ABSTRACT
During a cell's lifespan, DNA break formation is a common event, associated with many processes, from replication to apoptosis. Most of DNA breaks are readily repaired, but some are meant to persist in time, such as the chromosome ends, protected by telomeres. Besides them, eukaryotic genomes comprise shorter stretches of interstitial telomeric repeats. We assumed that the latter may also be associated with the formation of DNA breaks meant to persist in time. In zebrafish and mouse embryos, cells containing numerous breakage foci were identified. These breaks were not associated with apoptosis or replication, nor did they seem to activate DNA damage response machinery. Unlike short-living, accidental sparse breaks, the ones we found seem to be closely associated, forming discrete break foci. A PCR-based method was developed, allowing specific amplification of DNA regions located between inverted telomeric repeats associated with breaks. The cloning and sequencing of such DNA fragments were found to denote some specificity in their distribution for different tissue types and development stages.
Introduction
Maintenance of genome integrity is a prerequisite for the stable inheritance of genetic information over multiple cell generations. Accumulation of DNA lesions has impacts on aging, as well as on the development of cancer and other pathologies.Citation1,Citation2 Among others, telomere damage drastically affects many events, from the expression of many genes to major changes of nuclear landscape.Citation3 Cellular mechanisms involved in genome maintenance include the stabilization of chromosome ends by telomeres and a system of DNA-damage response (DDR) pathways that promptly detect double-stranded breaks.Citation4 Telomeres perform a wide variety of functions, including maintenance of chromosome ends, their elongation by telomerase, and the regulation of adjacent genes.Citation5,Citation6 Telomeric DNA consists of repetitive guanine-enriched sequences (TTAGGG in vertebrates). DNA damage signaling pathways, induced by lesions located at telomeres, often differ from the ones activated if chromosomes are damaged sporadically at internal sites.Citation7 Along with telomeric repeats at chromosome ends, interstitial telomeric or telomere-like repeats of variable length are present virtually in all eukaryotic genomes.Citation8 The opinions concerning the fact that they may beCitation9 or may not beCitation10 hot spots of DNA breakage are rather controversial.
Besides telomeres, there are other DNA strand breaks that normally should not induce the DDR, such as stalled replication forks at the borders of early and late-replicating chromatin domains or recombination sites.Citation5,Citation8,Citation10 Beyond that, repetitive elements in general and telomeric repeats in particular, including the interstitial ones as well, may require the recruitment of additional factors for efficient replication and for stabilization, especially in case of long tracks of repeats.Citation11 Given that telomeric repeats play a key role in DNA end protection,Citation5-7 we assumed that if, apart from chromosome ends, some stable DNA breaks arise at specific stages during cell cycle, or are associated with certain developmental events, they may be protected from immediate repair by telomeric repeats. Thus, non-random distribution of interstitial telomeric repeats relative to sites of specific DNA breakage can be hypothesized. In this work, we show that when stable DNA breaks can be detected in some cell types, such as liver or neural cells, these breakage sites appear to be distinct from the known ones, such as provisory breaks related to replication or transcription, they are not linked to apoptosis; also, that these breaks, at least in part, are associated with internal telomeric repeats.
Results
Tissue-specific detection of DNA breaks in situ in Danio rerio and mouse embryos
It is known that programmed cell death plays an important role in embryogenesis and is specifically induced at certain developmental stages.Citation12 Nevertheless, some DNA breaks not as massive as the ones occurring in apoptosis, but rather stable in time, may appear during normal cell differentiation and chromatin remodeling. To identify them, we developed an enhanced TUNEL procedure (ETUNEL), which utilizes rabbit anti-FITC antibodies, with subsequent detection with goat-anti rabbit FITC-labeled secondary antibodies for signal amplification. The increased sensitivity of the method allowed the identification of a cell subpopulation showing an intense non-uniform nuclear labeling, giving evidence that they contain foci of free DNA ends. These cells demonstrated non-random tissue specific distribution in vertebrates (). For zebrafish, the highest concentration of ETUNEL-positive cells was observed in the epithelia of both fins and body, and especially in the posterior lens (). Less intense labeling was observed in ganglion cell layer nuclei (). A rather heterogeneous population of ETUNEL-positive cells was detected in the forebrains and midbrains of 4dpf zebrafish embryos ().
Figure 1. ETUNEL staining of 4 dpf zebrafish and 11 dpf mice embryos. There is an uneven distribution of the cells with numerous DNA breakage foci within the body, these ones being particularly abundant in epithelia, but also present in the midbrain and forebrain (a). A rather intense ETUNEL signal can be observed in posterior lens, and is also detectable in ganglion cell layer (b). The ETUNEL signal shows a nonuniform distribution within nuclei, often forming small foci, from several to hundreds per nucleus(c). In mouse 11 dpf embryo head ETUNEL-positive cells are concentrated especially near the ventricular zone of the brain, in forming embryonic eyes and epithelia (d). Epithelial (e) as well as neural cells (f) show nonuniform nuclear labeling, with defined breakage foci.
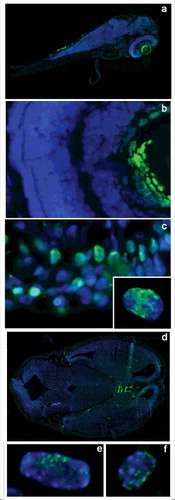
In most labeled cells, an intranuclear staining was distributed nonuniformly throughout the nuclei forming small, often coalescing foci 0.3–0.5 um in size (). The number of such foci varied from several dozen to hundreds per nucleus. Overall staining intensity also varied depending on tissue of origin, being most prominent in skin and posterior lens (). Rather similar picture has been observed in 11dpf mouse embryos (), where skin epithelial and neural cells showed prominent and nonuniform labeling ().
DNA breakage foci do not coincide with apoptosis-related DNA fragmentation, gammaH2AX repair foci, and seem to be not associated with replication
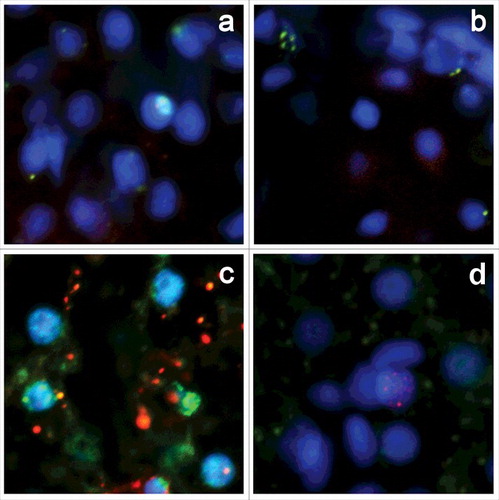
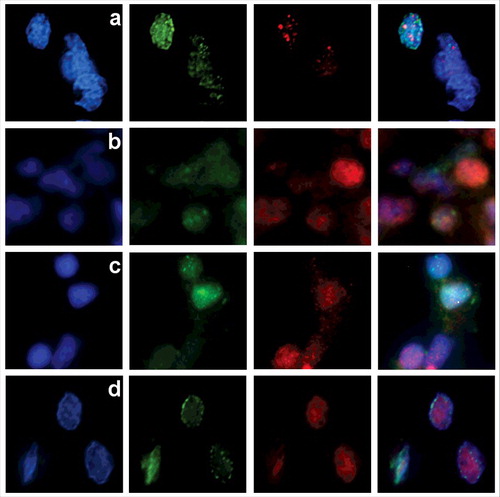
Most ETUNEL-positive cells display normal morphology without apparent signs of apoptotic death. Because some tissues contain a rather high amount of ETUNEL-positive cells, it is unlikely that all of them are committed to apoptosis. We performed double labeling of zebrafish embryo cryosections for ETUNEL, and apoptotic markers PARP and caspase 3 (). ETUNEL-positive cells, which have well-defined clusters of breaks, show no apparent colocalization with PARP fragments (), and do not express active caspase 3 (). To explore the proliferative status of ETUNEL-positive cells, we performed double labeling of zebrafish tissues with ETUNEL and antibodies against PCNA. No apparent colocalization between the ETUNEL () and PCNA signal was found, so the observed breaks are generally not related to normal replication. The formation of DNA breaks commonly triggers DNA damage repair pathway activation, which usually comes out in H2AX histone isoform accumulation, phosphorylated at Ser139 (γH2AX) on chromatin around DSB sites.Citation13 Zebrafish tissues showed virtually no γH2AX staining in both ETUNEL-positive and negative cells, with only a few γH2AX-positive cells being present, and no apparent colocalization of ETUNEL signal with γH2AX ().
Chromatin status near DNA breakage foci
Tissue-specific distribution of the ETUNEL foci in zebrafish and mice, as well as their relative abundance, indicates that they are related to some of the free DNA ends, present in nuclei, which are unlikely to coincide with chromosome ends. Several processes, including chromatin remodeling, can also be associated with DNA break formation, being related either to transitions between hetero- and euchromatin or to open chromatin stabilization.Citation14-16 To verify whether the observed breakage foci correspond to sites of active chromatin remodeling, a co-staining with SNF2 was performed, showing that ETUNEL signals are not located at chromatin remodeling centers (). To specify whether the observed foci of free DNA ends are assigned to euchromatin or heterochromatin regions, we analyzed the localization of the ETUNEL signal relative to euchromatin (H3K9-ac) and heterochromatin (H3K9–3me) markers (). H3K9-ac and ETUNEL signals demonstrated a high degree of colocalization (), while the H3K9-Me3 signal showed no significant overlapping with ETUNEL (). As open chromatin domains at regions of intense transcription could also be associated with breaks,Citation17-19 a co-staining with RNA polymerase II was made. We found that the ETUNEL signal location generally doesn't correspond to sites of the most active transcription ().
Figure 3. In 4dpf zebrafish embryo cells the distribution of ETUNEL signal (green) is different from the one of SNF2 (red) chromatin remodeling marker (a). There is substantial colocalization between ETUNEL (green) and euchromatin marker H3K9-ac (red) (b), and virtually no overlapping with histone H3K9-Me3 (red), specific to heterochromatic regions (c). ETUNEL sites (green) are not located at sites of intense transcription, marked by RNA polymerase II (red) (d).
Identification of interstitial telomeric repeats-associated DNA breaks (ITR-DB)
To identify these repeat-associated DNA breaks (both single- and double-stranded), we designed a PCR-based approach which includes a 2-step procedure. During the first step, all DNA ends are extended using terminal transferase reaction with dTTP. Next, a PCR reaction with a hybrid primer containing repetitive element sequence at the 3' end and polyA at the 5' end is used to amplify DNA stretches encompassed within oppositely directed repeat-associated breaks. We performed PCR with various primers specific to several types of repetitive sequences (telomeric repeats and 5 other types of repeats (Rep 1–5), which are oppositely directed and flank D.rerio transposons). Stretches of these transposon-flanking repeats are found approximately 130, 150, 660, 1220 and 5730 times per D.rerio genome, i.e. with similar frequencies as interstitial telomeric repeats (approximately 1100 times), and 3 of them are G-enriched (> = 50%). Analysis of the amplified fragments on agarose gel showed smear patterns for all tested primers, but we only observed a clear difference in fragment size distribution between terminal transferase-treated and control samples for telomere-specific primers (, ). These results reflect non-random distribution of DNA breaks with preference toward ITR-containing regions.
Figure 4. Gel-electrophoresis of the amplified DNA fragments, selected by association of the free 3′ DNA ends with telomeric repeats on both sides, obtained by PCR with Term1-Term4 primer mixture. The distribution by size of the amplified DNA fragments from zebrafish hardroe (line 2,5), 4 dpf embryos (line 3,6) and adult fish (line 4,7) shows a general lowering of the molecular weight of the amplified fragments from germline to the embryonic stage and adult organism (a). There are also differences in the predominant fragment sizes of amplified fragments, comprised between DNA break-associated telomeric repeats for different zebrafish tissues, as seen for fin (line 1,7), gill (line 2,8), brain (line3,9), liver (line 4,10), muscle (line 5,11) and hardroe (line 6,12), also showing the highest molecular weight for germline and the lowest – for muscle (b). In both cases a specific signal appears after TdT treatment (a, line 2–4), (b, line 1–6), and there is almost no specific signal without TdT treatment (a, line 5–7), (b, line 7–12).
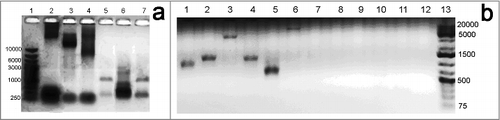
Figure 5. Gel-electrophoresis of the amplified DNA fragments, selected by possible association of the free 3′ DNA ends with other inverted repeats, originated from 5 types of D.rerio transposons, obtained by PCR with Rep1-Rep5 primers. The distribution of the amplified fragments appears to be constant and not dependent on fish age, neither it is associated with DNA breaks, as there is no apparent difference between samples, treated or not treated with TdT: 1,5 dpf embryos (lanes 1,6), 4 dpf embryos (lanes 2,7), 21 dpf zebrafish (lanes 3,8), adult female (lanes 4,9) and adult male (lanes 5,10).
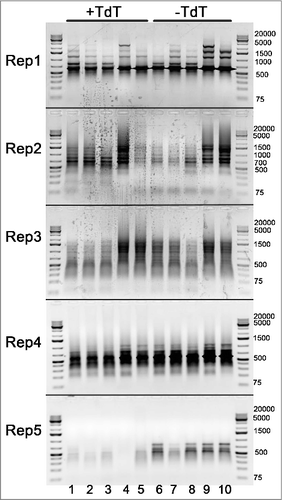
Since we observed tissue-specific patterns of DNA break distribution by ETUNEL, we performed the same PCR procedure with template zebrafish DNA isolated from various organs, and from organisms at different developmental stages. Interestingly, agarose gel fractionation of PCR products revealed several unusual features (). It was shown that the distribution of breakage sites varies depending on fish age, as shown by the germline 4 dpf embryo and adult fish, size dispersion of the amplified fragments gradually increasing from germline to embryo and finally to adult fish, shifting to lower molecular weight products (). The control and TdT-treated samples displayed very different patterns of PCR fragment size distribution between different organs, varying much from cells in roe to muscle tissues in adult fish (). These results prove that the genomic distribution of ITR-associated DNA breaks appears to be tissue-specific. In contrast, there is virtually no association of DNA breaks with 5 other types of zebrafish repeats (Rep1-Rep5) originated from transposons ().
To analyze the sequence features and genomic locations of break-associated regions, PCR fragments were cut out from gel, cloned into a pUC19 vector and sequenced. Our clone library contained 161 individual sequences, representing only a part of the amplified fragments. The sequences are available in the NCBI BioSample database (http://www.ncbi.nlm.nih.gov/biosample/) under accession number SAMN07285974. General features of the sequences in the clone library, including their length, position in the genome and identity percentage comparing to the annotated genome (assembly GRCz10) are given in . Sequence analysis revealed that clones were represented with different frequencies in the libraries, and could be aligned into homology groups, representing either the same sequences with polymorphism in the length of terminal telomeric repeats, or with additional polymorphism at internal sites (). Genomic localization of the sequences was rather similar for all of the analyzed fish tissues, notwithstanding apparent differences in fragment size distribution. The number of telomeric repeats next to the terminal stretches of poly-A varied from 3–4 to dozens, however no clear association with age or tissue type was found, regardless the differences in telomerase expression. However, it should be noted that even the enhanced TUNEL procedure did not allow us to detect individual break events, if they were not clustered together. Therefore, the presence of sparse breaks in all tissues cannot be excluded.Citation20 On the other hand, our ETUNEL procedure detects not only ITR-associated breaks, but all types of DNA breaks, although it is unlikely for the latter to persist in cell nuclei without being recognized by cell repair systems or telomerase, unless this recognition process could be trans-regulated and guided by break clustering.
Table 1. General features of the sequences, flanked by inverted telomeric repeats, associated with breaks.
Discussion
DNA breaks form constantly in living cells and can be caused by endogenous factors, normally accompanying such processes as replication, transcription, chromatin remodeling and recombination, or exogenous ones, such as ionizing radiation or oxidative agents. It is generally understood that if DNA breaks are left unrepaired, DNA damage response is immediately induced, with subsequent cell-cycle arrest, and the accumulation of DSB can lead to cell death, deregulation of cell growth,Citation21 or result in genome instability.Citation22
Being a regulated process, multiple DNA breaks form after programmed cell death is induced.Citation23,Citation24 Apoptosis-related formation of DNA breaks often accompanies differentiation.Citation25,Citation26 Consequently, developmentally regulated apoptotic events are observed in lens and limb development,Citation27 recombination during immune cell development Citation28 and neuron differentiation.Citation2 Nevertheless, occasionally, DNA breakage events are not associated with apoptosis, being linked to other processes, such as chromatin remodeling,Citation22 and this interconnection may be reciprocal.Citation29-31
In the development of certain organisms, from ciliates to vertebrates, massive DNA fragmentation may accompany cell differentiation, and is related to somatic cell specialization, being coupled with deletion of some genome regions and amplification of the others.Citation32 In these cases it usually accompanies terminal differentiation stages, and sometimes results in great differences between somatic and germline genomes.Citation33 However, in the majority of higher eukaryotes, the genome of somatic cells is rather stable, variations mainly affecting recombination during immune cell development or occurring in cancers, especially after genotoxic treatments.Citation34
The presence of tissue-specific, non-induced DNA breaks raises a question of whether these breaks occur at random or whether there is some sequence specificity for break formation. Some DNA sequences prone to breakage may correspond to recombination hotspots.Citation35-37 As has been shown by cloning and sequencing of DNA “forum” domainsCitation37,Citation38 or 50kb DNA fragments isolated from non-apoptotic human cells,Citation39 several types of repetitive sequences, which are prone to recombination, were found to associate with breakage sites. These include Alu repeats, the (TCAG)11 microsatellite, as well as CCAGCCTGG and AAAAAAAACAAAA motifs, the murine major satellite and the (TCCAA)n human satellite. Interstitial telomeric repeats (ITR) are another type of repetitive sequences in which numerous DNA lesions have been detected.Citation40-43
But, virtually in all of these cases, DNA fragmentation and subsequent healing is a rather rapid process, and, on the contrary, the accumulation of DNA breaks in differentiated cells is considered to be a consequence of their inability to efficiently repair DNA.Citation44
Our study demonstrates that besides transient DNA breaks, which sporadically arise and are promptly repaired during cell life or accompany cell death, there are foci of persistent DNA breaks which can be visualized in different cell types, such as neural or epithelial cells. The amount and distribution of free DNA end foci appears to be tissue-specific, so they may be associated with cell differentiation or may help to maintain specific chromatin architecture.
If, apart from chromosome ends, other stable DNA breaks exist in cells at some stages during a cell's lifecycle, or are associated with developmental events, it is conceivable that such breaks may be protected by telomeric repeats. Interstitial telomeric repeats, at least in part, may be linked to the protection of differentiation-related DNA breaks from repair or cell cycle checkpoint activation. Our findings suggest that certain interstitial telomeric repeats, as well as telomeres at chromosome ends, are associated with DNA breaks, limited to particular cell types during development. On the other hand, in certain cases, amplification of some internal chromosome regions could be hypothesized. Furthermore, if such amplification occurs, the generated short DNA molecules might also be capped by telomeric repeats, either added by telomerase, coming from internal genome regions or added by reverse transcription in a telomerase-independent manner.Citation45-47
There are different and controversial reports concerning whether the ITRs themselves are hot spots of breakage and recombination.Citation40,Citation48-50 Some support this idea,Citation51 while others extend it further, claiming that these potential breakage sites have a mutagenic impact on the adjacent regions.Citation40,Citation48,Citation50,Citation52 Other reports show that ITRs are not hot spots of breakage or recombination, but rather are associated with chromosome regions of higher stability.Citation10,Citation53 This can indicate that ITR are functionally different, their stability being dependent on length or genomic context. We found that some ITR are associated with stable DNA breaks organized in clusters and appear to be not a result of apoptotic events, recombination, replication or transcription by themselves, but might harbor mainly regulatory functions, from structural chromatin organization to involvement in epigenetic events. The fact that such repeats are present in rather distant species, such as fish and mice, can also point out that they might be involved in some evolutionary conservative regulatory mechanisms, and the variability in ITR number and location could reflect the plasticity of such processes.
Materials and methods
Cryosectioning
Adult zebrafish (line AB/TL) were dissected on ice, organs or whole embryos were placed in O.C.T. media (Fisher Scientific) and frozen in liquid nitrogen. Cryosections were made on CM3050 cryostat (Leica) within −18 to −25°C temperature range, with a slice thickness of 7 µm. Sections were transferred on polylysine coated slides and fixed in 4% paraformaldehyde in 1xPBS with 5 mM MgCl2 (PBS*) for 10 minutes, afterwards they were washed 4 times in PBS* and permeabilized with 1% Triton X-100 in PBS* for 15 minutes, washed again and used for subsequent manipulations or stored in 20% glycerol, in PBS* at 0°C.
ETUNEL
Coverslips containing permeabilized zebrafish or mouse cryosections in 20% glycerol were washed 3 times for 10 min with PBS*, equilibrated in a TdT (Terminal deoxynucleotidyl transferase) buffer, 40 μl/slide, for 30 min. The slides were treated with TdT, 0,5 U/μl, and FITC-dUTP, 2,5 μM at 37°C for 1 hour.Citation54 The samples were washed 3 times for 10 minutes with 0,05% Tween 20 in PBS*, and blocked with 2% BSA in PBS for 2 hours at 37°C. To amplify the FITC signal, the slides were incubated with rabbit anti-FITC antibodies (Invitrogen) 1:100, in 0,05% Tween 20 in PBS*, and 1% BSA overnight at 4°C. Afterwards the samples were washed 5 times for 15 minutes with 0,05% Tween 20 in PBS* and incubated for 2 hours with FITC-labeled goat anti-rabbit antibody (IMTEK, Russia) at 37°C, at 1:1000 dilution. After washing 5 times for 15 minutes with 0,05% Tween 20 in PBS* coverslips were mounted in Moviol with DAPI.
Immunohistochemistry
Slides with fixed cryosections were washed 3 times for 10 minutes in PBS*. Afterwards, samples were washed in PBS*, 0,05% Tween 20 and then incubated in the same solution, supplemented with 2% BSA for 2 hours at room temperature. Then the slides were incubated with primary antibodies, diluted 1:100 to 1:1000 in 0,05% Tween 20 in PBS* and 1% BSA overnight at 4°C. The following primary antibodies and dilutions were used: mouse anti-FITC (Thermo Fisher Scientific) or rabbit anti-FITC (Abcam), mouse anti-PCNA (Abcam) at 1:1000, rabbit anti-γ-H2AX (Abcam) at 1:500, rabbit anti-caspase 3 at 1:500 (Life Technologies), rabbit anti-RNA polymerase II (Abcam) at 1:100, rabbit anti-PARP (Abcam) at 1:100, rabbit anti-SNF2 (Abcam) at 1:200, rabbit anti-Ac-H3K9 (Abcam) at 1:500, and rabbit anti-H3K9-Me3 (Abcam) at 1:200 dilution. After that, samples were washed 4 times (15 min each) in PBS supplemented with 0.05% Tween 20, and treated with goat anti-rabbit FITC or goat anti-mouse Alexa Fluor 555 conjugated secondary antibodies (Life Technologies) at 1:1000 dilution, or, alternatively, with goat anti-mouse FITC (Abcam) and goat anti-rabbit Alexa Fluor 555 (Thermo Fisher Scientific) conjugated secondary antibodies at 1:500 dilution. After washing 4 times for 15 minutes with 0,05% Tween 20 in PBS*, the slides were mounted in Moviol supplemented with DAPI to counterstain the DNA.
Fluorescence microscopy
Slides were examined and photographed with an Eclipse Ti-E inverted fluorescent microscope (Nikon, Japan) equipped with a × 60/NA = 1.4 lens and iXon cooled EMCCD camera (Andor), under the control of NIS-Elements 4.0 software. The entire section area was scanned automatically using a x20/NA = 0.45 lens and images were stitched using NIS-Elements. Serial optical sections were deconvolved using the AutoQuant blind deconvolution algorithm included in the NIS-Elements package.
PCR amplification of regions, flanked with inverted telomere repeats coupled with DNA breaks
High molecular weight genomic DNA from D.rerio was isolated according to The zebrafish book protocol.Citation55 For each sample, organs from 5 fish were pooled, or 10–15 whole embryos were used.
To amplify the regions flanked with telomere repeats coupled with breaks, isolated genomic DNA samples were preliminary treated with terminal transferase (Thermo scientific) in a TdT buffer, in the presence of 100 nM dTTP at 37°C for 1 hour to add poly-T tails to all 3′ DNA ends. Then the enzyme was inactivated by heating at 70°C for 10 minutes. Control samples were incubated with the same reaction mixture without enzyme addition.
The PCR reaction with a primer mixture, consisting of primers complimentary to the junction of telomeric repeats and a poly-T tail (Term1–4), was performed under the following conditions: 1x Taq buffer with (NH4)2SO4, 3,5 mM MgCl2, 0,5 mM dNTPs, 1 mM primer mixture (), and ∼100 fMol genomic DNA, 2U/50 µl Taq polymerase. The reaction was performed as follows: initial denaturation at 95°C for 5′, then 38 reaction cycles (95°C for 30," 56°C for 20," 72°C for 5′), and final elongation at 72°C for 10′.
Table 2. Primers, used to identify the correlation between DNA breaks and telomeric or other types of repeats.
To show whether the DNA-breaks could be found in association with other types of repetitive elements with a genome location similar to those of internal telomeric repeats, primers to the junction of poly-T tail and 5 other types of zebrafish repeats were used (Rep 1–5). PCR conditions were similar to those described for telomeric repeats, except the use of one primer (Rep 1–5 respectively) at 0,5 mM concentration instead of primer mixture. Annealing temperature was 58°C for Rep 2 and 56°C for Rep 1, Rep 3, Rep 4 and Rep5.
The amplification products were analyzed in 1% agarose gel, or cloned in pUC19 plasmid and sequenced.
Analysis of regions, containing telomeric repeat coupled with DNA breaks
For cloning, regions flanked with telomere repeats were amplified using telomeric primers (Term1–4 mix), phosphorylated using T4 Polynucleotide Kinase (Fermentas) according to the manufacturer protocol. PCR products were purified from 1% agarose gel using a GeneJet gel extraction kit (Fermentas). Blunt-ended products were obtained using T4 DNA polymerase. For cloning, dephosphorylated pUC19 vector cut on SmaI was used. Ligation was performed with T4 DNA ligase (Fermentas) according to the manufacturer protocol, using a 1:3 vector to insert ratio. Then the ligation mix was transformed into E. coli JM-109 strain by electroporation, the colonies were grown on LB with ampicillin and inserts were analyzed by electrophoresis. The plasmids with inserts were purified using plasmid MiniPrep kit (Fermentas) and sequenced.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are thankful for support from the Russian Scientific Foundation (project 14–24–00061) for animal care and cryosectioning equipment and service and to the development program of MSU (Plan of research work 5.13) for the equipment provided.
Funding
We are grateful to the Rostok group of companies for their support. This work was partially supported by the Russian Foundation of basic research (project 13–04–40199).
References
- Sulli G, di Micco R, d'Adda di Fagagna F. Crosstalk between chromatin state and DNA damage response in cellular senescence and cancer. Nat. Rev. Cancer. 2012;12:709-720. doi:10.1038/nrc3344. PMID:22952011
- Martin LJ, Liu Z, Pipino J, Chestnut B, Landek MA. Molecular regulation of DNA damage-induced apoptosis in neurons of cerebral cortex. Cereb. Cortex N. Y. NY. 2009;19: 1273-1293. doi:10.1093/cercor/bhn167
- Chandra T, Ewels PA, Schoenfelder S, Furlan-Magaril M, Wingett SW, Kirschner K, Thuret J-Y, Andrews S, Fraser P, Reik W. Global reorganization of the nuclear landscape in senescent cells. Cell Rep. 2015;10:471-483. doi:10.1016/j.celrep.2014.12.055. PMID:25640177
- Nandakumar J, Cech TR. Finding the end: recruitment of telomerase to the telomere. Nat. Rev. Mol. Cell Biol. 2013;14:69-82. doi:10.1038/nrm3505. PMID:23299958
- Wood AM, Laster K, Rice EL, Kosak ST. A beginning of the end: new insights into the functional organization of telomeres. Nucleus. 2015;6:172-178. doi:10.1080/19491034.2015.1048407. PMID:25961132
- Blackburn EH. Telomeres and telomerase: Their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579:859-862. doi:10.1016/j.febslet.2004.11.036. PMID:15680963
- Chang S. Chromosome ends teach unexpected lessons on DNA damage signaling. EMBO J. 2012;31:3380-3381. doi:10.1038/emboj.2012.199. PMID:22842787
- Ruiz-Herrera A, Nergadze SG, Santagostino M, Giulotto E. Telomeric repeats far from the ends: mechanisms of origin and role in evolution. Cytogenet. Genome Res. 2008;122:219-228. doi:10.1159/000167807. PMID:19188690
- Bolzan AD. Chromosomal aberrations involving telomeres and interstitial telomeric sequences. Mutagenesis 2012; 27:1-15. doi:10.1093/mutage/ger052. PMID:21857006
- Galkina S, Lukina N, Zakharova K, Rodionov AV. Interstitial (TTAGGG)(n) sequences are not hot spots of recombination in the chicken lampbrush macrochromosomes 1–3. Chromosome Res. Int. J. Mol. Supramol. Evol. Asp. Chromosome Biol. 2005;13:551-557. doi:10.1007/s10577-005-0980-y
- Anand RP, Shah KA, Niu H, Sung P, Mirkin SM, Freudenreich CH. Overcoming natural replication barriers: differential helicase requirements. Nucleic Acids Res. 2012;40:1091-1105. doi:10.1093/nar/gkr836. PMID:21984413
- Juraver-Geslin HA, Durand BC. Early development of the neural plate: new roles for apoptosis and for one of its main effectors caspase-3. Genesis. 2015;53:203-224. doi:10.1002/dvg.22844. PMID:25619400
- Revet I, Feeney L, Bruguera S, Wilson W, Dong TK, Oh DH, Dankort D, Cleaver JE. Functional relevance of the histone gammaH2Ax in the response to DNA damaging agents. Proc Natl Acad Sci U S A. 2011;108:8663-8667. doi:10.1073/pnas.1105866108. PMID:21555580
- Price BD, D'Andrea AD. Chromatin remodeling at DNA double strand breaks. Cell. 2013; 1521344-1354.
- House NCM, Koch MR, Freudenreich CH. Chromatin modifications and DNA repair: beyond double-strand breaks. Front. Genet. 2014;5:296. doi:10.3389/fgene.2014.00296. PMID:25250043
- Liu B, Yip RK, Zhou Z. Chromatin remodeling, DNA damage repair and aging. Curr. Genomics. 2012;13:533-547. doi:10.2174/138920212803251373. PMID:23633913
- Bunch H, Lawney BP, Lin YF, Asaithamby A, Murshid A, Wang YE, Chen BP, Calderwood SK. Transcriptional elongation requires DNA break-induced signalling Nat. Commun. 2015;6:10191.
- Scicchitano DA, Mellon I. Transcription and DNA damage: a link to a kink. Environ. Health Perspect. 1997;105:145-153. doi:10.1289/ehp.97105s1145. PMID:9114283
- Hanawalt PC, Spivak G. Transcription-coupled DNA repair: two decades of progress and surprises. Nat. Rev. Mol. Cell Biol. 2008;9:958-970. doi:10.1038/nrm2549. PMID:19023283
- Szilágyi I, Varga T, Székvölgyi L, Hegedüs É, Goda K, Kaczur V, Bacsó Z, Nakayama Y, Pósafi J, Pongor S, et al. Non-random features of loop-size chromatin fragmentation. J Cell Biochem. 2003; 89:1193-1205. doi:10.1002/jcb.10591. PMID:12898517
- Gospodinov A, Herceg Z. Chromatin structure in double strand break repair. DNA Repair (Amst). 2013;12:800-810. doi:10.1016/j.dnarep.2013.07.006. PMID:23919923
- Jeggo PA, Downs JA. Roles of chromatin remodellers in DNA double strand break repair. Exp Cell Res. 2014;329:69-77. doi:10.1016/j.yexcr.2014.09.023. PMID:25278484
- Polo SE, Jackson SP. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev. 2011; 25:409-433. doi:10.1101/gad.2021311. PMID:21363960
- Darzynkiewicz Z, Zhao H, Halicka HD, Rybak P, Dobrucki J, Wlodkowic D. DNA damage signaling assessed in individual cells in relation to the cell cycle phase and induction of apoptosis. Crit Rev Clin Lab Sci. 2012;49:199-217. doi:10.3109/10408363.2012.738808. PMID:23137030
- Heyer BS, MacAuley A, Behrendtsen O, Werb Z. Hypersensitivity to DNA damage leads to increased apoptosis during early mouse development. Genes Dev. 2000;14:2072-2084. PMID:10950870
- Sherman MH, Bassing CH, Teitell MA. DNA damage response regulates cell differentiation. Trends Cell Biol. 2011;21:312-319. doi:10.1016/j.tcb.2011.01.004. PMID:21354798
- Cecconi F, Levine B. The role of autophagy in mammalian development. Dev Cell. 2008;15:344-357. doi:10.1016/j.devcel.2008.08.012. PMID:18804433
- Yabuki M, Ordinario EC, Cummings WJ, Fujii MM, Maizels N. E2A acts in cis in G1 phase of cell cycle to promote Ig gene diversification. J Immunol Baltim Md 1950. 2009;182:408-415.
- Oliver L, Hue E, Séry Q, Lafargue A, Pecqueur C, Paris F, Vallette FM. Differentiation-related response to DNA breaks in human mesenchymal stem cells. Stem Cells 2013;31:800-807. doi:10.1002/stem.1336. PMID:23341263
- Weiss CN, Ito K. DNA damage: a sensible mediator of the differentiation decision in hematopoietic stem cells and in leukemia. Int. J. Mol. Sci. 2015;16:6183-6201. doi:10.3390/ijms16036183. PMID:25789504
- Schneider L. Survival of neural stem cells undergoing DNA damage-induced astrocytic differentiation in self-renewal-promoting conditions in vitro. PLoS One. 2014;9:e87228. doi:10.1371/journal.pone.0087228. PMID:24475256
- Streit A. Silencing by throwing away: A role for chromatin diminution. Dev Cell. 2012;23:918-919. doi:10.1016/j.devcel.2012.10.022. PMID:23153488
- Grishanin A. Chromatin diminution in Copepoda (Crustacea): Pattern, biological role and evolutionary aspects. Comp Cytogenet. 2014;8:1-10. doi:10.3897/compcytogen.v8i1.5913. PMID:24744830
- Erenpreisa J, Huna A, Salmina K, Jackson TR, Cragg MS. Macroautophagy-aided elimination of chromatin: sorting of waste, sorting of fate? Autophagy. 2012;8:1877-1881. doi:10.4161/auto.21610. PMID:22935563
- Tan FJ, Hoang ML, Koshland D. DNA resection at chromosome breaks promotes genome stability by constraining non-allelic homologous recombination. PLoS Genet. 2012;8:e1002633. doi:10.1371/journal.pgen.1002633. PMID:22479212
- Cauwood JD, Johnson AL, Widger A, Cha RS. Recombinogenic conditions influence partner choice in spontaneous mitotic recombination. PLoS Genet. 2013;9:e1003931. doi:10.1371/journal.pgen.1003931. PMID:24244194
- Tchurikov NA, Krasnov AN, Ponomarenko NA, Golova YB, Chernov BK. Forum domain in Drosophila melanogaster cut locus possesses looped domains inside. Nucleic Acids Res. 1998;26:3221-3227. doi:10.1093/nar/26.13.3221. PMID:9628922
- Tchurikov NA, Kretova OV, Chernov BK, Golova YB, Zhimulev IF, Zykov IA. SuUR Protein binds to the boundary regions separating forum domains in drosophila melanogaster. J Biol Chem. 2004;279:11705-11710. doi:10.1074/jbc.M306191200. PMID:14702350
- Szilágyi I, Varga T, Székvölgyi L, Hegedüs É, Goda K, Kaczur V, Bacsó Z, Nakayama Y, Pósafi J, Pongor S, Szabó G Jr. Non-random features of loop-size chromatin fragmentation. J Cell Biochem. 2003;89:1193-205. doi:10.1002/jcb.10591. PMID:12898517
- Bosco N, de Lange T. A TRF1-controlled common fragile site containing interstitial telomeric sequences. Chromosoma. 2012;121:465-474. doi:10.1007/s00412-012-0377-6. PMID:22790221
- Nergadze SG, Rocchi M, Azzalin CM, Mondello C, Giulotto E. Insertion of telomeric repeats at intrachromosomal break sites during primate evolution. Genome Res. 2004;14:1704-1710. doi:10.1101/gr.2778904. PMID:15310657
- López-Fernández C, Arroyo F, Fernández JL, Gosálvez J. Interstitial telomeric sequence blocks in constitutive pericentromeric heterochromatin from Pyrgomorpha conica (Orthoptera) are enriched in constitutive alkali-labile sites. Mutat Res. 2006;599:36-44. doi:10.1016/j.mrfmmm.2006.01.004. PMID:16481011
- Rivero MT, Mosquera A, Goyanes V, Slijepcevic P, Fernández JL. Differences in repair profiles of interstitial telomeric sites between normal and DNA double-strand break repair deficient Chinese hamster cells. Exp Cell Res. 2004;295:161-72. doi:10.1016/j.yexcr.2003.12.031. PMID:15051499
- Nouspikel T, Hanawalt PC. DNA repair in terminally differentiated cells. DNA Repair. 2002;1:59-75. PMID:12509262
- Olovnikov AM. The redusome hypothesis of aging and the control of biological time during individual development. Biochemistry (Moscow). 2003;68:2-33. doi:10.1023/A:1022185100035
- Olovnikov AM. When creating an embryo, cells are synthesizing transitory. perichromosomal” DNA-containing organelles necessary for interpreting positional information. In: Burlakova E, Varfolomeev SD. (eds), Chemical and Biological Kinetics. New Horizons. In commemoration of Professor N.M. Emanuel's 90th Anniversary. Vol. 2: Biological Kinetics. Boca Raton, FL, USA: CRC Press. 2005;465-480.
- Olovnikov AM. Role of paragenome in development. Russian J Dev Biol. 2007;38:104-123. doi:10.1134/S1062360407020075
- Aksenova AY, Greenwell PW, Dominska M, Shishkin AA, Kim JC, Petes TD, Mirkin SM. Genome rearrangements caused by interstitial telomeric sequences in yeast. Proc Natl Acad Sci U S A. 2013;110:19866-19871. doi:10.1073/pnas.1319313110
- Azzalin CM, Nergadze SG, Giulotto E. Human intrachromosomal telomeric-like repeats: sequence organization and mechanisms of origin. Chromosoma. 2001;110:75-82. doi:10.1007/s004120100135. PMID:11453557
- Kilburn AE, Shea MJ, Sargent RG, Wilson JH. Insertion of a telomere repeat sequence into a mammalian gene causes chromosome instability. Mol Cell Biol. 2001;21:126-135. doi:10.1128/MCB.21.1.126-135.2001. PMID:11113187
- Schwertman P, Bekker-Jensen S, Mailand N. Regulation of DNA double-strand break repair by ubiquitin and ubiquitin-like modifiers. Nat Rev Mol Cell Biol. 2016;17:379-394. doi:10.1038/nrm.2016.58. PMID:27211488
- Damerla RR, Knickelbein KE, Kepchia D, Jackson A, Armitage BA, Eckert KA, Opresko PL. Telomeric repeat mutagenicity in human somatic cells is modulated by repeat orientation and G-Quadruplex stability. DNA Repair (Amst). 2010;9:1119-1129. doi:10.1016/j.dnarep.2010.07.014. PMID:20800555
- Rossi E, Floridia G, Casali M, Danesino C, Chiumello G, Bernardi F, Magnani I, Papi L, Mura M, Zuffardi O. Types, stability, and phenotypic consequences of chromosome rearrangements leading to interstitial telomeric sequences. J Med Genet. Nov. 1993;30(11):926-931. doi:10.1136/jmg.30.11.926
- Deng G, Wu R. Terminal transferase: use of the tailing of DNA and for in vitro mutagenesis. Methods Enzymol. 1983; 100:96-116. doi:10.1016/0076-6879(83)00047-6. PMID:6312266
- Westerfield M. The zebrafish book: A guide for the laboratory use of zebrafish Danio (Brachydanio) rerio. Eugene, OR: University of Oregon Press; 2000.