ABSTRACT
Cell migration through tight spaces can induce substantial deformations of the nucleus and cause nuclear envelope (NE) rupture, resulting in uncontrolled exchange of nuclear and cytosolic proteins. These events can cause DNA damage and, in severe cases, nuclear fragmentation, challenging the integrity of the genomic material. Cells overcome NE ruptures during interphase by repairing the NE using components of the endosomal sorting complexes required for transport (ESCRT) machinery. Paralleling the molecular mechanism used during NE reformation in late mitosis, ESCRT-III subunits and the associated AAA-ATPase VPS4B are recruited to NE rupture sites and help restore NE integrity. While these findings are common to many cell types, they are particularly relevant in the context of cancer metastasis, where nuclear deformation and rupture could drive genomic instability in invading cells and further promote cancer progression. At the same time, inhibiting NE repair may offer new therapeutic approaches to specifically target invasive cancer cells.
Introduction
In eukaryotic cells, chromosomes are separated from the cytoplasm by the nuclear envelope (NE), which consists of two phospholipid bilayer membranes, termed the inner and outer nuclear membranes (INM and ONM, respectively), nuclear membrane proteins, the nuclear lamina, and nuclear pore complexes (NPCs).Citation1 The INM and ONM join at the sites of NPCs, which regulate the macromolecular transport in and out of the nucleus.Citation2 For proteins larger than 30–60 kDa, a nuclear localization sequence (NLS) or nuclear export signal (NES) is required for the transport through nuclear pores.Citation2 Underlying the INM is the nuclear lamina, a dense intermediate filament meshwork that provides mechanical support to the nucleus.Citation3-5 The main nuclear lamina components in mammalian cells are A-type lamins (lamin A and C) and B-type lamins (lamin B1 and B2).Citation1
The NE is thought to physically protect the cell's genome integrity by creating a tightly controlled, intracellular compartment with distinct molecular composition that facilitates nuclear processes such as DNA replication, repair, and transcriptional regulation. Loss of NE integrity during interphase is likely to perturb cellular functions on multiple levels, including the RanGTP gradient across the NE required for directed protein transport through nuclear pores. Furthermore, cytoplasmic nucleases that normally protect cells from foreign DNA could potentially enter the nucleus and damage the endogenous genome. As such, it had long been assumed that NE rupture would be lethal for cells.
In recent years, however, multiple studies have observed transient NE ruptures in cultured cells during interphase that allow uncontrolled exchange of molecules between the cytoplasm and nucleoplasm.Citation6-9 NE ruptures can be readily visualized by live cell microscopy of fluorescent proteins fused to a nuclear localization signal (NLS), e.g., NLS-GFP or NLS-RFP. These fluorescent reporters are normally localized to the nucleus, but rapidly spill into the cytoplasm upon NE rupture and are then gradually re-imported into the nucleus over the course of 10–90 minutes ().Citation6-11 NE ruptures can also be detected by monitoring fluorescent proteins tagged with a nuclear export signal (NES), which only enter the nucleus during NE rupture and are exported back into the cytoplasm once NE integrity is restored.Citation6,10 In addition to these fluorescent reporters, evidence of NE rupture comes from mitochondria and ribosomes that become mislocalized to the nucleus, and promyelocytic leukemia (PML) nuclear bodies found in the cytoplasm of cells that exhibited NE rupture.Citation6,7 In cells cultured on rigid substrates, spontaneous NE rupture is observed in circa 5% of cells over a 24 h period.Citation6,7,9 Depletion of lamins or expression of disease-causing lamin mutants that impair nuclear lamina stability increases NE rupture rates.Citation6,7,9 Similarly, human cancer cell lines, which often have altered lamin levels,Citation12 exhibit more frequent NE rupture than non-tumorigenic controls.Citation7
Figure 1. Schematic depiction of NE rupture sequence during cell migrating through an engineered constriction (gray pillars) mimicking confining interstitial spaces. The nucleus containing NLS-GFP is indicated in green. Right side: close-up of the area indicated in the red dashed box in panel I, containing the leading edge of the nucleus where the NE rupture occurs. (I) The nucleus enters the constriction. (II) Formation of a nuclear membrane bleb at the site of a local defect in the nuclear lamina. (III) The membrane bleb expands and chromatin can enter the bleb through the nuclear lamina opening. (IV) NE rupture results in collapse of the nuclear bleb and spilling of NLS-GFP into the cytoplasm. (V) Following NE repair, NLS-GFP is gradually re-imported into the nucleus. A small ‘lamin scar’ remains at the location of NE rupture. (VI) The cell has passed the constriction and nucleo-cytoplasmic compartmentalization has been restored.
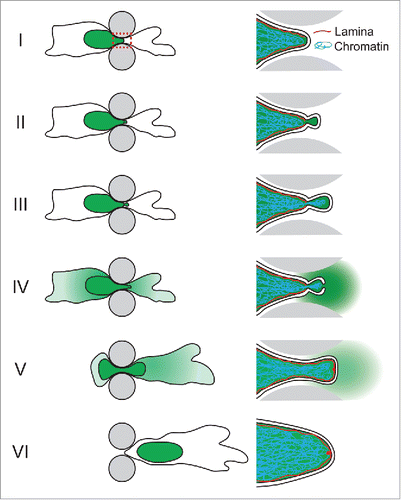
Confined migration causes increased nuclear envelope rupture
While earlier studies were limited to cells in 2D culture, researchers have recently begun to investigate the role of the nucleus in 3D cell migration and uncovered that nuclear deformation can become a rate-limiting factor during confined migration.Citation13-18 When cells encounter constrictions smaller than the nuclear cross section, the large and relatively rigid nucleus must deform substantially to pass through the available space.Citation13,14,19 Two recent studies found that the forces exerted on the nucleus during this process frequently rupture the NE.Citation10,11 Nuclear deformation and NE rupture were recorded in cells migrating through collagen matrices, through microfluidic devices with precisely controlled pore sizes, and through living tissues.Citation10,11 The incidence of NE rupture increased dramatically with increasing confinement, with up to 90% of cells exhibiting NE rupture rates over 24 h when moving through pores smaller than ∼6 μm2 in cross section.Citation10,11
Mechanics of nuclear envelope rupture
The formation of nuclear membrane blebs () that precede NE rupture and that collapse upon nuclear membrane ruptureCitation6-11 suggest that these events are driven by an increase in intranuclear pressure due to forces, possibly generated by the cytoskeleton, that compress the nucleus. Notably, even nuclei in unconfined cells cultured on 2D substrates experience compressive forces from the contractile actomyosin filaments spanning the nucleus.Citation8 Supporting a role for the cytoskeleton, treatment with either the myosin inhibitor blebbistatin or the actin polymerization inhibitor cytochalasin, which reduces the tension of the actin cytoskeleton, also reduces the NE rupture rates.Citation8,10 In contrast, when cytochalasin D treated cells are subjected to external vertical confinement to match nuclear compression levels of untreated cells, their NE rupture rate rises to that of unconfined cells. This finding is consistent with a model in which NE bleb formation—and subsequently rupture—arise from intranuclear pressure, rather than from local cytoskeletal pulling on nuclear membrane segments.Citation8
During confined migration, the nuclear membrane bulges out and blebs at sites of defects in the nuclear lamina.Citation7,10 It remains unclear whether these nuclear lamina defects, which precede nuclear membrane bleb formation, are caused by excessive nuclear deformation during migration or are pre-existing abnormalities in the nuclear envelope. Nuclear bleb formation predominantly occurs at the leading edge of the nucleus as it passes through a constriction, which is where the highest nuclear membrane curvature is typically found.Citation10,11,20 The C-terminal farnesyl group of B-type lamins, along with INM proteins, tether the INM and nuclear lamina and thereby stabilize the nuclear membrane. Thus, when the pressure in the nuclear interior increases and places additional stress on the NE, local defects in the nuclear lamina, particularly the B-type lamin network, could promote nuclear membrane detachment and bleb formation in these regions. This process may thus closely resemble the mechanics of bleb formation at the plasma membrane, where local defects in the underlying actin cortex can result in pressure-induced membrane expansion and blebbing.Citation21
Under continued confinement, nuclear membrane blebs progressively expand until NE rupture occurs, presumably once the critical area strain of lipid bilayers has been exceeded.Citation10 It remains unclear to what extent recruitment of membrane from the endoplasmic reticulum, which is continuous with the ONM, contributes to bleb expansion and delays NE rupture. The nuclear membrane blebs are initially devoid of most NE proteins and lack nuclear pores.Citation8,10,22 During the bleb expansion, chromatin can herniate through the nuclear lamina and protrude into the bleb.Citation6-11 In severe cases, chromatin segments can separate from the main nucleus as cells squeeze through tight constrictions.Citation10 Over time, lamin A frequently forms a new nuclear lamina in the bleb, whereas lamin B1 remains absent from the bleb.Citation10 Intriguingly, after NE rupture lamin A accumulates at the rupture site, forming ‘lamin A scars’ that remain visible for hours and that may locally protect the nuclear membrane from further rupture.Citation10,22
Consequences of nuclear envelope rupture
Micronuclei, composed of single chromosomes ‘lost’ from the main nucleus during improper mitotic segregation, have long been recognized as hotspots for DNA damage and chromothripsis, i.e., extensive chromatin fragmentation and rearrangements.Citation23 Recent work suggests that micronuclei are initially protected by an intact NE, but often lose the micronuclei membrane integrity during interphase, resulting in extensive DNA defects in the micronucleus.Citation23,24 Time-lapse microscopy and immunofluorescence staining of markers for DNA double strand breaks, such as γH2AX and 53BP1, reveal that NE rupture can similarly cause DNA damage in the main nucleus.Citation10,11 During cell migration through small constrictions, new GFP-53BP1 foci form rapidly after NE rupture in several cell lines.Citation10,11 In some cases, 53BP1 foci formation precedes NE rupture and may be caused by nuclear deformation.Citation10 Furthermore, compaction of the ‘sponge-like’ nuclear interior during migration through tight spaces can temporarily displace water and soluble molecules, including components of the DNA damage repair machinery, from the confined regions of the nucleus and thereby delay or impair DNA repair.Citation25 It is intriguing to speculate that DNA damage arising from nuclear deformation, fragmentation, and NE rupture during confined migration could contribute to the genomic instability of metastatic cancer cells.
ESCRT-III proteins mediate nuclear membrane repair
Stunningly, most of cells survive even repeated NE rupture and are able to restore NE integrity.Citation6-11 Clues into the responsible NE repair mechanism have come from studying NE reformation during late anaphase, where the ESCRT machinery serves as an essential component for resealing any remaining gaps in the nuclear envelope.Citation26-28 The ESCRT machinery was originally described as a vacuole-sorting complex,Citation29 and subsequently recognized to mediate many additional cellular processes, such as plasma membrane repair, cytokinetic abscission, viral budding at the plasma membrane, endocytosis and secretion, and NPC insertion into the NE.Citation30-32 In all of these processes, the ESCRT machinery mediates the membrane remodeling by promoting membrane curvature and, in most cases, driving membrane scission. The ESCRT machinery consists of multiple subunits, ranging from ESCRT-0 to ESCRT-III. ESCRT-0, -I, and –II subunits are often involved in cargo sorting at the vacuole and the recruitment of ESCRT-III components to this location. ESCRT-III subunits assemble into a spiral structure, in the process bending the membrane into a negative curvature and ultimately resulting in membrane scission.Citation30,31 VPS4B, an AAA-ATPase, is recruited to the spiral structure and disassembles the ESCRT subunits after membrane scission.Citation30,31 Intriguingly, only ESCRT-III members are required for nuclear membrane resealing during NE reformation and NE repair.Citation10,11,26,27
ESCRT dependent nuclear membrane repair following NE rupture () appears to parallel in major parts the molecular mechanism of NE sealing during mitosis.Citation9-11 When the NE ruptures, the newly created INM and ONM membrane ends likely fuse to each other to minimize exposure of hydrophobic fatty acid tails. This results in annular membrane channels (), similar in geometry to the nuclear membrane openings when the NE reforms around residual mitotic spindle microtubules in late mitosis.Citation10,26-28 In both cases, ESCRT-III subunits and VPS4B drive the membrane scission required to separate INM and ONM and to restore NE integrity ().
Figure 2. Illustration of the proposed model for ESCRT-mediated NE repair. (I) In the intact NE, membrane-bound CHMP7 is localized to the ER and ONM, whereas LEM-domain proteins are localized to the INM. (II) NE rupture creates exposed hydrophilic domains of the membrane phospholipids and opens the NE lumen to the cytoplasm and nucleoplasm. Given the thermodynamically unfavorable state of open membrane ends, this phase is likely extremely short-lived. (III) The exposed membrane ends of the INM and ONM fuse to each other, resulting in an annular channel connecting the nuclear interior and cytoplasm. The annular channel allows nuclear membrane proteins, including CHMP7 and LEM-domain proteins, to freely diffuse between the INM and ONM without restriction from the nuclear pore complex. Given that LEM-domain proteins interact with chromatin and intranuclear proteins, it is likely that the interaction between LEM-domain proteins and CHMP7 occurs at the INM. (IV) Interaction of CHMP7 with LEM-domain proteins at the annular fusion causes recruitment of CHMP4B to assemble into a spiral of ESCRT-III subunits. CHMP2A, which is incorporated into the ESCRT-III spiral structure, recruits the AAA-ATPase VPS4B. (V) Tightening of the ESCRT-III spiral drives closure of the annular membrane channel and ultimately membrane scission. This process may be mediated through partial disassembly by the recruited VPS4B, or involve sequential assembly of ESCRT-III proteins followed by VPS4B recruitment. (VI) After membrane scission and ESCRT-III complex disassembly have been completed, INM and ONM are separated and NE integrity is restored.
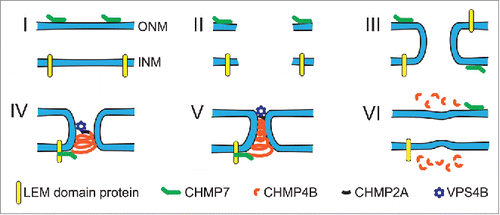
A number of recent publications have provided additional insights into the molecular details of the recruitment and function of ESCRT components at the NE. One of the key nucleators for ESCRT-mediated NE remodeling is CHMP7. CHMP7 is a fusion of an ESCRT-II and an ESCRT-III-like protein,Citation33 allowing it to bypass the upstream components of the canonical ESCRT pathway, in which ESCRT-II proteins recruit ESCRT-III subunits and VPS4B.Citation10,27 CHMP7 is normally localized to the membrane of the endoplasmic reticulum (ER) through its curvature sensitive N-terminal membrane binding domain, but accumulates at the NE during NE reformation.Citation28 CHMP7 initiates assembly of CHMP4B, the major ESCRT-III subunit, which ultimately drives nuclear membrane scission. Interaction of the ESCRT-III spiral with VPS4B is mediated by the ESCRT-III subunit CHMP2A, which is also incorporated into the ESCRT-III spiral.Citation34 Interestingly, CHMP4B and VPS4B both accumulate at the rupture site within 2 min of NE rupture, and remain there for ∼12 minutes.Citation10 If VPS4B were only required for disassembly of the ESCRT-III complex after the membrane scission event, one would expect VPS4B to be recruited to the rupture site following CHMP4B, and not at the same time. The apparently parallel recruitment may indicate that VPS4B could be involved in remodeling the ESCRT complex even before membrane scission, and thus directly mediate the scission event, rather than only promoting ESCRT-III disassembly after membrane scission. Regardless of these molecular details, depletion of either CHMP2, CHMP4B, or CHMP7 or expression of a dominant negative VPS4B mutant delays NE reformation and NE repair, demonstrating the functional importance of these proteins in restoring NE integrity.Citation9-11,26-28
The mechanisms by which CHMP7 is recruited to sites of NE rupture to initiate ESCRT-III mediate NE repair are only beginning to emerge. Recent work suggests that LEM-domain proteins, normally in the INM, can recruit CHMP7 to exposed sites during NE reformation.Citation35 In yeast, the LEM domain proteins Heh1 and Heh2 both bind to CHM7, the yeast homolog of CHMP7, and this complex is linked to nuclear membrane sealing and the quality control of the nuclear pore complex.Citation36 In interphase, CHMP7 is localized on the ER membrane,Citation28 which is continuous with the ONM. Thus, CHMP7 and LEM-domain proteins, which reside in the INM, are on opposite faces of the intact NE. During NE reformation or NE rupture, however, the membrane annulus formed by the fusion of INM and ONM membrane ends () could enable NPC-independent diffusion of membrane proteins between the INM and ONM, without control of the nuclear pore complex, and thus allow CHMP7 and the LEM-domain proteins to come into contact. The interaction between these proteins could then initiate ESCRT-III assembly and nuclear membrane remodeling.Citation10,26-28 An additional signal for ESCRT-III assembly could come from the release of calcium from the ER and nuclear lumen during NE rupture, as it is implicated during in vitro NE reformationCitation37 and nuclear pore formation,Citation38 as well as paralleling the role of calcium influx in promoting ESCRT-III plasma membrane repair.Citation39
Conclusions and future directions
Cell migration through confined spaces, such as those found in interstitial spaces or during transendothelial migration, requires substantial nuclear deformation. The nuclear deformation can lead to NE rupture, nuclear fragmentation, and DNA damage.Citation10,11 While cells rapidly repair the NE, enabling cell survival, the functional consequences of nuclear deformation and NE rupture remain to be assessed. In the short-term, nuclear deformation, fragmentation, and NE rupture could impact chromatin organization and transcriptional activity. In the long-term, they could promote genomic instability, particularly in proliferative cancer cells, whereas postmitotic immune cells may be less at risk. At the same time, inhibiting ESCRT-III mediated NE repair could present a novel therapeutic approach to target metastatic cancer cells. In proof-of-concept studies, treating cells with both a dominant negative VPS4B mutant to decrease NE repair efficiency and an ATM inhibitor to block DNA damage repair dramatically increased cell death after NE rupture.Citation10,11 Since each treatment alone did not significantly reduce cell viability,Citation10,11 it suggests that synthetic lethality was required for the effect. As both normal and cancer cells showed similar susceptibility to the treatment,Citation10,11 future work will be needed to determine whether metastatic cancer cells have evolved unique features that enable them to withstand NE rupture and that could be exploited for specific targeting. If successful, drugs targeting such pathways could ultimately reduce metastatic cancer spreading while sparing normal cells.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The authors are supported by awards from the National Institutes of Health (R01 HL082792 and U54 CA210184, to J.L.), the Department of Defense Breast Cancer Research Program (Breakthrough Award BC150580, to J.L.), and the National Science Foundation (CAREER Award CBET-1254846, to J.L.).
References
- Gruenbaum Y, Margalit A, Goldman RD, Shumaker DK, Wilson KL. The nuclear lamina comes of age. Nat Rev Mol Cell Biol 2005; 6(1):21-31; PMID:15688064; https://doi.org/10.1038/nrm1550
- Timney BL, Raveh B, Mironska R, Trivedi JM, Kim SJ, Russel D, Wente SR, Sali A, Rout MP. Simple rules for passive diffusion through the nuclear pore complex. J Cell Biol 2016; 215(10):57-76; PMID:27697925; https://doi.org/10.1083/jcb.201601004
- Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, Young SG, Lee RT. Lamins a and c but not lamin b1 regulate nuclear mechanics. J Biol Chem 2006; 281(35):25768-80; PMID:16825190; https://doi.org/10.1074/jbc.M513511200
- Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT. Lamin a/c deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest 2004; 113(3):370-78; PMID:14755334; https://doi.org/10.1172/JCI200419670
- Dahl KN, Kahn SM, Wilson KL, Discher DE. The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J Cell Sci 2004; 117(Pt 20):4779-86; PMID:15331638; https://doi.org/10.1242/jcs.01357
- De Vos WH, Houben F, Kamps M, Malhas A, Verheyen F, Cox J, Manders EM, Verstraeten VL, van Steensel MA, Marcelis CL, et al. Repetitive disruptions of the nuclear envelope invoke temporary loss of cellular compartmentalization in laminopathies. Hum Mol Genet 2011; 20(21):4175-86; PMID:21831885; https://doi.org/10.1093/hmg/ddr344
- Vargas JD, Hatch EM, Anderson DJ, Hetzer MW. Transient nuclear envelope rupturing during interphase in human cancer cells. Nucleus 2012; 3(1):88-100; PMID:22567193; https://doi.org/10.4161/nucl.18954
- Hatch EM, Hetzer MW. Nuclear envelope rupture is induced by actin-based nucleus confinement. J Cell Biol 2016d; 215(1):27-36; PMID:27697922; https://doi.org/10.1083/jcb.201603053
- Robijns J, Molenberghs F, Sieprath T, Corne TD, Verschuuren M, De Vos WH. In silico synchronization reveals regulators of nuclear ruptures in lamin a/c deficient model cells. Sci Rep 2016; 6:30325; PMID:27461848; https://doi.org/10.1038/srep30325
- Denais CM, Gilbert RM, Isermann P, McGregor AL, te Lindert M, Weigelin B, Davidson PM, Friedl P, Wolf K, Lammerding J. Nuclear envelope rupture and repair during cancer cell migration. Science 2016; 352(6283):353-58; PMID:27013428; https://doi.org/10.1126/science.aad7297
- Raab M, Gentili M, de Belly H, Thiam HR, Vargas P, Jimenez AJ, Lautenschlaeger F, Voituriez R, Lennon-Dumenil AM, Manel N, et al. Escrt iii repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science 2016; 352(6283):359-62; PMID:27013426; https://doi.org/10.1126/science.aad7611
- Foster CR, Przyborski SA, Wilson RG, Hutchison CJ. Lamins as cancer biomarkers. Biochem Soc Trans 2010; 38(Pt 1):297-300; PMID:20074078; https://doi.org/10.1042/BST0380297
- Harada T, Swift J, Irianto J, Shin JW, Spinler KR, Athirasala A, Diegmiller R, Dingal PC, Ivanovska IL, Discher DE. Nuclear lamin stiffness is a barrier to 3d migration, but softness can limit survival. J Cell Biol 2014; 204(5):669-82; PMID:24567359; https://doi.org/10.1083/jcb.201308029
- Wolf K, Te Lindert M, Krause M, Alexander S, Te Riet J, Willis AL, Hoffman RM, Figdor CG, Weiss SJ, Friedl P. Physical limits of cell migration: Control by ecm space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol 2013; 201(7):1069-84; PMID:23798731; https://doi.org/10.1083/jcb.201210152
- Friedl P, Wolf K, Lammerding J. Nuclear mechanics during cell migration. Curr Opin Cell Biol 2011; 23(1):55-64; PMID:21109415; https://doi.org/10.1016/j.ceb.2010.10.015
- Davidson PM, Denais C, Bakshi MC, Lammerding J. Nuclear deformability constitutes a rate-limiting step during cell migration in 3-d environments. Cell Mol Bioeng 2014; 7(3):293-306; PMID:25436017; https://doi.org/10.1007/s12195-014-0342-y
- Rowat AC, Jaalouk DE, Zwerger M, Ung WL, Eydelnant IA, Olins DE, Olins AL, Herrmann H, Weitz DA, Lammerding J. Nuclear envelope composition determines the ability of neutrophil-type cells to passage through micron-scale constrictions. J Biol Chem 2013; 288(12):8610-8; PMID:23355469; https://doi.org/10.1074/jbc.M112.441535
- Fu Y, Chin LK, Bourouina T, Liu AQ, VanDongen AM. Nuclear deformation during breast cancer cell transmigration. Lab on a chip 2012; 12(19):3774-8; PMID:22864314; https://doi.org/10.1039/c2lc40477j
- Davidson PM, Sliz J, Isermann P, Denais C, Lammerding J. Design of a microfluidic device to quantify dynamic intra-nuclear deformation during cell migration through confining environments. Integr Biol 2015; 7(12):1534-46; PMID:26549481; https://doi.org/10.1039/C5IB00200A
- Cao X, Moeendarbary E, Isermann P, Davidson PM, Wang X, Chen MB, Burkart AK, Lammerding J, Kamm RD, Shenoy VB. A chemomechanical model for nuclear morphology and stresses during cell transendothelial migration. Biophys J 2016; 111(7):1541-52; PMID:27705776; https://doi.org/10.1016/j.bpj.2016.08.011
- Charras GT, Yarrow JC, Horton MA, Mahadevan L, Mitchison TJ. Non-equilibration of hydrostatic pressure in blebbing cells. Nature 2005; 435(7040):365-9; PMID:15902261; https://doi.org/10.1038/nature03550
- Le Berre M, Aubertin J, Piel M. Fine control of nuclear confinement identifies a threshold deformation leading to lamina rupture and induction of specific genes. Integr Biol 2012; 4(11):1406-14; PMID:23038068; https://doi.org/10.1039/c2ib20056b
- Zhang CZ, Spektor A, Cornils H, Francis JM, Jackson EK, Liu S, Meyerson M, Pellman D. Chromothripsis from DNA damage in micronuclei. Nature 2015; 522(7555):179-84; PMID:26017310; https://doi.org/10.1038/nature14493
- Hatch EM, Hetzer MW. Linking micronuclei to chromosome fragmentation. Cell 2015; 161(7):1502-4; PMID:26091034; https://doi.org/10.1016/j.cell.2015.06.005
- Irianto J, Pfeifer CR, Bennett RR, Xia Y, Ivanovska IL, Liu AJ, Greenberg RA, Discher DE. Nuclear constriction segregates mobile nuclear proteins away from chromatin. Mol Biol Cell 2016; 27(15):4011-20; PMID:27798234; https://doi.org/10.1091/mbc.E16-06-0428
- Olmos Y, Hodgson L, Mantell J, Verkade P, Carlton JG. Escrt-iii controls nuclear envelope reformation. Nature 2015; 522(7555):236-9; PMID:26040713; https://doi.org/10.1038/nature14503
- Vietri M, Schink KO, Campsteijn C, Wegner CS, Schultz SW, Christ L, Thoresen SB, Brech A, Raiborg C, Stenmark H. Spastin and escrt-iii coordinate mitotic spindle disassembly and nuclear envelope sealing. Nature 2015; 522(7555):231-5; PMID:26040712; https://doi.org/10.1038/nature14408
- Olmos Y, Perdrix-Rosell A, Carlton JG. Membrane binding by chmp7 coordinates escrt-iii-dependent nuclear envelope reformation. Curr Biol 2016; 26(19):2635-41; PMID:27618263; https://doi.org/10.1016/j.cub.2016.07.039
- Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol 2002; 3(12):893-905; PMID:12461556; https://doi.org/10.1038/nrm973
- Henne WM, Buchkovich NJ, Emr SD. The escrt pathway. Dev Cell 2011; 21(1):77-91; PMID:21763610; https://doi.org/10.1016/j.devcel.2011.05.015
- Christ L, Raiborg C, Wenzel EM, Campsteijn C, Stenmark H. Cellular functions and molecular mechanisms of the escrt membrane-scission machinery. Trends Biochem Sci 2016; 42(1):42-56; PMID:27669649; https://doi.org/10.1016/j.tibs.2016.08.016
- Webster BM, Lusk CP. Escrts breach the nuclear border. Nucleus 2015; 6(3):197-202; PMID:25942571; https://doi.org/10.1080/19491034.2015.1035844
- Bauer I, Brune T, Preiss R, Kolling R. Evidence for a nonendosomal function of the saccharomyces cerevisiae escrt-iii-like protein chm7. Genetics 2015; 201(4):1439-52; PMID:26510789; https://doi.org/10.1534/genetics.115.178939
- Effantin G, Dordor A, Sandrin V, Martinelli N, Sundquist WI, Schoehn G, Weissenhorn W. Escrt-iii chmp2a and chmp3 form variable helical polymers in vitro and act synergistically during hiv-1 budding. Cell Microbiol 2013; 15(2):213-26; PMID:23051622; https://doi.org/10.1111/cmi.12041
- Gu M, LaJoie D, Chen OS, von Appen A, Ladinsky MS, Redd MJ, Nikolova L, Bjorkman PJ, Sundquist WI, Ullman KS et al. Lem2 recruits chmp7 for escrt-mediated nuclear envelope closure in fission yeast and human cells. Proc Natl Acad Sci U S A 2017; PMID:28242692; https://doi.org/10.1073/pnas.1613916114
- Webster BM, Thaller DJ, Jager J, Ochmann SE, Borah S, Lusk CP. Chm7 and heh1 collaborate to link nuclear pore complex quality control with nuclear envelope sealing. EMBO J 2016; 35(22):2447-67; PMID:27733427; https://doi.org/10.15252/embj.201694574
- Sullivan KM, Busa WB, Wilson KL. Calcium mobilization is required for nuclear vesicle fusion in vitro: Implications for membrane traffic and ip3 receptor function. Cell 1993; 73(7):1411-22; PMID:8391933; https://doi.org/10.1016/0092-8674(93)90366-X
- Macaulay C, Forbes DJ. Assembly of the nuclear pore: Biochemically distinct steps revealed with nem, gtp gamma s, and bapta. J Cell Biol 1996; 132(1-2):5-20; PMID:8567730; https://doi.org/10.1083/jcb.132.1.5
- Jimenez AJ, Maiuri P, Lafaurie-Janvore J, Divoux S, Piel M, Perez F. Escrt machinery is required for plasma membrane repair. Science 2014; 343(6174):1247136; PMID:24482116; https://doi.org/10.1126/science.1247136
