ABSTRACT
The alteration of the several roles that Lamin A/C plays in the mammalian cell leads to a broad spectrum of pathologies that – all together – are named laminopathies. Among those, the Emery Dreifuss Muscular Dystrophy (EDMD) is of particular interest as, despite the several known mutations of Lamin A/C, the genotype–phenotype correlation still remains poorly understood; this suggests that the epigenetic background of patients might play an important role during the time course of the disease. Historically, both a mechanical role of Lamin A/C and a regulative one have been suggested as the driving force of laminopathies; however, those two hypotheses are not mutually exclusive. Recent scientific evidence shows that Lamin A/C sustains the correct gene expression at the epigenetic level thanks to the Lamina Associated Domains (LADs) reorganization and the crosstalk with the Polycomb Group of Proteins (PcG). Furthermore, the PcG-dependent histone mark H3K27me3 increases under mechanical stress, finally pointing out the link between the mechano-properties of the nuclear lamina and epigenetics. Here, we summarize the emerging mechanisms that could explain the high variability seen in Emery Dreifuss muscular dystrophy.
Introduction
Complex organisms are formed of several specialized cell types, each having characteristic features. Their phenotypic diversity is due to the cell-specific expression pattern of gene subsets. At the single gene level, the transcriptional state is determined by epigenetic mechanisms of regulation that establish distinct layers of structural organization, including covalent modification of DNA and histones, packaging of DNA around nucleosomes, higher order chromatin interactions and nuclear positioning [Citation1]. The ensemble of epigenetic mechanisms determines the epigenome complexity, which establishes and maintains the cell identity in time and space without altering the DNA sequence. To achieve higher order configurations and/or to maintain the steady state of chromatin conformation, epigenetic factors cooperate with nuclear structures, such as nuclear pores and the nuclear lamina [Citation2]. In fact, recent evidence has described how, in several human pathologies, alterations of nuclear components can influence genome conformation with drastic consequences for gene regulation [Citation2]. For this reason, in these years, the study of the crosstalk between the epigenome and the nuclear architecture has attracted considerable interest. In this review, we will summarize the recent findings concerning genetic and epigenetic dysfunctions in Emery Dreifuss Muscular Dystrophy (EDMD), a human disease caused by mutations in the components of the nuclear lamina.
The nuclear lamina
The nuclear lamina (NL) is a branched protein meshwork beneath the inner nuclear membrane (INM). The principal components of the NL are lamins, intermediate filaments (IF) proteins of the V type. Lamin proteins exhibit a tripartite structure, constituted of an α-helical central rod domain flanked by N-terminal and C-terminal globular domains [Citation3–Citation5]. In vertebrates, lamins are divided into B-type, encoded by LMNB1 and LMNB2 genes, and A-type, encoded by LMNA gene [Citation6]. The alternative splicing of LMNA-derived transcripts generates two main proteins: Lamin A and Lamin C [Citation7,Citation8]. Lamin A is initially synthesized as a longer precursor, the prelamin A, which undergoes a series of post-translational modifications inside the nucleus: the CaaX motif at its C-terminus is farnesylated, partially cleaved and then carboxymethylated. Finally, an endoproteolytic cleavage produces the mature Lamin A protein [Citation9]. Mature Lamin A can dimerize with a parallel and in-register homolog via a coiled-coil structure which involves the four α-helical segments of their central rod domains [Citation10]. Anti-parallel homodimers then assemble in a head-to-tail fashion forming protofilaments, whose weaker lateral half-staggered interactions generate IF-like structures of heterogeneous diameter [Citation10,Citation11]. Transmission electron microscopy, in 1986, afforded the first structural view of nuclear lamins: nuclear envelopes isolated from Xenopus laevis oocytes revealed IF-like filaments with an approximate diameter of 10-nm forming a net, with the two orthogonal sets of filaments having a crossover spacing of about 52 nm [Citation12]. These observations were later confirmed by using cryoelectron and field emission scanning microscopies [Citation13,Citation14]. More recently, Turgay and colleagues used cryo-electron tomography (cryo-ET) in vimentin-null mouse embryonic fibroblasts to show that A- and B-type lamins localized under the INM exhibit a peculiar structure, assembling into tetrameric filaments of 3.5 nm thickness [Citation15]. This structure is different from other canonical cytoskeletal elements such as microtubules, vimentins and actin, that form thicker filaments. Three-dimensional structured illumination microscopy (3D-SIM) was also used to visualize lamin organization [Citation16] and highlighted a type-specific distribution of lamins, possibly suggesting that different lamin isoforms have distinct roles in maintaining the organization of the nuclear lamina. In line with these findings, previous works showed that lamin proteins are not exclusively present at the nuclear periphery, but also localize in the nucleoplasm [Citation17–Citation19]. Unfortunately, cryo-electron tomography studies were restricted to peripheral lamins [Citation15]. Thus, further studies, combining new imaging technologies, will hopefully elucidate the real structure of lamins in the nucleoplasm and will define in detail how the network of different lamin isoforms organized in distinct nuclear spaces.
The nuclear lamina as a chromatin organizer
Due to its structure and positioning underneath the INM, the nuclear lamina was initially thought as a static skeletal element involved in the organization of the nuclear envelope [Citation20,Citation21] and in the definition of higher order chromatin domains in interphase chromosomes [Citation21–Citation24]. Then came their suggested role as an assembly platform that could connect and coordinate the complex molecular machineries involved in a wide range of functions [Citation25]. This hypothesis is supported by a burst of experimental evidence that linked Lamin A/C to active cell processes, including cell migration [Citation26], signal transduction [Citation27–Citation29], RNA PolII-dependent transcription [Citation30], DNA replication [Citation31,Citation32], cell cycle [Citation33,Citation34] and cancer growth [Citation35-Citation37]. Lamin proteins are also involved in the epigenetic regulation of chromatin [Citation38–Citation41], as evidenced by the fact that nuclear periphery is widely recognized as a repressive environment [Citation42,Citation43] and lamins directly interact with the genome at specific DNA sequences called Lamina Associated Domains (LADs) [Citation44]. These domains, of a variable length from 0.1 to 10 Mb, create an environment that maintains genes repressed [Citation45,Citation46] and marked by H3K9me2 and H3K9me3 histone modifications [Citation47–Citation49]. The borders of LADs include gene promoters that often contain H3K27me3 histone modification, suggesting a peculiar lamin dependent chromatin organization where the transcriptional start sites of genes located in the facultative heterochromatin are adjacent to constitutive heterochromatin [Citation50]. Some LADs, called constitutive LADs (cLADs), are associated with the lamina across cell types and conserved between species, suggesting a role in the organization of chromosomal architecture [Citation44–Citation46]. Other LADs, the facultative LADs (fLADS), are cell-specific, pointing out a possible role in cell identity and differentiation. Repositioning of silent genes to the nuclear periphery [Citation51] and the crosstalk with developmentally regulated transcriptional factors in the nucleoplasm [Citation52,Citation53] both indicate that the nuclear lamina plays a role in transcriptional regulation and cell identity specification. For instance, during the differentiation of adipose stem cells (ASCs) into adipocytes, genes that regulate the adipogenesis are released from the lamina while genes that maintain the cells undifferentiated are retained near to it, in a repressive environment [Citation49]. Similarly, during the differentiation of embryonic stem cells (ESCs) into neural precursor cells (NPCs), “stemness genes” such as Nanog, Klf4 and Oct4, which are progressively repressed, exhibit significantly increased interactions with the nuclear lamina in NPCs compared to ESCs [Citation54]. This ability of lamins to influence the localization of genes suggests a role for nucleoplasmic Lamin A/C in pulling genomic regions toward the nuclear center [Citation49]. It is also possible that the interaction between nucleoplasmic Lamin A/C and LADs are dynamic and temporary acting in “intermittent molecular contact” as already shown for the peripheral Lamin A/C fraction [Citation47].
Emery dreifuss muscular dystrophy (EDMD)
Since Lamin A/C covers such a variety of roles, it is not surprising that mutations in the LMNA gene cause a wide set of pathologies, grouped all together under the term of Laminopathies [Citation55]. Lamin A-dependent diseases are tissue-specific and can affect the nervous system [Citation56], the striated muscle [Citation57–Citation59], the cardiac muscle [Citation60,Citation61] or the adipose tissue [Citation62,Citation63]. Other laminopathies lead to premature aging diseases as in the case of Hutchinson-Gilford Progeria Syndrome (HGPS) [Citation64] and atypical Werner syndrome (AWS) [Citation65]. In minor part, also B-type Lamin takes part to Laminopathies giving rise to neuropathy and lipodystrophy [Citation66].
Lamin A/C dependent Laminopathies affecting the striated muscles have been described as a continuous spectrum of successive phenotypes. A strong correlation between age of onset and the specific mutation, but also the severity of the phenotype has been described [Citation67]. In general, the early prenatal onset is associated with lethal fetal akinesia, late prenatal onset with severe lamin-related congenital muscular dystrophy (L-CMD), onset before 1 year with dropped head L-CMD, onset in childhood or young adulthood with classic EDMD, later onset with LGMD1B, and finally, the end of the spectrum where no skeletal muscle involvement is noted [Citation68,Citation69].
EDMD is thought to be the third most common dystrophy, following Duchenne and Becker muscular dystrophies. The prevalence of EDMD has been estimated at 0.13:100,000 – 0.2:100,000 [Citation67]. The vast majority of EDMD-causing variants present an autosomal dominant inheritance: they consist of single nucleotide mutations, short insertions or deletions in one of the two LMNA alleles (AD-EDMD). Some of them affect residues in domains required for intra- or intermolecular interactions; others introduce a premature stop codon yielding a truncated, dysfunctional protein [Citation68]. A smaller proportion of patients have an X-linked recessive form (XL-EDMD), associated with mutations in the EMD gene, coding for Emerin, an integral protein of the inner nuclear membrane located on the X chromosome [Citation70]. Among the mutations reported, most are predicted to cause loss of protein expression, while few of them are missense. At present, only five patients affected by autosomal recessive form (AR-EDMD) have been identified [Citation59,Citation71]. The first patient showed difficulties when he started to walk due to severe muscular dystrophy and joint contractures; he stopped walking by the age of 5 years but showed no cardiac involvement [Citation59]. The other four individuals have a homozygous c.674G>A LMNA pathogenic variant and belong to the same family, in which one sibling was accidentally diagnosed with AR-EDMD [Citation71]. These patients have a severe muscular dystrophy involving proximal muscles around the hips and shoulders (limb-girdle dystrophy); two of them – 25 and 35 years old – have joint contractures causing loss of ambulation; premature atrial and ventricular contractions and conduction defects have been diagnosed.
EDMD usually manifests itself in patients between mid-childhood and the second decade of life with slowly progressive muscular weakness, joint contractures, and cardiac disease [Citation70]. AD-EDMD and XL-EDMD are characterized by three diagnostic principles: i) early contractures affecting selectively the Achilles tendons, the elbow flexors and neck extensors; ii) a limitation of extension of the whole spine, due to the progressive development of spinal cervicodorsal; iii) lumbar contractures [Citation68]. In XL-EDMD, joint contractures are usually the first sign, whereas, in AD-EDMD, they may appear after the onset of muscle weakness [Citation68]. Variable slowly progressive muscle wasting and weakness occur, starting in humeroperoneal/scapulo-peroneal muscles and then extending to the lower legs. Loss of ambulation can occur in AD-EDMD but is rare in XL-EDMD [Citation68]. Cardiomyopathy at later stages of the disease is associated with conduction abnormalities. In AD-EDMD, the risk for ventricular tachyarrhythmia and dilated cardiomyopathy manifested by left ventricular dilation and dysfunction is higher than in XL-EDMD [Citation72]. The cardiac conduction system can be affected at all levels and can manifest itself as sick sinus syndrome, atrioventricular block or bundle branch blocks. This leads to the necessity for implantation of a pacemaker; furthermore, the presence of arrhythmias usually precedes the chamber enlargement [Citation73]. The course of the cardiomyopathy is aggressive and leads to premature death. By an age of 60 years, 55% of LMNA mutation carriers die of cardiovascular death or receive a heart transplant, compared with 11% of patients with idiopathic cardiomyopathy without LMNA mutation [Citation74].
Women heterozygous for mutations in EMD gene usually do not show any major clinical symptoms and are therefore named ‘healthy carriers’ of XL-EDMD. However, skin sections from healthy carriers showed a mosaic pattern of emerin expression in immunofluorescence assays and western blot analysis on their blood cells revealed reduced levels of emerin protein [Citation75]. Drastic reduction in emerin levels was initially associated with some cases of cardiac involvement among healthy carriers [Citation75-Citation78]. In fact, together with the cardiac phenotype, a great reduction in Emerin levels with respect to non-symptomatic females was observed. Methylation-sensitive restriction enzyme assay suggested that the clinical manifestation was due to uneven X-inactivation [Citation75]. However, more recently, Meinke and colleagues described a mild muscular and cardiac phenotype in healthy carriers not associated with X-inactivation defects [Citation79]. They excluded a dominant-negative effect of the mutant truncated peptide since its expression was undetectable in western blot of patient myoblasts. Furthermore, the patient's grandmother, a carrier in her own right, was unaffected. A second, modifying mutation was therefore hypothesized but, unfortunately, not identified. The concrete opportunity to analyze symptomatic and asymptomatic healthy carriers of EDMD could be a significant advance in the field and could be used to discover secondary mechanisms and/or mutations contributing to the disease phenotype. Moreover, despite the high number of identified pathological mutations, in more than 60% of EDMD cases, no genetic mutations were detected in EMD or LMNA genes [Citation70], suggesting the presence of other genes involved in EDMD dystrophy. We predict that in the coming years the number of patients diagnosed with AR-EDMD will increase, thanks to the increasing accessibility of next-generation sequencing in clinical medicine.
EDMD mouse models
The first Lamin A/C knockout mouse (Lmna −/−) was generated by the group of Brian Burke in 1999 [Citation80]; the region spanning from exon 8 to part of exon 11 was replaced by a Pgk-neomycin resistance cassette (). Though homozygous mice show no difference in phenotype compared to the wild type ones at birth, their growth rate decreases within 2–3 weeks and ceases by around 4 weeks. By then, mutant mice display an abnormal gait with a stiff walking posture; scoliosis/kyphosis are documented. By the 8th week, all homozygotes mice die. Although the rapid onset dystrophy is in contrast with lamin dependent dystrophy in humans, this being mainly autosomal dominant and having a slow progression, homozygous mice show several features of the human Emery Dreifuss muscular dystrophy. Microscopical analysis shows that the paravertebral muscles and some belonging to the hindlimb (more specifically, rectus femoris and semimembranosus) are dystrophic; the fibers proximal to the bone are the most severely impaired [Citation80]. Muscles of the head, tongue, and diaphragm are spared by the dystrophy. In the heart, the ventricular muscle is that most affected, though the involvement of myocytes is variable [Citation81]. In 2012, Jahn and colleagues [Citation82] discovered the presence of a lower amount of a truncated Lamin A in Lmna −/− mice. The strain, renamed Lmna Δ8-11, cannot be thus considered “null”. This C-terminally truncated Lamin A, although produced in very low amounts, could either be functionally hypoactive and able to carry out only certain functions but fail to complete others (loss of function model) or, alternatively, it could act as a toxic molecule with a dominant-negative effect (gain-of-function model).
Table 1. Mouse models of Emery Dreifuss Muscular Dystrophy.
To overcome artefact effects due to this truncated Lamin A form, another mouse strain, the Lmna GT −/−, was generated in 2011 [Citation83] by interruption of the endogenous Lamin A/C locus with a promoter-trap construct, which introduces an in-frame Lmna-βgeo fusion allele into Lmna intron 2 followed by β-galactosidase-neomycin cDNA (). Interestingly, by following β-galactosidase staining authors detected the activity of Lmna promoter in the heart, liver and embryonic somites at E11, thus dispelling the myth that Lamin A is expressed only after birth. Lmna GT −/− mice display a phenotype similar to the previous Lmna Δ8–11: hunched posture and abnormal gait, characterized by splayed hind legs from day P13 onwards. At the histological level, post-natal hypertrophy of cardiac myocytes and hypotrophy of the quadriceps are present. Lmna GT −/− mice die at 16–18 days after birth, much earlier than Lmna Δ8–11 mice, and representing the most severe loss-of-function mutants for A-type lamins to date. Consistent with findings obtained in these mouse models, homozygous LMNA nonsense mutation has been detected in a newborn patient leading to the complete absence of Lamin A/C; the child died at birth due to respiratory insufficiency and severe generalized muscular dystrophy [Citation84].
Another mouse model for EDMD was created in 2005 by Arimura and colleagues: the Lmna H222P/H222P knock-in mouse [Citation85] (). H222P is the first missense mutation discovered to be associated to EDMD. A transversion (CAT>CCT) in exon 4 of Lmna leads to a change from histidine to proline at codon 222 (c.665A>C, p.H222P). The mutant mice do not show any developmental anomaly and reach sexual maturity, but at adulthood, starting at approximately 16 weeks, male homozygous mice develop muscular dystrophy with abnormal stiff walking posture and all of them die by the 9th month. Dilative cardiomyopathy and hypokinesia with conduction defects are present; the cardiac involvement in female homozygous mice arises later compared to the male counterpart. A marked increase in fibrosis occurs in the heart of homozygous mice; degeneration and necrosis affect cardiac muscle cells in the ventricles. At the skeletal muscle level, variability in fiber size with hypertrophic muscle cells and numerous degenerative fibers can be observed in both diaphragm and soleus of male homozygous mice [Citation68]. However, in contrast with the Lmna null mouse, only a moderate phenotype was observed in gastrocnemius, quadriceps, triceps and tibialis anterior as reported by the author (data not shown). For the variable and late onset of muscular dystrophy, this mouse has mainly been used for studies on the heart.
In 2011, Bertrand and colleagues created the Lmna ΔK32 knock-in mouse; this strain harbors a severe congenital muscular dystrophy (L-CMD) mutation [Citation86] that leads to the loss of lysine 32 in the N-terminal domain of LMNA (). Though at birth Lmna ΔK32/ΔK32 mice do not show any difference from the wild type littermates, they display a severe reduction in the growth curve already at P5; by P15, only half of the homozygous mice survive and by P19 they are all dead. Homozygous mice show severe metabolic defects and a waddling gait with an increasing number of falls, probably due to hindquarter blockade. Microscopically, all muscles analyzed are affected, showing a reduction of fiber size associated with the presence of central myonuclei, as documented by the analysis of the gastrocnemius at P14, and indicating a delay in skeletal muscle maturation [Citation86].
Interestingly while all described mouse models carrying mutations or deletion of Lamin A do not show disease features in heterozygous, some evidence reported progressive electrophysiological cardiac abnormalities commencing around 4 weeks after birth and the emergence of late-onset cardiomyopathy in aged (50 weeks) heterozygous Lmna Δ8-11 mice, suggesting a cardiac pathology [Citation87]. Telemetric and in vivo electrophysiological studies in 10-week-old Lmna Δ8-11 +/− mice showed AV conduction defects and both atrial and ventricular arrhythmias, analogous to those observed in humans with heterozygous LMNA mutations. On the other hand, neonatal heterozygous Lmna Δ8-11 mice do not show conduction system defects suggesting that the heart development is not affected [Citation87]. A similar phenotype has been observed in heterozygous Lmna ΔK32mice [Citation88]. Haploinsufficiency of Lamin A in cardiomyopathy affected patients has also been described [Citation89,Citation90], suggesting that heterozygous mice can recapitulate some features of the human disease.
Taking into consideration that Lamin A is a key component of the nuclear environment and interacts with many other factors, it is not surprising that knock-out of known Lamin A-interactor in mouse, show muscular phenotypes similarly to lamin mutant mice. The study of these mouse models could shed a light on distinct Lamin A functions and their role in muscular dystrophy pathogenesis and progression. Lap2α is a nucleoplasmic protein implicated in cell cycle regulation through its interaction with A-type lamins and the retinoblastoma protein; a heterozygous mutation in Lap2α affecting its Lamin A/C interaction domain has also been linked to a cardiomyopathy [Citation91], symptomatically similar to Lamin A/C-linked ones [Citation92], suggesting a common disease mechanism of these two proteins. Lap2α −/− mice are not dystrophic and their heart is not affected histologically [Citation19]. However, Gotic and colleagues showed in primary myoblasts that the loss of Lap2α interferes with the process of the myofiber-type specification, though muscle morphology, function, and regeneration are not compromised in vivo [Citation93]. More specifically, slow muscles in Lap2α −/− exhibit a shift toward the fast fiber phenotype. Interestingly, fast fiber shifts were also observed in skeletal muscles of patients with chronic heart failure [Citation94]. Atrophy of slow fibers occurs also in Lmna H222P/H222P skeletal muscles and in some human striated muscle laminopathies where anomalies in heart structure and function are also present [Citation95,Citation96]. The predominantly oxidative metabolism of both slow skeletal and cardiac muscle 94 affected in these conditions suggests a role of lamins and their interaction partners in the regulation of oxidative metabolism of myofibers and postnatal muscle remodeling.
Molecular mechanisms underlying EDMD
In laminopathies, the symptomatology differs considerably among patients, even in the same family, so suggesting that the individual epigenetic background may play a major role in the disease development [Citation97,Citation98]. In line with this hypothesis, aberrations in the transcriptional control have been described in several laminopathies [Citation99]. Most lipodystrophies, for example, share impaired interaction of Lamin A with SREBP1, an important transcription factor for lipid homeostasis [Citation100]. On the other hand, lamin dependent muscular disorders show disruption of the transcriptional programs supported by the MAPK, pRb, MyoD, Wnt-β catenin and TGFβ altered pathways [Citation68, Citation101–Citation103]. Muscle regeneration and growth benefit from a small population of heterogeneous stem cells named Satellite Cells (SCs) that in normal condition rest in a quiescent state under the basal lamina of the adult muscle fibers [Citation104]. SCs express α7-integrin surface marker [Citation105,Citation106] and Pax7 (Paired Box Protein Pax-7) intranuclear transcription factor [Citation107–Citation109]. In response to various physiological or pathological stimuli, quiescent SCs are activated by expressing MyoD (myogenic determination protein) and can adopt divergent fate decisions that result in differentiation or self-renewal. The differentiation program ensures muscle regeneration, growth and myofibers turnover while the self-renewal preserves the muscle stem cell niche from the exhaustion [Citation110]. Epigenetic mechanisms sustain and influence both SCs fate choices being at the intersection of cell specification and identity. Key regulators of muscle differentiation are the Polycomb Group of proteins (PcG). These proteins are evolutionary conserved epigenetic repressors that act at different levels of epigenome complexity from histone modification to chromatin remodeling, ensuring the establishment and the maintenance of cell identity [Citation111]. PcG proteins aggregate into different complexes working together or separately on chromatin targets [Citation112]. Polycomb Repressive Complex 1 (PRC1) deposits the repressive histone mark H2aK119ub through the catalytic subunit Ring1a/b and Polycomb Repressive Complex 2 (PRC2) is responsible for H3K27me3 through the catalytic activity of Ezh2. At the microscopic level, PcG proteins form aggregates (PcG bodies) [Citation113,Citation114], an intranuclear architecture necessary for mediating the chromatin long-range interactions and the clustering of their targets [Citation52,Citation113,Citation115]. In muscle, PcG proteins finely regulate and coordinate muscle genes expression ensuring cell identity, differentiation and self-renewal [Citation116–Citation118]. In particular, PcG proteins maintain repression at myogenic markers in undifferentiated cells while, at the onset of differentiation, they relocate on stemness genes [Citation116,Citation119]. In muscle cells, chromatin relaxation and accessibility for transcription factors are also regulated by histone acetyltransferases (HATs) and deacetylases (HDACs) that respectively stimulate and repress gene transcription [Citation120]. During muscle differentiation, HATs and HDACs act analogously to PcG, relocating to the specific targets in a time-specific manner [Citation116,Citation121–Citation123]. In particular, during differentiation, the HAT p300 acetylates the regulatory element of MyoD gene, stimulating its expression [Citation124], essential for myogenesis [Citation125]. Interestingly, besides alternative PRC complexes binding to specific targets, plasticity in PRC complex composition also ensures proper maintenance of SCs transcriptional program and the response to environmental stress [Citation117,Citation118,Citation126–Citation128]. For instance, during the terminal differentiation, the proper timing for MyoG transcription is regulated by the Ezh1, the alternative catalytic subunit of PRC2, that replaces Ezh2 on MyoG promoter [Citation129]. Interestingly Ezh1 is also directly involved in the stress response in muscle: Bodega et colleagues described a novel isoform of Ezh1, the Ezh1β, that acts in the cytoplasm of post mitotic skeletal muscle cells as a stress-sensor and controls nuclear PRC2 activity [Citation126]. In response to atrophic oxidative stress, cytoplasmic Ezh1β releases the PRC2 subunit Eed from cytoplasm allowing its correct nuclear assembly with Suz12 and the canonical Ezh1 form, the Ezh1α, on myogenic promoters. This dynamic is necessary to prime an epigenetic response and to protect cell integrity.
Since Lamin A/C can directly bind DNA, shuffling the chromatin from repressive to permissive transcriptional environment and vice versa, it has been proposed that in laminopathies this epigenetic mechanism could be affected. Several studies have reinforced this hypothesis: i) an aberrant heterochromatin localization at the nuclear envelope and loss of the myogenic program have been found in murine Lmna-null cells [Citation41]; (ii) expression of the human EDMD LMNA in Caenorhabditis Elegans impairs tissue-specific reorganization of heterochromatin, with abnormal retention of a muscle-specific, transcriptionally silent gene at the nuclear periphery [Citation130]; iii) nuclear positioning of the PcG regulated FSHD (Facioscapulohumeral muscular dystrophy) locus, responsible for an autosomal dominant neuromuscular disorder, is altered in human Lamin A/C null cells [Citation131]; iv) the R482W mutation on Lamin A causing familial partial Dunnigan lipodystrophy (FPLD2) prevents Lamin A binding at MIR335 locus and mediates enhancers looping; this determines the aberrant transcription of the anti-adipogenic miR-335 with subsequent inhibition of adipogenic differentiation [Citation132]; v) Robson and colleagues showed that, during myogenesis, nuclear envelope transmembrane proteins (NETs) redirect the correct positioning of myogenic genes; in NET knockdowns such repositioning is affected and myotube formation is blocked [Citation133].
More recently, Perovanovic and colleagues, with a genome-wide approach based on DamID-seq technology [Citation134], investigated the mechanisms affecting cell differentiation in laminopathies [Citation135]. They found that R453W and R482W mutations, responsible for Emery Dreifuss Muscular Dystrophy (EDMD) and familial partial lipodystrophy (FPLD) respectively, disrupted the appropriate formation of Lamin A–associated heterochromatin domains in an allele-specific manner. Perturbations of the epigenomic transitions determine some recurrent aberrations at the transcriptional level, including lack of pluripotency and induction of myogenic loci, suggesting that in EDMD the altered formation of lamina-associated domains affects the epigenetic programming. Other studies investigated the direct crosstalk between Lamin and epigenetic factors. Super-resolution microscopy analysis revealed an interrelated distribution between PcG proteins and nuclear Lamin A/C [Citation52]. Their relative intranuclear localization is not random; rather Lamin A/C surrounds the PcG bodies ensuring their correct organization and positioning on target genes mirroring the correct gene expression. Knock down of Lamin A/C in muscular cells determines PcG bodies disassembly and an anticipated muscle differentiation [Citation52,Citation53]. Further studies in pathological models will hopefully elucidate how the mechanisms described here are involved in laminopathies and which is their interplay.
Epigenetics and mechanotransduction
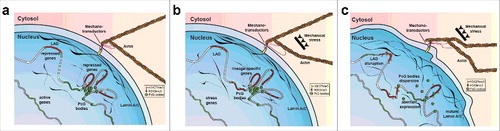
At the cellular level, Lamin A/C is one of the most important components of mechanotransduction, the mechanism by which external mechanical stimuli are converted into biochemical intracellular signals [Citation136,Citation137]. Studies on isolated nuclei showed that exposed to shear stress, the Ig fold domain of Lamin A is able to partially unfold, leading to stretching of the molecule [Citation138,Citation139]. Moreover, when the extracellular matrix increases its stiffness, Lamin A levels increase and the protein undergoes dephosphorylation of the S22 residue. The nucleoplasmic portion relocates to the nuclear lamina, providing increased nuclear stiffness and mechanical support [Citation136,Citation139–Citation141]. Considering these observations, for several years, scientists were convinced that lamin-dependent diseases were a consequence of aberrant mechanotransduction due to a higher sensitivity of the mutant to the mechanical stress [Citation142,Citation143]. This hypothesis was supported by evidence that, in the context of constant mechanical stress, mutant lamin protofilament could be more prone to dissociation [Citation80,Citation144–Citation146], determining muscular specific nuclear rupture, cell death and tissue deterioration [Citation147,Citation148]. In line with these observations, microarray analysis conducted on the hearts of Lmna H222P/H222P mice showed that the extracellular signal-regulated kinase 1/2 (ERK1/2) and the Jun N-terminal kinase (JNK) signaling pathways, both being branches of the stress-related, mitogen-activated protein kinase (MAPK) cascade, are abnormally activated [Citation149]. Furthermore, other signaling cascades, AKT/mTOR [Citation150], TGF-β, CTGF [Citation151] and p38α [Citation152], are involved in the pathogenesis of the EDMD cardiomyopathy. On the other hand, the mechanotransduction process implies the translation of external signals into transcriptional changes. Lamin A, using its direct association with DNA at Lamina-Associated Domains (LADs), could orchestrate the genome reprogramming, directly linking mechanotransduction to chromatin. This would explain the emerging findings that attribute some features of laminopathies to genome misregulation, from failure in stem cell maintenance to impaired differentiation [Citation45,Citation153–Citation156]. Interestingly, PcG proteins are also involved in the mechano-signal transduction mediated by Lamin A [Citation157]. As described above, mechanical stress induces a reshaping of the nuclear lamina composition, this triggering a decrease in H3K9me2,3 and an accumulation of H3K27me3 with inhibition of RNA Polymerase II [Citation157]. This observation paves the way for a new hypothesis: in lamin mutant cells, a defective Lamin A/PcG crosstalk and consequent programming dysfunctions could finally account for the higher sensitivity of these cells to mechanical stress. It is therefore tempting to speculate that, in Laminopathies, the uncoupled mechanotransduction axis and alterations at the chromatin level may be closely connected to common Lamin A dysfunctions.
Epigenetic therapies for muscle disorders
Epigenetic treatments for muscle disease are attractive opportunities to tackle these pathologies; indeed, some clinical trials are already ongoing with promising results. In particular, in Duchenne Muscular Dystrophy, an X-linked pathology related to mutation of Dystrophin (DMD), HDAC inhibitors have revealed beneficial effects [Citation158]. In particular, the HDAC inhibitor (HDACi) Givinostat has been tested on the mouse model of DMD showing an improvement in muscle homeostasis and regeneration [Citation159]. Mechanistically, HDACi directly prevents the deacetylation and inactivation of MyoD protein, thus enhancing muscle regeneration in DMD [Citation160–Citation162]. At the DNA level, HDACi prevents histone deacetylation, thus maintaining Myod1 and Mef2 (Myocyte enhancer factor 2) loci transcriptionally active. This will sustain the transcriptional cascade leading to muscle differentiation [Citation124,Citation163,Citation164]. Moreover, HDACi favors the expression of Follistatin, an antagonist of the muscle differentiation and growth inhibitor, Myostatin [Citation165-Citation167]. These encouraging results fostered the beginning of a clinical trial that is currently entering phase II [Citation168].
Signaling pathways are known to impact on chromatin conformation by targeting chromatin modifying proteins [Citation169]. Among the players involved in the EDMD cardiomyopathy, p38α is known to phosphorylate Ezh2 in satellite stem cells, thus promoting the repression of Pax7 during myogenesis [Citation119]. On the other hand, the PI3-AKT pathway both reduces the affinity of the PRC2 for histone H3 and increases the H3K27-specific acetyl-transferase activity of P300, thus contributing to the switch from a repressive to an activated state of lysine K27 residue of histone H3 [Citation169]. Several compounds involved in the aberrant signaling have been tested. On the basis of scientific findings on the Lmna H222P/H222P mice [Citation85] Muchir and colleagues treated H222P/H222P mice with small molecules inhibiting the phosphorylation of ERK and JNK (PD98059 and SP600125) before and after the onset of the cardiomyopathy; the same authors documented a delay in left ventricular dilation, an improvement in the ejection fraction (EF) and a decrease in myocardial fibrosis [Citation170–Citation172]. Unfortunately, those molecules are not suitable for use in patients due to problems with bioavailability and toxicity [Citation171]. Subsequently, Selumetinib was used to inhibit ERK1/2 signaling as it has been safely administered to human subjects in clinical trials for cancer [Citation173]; its use led to cardiac fractional shortening, improvement of survival and prolonged overall survival in Lmna H222P mice. In 2014, Selumetinib was used in conjunction with Benazepril, an angiotensin II converting enzyme (ACE) inhibitor that constitutes standard medical therapy for patients with heart failure. When combinatory treatment started at 16 weeks of age, after the onset of left ventricular dysfunction, a statistically significant increase in left ventricular fractional shortening at 20 weeks of age was documented in H222P/H222P mice [Citation174]. To our knowledge, no clinical trial to target LMNA cardiomyopathy with Selumetinib has been conducted to date.
The hyperactivation of a third branch of the MAP kinase cascade, p38α signaling, was identified in the hearts of Lmna H222P/H222P mice [Citation152]. These mice were treated with a p38α inhibitor, named ARRY-371797. In parallel, a placebo was administered to another group of H222P/H222P mice as negative control. Scientists found that ARRY-371797 treatment prevented left ventricular dilatation and deterioration of fractional shortening. A phase 2 clinical trial with ARRY-371797 started in February 2014 (NCT02057341) for patients with symptomatic genetic dilated cardiomyopathy due to Lamin A/C gene mutation (https://clinicaltrials.gov/ct2/show/NCT02057341?term = NCT02057341&recrs = abeh&rank = 1). The preliminary study is complete and a rollover one (NCT02351856) will be conducted until December 2018 (https://clinicaltrials.gov/ct2/show/NCT02351856?recrs = abeh&cond = ARRY-371797&rank = 8?). In 2017, a novel macrocyclic MEK1/2 inhibitor with improved pharmacological profile was synthesized and proved to be effective in ameliorating the outcome of the cardiomyopathy [Citation175].
The observation that AKT-mammalian target of rapamycin pathway is hyperactivated in the Lmna-mutation-caused cardiomyopathy has paved the way for the in vivo administration of the rapamycin-analog temsirolimus [Citation150]; the inhibition of mTORC1 inhibition reactivates autophagy and prevents cardiac damage in Lmna H222P/H222P mice. These data are reinforced by those of Ramos and colleagues on rapamycin treatment of Lmna −/− mice [Citation176]. Eventually, the activation of Wnt/β-catenin activity with BIO (6-bromoindirubin-3'-oxime), a reversible and ATP-competitive inhibitor for GSK-3α/β, improved cardiac contractility and ameliorated intraventricular conduction defects in Lmna H222P/H222P mice [Citation102]. New epigenetic studies are required in the mouse models of laminopathies, more specifically before and after treatment with the above-cited small molecules, in order to further elucidate the mechanisms downstream the alteration of the signaling pathways. This could allow both a “surgical” intervention on the single mechanism and combinatory approaches acting on several levels from signaling to gene regulation.
Conclusion
Decades of study have unveiled the multiple roles of Lamin A/C, suggesting how aberrant forms of this protein can cause tissue-specific laminopathies. Emerging findings describe the key role of Lamin A/C in transcriptional control, so opening the “Pandora's box” of the multiple epigenetic mechanisms involved in laminopathies. In parallel, basic research studies elucidate the epigenetic involvement in mechanotransduction, linking another Lamin A function with transcriptional control. Indeed, under mechanical stress, the rearrangement of nuclear lamina components mirrors the epigenetic accumulation of PcG mark H3K27me3 [Citation157]. All these findings progressively converge into a unique model by which the uncoupling of the mechanical-properties/epigenetic-factors/gene-regulation axis could drive the tissue-specific pathology (). Medical treatment aimed at reversing epigenetic aberrancy is a fascinating path that will hopefully lead to new cures for a whole variety of diseases. However, considering that, in an adult organism, epigenetic regulators are strictly required for the maintenance of the adult tissue self-renewal [Citation117], focused disease-specific studies describing how these regulators participate in the aberrant phenotype could open the way for compounds that specifically inhibit disease-related activity without affecting the other functions necessary for tissue homeostasis.
Figure 1. Schematic representation of Lamin dependent mechanotransduction. Nuclear lamina works in couple with the mechanotransductor machinery to convert mechanical stimuli into epigenetic changes including: Histone modification, LAD higher order structures and PcG organization (a, b) In EDMD, the compromised mechano-properties of aberrant form of Lamin A/C could increase the sensitivity of the nuclei to mechanical stress resulting in: loss of LAD conformation, PcG bodies dispersion and aberrant gene expression.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Prof. Martin Bennett for “proofreading work” of the manuscript. We thank the Italian network of Laminopathies and members of the laboratory for stimulating discussions and constructive criticisms. CL is supported by grants from the Italian Ministry of Research and University (Futuro in Ricerca RBFR106S1Z_001), the flagship CNR projects, (Epigen and Interomics), the Italian Minister of Health n. GR-2013-02355413, My First AIRC Grant (MFAG), n. 18535, AFM-Telethon n. 21030. Authors declare that there are no conflicts of interests.
Additional information
Funding
References
- Lanzuolo C, Orlando V. The function of the epigenome in cell reprogramming. Cell Mol Life Sci. 2007;64:1043–62.
- Bianchi A, Lanzuolo C. Into the chromatin world: Role of nuclear architecture in epigenome regulation. AIMS Biophysics. 2015;2:585–612.
- Fisher DZ, Chaudhary N, Blobel G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc Natl Acad Sci U S A. 1986;83:6450–4.
- Krohne G, Wolin SL, McKeon FD, et al. Nuclear lamin LI of Xenopus laevis: cDNA cloning, amino acid sequence and binding specificity of a member of the lamin B subfamily. EMBO J. 1987;6:3801–8.
- McKeon FD, Kirschner MW, Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature. 1986;319:463–8.
- Adam SA, Goldman RD. Insights into the differences between the A- and B-type nuclear lamins. Adv Biol Regul. 2012;52:108–13.
- Laliberte JF, Dagenais A, Filion M, et al. Identification of distinct messenger RNAs for nuclear lamin C and a putative precursor of nuclear lamin A. J Cell Biol. 1984;98:980–5.
- Lin F, Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem. 1993;268:16321–6.
- Barrowman J, Hamblet C, George CM, et al. Analysis of prelamin A biogenesis reveals the nucleus to be a CaaX processing compartment. Mol Biol Cell. 2008;19:5398–408.
- Strelkov SV, Schumacher J, Burkhard P, et al. Crystal structure of the human lamin A coil 2B dimer: implications for the head-to-tail association of nuclear lamins. J Mol Biol. 2004;343:1067–80.
- Heitlinger E, Peter M, Haner M, et al. Expression of chicken lamin B2 in Escherichia coli: characterization of its structure, assembly, and molecular interactions. J Cell Biol. 1991;113:485–95.
- Aebi U, Cohn J, Buhle L, et al. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986;323:560–4.
- Akey CW. Interactions and structure of the nuclear pore complex revealed by cryo-electron microscopy. J Cell Biol. 1989;109:955–70.
- Ris H. High-resolution field-emission scanning electron microscopy of nuclear pore complex. Scanning. 1997;19:368–75.
- Turgay Y, Eibauer M, Goldman AE, et al. The molecular architecture of lamins in somatic cells. Nature. 2017;543:261–4.
- Shimi T, Kittisopikul M, Tran J, et al. Structural organization of nuclear lamins A, C, B1, and B2 revealed by superresolution microscopy. Mol Biol Cell. 2015;26:4075–86.
- Hozak P, Sasseville AM, Raymond Y, et al. Lamin proteins form an internal nucleoskeleton as well as a peripheral lamina in human cells. J Cell Sci. 1995;108(Pt 2):635–44.
- Kolb T, Maass K, Hergt M, et al. Lamin A and lamin C form homodimers and coexist in higher complex forms both in the nucleoplasmic fraction and in the lamina of cultured human cells. Nucleus. 2011;2:425–33.
- Naetar N, Korbei B, Kozlov S, et al. Loss of nucleoplasmic LAP2alpha-lamin A complexes causes erythroid and epidermal progenitor hyperproliferation. Nat Cell Biol. 2008;10:1341–8.
- Aaronson RP, Blobel G. Isolation of nuclear pore complexes in association with a lamina. Proc Natl Acad Sci U S A. 1975;72:1007–11.
- Gerace L, Blum A, Blobel G. Immunocytochemical localization of the major polypeptides of the nuclear pore complex-lamina fraction. Interphase and mitotic distribution. J Cell Biol. 1978;79:546–66.
- Benyajati C, Worcel A. Isolation, characterization, and structure of the folded interphase genome of Drosophila melanogaster. Cell. 1976;9:393–407.
- Cook PR, Brazell IA. Conformational constraints in nuclear DNA. J Cell Sci. 1976;22:287–302.
- Gerace L, Comeau C, Benson M. Organization and modulation of nuclear lamina structure. J Cell Sci Suppl. 1984;1:137–60.
- Gruenbaum Y, Foisner R. Lamins: Nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. Annu Rev Biochem. 2015;84:131–6
- Lee JS, Hale CM, Panorchan P, et al. Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization, and migration. Biophys J. 2007;93:2542–52.
- Belaadi N, Aureille J, Guilluy C. Under PRESSURE: Mechanical stress management in the nucleus. Cells. 2016;5:E27
- Collard JF, Raymond Y. Phorbol esters induce transient changes in the accessibility of the carboxy-terminal domain of nuclear lamin A. Exp Cell Res. 1992;201:174–83.
- Osmanagic-Myers S, Dechat T, Foisner R. Lamins at the crossroads of mechanosignaling. Genes Dev. 2015;29:225–37.
- Spann TP, Goldman AE, Wang C, et al. Alteration of nuclear lamin organization inhibits RNA polymerase II-dependent transcription. J Cell Biol. 2002;156:603–8.
- Cobb AM, Murray TV, Warren DT, et al. Disruption of PCNA-lamins A/C interactions by prelamin A induces DNA replication fork stalling. Nucleus. 2016;7:498–511.
- Moir RD, Spann TP, Herrmann H, et al. Disruption of nuclear lamin organization blocks the elongation phase of DNA replication. J Cell Biol. 2000;149:1179–92.
- Dechat T, Shimi T, Adam SA, et al. Alterations in mitosis and cell cycle progression caused by a mutant lamin A known to accelerate human aging. Proc Natl Acad Sci U S A. 2007;104:4955–60.
- Qi R, Xu N, Wang G, et al. The lamin-A/C-LAP2alpha-BAF1 protein complex regulates mitotic spindle assembly and positioning. J Cell Sci. 2015;128:2830–41.
- Belt EJ, Fijneman RJ, van den Berg EG, et al. Loss of lamin A/C expression in stage II and III colon cancer is associated with disease recurrence. Eur J Cancer. 2011;47:1837–45.
- Capo-chichi CD, Aguida B, Chabi NW, et al. Lamin A/C deficiency is an independent risk factor for cervical cancer. Cell Oncol (Dordr). 2016;39:59–68.
- Kong L, Schafer G, Bu H, et al. Lamin A/C protein is overexpressed in tissue-invading prostate cancer and promotes prostate cancer cell growth, migration and invasion through the PI3K/AKT/PTEN pathway. Carcinogenesis. 2012;33:751–9.
- Goldman RD, Gruenbaum Y, Moir RD, et al. Nuclear lamins: building blocks of nuclear architecture. Genes Dev. 2002;16:533–47.
- Gruenbaum Y, Margalit A, Goldman RD, et al. The nuclear lamina comes of age. Nat Rev Mol Cell Biol. 2005;6:21–31.
- Shumaker DK, Kuczmarski ER, Goldman RD. The nucleoskeleton: lamins and actin are major players in essential nuclear functions. Curr Opin Cell Biol. 2003;15:358–66.
- Solovei I, Wang AS, Thanisch K, et al. LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell. 2013;152:584–98.
- Deniaud E, Bickmore WA. Transcription and the nuclear periphery: edge of darkness? Curr Opin Genet Dev. 2009;19:187–91.
- Meister P, Taddei A. Building silent compartments at the nuclear periphery: a recurrent theme. Curr Opin Genet Dev. 2013;23:96–103.
- van Steensel B, Belmont AS. Lamina-Associated Domains: Links with Chromosome Architecture, Heterochromatin, and gene repression. Cell. 2017;169:780–91.
- Collas P, Lund EG, Oldenburg AR. Closing the (nuclear) envelope on the genome: How nuclear lamins interact with promoters and modulate gene expression. Bioessays. 2014;36:75–83.
- Meuleman W, Peric-Hupkes D, Kind J, et al. Constitutive nuclear lamina-genome interactions are highly conserved and associated with A/T-rich sequence. Genome Res. 2013;23:270–80.
- Kind J, Pagie L, Ortabozkoyun H, et al. Single-cell dynamics of genome-nuclear lamina interactions. Cell. 2013;153:178–92.
- Kind J, van Steensel B. Genome-nuclear lamina interactions and gene regulation. Curr Opin Cell Biol. 2010;22:320–5.
- Lund E, Oldenburg A, Delbarre E, et al. Lamin A/C-promoter interactions specify chromatin state-dependent transcription outcomes. Genome Res. 2013;23(10):1580–9
- Guelen L, Pagie L, Brasset E, et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature. 2008;453:948–51.
- Reddy KL, Zullo JM, Bertolino E, et al. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature. 2008;452:243–7.
- Cesarini E, Mozzetta C, Marullo F, et al. Lamin A/C sustains PcG protein architecture, maintaining transcriptional repression at target genes. J Cell Biol. 2015;211:533–51.
- Marullo F, Cesarini E, Antonelli L, et al. Nucleoplasmic Lamin A/C and Polycomb group of proteins: an evolutionarily conserved interplay. Nucleus. 2016;7(2):103–11.
- Peric-Hupkes D, Meuleman W, Pagie L, et al. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol Cell. 2010;38:603–13.
- Zaremba-Czogalla M, Dubinska-Magiera M, Rzepecki R. Laminopathies: the molecular background of the disease and the prospects for its treatment. Cell Mol Biol Lett. 2011;16:114–48.
- De Sandre-Giovannoli A, Chaouch M, Kozlov S, Vallat JM, et al. Homozygous defects in LMNA, encoding lamin A/C nuclear-envelope proteins, cause autosomal recessive axonal neuropathy in human (Charcot-Marie-Tooth disorder type 2) and mouse. Am J Hum Genet. 2002;70:726–36.
- Bione S, Maestrini E, Rivella S, et al. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat Genet. 1994;8:323–7.
- Muchir A, Bonne G, van der Kooi AJ, et al. Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B). Hum Mol Genet. 2000;9:1453–9.
- Raffaele Di Barletta M, Ricci E, Galluzzi G, et al. Different mutations in the LMNA gene cause autosomal dominant and autosomal recessive Emery-Dreifuss muscular dystrophy. Am J Hum Genet. 2000;66:1407–12.
- Fatkin D, MacRae C, Sasaki T, et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med. 1999;341:1715–24.
- Renou L, Stora S, Yaou RB, et al. Heart-hand syndrome of Slovenian type: a new kind of laminopathy. J Med Genet. 2008;45:666–71.
- Cao H, Hegele RA. Nuclear lamin A/C R482Q mutation in canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum Mol Genet. 2000;9:109–12.
- Drac H, Madej-Pilarczyk A, Gospodarczyk-Szot K, et al. Familial partial lipodystrophy associated with the heterozygous LMNA mutation 1445G>A (Arg482Gln) in a Polish family. Neurol Neurochir Pol. 2010;44:291–6.
- De Sandre-Giovannoli A, Bernard R, Cau P, et al. Lamin a truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055.
- Chen L, Lee L, Kudlow BA, et al. LMNA mutations in atypical Werner's syndrome. Lancet. 2003;362:440–5.
- Hegele RA, Cao H, Liu DM, et al. Sequencing of the reannotated LMNB2 gene reveals novel mutations in patients with acquired partial lipodystrophy. Am J Hum Genet. 2006;79:383–9.
- Norwood FL, Harling C, Chinnery PF, et al. Prevalence of genetic muscle disease in Northern England: in-depth analysis of a muscle clinic population. Brain. 2009;132:3175–86.
- Bonne G, Mercuri E, Muchir A, et al. Clinical and molecular genetic spectrum of autosomal dominant Emery-Dreifuss muscular dystrophy due to mutations of the lamin A/C gene. Ann Neurol. 2000;48:170–80.
- Quijano-Roy S, Mbieleu B, Bonnemann CG, et al. De novo LMNA mutations cause a new form of congenital muscular dystrophy. Ann Neurol. 2008;64:177–86.
- Bonne G, Quijano-Roy S. Emery-Dreifuss muscular dystrophy, laminopathies, and other nuclear envelopathies. Handb Clin Neurol. 2013;113:1367–76.
- Jimenez-Escrig A, Gobernado I, Garcia-Villanueva M, et al. Autosomal recessive Emery-Dreifuss muscular dystrophy caused by a novel mutation (R225Q) in the lamin A/C gene identified by exome sequencing. Muscle Nerve. 2012;45:605–10.
- Becane HM, Bonne G, Varnous S, et al. High incidence of sudden death with conduction system and myocardial disease due to lamins A and C gene mutation. Pacing Clin Electrophysiol. 2000;23:1661–6.
- Lu JT, Muchir A, Nagy PL, et al. LMNA cardiomyopathy: cell biology and genetics meet clinical medicine. Dis Model Mech. 2011;4:562–8.
- Taylor MR, Fain PR, Sinagra G, et al. Natural history of dilated cardiomyopathy due to lamin A/C gene mutations. J Am Coll Cardiol. 2003;41:771–80.
- Manilal S, Sewry CA, Man N, et al. Diagnosis of X-linked Emery-Dreifuss muscular dystrophy by protein analysis of leucocytes and skin with monoclonal antibodies. Neuromuscul Disord. 1997;7:63–6.
- Dickey RP, Ziter FA, Smith RA. Emery-Dreifuss muscular dystrophy. J Pediatr. 1984;104:555–9.
- Emery AE. X-linked muscular dystrophy with early contractures and cardiomyopathy (Emery-Dreifuss type). Clin Genet. 1987;32:360–7.
- Hopkins LC, Jackson JA, Elsas LJ. Emery-dreifuss humeroperoneal muscular dystrophy: an x-linked myopathy with unusual contractures and bradycardia. Ann Neurol. 1981;10:230–7.
- Meinke P, Schneiderat P, Srsen V, et al. Abnormal proliferation and spontaneous differentiation of myoblasts from a symptomatic female carrier of X-linked Emery-Dreifuss muscular dystrophy. Neuromuscul Disord. 2015;25:127–36.
- Sullivan T, Escalante-Alcalde D, Bhatt H, et al. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol. 1999;147:913–20.
- Wehnert M, Muntoni F. 60th ENMC International Workshop: non X-linked Emery-Dreifuss Muscular Dystrophy 5–7 June 1998, Naarden, The Netherlands. Neuromuscul Disord. 1999;9:115–21.
- Jahn D, Schramm S, Schnolzer M, et al. A truncated lamin A in the Lmna −/− mouse line: implications for the understanding of laminopathies. Nucleus. 2012;3:463–74.
- Kubben N, Voncken JW, Konings G, et al. Post-natal myogenic and adipogenic developmental: defects and metabolic impairment upon loss of A-type lamins. Nucleus. 2011;2:195–207.
- van Engelen BG, Muchir A, Hutchison CJ, et al. The lethal phenotype of a homozygous nonsense mutation in the lamin A/C gene. Neurology. 2005;64:374–6.
- Arimura T, Helbling-Leclerc A, Massart C, et al. Mouse model carrying H222P-Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Hum Mol Genet. 2005;14:155–69.
- Bertrand AT, Renou L, Papadopoulos A, et al. DelK32-lamin A/C has abnormal location and induces incomplete tissue maturation and severe metabolic defects leading to premature death. Hum Mol Genet. 2012;21:1037–48.
- Wolf CM, Wang L, Alcalai R, et al. Lamin A/C haploinsufficiency causes dilated cardiomyopathy and apoptosis-triggered cardiac conduction system disease. J Mol Cell Cardiol. 2008;44:293–303.
- Cattin ME, Bertrand AT, Schlossarek S, et al. Heterozygous LmnadelK32 mice develop dilated cardiomyopathy through a combined pathomechanism of haploinsufficiency and peptide toxicity. Hum Mol Genet. 2013;22:3152–64.
- Gupta P, Bilinska ZT, Sylvius N, et al. Genetic and ultrastructural studies in dilated cardiomyopathy patients: a large deletion in the lamin A/C gene is associated with cardiomyocyte nuclear envelope disruption. Basic Res Cardiol. 2010;105:365–77.
- Zaragoza MV, Fung L, Jensen E, et al. Exome sequencing identifies a Novel LMNA splice-site mutation and multigenic heterozygosity of potential modifiers in a family with sick sinus syndrome, dilated cardiomyopathy, and sudden cardiac death. PLoS One. 2016;11:e0155421.
- Taylor MR, Slavov D, Gajewski A, et al. Thymopoietin (lamina-associated polypeptide 2) gene mutation associated with dilated cardiomyopathy. Hum Mutat. 2005;26:566–74.
- Worman HJ, Bonne G. “Laminopathies”: a wide spectrum of human diseases. Exp Cell Res. 2007;313:2121–33.
- Gotic I, Leschnik M, Kolm U, et al. Lamina-associated polypeptide 2alpha loss impairs heart function and stress response in mice. Circ Res. 2010;106:346–53.
- Ventura-Clapier R, Mettauer B, Bigard X. Beneficial effects of endurance training on cardiac and skeletal muscle energy metabolism in heart failure. Cardiovasc Res. 2007;73:10–8.
- Hong JS, Ki CS, Kim JW, et al. Cardiac dysrhythmias,cardiomyopathy and muscular dystrophy in patients with Emery-Dreifuss muscular dystrophy and limb-girdle muscular dystrophy type 1B. J Korean Med Sci. 2005;20:283–90.
- Mittelbronn M, Hanisch F, Gleichmann M, et al. Myofiber degeneration in autosomal dominant Emery-Dreifuss muscular dystrophy (AD-EDMD) (LGMD1B). Brain Pathol. 2006;16:266–72.
- Lanzuolo C. Epigenetic alterations in muscular disorders. Comp Funct Genomics. 2012;2012:256892.
- Mory PB, Crispim F, Freire MB, et al. Phenotypic diversity in patients with lipodystrophy associated with LMNA mutations. Eur J Endocrinol. 2012;167:423–31.
- Dubinska-Magiera M, Zaremba-Czogalla M, Rzepecki R. Muscle development, regeneration and laminopathies: how lamins or lamina-associated proteins can contribute to muscle development, regeneration and disease. Cell Mol Life Sci. 2013;70:2713–41.
- Lloyd DJ, Trembath RC, Shackleton S. A novel interaction between lamin A and SREBP1: implications for partial lipodystrophy and other laminopathies. Hum Mol Genet. 2002;11:769–77.
- Frock RL, Kudlow BA, Evans AM, et al. Lamin A/C and emerin are critical for skeletal muscle satellite cell differentiation. Genes Dev. 2006;20:486–500.
- Le Dour C, Macquart C, Sera F, et al. Decreased WNT/beta-catenin signalling contributes to the pathogenesis of dilated cardiomyopathy caused by mutations in the lamin a/C gene. Hum Mol Genet. 2017;26:333–43.
- Van Berlo JH, Voncken JW, Kubben N, et al. A-type lamins are essential for TGF-beta1 induced PP2A to dephosphorylate transcription factors. Hum Mol Genet. 2005;14:2839–49.
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–5.
- Gnocchi VF, White RB, Ono Y, et al. Further characterisation of the molecular signature of quiescent and activated mouse muscle satellite cells. PLoS One. 2009;4:e5205.
- Sacco A, Doyonnas R, Kraft P, et al. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–6.
- Sambasivan R, Yao R, Kissenpfennig A, et al. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138:3647–56.
- Seale P, Sabourin LA, Girgis-Gabardo A, et al. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–86.
- von Maltzahn J, Jones AE, Parks RJ, et al. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc Natl Acad Sci U S A. 2013;110:16474–9.
- Zammit PS, Golding JP, Nagata Y, et al. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol. 2004;166:347–57.
- Schuettengruber B, Bourbon HM, Di Croce L, et al. Genome Regulation by Polycomb and Trithorax: 70 years and counting. Cell. 2017;171:34–57.
- Lanzuolo C, Orlando V. Memories from the polycomb group proteins. Annu Rev Genet. 2012;46:561–89
- Lanzuolo C, Roure V, Dekker J, et al. Polycomb response elements mediate the formation of chromosome higher-order structures in the bithorax complex. Nat Cell Biol. 2007;9:1167–74.
- Saurin AJ, Shiels C, Williamson J, et al. The human polycomb group complex associates with pericentromeric heterochromatin to form a novel nuclear domain. J Cell Biol. 1998;142:887–98.
- Bantignies F, Roure V, Comet I, et al. Polycomb-dependent regulatory contacts between distant Hox Loci in Drosophila. Cell. 2011;144:214–26.
- Caretti G, Di Padova M, Micales B, et al. The Polycomb Ezh2 methyltransferase regulates muscle gene expression and skeletal muscle differentiation. Genes Dev. 2004;18:2627–38.
- Juan AH, Derfoul A, Feng X, et al. Polycomb EZH2 controls self-renewal and safeguards the transcriptional identity of skeletal muscle stem cells. Genes Dev. 2011;25:789–94.
- Juan AH, Kumar RM, Marx JG, et al. Mir-214-dependent regulation of the polycomb protein Ezh2 in skeletal muscle and embryonic stem cells. Mol Cell. 2009;36:61–74.
- Palacios D, Mozzetta C, Consalvi S, et al. TNF/p38alpha/polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell. 2010;7:455–69.
- Segales J, Perdiguero E, Munoz-Canoves P. Epigenetic control of adult skeletal muscle stem cell functions. FEBS J. 2015;282:1571–88.
- Mal A, Harter ML. MyoD is functionally linked to the silencing of a muscle-specific regulatory gene prior to skeletal myogenesis. Proc Natl Acad Sci U S A. 2003;100:1735–9.
- Ohkawa Y, Yoshimura S, Higashi C, et al. Myogenin and the SWI/SNF ATPase Brg1 maintain myogenic gene expression at different stages of skeletal myogenesis. J Biol Chem. 2007;282:6564–70.
- Sincennes MC, Brun CE, Rudnicki MA. Concise Review: Epigenetic regulation of Myogenesis in health and disease. Stem Cells Transl Med. 2016;5:282–90.
- Hamed M, Khilji S, Chen J, et al. Stepwise acetyltransferase association and histone acetylation at the Myod1 locus during myogenic differentiation. Sci Rep. 2013;3:2390.
- Sabourin LA, Rudnicki MA. The molecular regulation of myogenesis. Clin Genet. 2000;57:16–25.
- Bodega B, Marasca F, Ranzani V, et al. A cytosolic Ezh1 isoform modulates a PRC2-Ezh1 epigenetic adaptive response in postmitotic cells. Nat Struct Mol Biol. 2017;24:444–52.
- Marasca F, Bodega B, Orlando V. How Polycomb-Mediated cell memory deals with a changing environment: Variations in PcG complexes and proteins assortment convey plasticity to epigenetic regulation as a response to environment. Bioessays. 2018;40(4):e1700137
- Woodhouse S, Pugazhendhi D, Brien P, et al. Ezh2 maintains a key phase of muscle satellite cell expansion but does not regulate terminal differentiation. J Cell Sci. 2013;126:565–79.
- Stojic L, Jasencakova Z, Prezioso C, et al. Chromatin regulated interchange between polycomb repressive complex 2 (PRC2)-Ezh2 and PRC2-Ezh1 complexes controls myogenin activation in skeletal muscle cells. Epigenetics Chromatin. 2011;4:16.
- Mattout A, Pike BL, Towbin BD, et al. An EDMD mutation in C. elegans lamin blocks muscle-specific gene relocation and compromises muscle integrity. Curr Biol. 2011;21:1603–14.
- Masny PS, Bengtsson U, Chung SA, et al. Localization of 4q35.2 to the nuclear periphery: is FSHD a nuclear envelope disease? Hum Mol Genet. 2004;13:1857–71.
- Oldenburg A, Briand N, Sorensen AL, et al. A lipodystrophy-causing lamin A mutant alters conformation and epigenetic regulation of the anti-adipogenic MIR335 locus. J Cell Biol. 2017;216:2731–43.
- Robson MI, de Las Heras JI, Czapiewski R, et al. Tissue-specific gene repositioning by muscle nuclear membrane proteins enhances repression of critical developmental genes during Myogenesis. Mol Cell. 2016;62:834–47.
- van Steensel B, Delrow J, Henikoff S. Chromatin profiling using targeted DNA adenine methyltransferase. Nat Genet. 2001;27:304–8.
- Perovanovic J, Dell'Orso S, Gnochi VF, et al. Laminopathies disrupt epigenomic developmental programs and cell fate. Sci Transl Med. 2016;8:335.ra58.
- Isermann P, Lammerding J. Nuclear mechanics and mechanotransduction in health and disease. Curr Biol. 2013;23:R1113–21.
- Lammerding J, Schulze PC, Takahashi T, et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–8.
- Bera M, Kotamarthi HC, Dutta S, et al. Characterization of unfolding mechanism of human lamin A Ig fold by single-molecule force spectroscopy-implications in EDMD. Biochemistry. 2014;53:7247–58.
- Swift J, Ivanovska IL, Buxboim A, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104.
- Buxboim A, Swift J, Irianto J, et al. Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin. Curr Biol. 2014;24:1909–17.
- Guilluy C, Burridge K. Nuclear mechanotransduction: forcing the nucleus to respond. Nucleus. 2015;6:19–22.
- Scharner J, Gnocchi VF, Ellis JA, et al. Genotype-phenotype correlations in laminopathies: how does fate translate? Biochem Soc Trans. 2010;38:257–62.
- Simon DN, Zastrow MS, Wilson KL. Direct actin binding to A- and B-type lamin tails and actin filament bundling by the lamin A tail. Nucleus. 2010;1:264–72.
- Charniot JC, Pascal C, Bouchier C, et al. Functional consequences of an LMNA mutation associated with a new cardiac and non-cardiac phenotype. Hum Mutat. 2003;21:473–81.
- Raharjo WH, Enarson P, Sullivan T, et al. Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery-Dreifuss muscular dystrophy. J Cell Sci. 2001;114:4447–57.
- Vaughan A, Alvarez-Reyes M, Bridger JM, et al. Both emerin and lamin C depend on lamin A for localization at the nuclear envelope. J Cell Sci. 2001;114:2577–90.
- Davidson PM, Lammerding J. Broken nuclei–lamins, nuclear mechanics, and disease. Trends Cell Biol. 2014;24:247–56.
- Gangemi F, Degano M. Disease-associated mutations in the coil 2B domain of human lamin A/C affect structural properties that mediate dimerization and intermediate filament formation. J Struct Biol. 2013;181:17–28.
- Muchir A, Pavlidis P, Decostre V, et al. Activation of MAPK pathways links LMNA mutations to cardiomyopathy in Emery-Dreifuss muscular dystrophy. J Clin Invest. 2007;117:1282–93.
- Choi JC, Muchir A, Wu W, et al. Temsirolimus activates autophagy and ameliorates cardiomyopathy caused by lamin A/C gene mutation. Sci Transl Med. 2012;4:144ra02.
- Chatzifrangkeskou M, Le Dour C, Wu W, Morrow JP, et al. ERK1/2 directly acts on CTGF/CCN2 expression to mediate myocardial fibrosis in cardiomyopathy caused by mutations in the lamin A/C gene. Hum Mol Genet. 2016;25:2220–33.
- Muchir A, Wu W, Choi JC, et al. Abnormal p38alpha mitogen-activated protein kinase signaling in dilated cardiomyopathy caused by lamin A/C gene mutation. Hum Mol Genet. 2012;21:4325–33.
- Cohen M, Lee KK, Wilson KL, et al. Transcriptional repression, apoptosis, human disease and the functional evolution of the nuclear lamina. Trends Biochem Sci. 2001;26:41–7.
- Fairley EA, Riddell A, Ellis JA, et al. The cell cycle dependent mislocalisation of emerin may contribute to the Emery-Dreifuss muscular dystrophy phenotype. J Cell Sci. 2002;115:341–54.
- Fidzianska A, Toniolo D, Hausmanowa-Petrusewicz I. Ultrastructural abnormality of sarcolemmal nuclei in Emery-Dreifuss muscular dystrophy (EDMD). J Neurol Sci. 1998;159:88–93.
- Liu J, Rolef Ben-Shahar T, Riemer D, et al. Essential roles for Caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes. Mol Biol Cell. 2000;11:3937–47.
- Le HQ, Ghatak S, Yeung CY, et al. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat Cell Biol. 2016;18:864–75.
- Minetti GC, Colussi C, Adami R, et al. Functional and morphological recovery of dystrophic muscles in mice treated with deacetylase inhibitors. Nat Med. 2006;12:1147–50.
- Consalvi S, Mozzetta C, Bettica P, et al. Preclinical studies in the mdx mouse model of duchenne muscular dystrophy with the histone deacetylase inhibitor givinostat. Mol Med. 2013;19:79–87.
- Di Padova M, Caretti G, Zhao P, et al. MyoD acetylation influences temporal patterns of skeletal muscle gene expression. J Biol Chem. 2007;282:37650–9.
- Duquet A, Polesskaya A, Cuvellier S, et al. Acetylation is important for MyoD function in adult mice. EMBO Rep. 2006;7:1140–6.
- Sartorelli V, Puri PL, Hamamori Y, et al. Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle program. Mol Cell. 1999;4:725–34.
- Angelelli C, Magli A, Ferrari D, et al. Differentiation-dependent lysine 4 acetylation enhances MEF2C binding to DNA in skeletal muscle cells. Nucleic Acids Res. 2008;36:915–28.
- Ma K, Chan JK, Zhu G, et al. Myocyte enhancer factor 2 acetylation by p300 enhances its DNA binding activity, transcriptional activity, and myogenic differentiation. Mol Cell Biol. 2005;25:3575–82.
- Bogdanovich S, Krag TO, Barton ER, et al. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420:418–21.
- Iezzi S, Di Padova M, Serra C, et al. Deacetylase inhibitors increase muscle cell size by promoting myoblast recruitment and fusion through induction of follistatin. Dev Cell. 2004;6:673–84.
- Nakatani M, Takehara Y, Sugino H, et al. Transgenic expression of a myostatin inhibitor derived from follistatin increases skeletal muscle mass and ameliorates dystrophic pathology in mdx mice. FASEB J. 2008;22:477–87.
- Bettica P, Petrini S, D'Oria V, et al. Histological effects of givinostat in boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2016;26:643–9.
- Fagnocchi L, Mazzoleni S, Zippo A. Integration of Signaling Pathways with the Epigenetic Machinery in the Maintenance of Stem Cells. Stem Cells Int. 2016;2016:8652748.
- Muchir A, Shan J, Bonne G, et al. Inhibition of extracellular signal-regulated kinase signaling to prevent cardiomyopathy caused by mutation in the gene encoding A-type lamins. Hum Mol Genet. 2009;18:241–7.
- Wu W, Muchir A, Shan J, et al. Mitogen-activated protein kinase inhibitors improve heart function and prevent fibrosis in cardiomyopathy caused by mutation in lamin A/C gene. Circulation. 2011;123:53–61.
- Wu W, Shan J, Bonne G, et al. Pharmacological inhibition of c-Jun N-terminal kinase signaling prevents cardiomyopathy caused by mutation in LMNA gene. Biochim Biophys Acta. 2010;1802:632–8.
- Muchir A, Reilly SA, Wu W, et al. Treatment with selumetinib preserves cardiac function and improves survival in cardiomyopathy caused by mutation in the lamin A/C gene. Cardiovasc Res. 2012;93:311–9.
- Muchir A, Wu W, Sera F, et al. Mitogen-activated protein kinase kinase 1/2 inhibition and angiotensin II converting inhibition in mice with cardiomyopathy caused by lamin A/C gene mutation. Biochem Biophys Res Commun. 2014;452:958–61.
- Wu W, Chordia MD, Hart BP, et al. Macrocyclic MEK1/2 inhibitor with efficacy in a mouse model of cardiomyopathy caused by lamin A/C gene mutation. Bioorg Med Chem. 2017;25:1004–13.
- Ramos FJ, Chen SC, Garelick MG, et al. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med. 2012;4:144ra03.
