ABSTRACT
The first step of gene expression results in the production of mRNA ribonucleoparticles (mRNPs) that are exported to the cytoplasm via the NPC for translation into the cytoplasm. During this process, the mRNA molecule synthesized by RNA polymerase II (Pol II) undergoes extensive maturation, folding and packaging events that are intimately coupled to its synthesis. All these events take place in a chromatin context and it is therefore not surprising that a growing number of studies recently reported specific contributions of chromatin dynamics to various steps of mRNP biogenesis. In this extra view, we replace our recent findings highlighting the contribution of the yeast chromatin remodeling complex ISW1 to nuclear mRNA quality control in the context of the recent literature.
Introduction
In eukaryotes, mRNA biogenesis in the nucleus and their translation in the cytoplasm are physically separated by the nuclear envelope that controls molecular exchanges between these 2 compartments through a specialized and evolutionary conserved structure, the nuclear pore complex (NPC).Citation1 The accurate formation of export-competent mRNPs results from the precise spatio-temporal orchestration of highly coordinated processing reactions and is followed by their transport into the cytoplasm by the heterodimeric mRNA export receptor Mex67-Mtr2 (TAP/NXF1 in metazoan). Transcripts generated by Pol II undergo several processing steps including 5′-end capping, splicing, 3′-end cleavage and polyadenylation that initiate co-transcriptionally. The C-terminal domain (CTD) of the large subunit of Pol II is the conductor of this tight coupling between transcription and pre-mRNA processing, which coordinates the recruitment of the biogenesis factors according to its phosphorylation state.Citation2 In parallel of these maturation events, the nuclear export machinery -including Mex67 and its mRNA-binding adaptor proteins such as the SR protein Npl3, the polyA RNA binding protein Nab2, the TREX complex (THO complex, Yra1, Sub2) or the TREX2 complex - is also recruited thereby ensuring an optimal coordination of transcription elongation with mRNP maturation, packaging and export.Citation3-5 This co-transcriptional assembly of mRNPs also allows early protection from nucleases and may provide sequentiality to the association of RNA binding proteins with the nascent transcript, thus guaranteeing the accuracy of the biogenesis process. In addition, nuclear mRNA biogenesis is under rigorous surveillance to ensure that only correctly processed and packaged mRNPs are exported to the cytoplasm. However, sub-optimal mRNPs are believed not to be detected as such by a dedicated machinery. Instead, a currently accepted model proposed by Jensen et al. postulates that mRNA quality control results from a subtle balance between biogenesis and degradation.Citation6 Hence, transcripts that do not mature during a given time window undergo degradation by the 3′-5′ nuclear exonuclease exosome complex, the major 5′-3′ exonuclease Xrn26 or the NPC localized nuclease Swt1.Citation7 Moreover, nuclear retention of erroneous transcripts is another aspect of quality control that has been observed in yeast, fly and mammals and proposed to allow a protective function, by offering defective mRNPs extending time to mature.Citation6 Yeast mutants impaired in mRNA maturation, packaging or export all exhibit transcripts retention in nuclear foci located close to the transcription site that depends on the Rrp6 catalytic subunit of nuclear exosome. Furthermore, in S. cerevisiae, unspliced pre-mRNAs are retained at the nuclear side of the NPC, in a functional microenvironment organized by the perinuclear and NPC-associated Mlp proteins.Citation8-10
The synthesis, maturation, packaging and quality control events leading to the formation of export-competent mRNPs proceed co-transcriptionally, and therefore in a chromatin environment. While the dynamics of chromatin structure - which is influenced by post-translational modifications of histone tails and ATP-dependent chromatin remodeling events - is recognized as playing an essential role in the control of DNA-associated processes (transcription, replication, repair), an increasing number of studies during the last decade revealed that chromatin also influences mRNP formation and fate.
Chromatin dynamics influences splicing
Splicing is the best-documented and evolutionary conserved pre-RNA maturation event whose regulation is impacted by the local chromatin status.Citation11,12 Although no simple relationship exists between the chromatin state and splice site recognition or choice (in case of alternative splicing), both the modulation of Pol II elongation rate and the recruitment of spliceosome components and splicing factors by specific epigenetic marks are known to affect splicing. The following section describes few emblematic examples that illustrate the tight regulation of splicing by chromatin dynamics.
Histone marks and splicing
Several covalent histone marks associated with active transcription, such as SETD2-deposited H3K36me3, have been implicated in the regulation of splicing, independently of, or additionally to, their effect on transcription elongation. Furthermore, exons were shown to be preferentially marked with H3K36me3 relative to introns.Citation13,14 Kidney tumors mutated for SETD2 show intron retention and aberrant splicing, in 25% of all expressed genes.Citation15 Further evidence for a role of histone modification in alternative splicing is the depolarization-induced increase in H3K36 methylation and H3K9 acetylation around the alternatively spliced exon 18 of NCAM (neural cell adhesion molecule), which induces exon skipping.Citation16
Besides multiple examples that correlate histone marks and splicing efficiency and specificity, a direct role for histone marks in the control of alternative splicing is remarkably illustrated by work from the Misteli laboratory based on the human fibroblast growth factor receptor 2 gene (FGFR2) as a model for alternative splicing. FGFR2 exhibits tissue-specific expression of 2 mutually exclusive isoforms FGFR2-IIIb and -IIIc. In mesenchymal cells, H3K36me3 and H3K4me1 are enriched over FGFR2 and exon IIIb is skipped while epithelial cells express exon IIIb and show enrichment for H3K23me3 and H3K4me3. Strikingly, the genetically engineered inversion of these tissue-specific histones marks between both cell types results in an inversion of the alternative splicing outcome. In mesenchymal stem cells, a splicing inhibitor is recruited to chromatin by a “reader” of the H3K36me3 histone mark, thereby preventing exon IIIb inclusion.Citation17 In epithelial cells, a long noncoding RNA (lncRNA) expressed by the FGFR2 locus prevents the recruitment of the splicing inhibitor to chromatin by creating an unfavorable chromatin environment via the recruitment of histone-modifying enzymes, thus leading to the expression of the FGFR2-IIIb isoform.Citation18
Studies in the budding yeast also revealed a tight dependency of pre-mRNA splicing on the dynamics of histone H3 acetylation. Gunderson et al. indeed reported that while the recruitment of the U2 snRNP depends on the histone acetyltransferase activity of the Gcn5 subunit of the SAGA complex, the subsequent exchange of the U2 snRNP for the U5 snRNP relies on the deacetylase activity of Hos2 and Hos3. Hence the co-transcriptional spliceosome assembly and its remodeling onto pre-mRNA are coupled to the Gcn5/Hos2–3-mediated histone H3 acetylation/deacetylation.Citation19,20
Finally, monoubiquitylation of histone H2B (H2B-Ub) is another histone mark implicated in the control of co-transcriptional splicing. Depletion of USP49, a histone H2B deubiquitylase, results in extensive changes in alternative splicing characterized by preferential skipping of exons with high H2B-Ub and decreased association of the U1 and U2 snRNPs with nascent transcripts.Citation21 In S. cerevisiae, H2B-Ub is predominantly localized across gene coding regions but reflects intron/exon structure.Citation22 Interestingly H2B-Ub is required for efficient co-transcriptional recruitment of U1 and U2 snRNPs and H2B-Ub machinery interacts with the SR-like protein Npl3 that also influences recruitment of U1 and U2.Citation23,24 Together these results suggest that H2B ubiquitylation/deubiquitylation could be a universal strategy to control the appropriate recruitment of the early splicing machinery to nascent transcripts.
Chromatin remodelers and splicing
Nucleosome positioning and chromatin remodelers can also impact the splicing outcome.Citation25 The ATPase catalytic subunit Brahma (Brm) of the human SWI/SNF chromatin remodeler was reported to favor inclusion of variant exons in CD44, independently of its catalytic activity. This is mediated by its ability to slow down Pol II elongation rate, and also by its interaction with the spliceosome or with Sam68, an hnRNP-like protein that influences exon recognition. Altogether, this would in turn favor the recruitment of Brm to suboptimal splice sites.Citation26 The influence of SWI/SNF on alternative splicing was also investigated in the dipteran Chironomus tentans, whose Balbani rings (BR) - giant puffs formed by active transcription of the BR genes of the salivary gland cells polytene chromosomes- allowed the remarkable in situ detection of a direct association between SWI/SNF and nascent mRNPs. Based on these data, it has been proposed that SWI/SNF would be incorporated into nascent pre-mRNPs and thus would post-transcriptionally regulate the type of alternative transcript produced.Citation27 In another example, the chromodomain protein CHD1 also controls splicing. Its overexpression but also its depletion affect alternative splicing in vitro and in vivo and lead to impaired association of U2 snRNP components with pre-mRNA. CHD1 would act as a bridge between H3K4me3 and the growing spliceosome therefore facilitating pre-mRNA maturation.Citation28
Other mRNP biogenesis events influenced by chromatin events
Studies linking chromatin structure and epigenetic marks to other mRNA biogenesis events than splicing are relatively sparse. 3′- end processing is one of the mRNA maturation event influenced by the local chromatin structure. Transcription termination and polyadenylation sites (PASs) display distinct nucleosome occupancy patterns. In particular, genomic regions surrounding PAS are strongly depleted of nucleosomes, while the regions immediately downstream of PAS are enriched in nucleosomes.Citation13 Moreover, in genes undergoing alternative polyadenylation (APA) - a process that has recently emerged as a major player of gene regulationCitation11 -, the usage of individual PAS is correlated with the nucleosome occupancy downstream of the PAS, suggesting a role of the chromatin structure in the regulation of polyadenylation. Although to date the precise chromatin-linked mechanisms influencing APA are poorly understood, the Brm catalytic subunit of the human SWI/SNF, was recently reported to control APA by favoring the inclusion of distal terminal exons. Brm indeed interacts with BRCA1/BARD1, which ubiquitinates the 3′-end processing factor Cstf (Cleavage stimulation factor), thus inhibiting transcript cleavage at proximal terminal sites. Upon Brm depletion, which can result from environmental conditions such as oxidative stress, Cstf inhibition is released and the inclusion of proximal last exon favored.Citation29
mRNA export, the last step in the nuclear mRNP biogenesis process, is also influenced by the local chromatin state. In higher eukaryotes, the transcription-elongation factor SPT6 recruits IWS1 (Interact with SPT6) to nascent transcripts, thereby bridging the mRNA export factor ALY/REF (Yra1 in yeast) to the pre-mRNA.Citation30 Similarly, the histone chaperone FACT can mediate recruitment of UAP56 (Sub2 in yeast) to maturing transcripts via an interaction with UIF (UPA56 Interacting Factor), thus assisting the loading of the export machinery to mRNAs.Citation31 In budding yeast, ubiquitylation of histone H2B regulates the formation of export-competent mRNPs. Preventing ubiquitylation of H2B indeed impairs ubiquitylation of Swd2 - a common subunit of the Set1c histone H3K4 methyltransferase complex and the cleavage and polyadenylation complex- resulting in a defective recruitment of the Mex67 adaptors Yra1 and Nab2 to the mRNP and thereby in a mRNA export defect.Citation32
ISW1 and quality control of nuclear mRNP biogenesis
The ISWI type of proteins is a conserved family of chromatin remodeling ATPases that uses DNA translocation to mobilize nucleosomes. The nucleosome sliding activity of ISWI generates regularly spaced nucleosome arrays thought to restrict access to DNA.Citation33-35 The yeast Saccharomyces cerevisiae features 2 non-essential members of this family, Isw1 and Isw2 which are homologous to human hSNF2-H and hSNF2-L. Isw1 is the ATPase subunit for distinct remodeling complexes formed by its association with the non-essential Ioc2, Ioc3 and Ioc4 co-factorsCitation36,37 that have been proposed to confer distinct genome-wide positional specificities on Isw1.Citation38 Additionally, genome-wide mapping of nucleosomes in yeast cells inactivated for ISW1 identified a widespread role for Isw1 in nucleosome positioning over coding regions.Citation34 Functionally however, the chromatin perturbations associated with loss of Isw1 are uncorrelated with changes in mRNA abundance in the mutant: cells inactivated for ISW1 exhibit a modest derepression of relatively few genes and an increase in cryptic transcription.Citation37,39 Instead, Isw1 was proposed to maintain chromatin integrity genome-wide during transcription elongation by RNA Pol II.Citation34,38,40,41
The first hint toward a function for the yeast chromatin remodeling complex ISW1 in mRNP biogenesis came with our recent discovery of its interaction with the mRNA export machinery revealed by a 2-hybrid screen.Citation42 While cells inactivated for ISW1 do not present a nuclear mRNA export defect, genetic investigations led us to propose a novel function for this chromatin remodeler in the quality control of nuclear mRNP biogenesis. In normal conditions, chromatin localized Isw1 is able to transiently interact with nuclear mRNPs, delaying their export to the cytoplasm. Upon defective mRNP formation, the interaction of Isw1 with mRNPs is prolonged, leading to their nuclear retention. As such, Isw1 may stand as one of the previously postulated (6) but never identified actors of nuclear mRNP QC that contributes, together with the nuclear exosome subunit Rrp6 and potentially with other unknown components, to the chromatin tethering of export-incompetent mRNPs to the gene locus. Inactivation of the catalytic activity of Isw1 was unable to release chromatin- retained transcripts, indicating that chromatin remodeling and mRNPs retention are 2 distinct activities of the complex. In support of this, no correlation was found between the transcripts bound by Isw1 and the genes identified as recruiting Isw1 by chromatin immunoprecipitation. Isw1 can be co-immunoprecipitated with mRNP protein components in an RNA-independent manner but was also found to directly interact with mRNA using in vivo crosslinking approaches. However, the mechanism underying the Isw1-dependent selective nuclear retention of defective mRNPs requires further investigation. In particular, UV crosslinking experiments not only revealed a direct interaction of RNA with Isw1 but also with Ioc2 and Ioc3 (Citation42 and unpublished result). Although none of these subunits possess a previously described RNA-binding domain, it is possible that each of them is able to bind RNA independently or that the RNA-binding site results from the complex formation. In support of this hypothesis, the combined depletion of ISW1 subunits displayed additive effects toward the rescue of the phenotypes of mRNPs biogenesis mutants (unpublished results). Alternatively, we cannot formally exclude that only one subunit of the ISW1 complex binds the RNA and would bring the others in close contact to RNA, thereby bridging their interaction with RNA.
Whether Isw1 binding to mRNPs is co- or post-transcriptional and whether Isw1-bound mRNPs are still attached to Pol II remain major questions to be further analyzed. It is conceivable that the Isw1/Rrp6-dependant “retention dot” detected in proximity to the site of transcription in mRNA biogenesis mutants by fluorescent in situ hybridization using probes against specific transcripts, consists in large granules, reminiscent of nuclear bodies (). In this regard, in Drosophila melanogaster, Isw1 is an essential component of the omega speckle compartment (ω-speckle) associated with the long non-coding RNA heat shock responsive ω (hsrω).Citation43 This specialized compartment is believed to play crucial roles in storage and sequestration of heterogeneous ribonucleoparticles as well as other RNA processing factors and thus to contribute to the regulation of gene expression. Although never described so far in yeast, “retention dots” could be a variation of these nuclear bodies. Importantly, inactivation of ISW1 triggers the release of chromatin-tethered mRNPs produced in mutants of Mex67 and its RNA-binding adaptors but not in mutants of the TREX-2 complex or of the 3′-end processing machinery, thus restricting the Isw1 function to a certain window in mRNP formation.
Figure 1. Non-exclusive models for sub-optimal mRNP nuclear retention by ISW1 in yeast. ISW1 retains export-incompetent mRNPs in proximity to the transcription site via interactions with protein components of the mRNPs but also via direct protein-RNA interactions. These interactions could occur during transcription while transcripts are still attached to Pol II (left) and/or post-transcriptionally, in nuclear «retention dots» that may represent RNA granules (right).
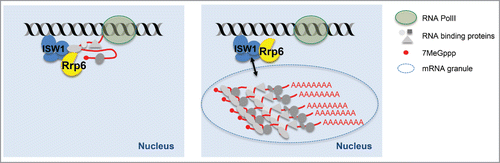
In this context, it is important to note that ISW1 inactivation rescues the transcription-dependent hyper-recombination phenotype of these mutants.Citation42 Indeed, accurate RNA packaging is crucial to maintain genome integrity as it counteracts the appearance of transcription-associated DNA damage by preventing the formation of RNA::DNA hybrids (R-loops) between the nascent transcript and its template.Citation44 Mutants affecting the loading of RNA binding proteins onto RNA have been proposed to uncoat the transcripts and thus to favor inappropriate interactions with target duplex DNA.Citation45 ISW1 inactivation could counteract such noxious effects by inducing export of these defective mRNPs to the cytoplasm. Along the same lines, inactivation of ISW1 is able to rescue the growth defects induced by the heterologous expression of the bacterial transcription termination factor Rho (unpublished results). The loading of this RNA-dependent helicase/translocase onto nascent transcripts was previously proposed to compete with mRNP biogenesis factors, thus leading to the production of aberrant transcripts, degraded by the nuclear exosome.Citation46 Taken together, these results argue for a co-transcriptional activity of Isw1 during mRNA biogenesis. Importantly however, co- and post-transcriptional activities of Isw1 are not mutually exclusive and careful investigation of Isw1 target transcripts will be required in the near future to fully unravel this issue.
The repertoire of mRNA-binding proteins, in both yeast and mammals, has recently greatly expanded owing to the generalization of approaches consisting in the purification of mRNPs under denaturing conditions after in vivo cross-linking and mass-spectrometry protein identification.Citation47-49 These allowed the identification of proteins with known mRNA-binding domains but also numbers of new RNA-binding proteins, including enzymes or chromatin factors for which there is a crucial need for further investigations to fully understand their functional significance.
Disclosure of potential conflicts of interests
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Who am I? laboratory of excellence (ANR-11-LABX-0071) funded by the “Investments for the Future” program operated by The French National Research Agency, the Association de Recherche contre le Cancer, the Fondation pour la recherche Médicale (Equipe labellisée).
References
- Strambio-De-Castillia C, Niepel M, Rout MP. The nuclear pore complex: bridging nuclear transport and gene regulation. Nat Rev Mol Cell Biol 2010; 11(7):490-501
- Bentley DL. Coupling mRNA processing with transcription in time and space. Nat Rev Genet 2014; 15(3):163-75
- Babour A, Dargemont C, Stutz F. Ubiquitin and assembly of export competent mRNP. Biochim Et Biophys Acta 2012; 1819(6):521-30; PMID:22240387
- Nino CA, Herissant L, Babour A, Dargemont C. mRNA nuclear export in yeast. Chem Rev 2013; 113(11):8523-45; PMID:23731471
- Tutucci E, Stutz F. Keeping mRNPs in check during assembly and nuclear export. Nat Rev Mol Cell Biol 2011; 12(6):377-84; PMID:21602906
- Schmid M, Jensen TH. Transcription-associated quality control of mRNP. Biochim Et Biophys Acta 2013; 1829(1):158-68; PMID:22982197
- Skruzny M, Schneider C, Racz A, Weng J, Tollervey D, Hurt E. An endoribonuclease functionally linked to perinuclear mRNP quality control associates with the nuclear pore complexes. PLoS Biol 2009; 7(1):e8; PMID:19127978
- Galy V, Gadal O, Fromont-Racine M, Romano A, Jacquier A, Nehrbass U. Nuclear retention of unspliced mRNAs in yeast is mediated by perinuclear Mlp1. Cell 2004; 116(1):63-73; PMID:14718167
- Palancade B, Zuccolo M, Loeillet S, Nicolas A, Doye V. Pml39, a novel protein of the nuclear periphery required for nuclear retention of improper messenger ribonucleoparticles. Mol Biol Cell 2005; 16(11):5258-68; PMID:16162818
- Vinciguerra P, Iglesias N, Camblong J, Zenklusen D, Stutz F. Perinuclear Mlp proteins downregulate gene expression in response to a defect in mRNA export. EMBO J 2005; 24(4):813-23; PMID:15692572; https://doi.org/10.1038/sj.emboj.7600527
- Naftelberg S, Schor IE, Ast G, Kornblihtt AR. Regulation of alternative splicing through coupling with transcription and chromatin structure. Annu Rev Biochem 2015; 84:165-98; PMID:26034889; https://doi.org/10.1146/annurev-biochem-060614-034242
- Saldi T, Cortazar MA, Sheridan RM, Bentley DL. Coupling of RNA Polymerase II Transcription Elongation with Pre-mRNA Splicing. J Mol Biol 2016; 428(12):2623-35; PMID:27107644; https://doi.org/10.1016/j.jmb.2016.04.017
- Spies N, Nielsen CB, Padgett RA, Burge CB. Biased chromatin signatures around polyadenylation sites and exons. Mol Cell 2009; 36(2):245-54; PMID:19854133; https://doi.org/10.1016/j.molcel.2009.10.008
- Kolasinska-Zwierz P, Down T, Latorre I, Liu T, Liu XS, Ahringer J. Differential chromatin marking of introns and expressed exons by H3K36me3. Nat Genet 2009; 41(3):376-81; PMID:19182803; https://doi.org/10.1038/ng.322
- Simon JM, Hacker KE, Singh D, Brannon AR, Parker JS, Weiser M, Ho TH, Kuan PF, Jonasch E, Furey TS, et al. Variation in chromatin accessibility in human kidney cancer links H3K36 methyltransferase loss with widespread RNA processing defects. Genome Res 2014; 24(2):241-50; PMID:24158655; https://doi.org/10.1101/gr.158253.113
- Schor IE, Rascovan N, Pelisch F, Allo M, Kornblihtt AR. Neuronal cell depolarization induces intragenic chromatin modifications affecting NCAM alternative splicing. Proc Natl Acad Sci U S A 2009; 106(11):4325-30; PMID:19251664; https://doi.org/10.1073/pnas.0810666106
- Luco RF, Pan Q, Tominaga K, Blencowe BJ, Pereira-Smith OM, Misteli T. Regulation of alternative splicing by histone modifications. Science 2010; 327(5968):996-1000; PMID:20133523; https://doi.org/10.1126/science.1184208
- Gonzalez I, Munita R, Agirre E, Dittmer TA, Gysling K, Misteli T, Luco RF. A lncRNA regulates alternative splicing via establishment of a splicing-specific chromatin signature. Nat Structural Mol Biol 2015; 22(5):370-6
- Gunderson FQ, Johnson TL. Acetylation by the transcriptional coactivator Gcn5 plays a novel role in co-transcriptional spliceosome assembly. PLoS Genet 2009; 5(10):e1000682; PMID:19834536; https://doi.org/10.1371/journal.pgen.1000682
- Gunderson FQ, Merkhofer EC, Johnson TL. Dynamic histone acetylation is critical for cotranscriptional spliceosome assembly and spliceosomal rearrangements. Proc Natl Acad Sci U S A 2011; 108(5):2004-9; PMID:21245291; https://doi.org/10.1073/pnas.1011982108
- Zhang Z, Jones A, Joo HY, Zhou D, Cao Y, Chen S, Erdjument-Bromage H, Renfrow M, He H, Tempst P, et al. USP49 deubiquitinates histone H2B and regulates cotranscriptional pre-mRNA splicing. Genes Dev 2013; 27(14):1581-95; https://doi.org/10.1101/gad.211037.112
- Shieh GS, Pan CH, Wu JH, Sun YJ, Wang CC, Hsiao WC, Lin CY, Tung L, Chang TH, Fleming AB, et al. H2B ubiquitylation is part of chromatin architecture that marks exon-intron structure in budding yeast. BMC Genomics 2011; 12:627; PMID:22188810; https://doi.org/10.1186/1471-2164-12-627
- Herissant L, Moehle EA, Bertaccini D, Van Dorsselaer A, Schaeffer-Reiss C, Guthrie C, et al. H2B ubiquitylation modulates spliceosome assembly and function in budding yeast. Biol Cell 2014; 106(4):126-38; https://doi.org/10.1111/boc.201400003
- Moehle EA, Ryan CJ, Krogan NJ, Kress TL, Guthrie C. The yeast SR-like protein Npl3 links chromatin modification to mRNA processing. PLoS Genet 2012; 8(11):e1003101; PMID:23209445; https://doi.org/10.1371/journal.pgen.1003101
- Allemand E, Myers MP, Garcia-Bernardo J, Harel-Bellan A, Krainer AR, Muchardt C. A broad set of chromatin factors influences splicing. PLoS Genet 2016; 12(9):e1006318; PMID:27662573; https://doi.org/10.1371/journal.pgen.1006318
- Batsche E, Yaniv M, Muchardt C. The human SWI/SNF subunit Brm is a regulator of alternative splicing. Nat Structural Mol Biol 2006; 13(1):22-9; https://doi.org/10.1038/nsmb1030
- Tyagi A, Ryme J, Brodin D, Ostlund Farrants AK, Visa N. SWI/SNF associates with nascent pre-mRNPs and regulates alternative pre-mRNA processing. PLoS Genet 2009; 5(5):e1000470; PMID:19424417; https://doi.org/10.1371/journal.pgen.1000470
- Sims RJ, 3rd, Millhouse S, Chen CF, Lewis BA, Erdjument-Bromage H, Tempst P, Manley JL, Reinberg D. Recognition of trimethylated histone H3 lysine 4 facilitates the recruitment of transcription postinitiation factors and pre-mRNA splicing. Mol Cell 2007; 28(4):665-76; PMID:18042460; https://doi.org/10.1016/j.molcel.2007.11.010
- Fontana GA, Rigamonti A, Lenzken SC, Filosa G, Alvarez R, Calogero R, Bianchi ME, Barabino SM. Oxidative stress controls the choice of alternative last exons via a Brahma-BRCA1-CstF pathway. Nucleic Acids Res 2017; 45(2):902-14; PMID:27591253; https://doi.org/10.1093/nar/gkw780
- Yoh SM, Cho H, Pickle L, Evans RM, Jones KA. The Spt6 SH2 domain binds Ser2-P RNAPII to direct Iws1-dependent mRNA splicing and export. Genes Dev 2007; 21(2):160-74; https://doi.org/10.1101/gad.1503107
- Hautbergue GM, Hung ML, Walsh MJ, Snijders AP, Chang CT, Jones R, Ponting CP, Dickman MJ, Wilson SA. UIF, a New mRNA export adaptor that works together with REF/ALY, requires FACT for recruitment to mRNA. Curr Biol 2009; 19(22):1918-24; PMID:19836239; https://doi.org/10.1016/j.cub.2009.09.041
- Vitaliano-Prunier A, Babour A, Herissant L, Apponi L, Margaritis T, Holstege FC, Corbett AH, Gwizdek C, Dargemont C. H2B ubiquitylation controls the formation of export-competent mRNP. Mol Cell 2012; 45(1):132-9; PMID:22244335; https://doi.org/10.1016/j.molcel.2011.12.011
- Bartholomew B. ISWI chromatin remodeling: one primary actor or a coordinated effort? Curr Opin Struct Biol 2014; 24:150-5; PMID:24561830; https://doi.org/10.1016/j.sbi.2014.01.010
- Tirosh I, Sigal N, Barkai N. Widespread remodeling of mid-coding sequence nucleosomes by Isw1. Genome Biol 2010; 11(5):R49; PMID:20459718; https://doi.org/10.1186/gb-2010-11-5-r49
- Whitehouse I, Tsukiyama T. Antagonistic forces that position nucleosomes in vivo. Nat Structural Mol Biol 2006; 13(7):633-40; https://doi.org/10.1038/nsmb1111
- Mellor J, Morillon A. ISWI complexes in Saccharomyces cerevisiae. Biochim Et Biophys Acta 2004; 1677(1–3):100-12; PMID:15020051; https://doi.org/10.1016/j.bbaexp.2003.10.014
- Vary JC, Jr, Gangaraju VK, Qin J, Landel CC, Kooperberg C, Bartholomew B, Tsukiyama T. Yeast Isw1p forms two separable complexes in vivo. Mol Cell Biol 2003; 23(1):80-91; PMID:12482963; https://doi.org/10.1128/MCB.23.1.80-91.2003
- Yen K, Vinayachandran V, Batta K, Koerber RT, Pugh BF. Genome-wide nucleosome specificity and directionality of chromatin remodelers. Cell 2012; 149(7):1461-73; PMID:22726434; https://doi.org/10.1016/j.cell.2012.04.036
- Lenstra TL, Benschop JJ, Kim T, Schulze JM, Brabers NA, Margaritis T, van de Pasch LA, van Heesch SA, Brok MO, Groot Koerkamp MJ, et al. The specificity and topology of chromatin interaction pathways in yeast. Mol Cell 2011; 42(4):536-49; PMID:21596317; https://doi.org/10.1016/j.molcel.2011.03.026
- Gkikopoulos T, Schofield P, Singh V, Pinskaya M, Mellor J, Smolle M, Workman JL, Barton GJ, Owen-Hughes T. A role for Snf2-related nucleosome-spacing enzymes in genome-wide nucleosome organization. Science 2011; 333(6050):1758-60; PMID:21940898; https://doi.org/10.1126/science.1206097
- Smolle M, Venkatesh S, Gogol MM, Li H, Zhang Y, Florens L, Washburn MP, Workman JL. Chromatin remodelers Isw1 and Chd1 maintain chromatin structure during transcription by preventing histone exchange. Nat Structural Mol Biol 2012; 19(9):884-92; https://doi.org/10.1038/nsmb.2312
- Babour A, Shen Q, Dos-Santos J, Murray S, Gay A, Challal D, Fasken M, Palancade B, Corbett A, Libri D, et al. The Chromatin Remodeler ISW1 Is a Quality Control Factor that Surveys Nuclear mRNP Biogenesis. Cell 2016; 167(5):1201-14 e15; https://doi.org/10.1016/j.cell.2016.10.048
- Lo Piccolo L, Attardi A, Bonaccorso R, Li Greci L, Giurato G, Ingrassia AM, Onorati MC. ISWI ATP-dependent remodeling of nucleoplasmic omega-speckles in the brain of Drosophila melanogaster. J Genet Genomics 2017; 44(2):85-94; PMID:28209301; https://doi.org/10.1016/j.jgg.2016.12.002
- Santos-Pereira JM, Aguilera A. R loops: new modulators of genome dynamics and function. Nat Rev Genet 2015; 16(10):583-97; PMID:26370899; https://doi.org/10.1038/nrg3961
- Wahba L, Amon JD, Koshland D, Vuica-Ross M. RNase H and multiple RNA biogenesis factors cooperate to prevent RNA:DNA hybrids from generating genome instability. Mol Cell 2011; 44(6):978-88; PMID:22195970; https://doi.org/10.1016/j.molcel.2011.10.017
- Honorine R, Mosrin-Huaman C, Hervouet-Coste N, Libri D, Rahmouni AR. Nuclear mRNA quality control in yeast is mediated by Nrd1 co-transcriptional recruitment, as revealed by the targeting of Rho-induced aberrant transcripts. Nucleic Acids Res 2011; 39(7):2809-20; PMID:21113025; https://doi.org/10.1093/nar/gkq1192
- Castello A, Fischer B, Eichelbaum K, Horos R, Beckmann BM, Strein C, Davey NE, Humphreys DT, Preiss T, Steinmetz LM, et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell 2012; 149(6):1393-406; PMID:22658674; https://doi.org/10.1016/j.cell.2012.04.031
- Kwon SC, Yi H, Eichelbaum K, Fohr S, Fischer B, You KT, Castello A, Krijgsveld J, Hentze MW, Kim VN. The RNA-binding protein repertoire of embryonic stem cells. Nat Structural Mol Biol 2013; 20(9):1122-30; https://doi.org/10.1038/nsmb.2638
- Mitchell SF, Jain S, She M, Parker R. Global analysis of yeast mRNPs. Nat Structural Mol Biol 2013; 20(1):127-33; https://doi.org/10.1038/nsmb.2468
