240104; PMID:23990565; http://dx.doi.org/10.1126/science.1240104
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006; 126:677-89; PMID:16923388; http://dx.doi.org/10.1016/j.cell.2006.06.044
- Buxboim A, Swift J, Irianto J, Spinler KR, Dingal PC, Athirasala A, Kao YR, Cho S, Harada T, Shin JW, Discher DE. Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin. Curr Biol 2014; 24:1909-17; PMID:25127216; http://dx.doi.org/10.1016/j.cub.2014.07.001
- Kochin V, Shimi T, Torvaldson E, Adam SA, Goldman A, Pack CG, Melo-Cardenas J, Imanishi SY, Goldman RD, Eriksson JE. Interphase phosphorylation of lamin A. J Cell Sci 2014; 127:2683-96; PMID:24741066; http://dx.doi.org/10.1242/jcs.141820
- Mitchell MJ, Denais C, Chan M, Wang Z, Lammerding J, King MR. Lamin A/C Deficiency Reduces Circulating Tumor Cell Resistance to Fluid Shear Stress. Am J Physiol Cell Physiol. 2015 Dec 1; 309(11):C736-46. PMID: 26447202; http://dx.doi.org/10.1152/ajpcell.00050.2015
- Wolf K, Te Lindert M, Krause M, Alexander S, Te Riet J, Willis AL, Hoffman RM, Figdor CG, Weiss SJ, Friedl P. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol 2013; 201:1069-84; PMID:23798731; http://dx.doi.org/10.1083/jcb.201210152
- Davidson PM, Denais C, Bakshi MC, Lammerding J. Nuclear deformability constitutes a rate-limiting step during cell migration in 3-D environments. Cell Mol Bioeng 2014; 7:293-306; PMID:25436017; http://dx.doi.org/10.1007/s12195-014-0342-y
- Harada T, Swift J, Irianto J, Shin JW, Spinler KR, Athirasala A, Diegmiller R, Dingal PC, Ivanovska IL, Discher DE. Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. J Cell Biol 2014; 204:669-82; PMID:24567359; http://dx.doi.org/10.1083/jcb.201308029
- Denais CM, Gilbert RM, Isermann P, McGregor AL, Te Lindert M, Weigelin B, Davidson PM, Friedl P, Wolf K, Lammerding J. Nuclear envelope rupture and repair during cancer cell migration. Science. 2016 Apr 15; 352(6283):353-8. PMID: 27013428; http://dx.doi.org/10.1126/science.aad7297
- Vargas JD, Hatch EM, Anderson DJ, Hetzer MW. Transient nuclear envelope rupturing during interphase in human cancer cells. Nucleus 2012; 3:88-100; PMID:22567193; http://dx.doi.org/10.4161/nucl.18954
- Raab M, Gentili M, de Belly H, Thiam HR, Vargas P, Jimenez AJ, Lautenschlaeger F, Voituriez R, Lennon-Dumenil AM, Manel N, Piel M. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science 2016; 352:359-62; PMID:27013426; http://dx.doi.org/10.1126/science.aad7611
- Petrie RJ, Koo H, Yamada KM. Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science 2014; 345:1062-5; PMID:25170155; http://dx.doi.org/10.1126/science.1256965
- Berk JM, Maitra S, Dawdy AW, Shabanowitz J, Hunt DF, Wilson KL. O-Linked β-N-acetylglucosamine (O-GlcNAc) regulates emerin binding to barrier to autointegration factor (BAF) in a chromatin- and lamin B-enriched “niche.” J Biol Chem 2013; 288:30192-209; PMID:24014020; http://dx.doi.org/10.1074/jbc.M113.503060
- Hart GW, Akimoto Y. The O-GlcNAc Modification. In Essentials of Glycobiology. 2nd edition. Edited by Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. Cold Spring Harbor, NY; 2009
- Bond MR, Hanover JA. A little sugar goes a long way: the cell biology of O-GlcNAc. J Cell Biol 2015; 208:869-80; PMID:25825515; http://dx.doi.org/10.1083/jcb.201501101
- Ronningen T, Shah A, Oldenburg AR, Vekterud K, Delbarre E, Moskaug JO, Collas P. Prepatterning of differentiation-driven nuclear lamin A/C-associated chromatin domains by GlcNAcylated histone H2B. Genome Res 2015; 25:1825-35; PMID:26359231; http://dx.doi.org/10.1101/gr.193748.115
- Sanchez P, Infante A, de Eguino GR, Fuentes-Maestre JA, Garcia-Verdugo JM, Rodriguez CI. Age-Related Lipid Metabolic Signature in Human LMNA-Lipodystrophic Stem Cell-Derived Adipocytes. J Clin Endocrinol Metab 2015; 100:E964-973; PMID:25961135; http://dx.doi.org/10.1210/jc.2014-4528
- Ruiz de Eguino G, Infante A, Schlangen K, Aransay AM, Fullaondo A, Soriano M, Garcia-Verdugo JM, Martin AG, Rodriguez CI. Sp1 transcription factor interaction with accumulated prelamin a impairs adipose lineage differentiation in human mesenchymal stem cells: essential role of sp1 in the integrity of lipid vesicles. Stem Cells Transl Med 2012; 1:309-21; PMID:23197810; http://dx.doi.org/10.5966/sctm.2011-0010
- Ragnauth CD, Warren DT, Liu Y, McNair R, Tajsic T, Figg N, Shroff R, Skepper J, Shanahan CM. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation 2010; 121:2200-10; PMID:20458013; http://dx.doi.org/10.1161/CIRCULATIONAHA.109.902056
- Liu Y, Drozdov I, Shroff R, Beltran LE, Shanahan CM. Prelamin A accelerates vascular calcification via activation of the DNA damage response and senescence-associated secretory phenotype in vascular smooth muscle cells. Circ Res 2013; 112:e99-109; PMID:23564641; http://dx.doi.org/10.1161/CIRCRESAHA.111.300543
- Warren DT, Tajsic T, Porter LJ, Minaisah RM, Cobb A, Jacob A, Rajgor D, Zhang QP, Shanahan CM. Nesprin-2-dependent ERK1/2 compartmentalisation regulates the DNA damage response in vascular smooth muscle cell ageing. Cell Death Differ 2015; 22:1540-50; PMID:25744025; http://dx.doi.org/10.1038/cdd.2015.12
- Villa-Bellosta R, Rivera-Torres J, Osorio FG, Acin-Perez R, Enriquez JA, Lopez-Otin C, Andres V. Defective extracellular pyrophosphate metabolism promotes vascular calcification in a mouse model of Hutchinson-Gilford progeria syndrome that is ameliorated on pyrophosphate treatment. Circulation 2013; 127:2442-51; PMID:23690466; http://dx.doi.org/10.1161/CIRCULATIONAHA.112.000571
- Gonzalez-Granado JM, Silvestre-Roig C, Rocha-Perugini V, Trigueros-Motos L, Cibrian D, Morlino G, Blanco-Berrocal M, Osorio FG, Freije JM, Lopez-Otin C, et al. Nuclear envelope lamin-A couples actin dynamics with immunological synapse architecture and T cell activation. Sci Signal 2014; 7:ra37; PMID:24757177
- Gonzalo S, Kreienkamp R. DNA repair defects and genome instability in Hutchinson-Gilford Progeria Syndrome. Curr Opin Cell Biol 2015; 34:75-83; PMID:26079711; http://dx.doi.org/10.1016/j.ceb.2015.05.007
- Chatzifrangkeskou M, Bonne G, Muchir A. Nuclear envelope and striated muscle diseases. Curr Opin Cell Biol 2015; 32:1-6; PMID:25290386; http://dx.doi.org/10.1016/j.ceb.2014.09.007
- Cattin ME, Bertrand AT, Schlossarek S, Le Bihan MC, Skov Jensen S, Neuber C, Crocini C, Maron S, Laine J, Mougenot N, et al. Heterozygous LmnadelK32 mice develop dilated cardiomyopathy through a combined pathomechanism of haploinsufficiency and peptide toxicity. Hum Mol Genet 2013; 22:3152-64; PMID:23575224; http://dx.doi.org/10.1093/hmg/ddt172
- Banerjee I, Zhang J, Moore-Morris T, Pfeiffer E, Buchholz KS, Liu A, Ouyang K, Stroud MJ, Gerace L, Evans SM, et al. Targeted ablation of nesprin 1 and nesprin 2 from murine myocardium results in cardiomyopathy, altered nuclear morphology and inhibition of the biomechanical gene response. PLoS Genet 2014; 10:e1004114; PMID:24586179; http://dx.doi.org/10.1371/journal.pgen.1004114
- Scharner J, Figeac N, Ellis JA, Zammit PS. Ameliorating pathogenesis by removing an exon containing a missense mutation: a potential exon-skipping therapy for laminopathies. Gene Ther 2015; 22:503-15; PMID:25832542; http://dx.doi.org/10.1038/gt.2015.8
- Gordon LB, Massaro J, D'Agostino RB, Sr., Campbell SE, Brazier J, Brown WT, Kleinman ME, Kieran MW, Progeria Clinical Trials C. Impact of farnesylation inhibitors on survival in Hutchinson-Gilford progeria syndrome. Circulation 2014; 130:27-34; PMID:24795390; http://dx.doi.org/10.1161/CIRCULATIONAHA.113.008285
- Ibrahim MX, Sayin VI, Akula MK, Liu M, Fong LG, Young SG, Bergo MO. Targeting isoprenylcysteine methylation ameliorates disease in a mouse model of progeria. Science 2013; 340:1330-3; PMID:23686339; http://dx.doi.org/10.1126/science.1238880
- Cao K, Graziotto JJ, Blair CD, Mazzulli JR, Erdos MR, Krainc D, Collins FS. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson-Gilford progeria syndrome cells. Sci Transl Med 2011; 3:89ra58; PMID:21715679
- Cenni V, Capanni C, Columbaro M, Ortolani M, D'Apice MR, Novelli G, Fini M, Marmiroli S, Scarano E, Maraldi NM, et al. Autophagic degradation of farnesylated prelamin A as a therapeutic approach to lamin-linked progeria. Eur J Histochem 2011; 55:e36; PMID:22297442; http://dx.doi.org/10.4081/ejh.2011.e36
- Gabriel D, Roedl D, Gordon LB, Djabali K. Sulforaphane enhances progerin clearance in Hutchinson-Gilford progeria fibroblasts. Aging Cell 2015; 14:78-91; PMID:25510262; http://dx.doi.org/10.1111/acel.12300
- Liu B, Ghosh S, Yang X, Zheng H, Liu X, Wang Z, Jin G, Zheng B, Kennedy BK, Suh Y, et al. Resveratrol rescues SIRT1-dependent adult stem cell decline and alleviates progeroid features in laminopathy-based progeria. Cell Metab 2012; 16:738-50; PMID:23217256; http://dx.doi.org/10.1016/j.cmet.2012.11.007
- Pekovic V, Gibbs-Seymour I, Markiewicz E, Alzoghaibi F, Benham AM, Edwards R, Wenhert M, von Zglinicki T, Hutchison CJ. Conserved cysteine residues in the mammalian lamin A tail are essential for cellular responses to ROS generation. Aging Cell 2011; 10:1067-79; PMID:21951640; http://dx.doi.org/10.1111/j.1474-9726.2011.00750.x
- Sieprath T, Darwiche R, De Vos WH. Lamins as mediators of oxidative stress. Biochem Biophys Res Commun 2012; 421:635-9; PMID:22538370; http://dx.doi.org/10.1016/j.bbrc.2012.04.058
- Rivera-Torres J, Acin-Perez R, Cabezas-Sanchez P, Osorio FG, Gonzalez-Gomez C, Megias D, Camara C, Lopez-Otin C, Enriquez JA, Luque-Garcia JL, Andres V. Identification of mitochondrial dysfunction in Hutchinson-Gilford progeria syndrome through use of stable isotope labeling with amino acids in cell culture. J Proteomics 2013; 91:466-77; PMID:23969228; http://dx.doi.org/10.1016/j.jprot.2013.08.008
- Richards SA, Muter J, Ritchie P, Lattanzi G, Hutchison CJ. The accumulation of un-repairable DNA damage in laminopathy progeria fibroblasts is caused by ROS generation and is prevented by treatment with N-acetyl cysteine. Hum Mol Genet 2011; 20:3997-4004; PMID:21807766; http://dx.doi.org/10.1093/hmg/ddr327
- Lattanzi G, Marmiroli S, Facchini A, Maraldi NM. Nuclear damages and oxidative stress: new perspectives for laminopathies. Eur J Histochem 2012; 56:e45; PMID:23361241; http://dx.doi.org/10.4081/ejh.2012.e45
- Blondel S, Jaskowiak AL, Egesipe AL, Le Corf A, Navarro C, Cordette V, Martinat C, Laabi Y, Djabali K, de Sandre-Giovannoli A, et al. Induced pluripotent stem cells reveal functional differences between drugs currently investigated in patients with hutchinson-gilford progeria syndrome. Stem Cells Transl Med 2014; 3:510-9; PMID:24598781; http://dx.doi.org/10.5966/sctm.2013-0168
- Larrieu D, Britton S, Demir M, Rodriguez R, Jackson SP. Chemical inhibition of NAT10 corrects defects of laminopathic cells. Science 2014; 344:527-32; PMID:24786082; http://dx.doi.org/10.1126/science.1252651
- Pellegrini C, Columbaro M, Capanni C, D'Apice MR, Cavallo C, Murdocca M, Lattanzi G, Squarzoni S. All-trans retinoic acid and rapamycin normalize Hutchinson Gilford progeria fibroblast phenotype. Oncotarget 2015; 6:29914-28; PMID:26359359
- Das A, Grotsky DA, Neumann MA, Kreienkamp R, Gonzalez-Suarez I, Redwood AB, Kennedy BK, Stewart CL, Gonzalo S. Lamin A Deltaexon9 mutation leads to telomere and chromatin defects but not genomic instability. Nucleus 2013; 4:410-9; PMID:24153156; http://dx.doi.org/10.4161/nucl.26873
- Gonzalez-Suarez I, Redwood AB, Grotsky DA, Neumann MA, Cheng EH, Stewart CL, Dusso A, Gonzalo S. A new pathway that regulates 53BP1 stability implicates cathepsin L and vitamin D in DNA repair. EMBO J 2011; 30:3383-96; PMID:21750527; http://dx.doi.org/10.1038/emboj.2011.225
- Gonzalo S. DNA damage and lamins. Adv Exp Med Biol 2014; 773:377-99; PMID:24563357; http://dx.doi.org/10.1007/978-1-4899-8032-8_17
- Kubben N, Brimacombe KR, Donegan M, Li Z, Misteli T. A high-content imaging-based screening pipeline for the systematic identification of anti-progeroid compounds. Methods. 2016 Mar 1; 96:46-58. PMID: 26341717; http://dx.doi.org/10.1016/j.ymeth.2015.08.024
- Dittmer TA, Sahni N, Kubben N, Hill DE, Vidal M, Burgess RC, Roukos V, Misteli T. Systematic identification of pathological lamin A interactors. Mol Biol Cell 2014; 25:1493-510; PMID:24623722; http://dx.doi.org/10.1091/mbc.E14-02-0733
- Pegoraro G, Kubben N, Wickert U, Gohler H, Hoffmann K, Misteli T. Ageing-related chromatin defects through loss of the NURD complex. Nat Cell Biol 2009; 11:1261-7; PMID:19734887; http://dx.doi.org/10.1038/ncb1971
- Kudlow BA, Stanfel MN, Burtner CR, Johnston ED, Kennedy BK. Suppression of proliferative defects associated with processing-defective lamin A mutants by hTERT or inactivation of p53. Mol Biol Cell 2008; 19:5238-48; PMID:18843043; http://dx.doi.org/10.1091/mbc.E08-05-0492
- Cao K, Blair CD, Faddah DA, Kieckhaefer JE, Olive M, Erdos MR, Nabel EG, Collins FS. Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J Clin Invest 2011; 121:2833-44; PMID:21670498; http://dx.doi.org/10.1172/JCI43578
- Chojnowski A, Ong PF, Wong ES, Lim JS, Mutalif RA, Navasankari R, Dutta B, Yang H, Liow YY, Sze SK, et al. Progerin reduces LAP2alpha-telomere association in Hutchinson-Gilford progeria. Elife. 2015 Aug 27; 4:1-21. PMID: 26312502; http://dx.doi.org/10.7554/eLife.07759
- Zhang W, Li J, Suzuki K, Qu J, Wang P, Zhou J, Liu X, Ren R, Xu X, Ocampo A, et al. Aging stem cells. A Werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging. Science 2015; 348:1160-3; PMID:25931448; http://dx.doi.org/10.1126/science.aaa1356
- Redondo-Munoz J, Perez-Garcia V, Rodriguez MJ, Valpuesta JM, Carrera AC. Phosphoinositide 3-kinase β protects nuclear envelope integrity by controlling RCC1 localization and Ran activity. Mol Cell Biol 2015; 35:249-63; PMID:25348717; http://dx.doi.org/10.1128/MCB.01184-14
- Toyama BH, Savas JN, Park SK, Harris MS, Ingolia NT, Yates JR, 3rd, Hetzer MW. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell 2013; 154:971-82; PMID:23993091; http://dx.doi.org/10.1016/j.cell.2013.07.037
- Salpingidou G, Smertenko A, Hausmanowa-Petrucewicz I, Hussey PJ, Hutchison CJ. A novel role for the nuclear membrane protein emerin in association of the centrosome to the outer nuclear membrane. J Cell Biol 2007; 178:897-904; PMID:17785515; http://dx.doi.org/10.1083/jcb.200702026
