Abstract
We studied and compared the age structure, body size, and growth rates of field populations of two Korean salamander species (Hynobius yangi and Hynobius quelpaertensis) to elucidate important aspects of basic population dynamics of these two endemic Hynobius species. In both populations, females were sexually mature at three years of age, while H. yangi and H. quelpaertensis males matured at two and three years of age, respectively. Both males and females of H. yangi and H. quelpaertensis attained a maximum age of 11 years and 10 years, respectively[0]. In both species, the snout-vent length (SVL) and body weight (BW) of the females were greater than those of the males. The SVL, BW, and asymptotic SVL of both male and female H. yangi were smaller than those of H. quelpaertensis. The adult growth rates after sexual maturation of male and female H. yangi were lower than those of H. quelpaertensis, possibly resulting in the smaller body size of the former, although overall growth coefficients were not significantly different between the two species. We also compared the age structure and growth rates of three Korean and three Japanese species of Hynobius.
Introduction
Life history traits such as the age structure and growth rates of amphibian populations can assist us in understanding basic population dynamics (Stearns Citation1989; Misawa and Matsui Citation1999). In Urodela, age at maturity and growth rate are determined by the combined effects of environmental factors including food resources, competition, temperature, latitude and altitude and genetic factors such as developmental constraints (Olgun et al. Citation2001; Cogălniceanu and Miaud Citation2003). In Urodela, females are often larger than males, and female body size is usually correlated with fecundity. This sexual size dimorphism may be caused by the slower growth rate and delayed sexual maturation of females (Trivers Citation1972; Caetano and Leclair Citation1996; Marzona et al. Citation2004).
Several methods are available for determining the age of individual amphibians. One can use the relationship between age and the size frequency distributions of individuals, conduct a mark and recapture study, or initiate a skeletochronology study (Halliday and Verrell Citation1988; Eden et al. Citation2007; Matsuki and Matsui Citation2009). Of these techniques, skeletochronology has been used most frequently because it provides reliable data about the sexual maturity, growth rate, and longevity of a species (Castanet and Smirina Citation1990; Cheong et al. Citation2007; Lee and Park Citation2008). In Urodela, one can determine the age of a specimen by counting the number of lines of arrested growth (LAGs) in the periosteal bones of the phalanges.
In South Korea, there are five different urodele species. The largest genus, Hynobius, includes three endemic species, H. leechii, H. yangi, and H. quelpaertensis (Kim Citation2009). Hynobius leechii is distributed over most of South Korea, with the exception of regions where H. yangi and H. quelpaertensis are found. Age structure and growth in a population of H. leechii were previously studied in Chuncheon, Kangwon (Lee and Park Citation2008). Hynobius yangi is primarily distributed in Gijang-gun near Busan and in surrounding areas. Hynobius quelpaertensis is mainly found on Jeju Island and in parts of the southern Korean peninsula (Yang et al. Citation2001). The age and growth of H. yangi and H. quelpaertensis have not been investigated in any field population. It has been reported that H. yangi has a smaller body size than H. quelpaertensis (Kim et al. Citation2003), but the factors responsible for the smaller size are not known.
In this study, we determined the age structure and growth rates of H. yangi and H. quelpaertensis in a field population of each species and compared the results for the two species. This work was undertaken in order to elucidate important aspects of basic population dynamics of these two Korean endemic Hynobius species and to identify the factors responsible for the small body size of H. yangi. We also compared the age structure and growth rates of three Korean and three Japanese species of Hynobius.
Materials and methods
Animal collection
Between 22 February and 10 April 2006, we collected 508 H. yangi (288 males and 220 females) from a breeding population present in a mountain wetland (N 35°19′55.6?, E 129°19′28.0?, 10 m long×10 m wide×0.3 m deep, altitude 40 m) near Hyoam-ri, Jangan, Busan, South Korea. The animals were collected by setting up a drift fence with pitfall traps surrounding the wetland. The wetland is often dry in mid-summer. On Jeju Island between 16 February and 19 March 2008, we collected 123 H. quelpaertensis (76 males and 47 females) from a breeding population formed in a mountain pond (N 33°31′06.3?, E 126°42′53.7?, 5 m long×4 m wide×1 m deep, altitude 150 m) located at Deokcheon-ri, BukJeju, Jeju, South Korea. Most of the salamanders were found under rocks and among water plants and fallen leaves. The pond was located in a deciduous forest and does not dry up on a yearly basis.
We anesthetized the salamanders by submerging them in 0.1% MS-222 (3-aminobenzoic acid ethyl ester, Sigma) for 5–10 min. We measured the snout-vent length (SVL, the distance from the tip of the snout to the posterior margin of the cloaca) and body weight (BW) of each individual to an accuracy of 0.1 mm using digital calipers (CD-15CPX, Mitutoyo, NV) or to an accuracy of 0.1 g using a digital field balance (CB-1200, Sartorius, MA), respectively. For purposes of the skeletochronology study, two joints of two digits (approximately 1–3 mm of each digit) were removed from each animal and individually preserved in 10% neutral formalin (Junsei Chemical). To reduce the probability of infection, antiseptic solution (Povidone iodine topical solution, Green Pharmacy Co., Korea) was applied to the digits after cutting. Though they did not directly study the adverse effects of toe clipping in the field, Lee and Park (Citation2008) successfully used toe clipping for individual identification in their field study of H. leechii. The salamanders were released into the wetland or pond after they had recovered from the anesthetic.
Skeletochronology
The skeletochronology study was conducted following the methods of Cheong et al. (Citation2007) and Lee and Park (Citation2008). We washed the preserved digits in tap water for 24 h, decalcified the bone by submerging the digits in 5% nitric acid (Daejung Chemical, Seoul) for 2–3 h, and washed the digits again in tap water for 24 h. The digit samples were then dehydrated, paraffin-embedded, sectioned (8–10 µm, Microtome-530, Erma), stained using the Harris eosin-hematoxylin method (Presnell and Schreibman Citation1997), and observed under a microscope (X 400, Eclipse-50i, Nikon).
Because endosteal bone resorption was observed in both H. yangi and H. quelpaertensis, we determined the age of these species using a back calculation method (Castanet et al. Citation1993). Applying this method was necessary because in specimens in which such resorption occurred, we were unable to detect a metamorphosis line. To adjust the age in such individuals, we compared the mean diameter of the first LAG in individuals that had a metamorphosis line with the diameter of the first LAG in an individual that had lost the metamorphosis line. If the first LAG diameter of the latter individual was larger than that of the former, we added an additional year to the estimate of the first animal's age (Rozenblut and Ogielska Citation2005; Üzüm Citation2009).
Annual survivorship and adult life expectancy
Annual survivorship and adult life expectancy are important components of basic population dynamics. We calculated the annual adult survival rate (S) based on Robson and Chapman (Citation1961), as follows: S=T/(R+T - 1), where T=N1+2N2+3N3+…, R=Σ N i , and N i =the number of individuals in age group i. The adult life expectancy (LEP, the expected total longevity of individuals that have reached maturity) was calculated for each population of H. yangi and H. quelpaertensis using Seber's (Citation1973) formula: ESP=0.5+1/(1 - S).
Growth curve
To calculate a growth curve, we fitted the SVL and age data of H. yangi and H. quelpaertensis to the von CitationBertalanffy equation (1938): S t =S m - (S m - S 0 ) e − k(t - t0), where S t =the average SVL at age t, S m =the asymptotic SVL, S 0 =the SVL at metamorphosis, t=the number of growing seasons experienced, t 0 =the age at metamorphosis, and K=the growth coefficient (the shape of the growth curve). To obtain data for S 0 and t 0 , we collected one clutch of eggs of each species that had been newly oviposited at the wetland or pond in March 2006 and 2008, respectively. The eggs were hatched and reared in a laboratory aquarium (90 cm long×30 cm wide×45 cm deep, 5 L water) that included both aquatic and terrestrial conditions. Fallen leaves were provided in the aquarium as shelter for the animals; the air temperature and photoperiod were not manipulated. Blood worms were provided for food and half of the water was changed weekly. A total of 16 larvae of H. yangi and 22 larvae of H. quelpaertensis successfully metamorphosed. We measured the SVL of each metamorphosed individual after anesthetizing the animals as described above.
Data analysis
The SVL of recently metamorphosed and adult individuals and the BW of male and female H. yangi and H. quelpaertensis did not pass the normality test (Shapiro-Wilk normality test, P<0.05). Comparisons of these parameters between sexes in each species and between the two species were therefore assessed using a more conservative Mann-Whitney U test. To compare the growth coefficient and the asymptotic SVL between sexes and between species, we used the independent sample t-test. All analyses were performed with SPSS (ver. 11.0); the data are presented as mean±SE.
Results
Endosteal resorption, age structure, annual survivorship, and adult life expectancy
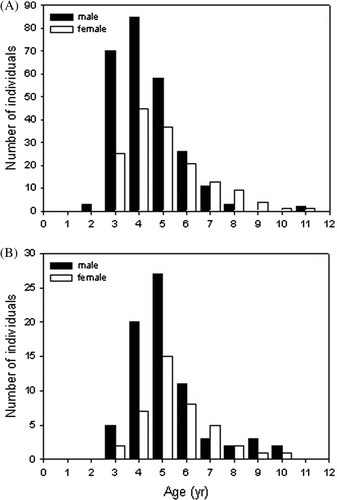
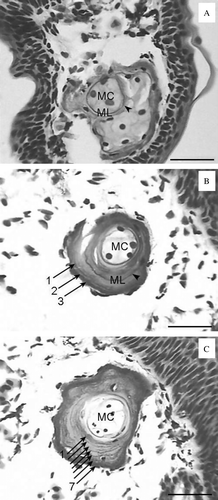
We determined the age of 414 of 508 H. yangi salamanders and of 114 of 123 H. quelpaertensis salamanders (). Endosteal resorption was observed in 397 H. yangi (95.9%) and 107 H. quelpaertensis (93.9%, C). We observed both a metamorphosis line (A) and the first LAG (B) in 17 H. yangi and 7 H. quelpaertensis; the mean diameters of the first LAG of these specimens were 85.1±12.1 µm (n=17, range=66.5–106.5) and 86.4±14.7 µm (n=8, range=69.0–106.1), respectively. Comparing these mean values to the diameter of the first LAG in each individual, we added one year to the calculated age of 27 H. yangi and 13 H. quelpaertensis whose first LAG diameter was larger than that of individuals having both a metamorphosis line and a first LAG.
Figure 1. Phalangeal cross-sections of Hynobius yangi and Hynobius quelpaertensis. An arrowhead indicates a metamorphosis line, and arrows indicate the lines of arrested growth (LAGs). MC=marrow cavity, ML=metamorphosis line. (A) A juvenile (SVL=23.8 mm) of H. quelpaertensis caught in February 2008. Only a metamorphosis line was observed. (B) A female of H. yangi (SVL=59.2 mm) caught in March 2006. A metamorphosis line and three LAGs were observed. This individual was three years old. (C) A male of H. quelpaertensis (SVL=63.3 mm) caught in March 2008. Seven LAGs were observed. This individual was seven years old. The scale bars represent 50 µm.
Hynobius yangi males and females become sexually mature at 2 and 3 years of age, respectively; both sexes commonly attain a maximum age of 11 years (A). In our sample, the mean age of females was higher than that of males (Mann-Whitney U test: Z=-4.18, P<0.01, ). Hynobius quelpaertensis males and females most often mature sexually at three years of age and attain a maximum age of ten years (B). The mean ages of males and females were not significantly different (Mann-Whitney U test: Z=-1.52, P=0.12, ). The mean ages of both male and female H. yangi were lower than those of H. quelpaertensis (Mann-Whitney U test: Z=-4.55, P<0.01 for males; Mann-Whitney U test: Z=-2.19, P=0.028 for females).
Table 1. Snout-vent length (SVL), body weight (BW), mean age, age at maturity, adult survivorship (S), life expectancy (LEP), asymptotic SVL, and growth coefficient in populations of Hynobius yangi, Hynobius quelpaertensis, and Hynobius leechii. The data for H. leechii were calculated from published data (Lee and Park 2008) for comparison among three Korean species in the genus Hynobius.
The annual survivorship and adult life expectancy of male H. yangi were 0.70 and 3.87, respectively, while those of females were 0.67 and 3.55. The annual survivorship and adult life expectancy of male H. quelpaertensis were 0.69 and 3.74, respectively; those of the females were 0.72 and 4.10.
Body size and growth
In both species, females were longer and heavier than males (Mann-Whitney U test: Z=-9.59, P<0.01 for SVL; Mann-Whitney U test: Z=-5.61, P<0.01 for BW in H. yangi; Mann-Whitney U test: Z=-2.04, P=0.041 for SVL; Mann-Whitney U test: Z=-3.01, P<0.003 for BW in H. quelpaertensis; ). The SVL and BW of both male and female H. yangi were less than those of male and female H. quelpaertensis (Mann-Whitney U test: Z=-11.38, P<0.01 for SVL; Mann-Whitney U test: Z=-8.85, P<0.01 for BW between males; Mann-Whitney U test: Z=-7.84, P<0.01 for SVL; Mann-Whitney U test: Z=-6.86, P<0.01 for BW between females; ).
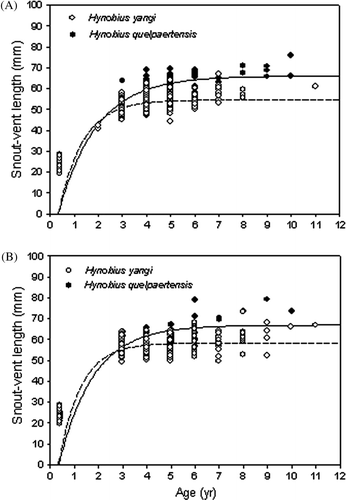
The larvae of both species metamorphosed approximately three months after hatching. The mean SVLs of recently metamorphosed H. yangi and H. quelpaertensis were 23.5±0.2 mm (n=16, range=19.4–28.5) and 23.3±0.8 mm (n=22, range=20.9–25.3), respectively; the difference was not significant (Mann-Whitney U test: Z=-0.59, P=0.55). Therefore, in applying the von Bertalanffy growth equation, we used t 0 =0.3 and S 0 =2.3 for both H. yangi and H. quelpaertensis.
The growth coefficients of males and females were not different in either species (t=0.41, df=412, P>0.05 in H. yangi; t=0.24, df=112, P>0.05 in H. quelpaertensis, ). The asymptotic SVL of H. yangi females was greater than that of males (t=3.23, df=412, P<0.05), but there was no difference in this variable between genders in H. quelpaertensis (t=0.16, df=112, P>0.05). The growth coefficients of males and females did not differ between the two species (t=0.83, df=195, P>0.05 between males; t=1.86, df=329, P>0.05 between females, ). However, the asymptotic SVLs of both male and female H. yangi were smaller than those of male and female H. quelpaertensis (t=-3.15, df=195, P<0.05 between males; t=-5.70, df=329, P<0.05 between females, ).
Discussion
In Urodela, more than 60% of the species show larger SVLs in females than in males (Shine Citation1979), and large SVL in females is directly related to high fecundity (Trivers Citation1972; Kaplan and Salthe Citation1979). Body size dimorphism between the sexes can be influenced by several factors including the growth rate before metamorphosis, the adult growth rate (different energy allocations between growth and reproduction), age at sexual maturity, and the age structure (mean age) of the population (Marzona et al. Citation2004). In several salamander species including H. leechii and H. kimurae, low sexual maturation and high mean age of a population have been shown to contribute to large female SVLs (Misawa and Matsui Citation1997, Citation1999; Miaud et al. Citation2001; Lee and Park Citation2008). However, other factors cannot be neglected as possible causes of this type of dimorphism.
In Alpine newts (Triturus alpestris), large SVLs of females have been shown to result from a higher growth rate in females than in males before sexual maturation (Miaud et al. Citation2000). In our study, we could not discriminate the sex of recently metamorphosed H. yangi and H. quelpaertensis. Although the growth rate of female H. yangi tended to be lower than that of the males, this is unlikely to be the primary causative factor of large female SVLs in this species because the differences in growth rates between the sexes were not significant. Instead, the slower sexual maturation and higher mean age of female H. yangi may be responsible for the larger SVLs and BWs observed in females. In H. quelpaertensis, on the other hand, the causes of the larger SVLs observed in females are not clear because the mean age and age at sexual maturity were not significantly different for the two sexes.
Another question we addressed in this study was why the body sizes (SVL and BW) of male and female H. yangi are smaller than those of their H. quelpaertensis counterparts. The earlier sexual maturation and lower growth rate after sexual maturation (i.e. greater K) of male and female H. yangi might cause these differences. Several previously mentioned factors that determine body size, including a greater growth coefficient and a lowered growth rate after sexual maturation, might explain the smaller body size in H. yangi. Although the differences were not statistically significant, the growth coefficients (K) of male and female H. yangi were greater (1.0 and 1.1) than those of male and female H. quelpaertensis (0.6 and 0.7). However, the growth rates of male and female H. yangi after sexual maturation were lower than those of their H. quelpaertensis counterparts. If the growth rates of the two species before sexual maturation are not different but the overall growth coefficient is greater in one species than in the other, the adult growth rate after sexual maturation in the species with the lower growth coefficient should be smaller, resulting in a smaller body size. Similar growth patterns have been reported in several amphibian species including H. nebulosus, Bombina bombina, and Hyla annectans chuanxiensis (Ento and Matsui Citation2002; Cogălniceanu and Miaud Citation2003; Liao and Lu Citation2010).
Amphibians show indeterminate growth patterns in which an individual grows continuously, even after sexual maturity (Kozlowski and Uchmanski Citation1987; Cichon Citation1999). The observation that the growth rate of H. yangi decreases after sexual maturation suggests that adult H. yangi allocate more energy to reproduction than to growth (Kozlowski and Uchmanski Citation1987; Hemelaar Citation1988). Although we do not know the clutch size of H. quelpaertensis, the clutch size of female H. yangi was found to be the same as that of H. leechii (66 eggs per clutch), despite the fact that the SVL of female H. leechii was greater than that of female H. yangi (Park and Park Citation2000; Lee Citation2007). This difference indicates that the smaller H. yangi might channel relatively more energy towards reproduction than growth. Similar results have been reported in B. bombina (Cogălniceanu and Miaud Citation2003, Citation2004), a species that has a low growth rate after sexual maturation because females put greater energy into reproduction, laying more than 500 eggs per year. It is known that individuals in a species will channel less energy towards growth when adult survivorship is low due to limited food resources, high predator pressure, reproductive competition, and highly fluctuating environmental conditions (Diaz-Paniagua et al. Citation1996; Olgun et al. Citation2001). However, our results are not easily explained by this phenomenon because the annual survivorship rates of H. yangi and H. quelpaertensis were not significantly different. In animals, the intrinsic growth rate of a species can be determined by the combined effects of both environmental and genetic variables including nutrient stress, temperature, and developmental constraints (Arendt Citation1997). To elucidate which factors contribute to the low growth rate of H. yangi after sexual maturation, further studies focusing on both environmental and genetic factors should be conducted.
In our analysis, the mean age and growth rate of all three Korean species in the genus Hynobius (H. leechii, H. yangi, and H. quelpaertensis) were determined for a field population of each species. Males and females of Korean Hynobius species mature sexually at 1–3 years and 3 years, respectively, and attain a maximum age of 9–11 years. The females of Korean Hynobius species tend to have larger SVLs than males. In Japan, the age and growth rate of three species in the genus Hynobius (H. nebulosus, H. tokyoensis, and H. kimurae) have been studied. The females of all three Japanese species show later sexual maturation and greater mean age than the males. Male and female H. nebulosus mature sexually at 3 and 4 years, respectively, and attain maximum ages of 10 and 6 years, respectively (Ento and Matsui Citation2002). In H. tokyoensis, both males and females start to breed at 4 years of age, and longevity ranges from 13 to 21 years (Kusano et al. Citation2006). Male and female H. kimurae mature sexually at 5–6 and 7 years respectively and attain a maximum age of 12 to 20 years (Misawa and Matsui Citation1999). Unlike Korean Hynobius, in which the females invariably have larger SVLs, sexual dimorphism as measured by SVL is not consistent in the Japanese speciese. Male H. nebulosus have larger SVLs than the females, the SVLs of male H. tokyoensis do not differ from those of the females, and female H. kimurae are larger than their male counterparts. Previous studies suggest that variability in sexual size dimorphism may be caused by several different factors including differences between lentic and lotic habitats (Ento and Matsui Citation2002), differences in air temperature at various latitudes and altitudes (Caetano and Castanet Citation1993; Üzüm and Olgun Citation2009; Liao and Lu Citation2010), and different mortality rates resulting from variable environmental conditions (Shine Citation1979; Olgun et al. Citation2001). Overall, Korean Hynobius species begin to breed early, at 1–4 years of age, but their longevity is similar to or shorter than that of Japanese Hynobius species by approximately 10 years.
Acknowledgements
We thank Y.M. Ko, D.I. Kim, and N.Y. Ra for their assistance in the field. This work was supported by a Korean Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2007-313-2-C00503) and by the Korea Hydro & Nuclear Power (KHNP) (# H07S016000).
Additional information
Notes on contributors
Jung-Hyun Lee
These authors contributed equally to this workReferences
- Arendt , JD. 1997 . Adaptive intrinsic growth rates: an integration across taxa . Q Rev Biol. , 72 : 149 – 177 .
- Bruce , RC. 2009 . Life-history contributions to miniaturization in the salamander genus Desmognathus (Urodela: Plethodontidae) . Copeia. , 2009 : 714 – 723 .
- Caetano , MH and Castanet , J. 1993 . Variability and microevolutionary patterns in Triturus marmoratus from Portugal: age, size, longevity and individual growth . Amphib- reptil. , 14 : 117 – 129 .
- Caetano , MH and Leclair , R. 1996 . Growth and population structure of red-spotted newts (Notophthalmus viridescens) in permanent lakes of the Laurentian shield, Quebec . Copeia. , 1996 : 866 – 874 .
- Castanet , J and Smirina , EM. 1990 . Introduction to the skeletochronological method in amphibians and reptiles . Ann Sci Nat Zool. , 11 : 191 – 196 .
- Castanet J Francillon-Vieillot H Meunier FJ de Ricqles A. 1993 Bone and individual aging In: Hall BBK editor. Bone growth Boca Raton CRC Press. 245 283
- Cheong , SH , Park , D , Sung , HC , Lee , JH and Park , SR. 2007 . Skeletochronological age determination and comparative demographic analysis of two populations of gold-spotted pond frog (Rana chosenica) . J Ecol Field Biol. , 30 : 57 – 62 .
- Cichon , M. 1999 . Growth after maturity as a sub-optimal strategy . Acta Oecol. , 20 : 25 – 28 .
- Cogălniceanu , D and Miaud , C. 2003 . Population age structure and growth in four syntopic amphibian species inhabiting a large river floodplain . Can J Zool. , 81 : 1096 – 1106 .
- Cogălniceanu , D and Miaud , C. 2004 . Variation in life history traits in Bombina bombina from the lower Danube floodplain . Amphib-reptil. , 25 : 115 – 119 .
- Diaz-Paniagua , C , Mateo , JA and Andreu , AC. 1996 . Age and size structure of populations of small marbled newts (Triturus marmoratus pygmaeus) from Donana national park (SW Spain): a case of dwarfism among dwarfs . J Zool. , 239 : 83 – 92 .
- Eden , CJ , Whiteman , HH , Gray , LD and Wissinger , SA. 2007 . Accuracy assessment of skeletochronology in the Arizona tiger salamander (Ambystoma tigrinum nebulosum) . Copeia. , 2007 : 471 – 477 .
- Ento , K and Matsui , M. 2002 . Estimation of age structure by skeletochronology of a population of Hynobius nebulosus in a breeding season (Amphibia, Urodela) . Zool Sci. , 19 : 241 – 247 .
- Halliday , TR and Verrell , PA. 1988 . Body size and age in amphibians and reptiles . J Herpetol. , 22 : 253 – 265 .
- Hemelaar , A. 1988 . Age, growth, and other characteristics of Bufo bufo from different latitudes and altitudes . J Herpetol. , 22 : 369 – 388 .
- Kaplan , RH and Salthe , SN. 1979 . The allometry of reproduction: an empirical view in salamanders . Am Nat. , 113 : 671 – 689 .
- Kim , JB. 2009 . Taxonomic list and distribution of Korean amphibians . Korean J Herpetol. , 1 : 1 – 13 .
- Kim , JB , Min , MS and Matsui , M. 2003 . A new species of lentic breeding Korean salamander of the genus Hynobius (Amphibia, Urodela) . Zool Sci. , 20 : 1163 – 1169 .
- Kozlowski , J and Uchmanski , J. 1987 . Optimal individual growth and reproduction in perennial species with indeterminate growth . Evol Ecol. , 1 : 214 – 230 .
- Kusano , T , Ueda , T and Nakagawa , H. 2006 . Body size and age structure of breeding populations of the salamander, Hynobius tokyoensis (Caudata: Hynobiidae) . Curr Herpetol. , 25 : 71 – 78 .
- Lee JH 2007 Reproductive ecology and age structure of the Gori salamander (Hynobius yangi) [unpublished M.E. thesis] Kangwon National University
- Lee , JH and Park , D. 2008 . Effects of physical parameters and age on the order of entrance of Hynobius leechii to a breeding pond . J Ecol Field Biol. , 31 : 183 – 191 .
- Liao , WB and Lu , X. 2010 . Age structure and body size of the Chuanxi tree frog Hyla annectans chuanxiensis from two different elevations in Sichuan (China) . Zool Anz. , 248 : 255 – 263 .
- Marzona , E , Seglie , D and Giacoma , C. 2004 . Sexual dimorphism in body size and life-history traits in a population of Triturus alpestris alpestris . Ital J Zool Suppl. , 1 : 117 – 120 .
- Matsuki , T and Matsui , M. 2009 . The validity of skeletochronology in estimating ages of Japanese clouded salamander, Hynobius nebulosus (Amphibia, Caudata) . Curr Herpetol. , 28 : 41 – 48 .
- Miaud , C , Guyétant , R and Faber , H. 2000 . Age, size, and growth of the Alpine newt, Triturus alpestris (Urodela: Salamandridae), at high altitude and a review of life-history trait variation throughout its range . Herpetologica. , 56 : 135 – 144 .
- Miaud , C , Andreone , F , Riberon , A , De Michelis , S , Clima , V , Castanet , J , Francillon-Vieillot , H and Guyétant , R. 2001 . Differences in age, size at maturity and gestation duration among two neighboring populations of the Alpine salamander Salamandra lanzai . J Zool. , 254 : 251 – 260 .
- Misawa , Y and Matsui , M. 1997 . Larval life history variation in two populations of the Japanese salamander, Hynobius kimurae (Amphibia, Urodela) . Zool Sci. , 14 : 257 – 262 .
- Misawa , Y and Matsui , M. 1999 . Age determination by skeletochronology of the Japanese salamander Hynobius kimurae (Amphibia, Urodela) . Zool Sci. , 16 : 845 – 851 .
- Olgun , K , Miaud , C and Gautier , P. 2001 . Age, growth, and survivorship in the viviparous salamander Mertensiella luschani from southwestern Turkey . Can J Zool. , 79 : 1559 – 1567 .
- Park , D and Park , SR. 2000 . Multiple insemination and reproductive biology of Hynobius leechii . J Herpetol. , 34 : 594 – 598 .
- Presnell , JK and Schreibman , MP. 1997 . Humanson's animal tissue techniques , Baltimore : Johns Hopkins University Press .
- Robson , DS and Chapman , DG. 1961 . Catch curves and mortality rates . T Am Fish Soc. , 90 : 181 – 189 .
- Rozenblut , B and Ogielska , M. 2005 . Development and growth of long bones in European water frogs (Amphibia: Anura: Ranidae), with remarks on age determination . J Morphol. , 265 : 304 – 317 .
- Seber , GAF. 1973 . The estimation of animal abundance and related parameters , London : Blackburn Press .
- Shine , R. 1979 . Sexual selection and sexual dimorphism in the amphibia . Copeia. , 1979 : 297 – 306 .
- Stearns , SC. 1989 . The evolutionary significance of phenotypic plasticity . Bioscience. , 39 : 436 – 435 .
- Trivers RL. 1972 Parental investment and sexual selection Campbell B Sexual selection and the descent of man, 1871–1971 Chicago Aldine Publishing Company. 136 179
- Üzüm , N. 2009 . A skeletochronological study of age, growth and longevity in a population of the Caucasian salamander, Mertensiella caucasica (Waga 1876) (Caudata: Salamandridae) from Turkey . North-West J Zool. , 5 : 74 – 84 .
- Üzüm , N and Olgun , K. 2009 . Age and growth of the southern crested newt, Triturus karelinii (Strauch, 1870), in a lowland population from Northwest Turkey . Acta Zool Hung. , 55 : 55 – 65 .
- von Bertalanffy , L. 1938 . A quantitative theory of organic growth . Hum Biol. , 10 : 181 – 213 .
- Yang , SY , Kim , JB , Min , MS , Suh , JH and Kang , YJ. 2001 . Monograph of Korean amphibia , Seoul : Academy Press .