Abstract
The larvae of Scyra acutifrons are described and illustrated for the first time. The larval stage consists of two zoeal and a megalopal stages. The zoea of S. acutifrons is compared with those of other known species of the Epialtidae from the northern Pacific. The zoea of Scyra acutifrons can be easily distinguished from that of S. compressipes by having a longer rostral carapace spine and an endopod of maxillule with three setae. It is found that the genus Scyra (Pisinae) shows a great similarity to Pisoides bidentatus (Pisinae) and the genus Pugettia (Epialtinae) in the family Epialtidae; especially, S. acutidens coincides well with two Pugettia species (Pugettia incisa and P. gracilis) in the characteristics of the zoeal mouthpart appendages. To facilitate the study of plankton-collected material, a provisional key to the known zoeae of the Epialtidae from the northern Pacific is provided.
Introduction
The majoid family Epialtidae contains four subfamilies; Epialtinae, Pisinae, Pliosomatinae, and Tychinae (see Ng et al. Citation2008). The larval descriptions have been known for 16 species of the subfamilies Epialtinae and Pisinae from the northern Pacific (). The pisinid genus Scyra Dana, 1852, includes three species (Griffin and Tranter Citation1986): Scyra acutifrons Dana, 1852, Scyra compressipes Stimpson, 1857, and Scyra tuberculata Yokoya, 1933. Of these, the sharpnose crab, S. acutifrons, is found in the northeastern Pacific of America from Alaska to California (Jensen Citation1995). S. compressipes and S. tuberculata are known to occur in the northwestern Pacific of Korea and Japan (Kim Citation1973; Sakai Citation1976).
Table 1. Species, stages and sources of known larval descriptions of the family Epialtidae from the northern Pacific (Z and M = zoeal and megalopal stages; * = brief description)
The larval development of S. compressipes has been reported by Kim and Hong (Citation1999), but no larval stages of S. acutifrons have been described yet. Therefore, the aims of this paper are to describe the larval stage of S. acutifrons, compare its morphology to previously described larvae of the family, and provide a key to the known zoeae of the Epialtidae from the northern Pacific.
Materials and methods
Ovigerous females of Scyra acutifrons were collected by trawl in the Burrow Bay (48°28'N, 122°40'W), Anacortes, WA, USA, on 2 Febuary 2004. On 12 April the zoeae hatched in the laboratory. They were reared individually at a water temperature of 15±1° and salinity of 29.7±0.6‰. Each of the individually reared zoeae was held in a plastic well containing 5–6 ml of seawater for the first and second stages. From the megalopal stage, they were transferred to a larger well containing 15–16 ml of seawater. The water was changed daily and each larva was provided with newly hatched Artemia nauplii once per day. The mass culture consisted of three dishes, each containing 150 zoeae in 200 ml of seawater, which was also changed daily. Both individual and mass cultures were checked daily for exuviae and dead through the megalopal stage. Molts and dead larvae were fixed and preserved in 10% buffered formalin for later examination. Dissected appendages were examined and drawn using a Leitz Laborlux S Microscope with camera lucida. Setal counts on appendages and measurements were based on the mean of ten specimens per larval stage. The sequence of the larval description is based on the malacostracan somite plan and described from anterior to posterior (Clark et al. Citation1998). Setal armature on appendages is described from proximal to distal segments and in order of endopod to exopod. The long plumose natatory setae of the first and second maxillipeds were drawn truncated. A micrometer was used for measurements: CL (carapace length) was measured from base of the rostral spine to the most posterior carapace margin and SL (spine to spine length) was from tip of the rostral to tip of the dorsal spine. The classification follows that of Ng et al. (Citation2008). The larvae and the spent females were deposited in Silla University, Korea (SUZCr103258).
Results
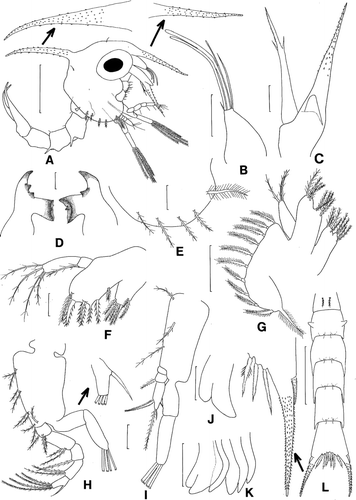
First zoea ()
Figure 1. Scyra acutifrons, first zoeal stage. A, lateral view; B, antennule; C, antenna; D, mandibles; E, lateral expansion of carapace; F, maxillule; G, maxilla; H, first maxilliped; I, second maxilliped; J, third maxilliped; K, chela and pereopods; L, dorsal view of abdomen and telson. Scale bars=0.5 mm (A, L), 0.1 mm (B, C, E, G–I, K) and 0.05 mm (D, F, J).
Size. CL 0.83±0.05 mm. SL 1.95±0.13 mm.
Carapace (A, E). Dorsal spine spinulate, slightly curved, slightly longer than rostral spine; rostral spine spinulate, approximately equal in length to antennal protopod; lateral spine absent; pair of posterodorsal setae present; each ventral margin with one plumose anterior and four posterior setae; eyes stalked.
Antennule (B). Uniramous; endopod absent; exopod with two long, stout aesthetascs, one shorter, thinner aesthetasc, and one long and one shorter setae, all terminal.
Antenna (C). Endopod bud present; protopod spinulate; exopod about 3/4 length of protopod, with two subterminal setae, bearing minute spinules distally.
Mandibles (D). Asymmetrical; right molar process with two teeth and left molar process with one tooth, confluent with incisor processes; endopod palp absent.
Maxillule (F). Coxal endite with seven plumodenticulate setae; basial endite with seven plumodenticulate setae; endopod 2-segmented, proximal segment with one plumose seta, distal segment with four terminal plumose setae.
Maxilla (G). Coxal endite bilobed, with 4+4 plumose setae; basial endite bilobed, with 5+5 plumose setae; endopod with 3 plumose setae; exopod (scaphognathite) margin with 10 plumose setae and one distal stout plumose process.
First maxilliped (H). Coxa without seta; basis with nine plumose setae arranged 2+2+2+3 (9); endopod 5-segmented with 3, 2, 1, 2, 5 (one strong subterminal spinous seta+four terminal plumose setae) setation; exopod 2-segmented, distal segment with four terminal plumose natatory setae.
Second maxilliped (I). Coxa unarmed; basis with 1+1+1 (3) plumose setae; endopod 3-segmented, with 0, 1, 4 (two subterminal plumose setae+two terminal plumose setae) setation; exopod 2-segmented, distal segment with four terminal plumose natatory setae.
Third maxilliped (J). Biramous.
Pereopods (K). Present, cheliped bilobed.
Abdomen (A, L). With five somites; somite 1 with two dorsomedial setae; somite 2 with pair of lateral processes, somites 2–5 each with pair of posterodorsal setae, somites 3–5 with posterolateral processes; pleopod buds present as buds.
Telson (L). Posterior margin with three serrated setae. Each fork long, distally spinulate, with one lateral spine.
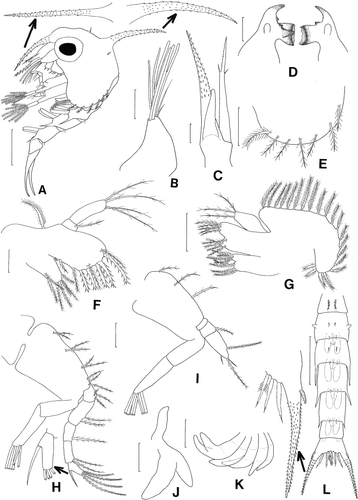
Second zoea ()
Figure 2. Scyra acutifrons, second zoeal stage. A, lateral view; B, antennule; C, antenna; D, mandibles; E, lateral expansion of carapace; F, maxillule; G, maxilla; H, first maxilliped; I, second maxilliped; J, third maxilliped; K, chela and pereopods; L, dorsal view of abdomen and telson. Scale bars=0.5 mm (A, L), 0.25 mm (E, K), 0.1 mm (B–D, G–J) and 0.05 mm (F).
Size. CL 1.03±0.06 mm. SL 2.30±0.09 mm.
Carapace (A, E). Each ventral margin with one plumose anterior and six posterior setae.
Antennule (B). Exopod with six long aesthetascs and two setae, all terminal.
Antenna (C). Endopod bud about 1/2 length of exopod.
Mandibles (D). Palps present.
Maxillule (F). Epipod with plumose seta; coxal endite with seven setae; basial endite with five plumodenticulate and five plumose setae.
Maxilla (G). Coxal endite with 4+4 plumose setae; basial endite with 5+5 plumose setae; exopod (scaphognathite) margin with 21 plumose setae.
First maxilliped (H). Exopod 2-segmented, distal segment with six terminal plumose natatory setae.
Second maxilliped (I). Exopod 2-segmented, distal segment with six terminal plumose natatory setae.
Third maxilliped (J). More developed.
Pereopods (K). More developed, segments differentiated.
Abdomen (A, L). With six somites; pleopod buds more developed, those on somites 2–5 biramous; pair of dorsomedial setae now present on somites 2, 3.
Telson (L). Unchanged.
Megalopa (, )
Figure 3. Scyra acutifrons, megalopal stage. A, dorsal view; B, antennule; C, antenna; D, mandible; E, maxillule; F, maxilla; G, first maxilliped; H, second maxilliped; I, third maxilliped. Scale bars=0.5 mm (A) and 0.1 mm (B–I).
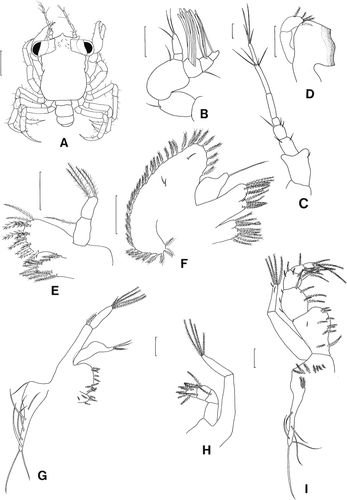
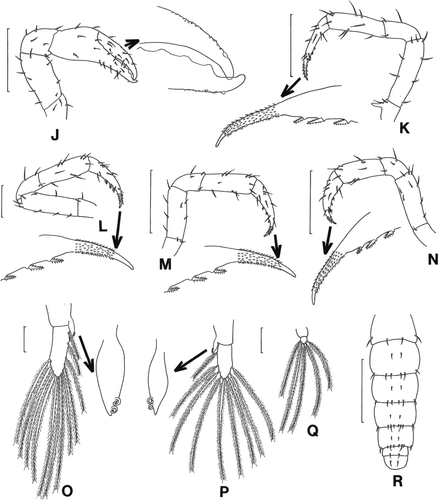
Figure 4. Scyra acutifrons, megalopal stage. J, chela; K–N, pereopods 1–4; O, pleopod 2; P, pereopod 4; Q, pereopod 5; R, dorsal view of abdomen and telson. Scale bars=0.5 mm (J, K, M, N, R), 0.25 mm (L) and 0.1 mm (O–Q).
Size. Carapace width 0.95±0.04 mm. Carapace length 1.48±0.08 mm.
Carapace (A). Subquadrate, longer than wide, with one short rostral process, pair of processes anterior to eye stalks.
Antennule (B). Peduncle 3-segmented, segments 2, 3 each with one seta; endopod 2-segmented, with one subterminal and two terminal setae; exopod 3-segmented, segment one with five aesthetascs and one short seta, segment 2 with four aesthetascs, segment 3 with one subterminal aesthetasc.
Antenna (C). Seven-segmented, each with 1, 2, 3, 0, 0, 4, and 4 setae.
Mandible (D). Endopod palp 2-segmented, distal segment with five marginal plumose setae.
Maxillule (E). Coxal endite with nine plumose setae; basial endite with 11 plumodenticulate and four plumose setae; endopod 2-segmented, proximal with one plumose seta, distal with four terminal plumose setae.
Maxilla (F). Coxal endite bilobed, with 6+4 plumose setae; basial endite bilobed, with 6+7 plumose setae; endopod with one terminal simple seta; exopod (scaphognathite) with 39 marginal plumose setae and three surface simple setae.
First maxilliped (G). Epipod with eight long simple setae; coxal and basial endites each with six and 10 plumose setae; endopod with four terminal simple setae; exopod 2-segmented, proximal segment with one distal plumose setae, distal segment with four long terminal plumose and one simple setae.
Second maxilliped (H). Epipod unarmed; coxa and basis not differentiated; endopod 4-segmented, with 0, 1, 4, 5 setation; exopod 2-segmented, proximal segment without seta, distal segment with four long terminal plumose setae.
Third maxilliped (I). Epipod with six long simple setae and nine proximal plumose setae; coxa and basis not differentiated; endopod 5-segmented, with 12, 8, 5, 6, 4 setation; exopod 2-segmented, proximal segment without seta, distal segment with four long terminal plumose and two short subterminal simple setae.
Chela (J). All segments with a few short setae; outer surface of finger with a few granules, tip slightly hooked.
Pereopods 1–4 (K–N). All segments well differentiated and sparsely armed with setae; only pereopod 1 with distinct ischial spine; dactylus with three spines on inner margin, tip spinulate, with a few granules on inner margin, sharp pointed.
Pleopods (O-Q). Endopod with two hooks except in pleopod 5; pleopods 1–5 with 12, 12, 12, 9, 5 plumose setae on distal segment.
Abdomen and telson (R). Abdomen 6-segmented; somites 1–6 with 4, 8, 8, 10, 10, 2 setae on dorsal surface; broad, rounded telson with two dorsomedial setae.
Discussion
The morphological differences between the larvae of Scyra acutifrons and those of S. compressipes described by Kim and Hong (Citation1999) are shown in . The first and second zoeae of S. acutifrons are larger in size, the length of the rostral carapace spine being approximately equal to that of the antennal protopod, an endopod of the maxilla has three setae, and a distal segment of an endopod of the first maxilliped has a strong spinous subterminal seta; whereas those of S. compressipes are smaller in size, the length of the rostral carapace spine being approximately half that of the antennal protopod, an endopod of the maxilla has four setae, and a distal segment of an endopod of the first maxilliped has a plumose subterminal seta. In megalopa S. acutifrons is larger in size and has more setae or aesthetascs on the appendages except in a coxal endite of the maxillule.
Table 2. Differences in the larval characteristics between Scyra compressipes described by Kim and Hong (1999) and Scyra acutifrons in the present study
Santana et al. (Citation2004) stated there was no apparent larval character that differentiated pisinid larvae after reviewing larvae of 28 pisinid species. According to , the family Epialtidae must be heterogeneous based on characteristics of the zoeal mouthpart appendages. Based on the adult morphology, Griffin and Tranter (Citation1986) reported that the Scyra could be separated from a subfamily Pisinae because of a truncate male first pleopod with widely flared tip. Kornienko and Korn (Citation2007) reported that zoeae of Pisoides bidentatus and Pugettia quadridens were identical, so they should be assigned to one genus. As shown in , zoeae of Scyra acutifrons (Pisinae) coincide well with those of Pugettia gracilis and P. incisa (Epialtinae) in having an endopod of the maxilla with three setae. Hence, it is found that a distinct group including the genera Pisoides, Scyra, and Pugettia exists in the Epialtidae. This group possesses common characteristics such as endopods of the maxillule and the maxilla with 1, 4 and 3 or 4 setae, a basis of the first maxilliped with 2+2+2+3 (9) setae, and an endopod of the second maxilliped with 0, 1, 4 setation. Therefore, it is suggested that the taxonomic status of the genera Pisoides, Scyra, and Pugettia should be re-examined in the family Epialtidae.
Table 3. Comparisons of the setations of the mouthpart appendages in the known zoeae of the subfamilies Epialtinae and Pisinae (Epialtidae) from the northern Pacific
Rice (Citation1980) provided a key to the brachyuran families based on the zoeal characters. Using his key, the majoid zoeae could be distinguished from most other brachyurans in having no lateral carapace spine, no lateral process on the third abdominal somite, and a telson fork with a single lateral spine. Also, Ko (Citation1998) summarized zoeal characteristics for zoeae of the subfamily Epialtinae as follows: lateral carapace spines absent, an exopod of the antenna nearly equal to the protopodal process with two unequal subterminal setae; a proximal segment of the endopod of the maxillule with or without a seta; setations of the endopod and basis of the first maxilliped as 3, 2, 1, 2, 5 and 2+2+2 (rarely 3)+3; setations of the endopod and basis of the second maxilliped as 0, 1, 4 and 1+1+1; the first abdominal somite with one pair of dorsomedial setae; a lateral process present on the second abdominal somite; each fork of telson spinulate, with a lateral spine. For the identification of the known zoeae in the family Epialtidae the following provisional key is provided from the northern Pacific. The characteristics employed are usually consistent during the zoeal development.
A key to zoeae of 17 known epialtid species from northern Pacific
Proximal segment of endopod of maxillule without seta ------------------------------ 2
– Proximal segment of endopod of maxillule with 1 seta --------------------------------- 4
Dorsal carapace spine present ------------------------------------------------------------- 3
– Dorsal carapace spine absent -------------------------------------- Menaethius monoceros
Distal segment of endopod of maxillule with 2 setae ------ Goniopugettia sagamiensis
– Distal segment of endopod of maxillule with 1+4 setae ---------------- Huenia proteus
Endopod of maxillule with 1, 4 setae ---------------------------------------------------- 5
– Endopod of maxillule with 1, 5 or 6 setae ----------------------------------------------- 13
Endopod of maxilla with 3 setae ---------------------------------------------------------- 6
– Endopod of maxilla with 4 setae ----------------------------------------------------------- 8
Subterminal seta on distal segment of endopod of maxilliped I spinous ------------ 7
– Subterminal seta on distal segment of endopod of maxilliped I plumose --- Pugettia gracilis
Rostral carapace spine ≤ 1/2 length to antennal protopod --------------- Pugettia incisa
– Rostral carapace spine equal length to antennal protopod -------------- Scyra acutifrons
Subterminal seta on distal segment of endopod of maxilliped I spinous ------------ 9
– Subterminal seta on distal segment of endopod of maxilliped I plumose -- Scyra compressipes
Dorsal carapace spine>carapace length ----------------------------- Pisoides ortmanni
– Dorsal carapace spine<carapace length -------------------------------------------------- 10
Anterior base of dorsal carapace spine with chromatophore ------------------------- 11
– Anterior base of dorsal carapace spine without chromatophore --- Pugettia marissinica
Middle of dorsal carapace spine without chromatophore ----------------------------- 12
– Middle of dorsal carapace spine with chromatophore -------------- Pugettia quadridens
Lateral to eye with chromatophore --------------------------------- Pugettia intermedia
– Lateral to eye without chromatophore -------------- Pugettia similis, Pisoides bidentatus
Endopod of maxillule with 1, 6 setae --------------------------------------------------- 14
– Endopod of maxillule with 1, 5 setae -------------------------------- Hyastenus diacanthus
Endopod of maxilliped 2 with 0, 1, 5 setae ----------------------- Hyastenus elongatus
– Endopod of maxilliped 2 with 0, 1, 4 setae ----------------------------------------------- 15
Rostral carapace spine 1/5 length to antennal protopod ------------------- Doclea ovis
– Rostral carapace spine ≤1/2 length to antennal protopod ----------- Phalangipus hystrix
Acknowledgements
H.S.K. gratefully acknowledges Dr. Stephen Sulkin for an invitation as a visiting researcher to Shannon Point Marine Center, USA, from 2003 to 2004. The authors wish to thank Mrs. Se Jin Ok for help with drawings.
References
- Aikawa H. 1929 On larval forms of some Brachyura. Rec Oceanogr Wks Japan 2 : 17 55
- Aikawa H. 1937. Further notes on brachyuran larvae. Rec Oceanogr Wks Japan 9 87 162
- Clark PF Calazans DK Pohle GW 1998 Accuracy and standardization of brachyuran larval descriptions. Invert Reprod Develop 33 127 144
- Gohar HAF Al-Kholy AA 1957 The larvae of four Decapod Crustacea (from the Red Sea). Publ mar boil Sta Al Ghardaqa Egypt 9 177 202
- Griffin DJG Tranter HA 1986 The Decapoda Brachyura of the Siboga Expedition. Part VIII. Majidae. Leiden: Siboga-Expeditie (Monograph) 39, C4, Livraison 1 335
- Jensen GC 1995 Pacific coast crabs and shrimps. Sea Challengers Publication 1 87 , figs 1–163
- Kim HS 1973 Anomura, Brachyura. Illustrated encyclopedia of fauna and flora of Korea. The Ministry of Education, Korea (in Korean) 458 506
- Kim ND Hong SY 1999 Larval development of Scyra compressipes (Decapoda: Brachyura: Majidae: Pisinae) reared in the laboratory. J Crust Biol 19 782 791
- Ko HS 1997 The first zoeal stage of Hyastenus elongatus (Ortmann, 1893) (Decapoda, Brachyura, Majidae). Korean J Syst Zool 13 1 8
- Ko HS 1998 Zoeal development of three species of Pugettia (Decapoda: Majidae), with a key to the known zoeas of the subfamily Epialtinae. J Crust Biol. 18 : 499 510
- Ko HS Hwang SG 1997 The megalopal stages of three Pugettia species (Crustacea: Decapoda: Majidae) reared in the laboratory. Korean J Syst Zool 13 261 270
- Kornienko ES Korn OM 2007 The larvae of the spider crab Pisoides bidentatus (A. Milne-Edwards, 1873) (Decapoda: Majoidea: Pisinae) reared under laboratory conditions. J Plankton Res. 29 : 605 617
- Kurata H. 1969 Larvae of decapod Brachyura of Arasaki, Sagami Bay. IV. Majidae. Bull Tokai Reg Fish Res Lab. 57 : 81 127
- Mohan R Kannupandi T 1985 Life history of laboratory reared spider crab Doclea ovis (Herbst). Indian J Mar Sci 14 24 30
- Ng PKL Guinot DL Davie PJF 2008 Systema Brachyurorum: Part 1. An annotated checklist of extant brachyuran crabs of the world. Raffles Bull Zool 17 1 286
- Oh SM Ko HS 2007 First zoea of Pugettia gracilis (Crustacea: Decapoda: Majidae) reared in the laboratory. Integrative Biosci 11 51 54
- Rice AL 1980 Crab zoeal morphology and its bearing on the classification of the Brachyura. Trans Zool Soc London 25 271 424
- Sakai T. 1976 Crabs of Japan and the adjacent seas Tokyo Kodansa 1 773 pls 1–251
- Santana W Pohle G Marques F 2004 Larval development of Apiomithrax violaceus (A. Milne Edwards, 1868) (Decapoda: Brachyura: Majoidea: Pisidae) reared in laboratory conditions, and a review of larval characters of Pisidae. J Nat Hist 38 : 1773 1797
- Taishaku H Konishi K 2001 Lecithotrophic larval development of the spider crab Goniopugettia sagamiensis (Gordon, 1931) (Decapoda: Brachyura: Majidae) collected from the continental shelf break J Crust Biol 21 748 759
- Terada M. 1981 Zoeal development of six species of crabs in the subfamily Acanthonychinae Res Crust 11 77 85
- Terada M. 1983 Larval development of four Japanese spider crabs (Brachyura, Majidae, Pisinae). Proc Jap Soc Syst Zool Tokyo 25 : 18 30