Abstract
Chunghyul-dan (CHD) is a combinatorial drug known to exert anti-inflammatory effects in endothelial cells. In this study, we employed global transcriptional profiling using cDNA microarrays to identify molecular mechanisms responsible for the anti-inflammatory activity of CHD in endothelial cells. An analysis of the microarray data revealed that transcript levels of monocyte chemotactic protein-1 (MCP-1), vascular cell-adhesion molecule-1 (VCAM-1) and activated leukocyte cell-adhesion molecule were dramatically altered in CHD-treated endothelial cells. These changes in gene expression were confirmed by RT-PCR, Western blotting and ELISA. Chronic CHD treatment also appeared to decrease MCP-1 secretion, probably as a result of decreased MCP-1 expression. In addition, we determined that chronic CHD treatment inhibited lipopolysaccharide-stimulated adhesion of THP-1 leukocytes to endothelial cells. The inhibitory effect of CHD on LPS-stimulated adhesion resulted from down-regulation of VCAM-1 expression. Transmigration of THP-1 leukocytes through endothelial cells was also inhibited by chronic CHD treatment. In conclusion, CHD controls a variety of inflammatory activities by regulating MCP-1 and VCAM-1 gene expression.
Introduction
Chunghyul-dan (CHD) is a combinatorial drug composed of Rhei rhizoma (rhubarb)-derived purgative drugs and orengedokuto (OT), which is composed of Scutellariae radix, Coptidis rhizoma, Phellodendri cortex and Gardeniae fructus. OT is known to improve cerebral blood flow, control blood pressure and possess anti-inflammatory and vasorelaxant activities (Suenaga et al. Citation1991; Kim et al. Citation2002). In previous studies on endothelial cells, CHD has been shown to exert an anti-apoptotic effect and act as a cell-cycle-progression- and cell-migration-promoting agent (Cho et al. Citation2004). These established effects are probably related to the regulation of inflammation, although the molecular mechanisms responsible for the efficacy of CHD in endothelial inflammation have not yet been elucidated.
Inflammatory responses are associated with cardiovascular disease, particularly atherosclerosis. When tissues are wounded by injury or transient infection by microorganisms, a variety of inflammatory responses are triggered in the human body (Ramos et al. Citation1999). During the initial stages of the inflammatory response, leukocytes are recruited to an injured area, where they adhere to the arterial walls and subsequently invade damaged tissues. These early inflammatory responses, including chemotaxis, leukocyte–endothelium adhesion and transmigration of leukocytes, are critical steps in the development of atherosclerosis (Ramos et al. Citation1999; Eriksson et al. Citation2000, Citation2001).
Inflammatory responses are regulated by various chemokines and cytokines, which are found at the site of vascular injury or inflammatory lesions and act as modulators to attract monocytes, leukocytes and/or microphages. Among the chemoattractant molecules known to play a role in inflammatory responses are tumor necrosis factor alpha (TNFα), monocyte chemotactic protein-1 (MCP-1) and macrophage inflammatory protein 1 alpha (MIP-1α) (Charo and Taubman Citation2004). TNFα also plays a role in regulating leukocytic adhesion to the endothelium, as do other pro-inflammatory factors such as interleukin-8 and lipopolysaccharides (LPS) (Mahalinagam and Karupiah Citation1999). Thus, their role in controlling initial inflammatory steps makes such chemokines and cytokines important players in early atherosclerotic development.
Transendothelial leukocytic migration is one essential step in the multistep inflammatory process that leads to atherosclerosis. Endothelial cells form a continuous layer lining blood vessels, separating the vascular walls from the vessel lumen. Owing to their location, endothelial cells are directly exposed to various circulating blood cells, including leukocytes and erythrocytes. Before leukocytes can infiltrate into tissue, they must first adhere to endothelial cells. Endothelial adherence is itself a multistep process that involves rolling, arrest and spreading; it is followed by secretion of proteases that degrade the basement membrane, a process known as diapedesis (Charo and Taubman Citation2004). Decades of research have spawned numerous efforts to develop therapeutic drugs that prevent one or more of the multiple atherosclerotic developmental steps. However, few preventive medicines capable of inhibiting atherosclerotic development have been developed.
Here, we used a variety of experimental approaches to understand the cellular and molecular bases for the anti-inflammatory effects of CHD. This study may provide mechanistic insights that facilitate the development of more effective medicines to prevent chronic inflammatory vascular diseases.
Materials and methods
Cell culture
Bovine aortic endothelial cells (BAECs) obtained from descending thoracic aortas were cultured in Dulbecco's Modified Essential Medium (DMEM 1 g/L glucose, WelGENE Inc.) containing 20% fetal bovine serum (FBS, WelGENE Inc.) and antibiotics (Jo et al. Citation1997; Karin et al. Citation2006). Human umbilical vein endothelial cells (HUVEC, Cambrex) were plated on gelatin (0.2% w/v)-coated dishes (Sigma) and grown to ~90% confluency in endothelial cell basal medium (EBM-2, Cambrex) containing 10% FBS and endothelial growth factors (Cambrex). Human acute monocytic leukemia cells (THP-1) were grown in RPMI1640 medium (WelGENE Inc.) supplemented with 10% FBS and antibiotics (Shin et al. Citation2006). All cells in this study were maintained at 37°C in a humidified 5% CO2 atmosphere and used between passages three and ten.
DNA microarray
Confluent HUVECs were serum-starved for at least 16 h, and then treated with 2 µg/mL CHD for 0.5–4 h. Total RNA was then isolated using the TRIzol reagent, as described by the manufacturer (Invitrogen), and 5 µg of purified RNA was reverse transcribed to produce cDNA. Biotin-labeled cRNA was synthesized from cDNA using the Illumina Amplification Kit (Ambion), purified with RNeasy kit (Qiagen), and hybridized onto Sentrix Human Ref-6-V2 Expression Bead Chips (Illumina) containing gene-specific oligonucleotide probes (50-mers) for more than 45,000 genes. Hybridization and other microarray processes were performed as described in the Illumina BeadStation 500 X Manual. Chips were scanned using a confocal laser scanner (Illumina BeadArray Reader) and signal values were normalized using the quantile normalization method. Gene profiles were analyzed using BeadStudio software, and Avadis Prophetic version 3.3 (Strand Genomics) was used for statistical analyses.
Reverse transcription-polymerase chain reaction (RT-PCR)
To confirm the cDNA microarray data, total RNA was extracted from BAECs and reversed transcribed, as described above. After reverse transcription, cDNAs were amplified by PCR using the following conditions: denaturation at 95°C for 30 s, annealing at 65°C for 30 s, and extension at 72°C for 30 s. The following specific primers were used:
VCAM-1: 5′-GAGCCCAGTGAGTTTTGAGAACGA-3′ (forward)
5′-CAGCTTACAGTGACTGGGTTTCCA-3′ (reverse)
MCP-1: 5′-AAGGAGTTATGTGCAGACCCCAAG-3′ (forward)
5′-TATCAGAGGGCAGTTAGGGAAAGC-3′ (reverse)
ALCAM: 5′-TTCCGAAGCCAGCTATACAGTGGA-3′ (forward)
5′-TATCTCGTCTGCCTCATCGTGTTC-3′ (reverse)
GAPDH: 5′-CCAACGTGTCTGTTGTGGATCTGA-3′ (forward)
5′-CAACCTGGTCCTCAGTGTAGCCTA-3′ (reverse)
GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA was used as an internal control.
Preparation of cell lysates
Endothelial cells were washed with ice-cold phosphate-buffered saline (PBS), scraped into 0.25 ml lysis buffer (10 mM Tris, pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS), and solubilized for 60 min as previously described (Park et al. Citation1998). The entire solubilization procedure was performed at 4°C and total protein concentrations in soluble cell lysates were measured using a Bio-Rad DC assay kit (Bio-Rad).
Western blotting
To determine changes in expression levels, endothelial cells grown to confluence and serum starved for 15 h were treated with 2 µg/mL CHD in serum-free media, harvested and lysed. Soluble lysates (25 µg each) were resolved by SDS-PAGE on 10% gels, transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad), and probed with VCAM-1 antibody (R&D Systems) as previously described (Park et al. Citation1998). Horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG; Santa Cruz) was used as a secondary antibody. Reactive proteins were visualized using a chemiluminescence detection method (Park et al. Citation1998).
Enzyme-linked immunosorbent assay (ELISA)
To determine secretion of MCP-1 and ALCAM, endothelial cells were grown, serum starved, and treated with 2 µg/mL CHD as described above, and the medium was collected and concentrated using an Amicon ultra-centrifugal filter device (Millipore). ELISAs were performed according to the manufacturer's instructions (Santa Cruz, R&D Systems). Antibodies against MCP-1 (Santa Cruz) and ALCAM (R&D Systems) were used as primary antibodies, and horseradish peroxidase-conjugated goat anti-mouse or mouse anti-goat antibodies were used as secondary antibodies. After incubating protein-bound secondary antibodies with a TMB (tetramethyl benzidine) substrate solution (GenDEPOT) for 60 min, protein concentrations were determined spectrophotometrically by measuring optical density at 650 nm using a microplate reader (Bio-Rad).
Cell adhesion
Endothelial cells were grown to ~90% confluency in 6-well plates and cultured in starvation medium for 6 h. CHD (2 µg/mL) and/or LPS (1 µg/mL) was then added and cells were incubated for an additional 6 h at 37°C in a humidified 5% CO2 atmosphere. The endothelial cells were then thoroughly washed with warm PBS, and 5 µg/mL antibodies (R&D Systems) specific for VCAM-1 or ALCAM, or non-immune immunoglobulins (IgG) were added. After incubating endothelial cells with antibodies for 1 h, THP-1 cells, previously grown to 5–6×106 cells/mL and stained with 10 µM calcein AM (Sigma) for 45 min according to the method of Shin et al. (Citation2006), were added to the endothelial cells and incubated for an additional 1 h. Unattached cells were then removed by thoroughly washing, and adhesive cells were observed under a fluorescence microscope (Zeiss Autoplan2). Adherence was quantified by counting the adhesive cells in a representative area using a modification of the method of Takeda et al. (Shin et al. Citation2006). The degree of adherence was expressed as a percentage of adhesive THP-1 cells.
Transmigration of THP-1
Cell migration assays were performed using a Transwell apparatus (24-well chamber) with polycarbonate membranes (8.0 µm pore size, Costar) as previously described, with minor modifications (Park et al. Citation2002). Endothelial cells suspended in growth medium were added to the upper chamber at 1×105 cells/well and incubated for 5–36 h. After serum starving for 16 h, various amounts of CHD were added to the upper chambers and cells were incubated for an additional 4 h. Endothelial cells were then thoroughly washed with serum-free media and mixed with THP-1 cells suspended in serum-free RPMI medium. THP-1 cells were allowed to migrate for 7 h, and then migrant cells (i.e. those attached to the lower surface) were fixed with 100% methanol, visualized by hematoxylin (Sigma) staining, and counted under a microscope. All incubations were performed at 37°C in a humidified 5% CO2 incubator.
Statistical analysis
Statistical analyses were conducted using SPSS version 13.0 (SPSS, Inc.). ANOVA and Duncan's multiple range tests were used to determine differences among three or more groups. When two groups were compared, unpaired t-tests were used.
Results
DNA microarray analysis suggests that CHD acts as an anti-inflammatory drug
To determine whether CHD modulates the expression of genes involved in vascular functions, we compared the transcriptional profiles of CHD-treated endothelial cells with those of untreated controls using a cDNA microarray. Preliminary time-course experiments revealed significant differences in the expression of multiple genes in HUVEC cells incubated with CHD for>4 h (unpublished data). Classification of these expression changes into functional groups using the PANTHER Classification System (Thomas et al. Citation2003; Mi et al. Citation2007) showed that there were no unique expressional alterations in certain functional groups (unpublished data). Thus we selected genes for further study from among those chemokines involved in controlling chemotaxis in the vascular system, grouped on the basis of previously published classifications (Ikeda Citation2003; Sheikine and Hansson Citation2004; Ardigo et al. Citation2007; Sertic et al. Citation2007). Among the genes in this group with the greatest change in expression () was chemokine (C-C motif) ligand 2 (CCL2)/MCP-1. We also assessed transcriptional levels in the cell adhesion molecule (CAM)-associated functional group classified by the PANTHER Classification System (). Cell-to-cell adhesion is an essential process in the initial inflammatory response, so an assessment of transcriptional changes in the CAM-associated group would potentially reveal molecular mechanisms that underlie the vascular anti-inflammatory efficacy of CHD. Among the genes in the CAM-associated functional group most altered by chronic CHD treatment were activated leukocyte cell adhesion molecule (ALCAM) and vascular cell adhesion molecule 1 (VCAM-1), transcript levels of which decreased by 2.5- and 2.0-fold, respectively (). Collectively, these data suggest that the anti-inflammatory activities of CHD are due to its regulation of gene expression.
Table 1. Chunghyul-dan (CHD)-dependent fold-changes of genes in vascular function-related chemokines and cytokines.
Table 2. Chunghyul-dan (CHD)-dependent fold-changes of genes in a CAM family adhesion molecules.
Decreases in the levels of MCP-1, ALCAM and VCAM-1 were confirmed by RT-PCR, Western blotting and ELISA analyses, which showed that expression was decreased at both the mRNA and protein levels (). The levels of MCP-1, ALCAM and VCAM-1 proteins detected in cell lysates were decreased by 30–50% in endothelial cells pretreated with CHD.
Figure 1. CHD down-regulates the expression of genes responsible for inflammatory responses. Confluent HUVECs and BAECs were incubated with or without CHD (2 µg/mL) for 4–12 h. (A) BAECs untreated or treated with CHD for 4 h were lysed and mRNAs was extracted and used for RT-PCR. (B–D) Cells untreated or treated with CHD for 12 h were lysed, and protein levels were determined by ELISA (B, MCP-1; C, ALCAM) and Western blotting (D, VCAM-1). In panels B–D, expression levels are represented as bar graphs (mean±SE, n=3, *P<0.01).
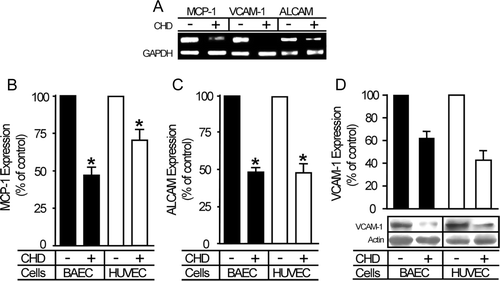
These analyses thus identify MCP-1, VCAM-1 and ALCAM as candidate molecular mediators of CHD efficacy in inflammatory diseases. Accordingly, CHD may exert its inhibitory effect on inflammation in the vascular system by down-regulating MCP-1, ALCAM and VCAM-1, which have important roles in both monocytic recruitment and adhesion to vascular injury sites (Schepers et al. Citation2006).
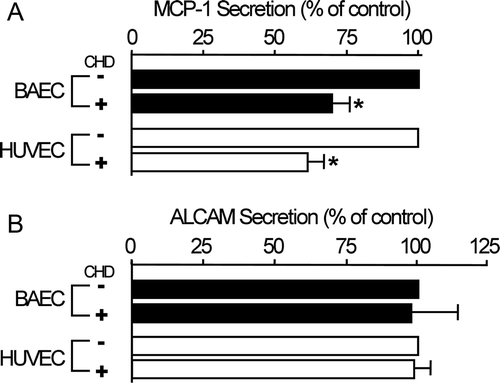
MCP-1 secretion is diminished by chronic CHD treatment
We then tested whether decreases in MCP-1 expression were linked to MCP-1 secretion. ELISA assays for two secretory proteins (MCP-1 and ALCAM) among previous target molecules revealed that chronic CHD treatment reduced the amount of extracellular MCP-1 by 30–40%, but had no effect on extracellular ALCAM levels (). Secretion of the secretory protein ALCAM (Masedunskas et al. Citation2006) was not changed by chronic CHD treatment, so these results indicate that CHD-dependent inhibition of MCP-1 secretion is not due to cell death. Although the detailed mechanism by which CHD inhibits MCP-1 secretion remains unknown, these findings nonetheless suggest that CHD-dependent regulation of MCP-1 expression may contribute to a decrease in MCP-1 secretion. This model is supported by the observation that acute CHD treatment (5–30 min) had no effect on the level of secreted MCP-1 (data not shown), which suggests that CHD-induced reduction of extracellular MCP-1 is chronically attained by unknown mechanisms. The fact that MCP-1 secretion appeared to be reduced to a lesser extent than MCP-1 expression suggests that a reduction in MCP-1 expression possibly plays a part in the CHD-induced reduction in MCP-1 secretion.
Figure 2. Extracellular MCP-1 levels are decreased by CHD treatment. Confluent HUVECs and BAECs were incubated with or without CHD (2 µg/mL) in serum-free media for 16 h. Extracellular media were harvested and concentrated, and MCP-1 and ALCAM levels were determined by ELISA. Data were obtained from three independent experiments (mean±SE, n=3, *P<0.01).
CHD blocks LPS-induced adhesion of leukocytes to endothelial cells
Both VCAM-1 and ALCAM are known as cell adhesion molecules (Masedunskas et al. Citation2006) and the expression of both was regulated by CHD, as shown in . Thus, it is highly possible that VCAM-1 and/or ALCAM play an important role in leukocytic adhesion. To test this possibility, we evaluated the effect of CHD on the adhesion of leukocytes to endothelial cells in the presence or absence of the pro-inflammatory agent LPS. In the absence of LPS pre-treatment, chronic CHD treatment had little or no effect on leukocytic adhesion (). However, LPS-induced adhesion of THP-1 cells to endothelial cells was substantially inhibited by chronic CHD treatment (A–C).
Figure 3. CHD inhibits LPS-induced adhesion of leukocytes to endothelial cells. BAECs or HUVECs were serum-starved for at least 6 h and then cells were incubated with or without 1 µg/mL LPS alone or co-treated with the indicated concentrations of CHD for 6 h. (A) After staining with 10 µM calcein AM, THP-1 cells (5–6×106 cells/mL) were added to LPS-pretreated or untreated BAECs incubated with or without 2 µg/mL CHD. After incubating for 1 h, adherent cells were observed under a fluorescence microscope. Bar graphs in panels B (BAECs) and C (HUVECs) show percent adhesion (mean±SE, n=6, *P<0.01). (D) Proteins in BAEC cell lysates were resolved by SDS-PAGE and transferred to a PVDF membrane. VCAM-1 was monitored by Western blotting using antibodies for VCAM-1.
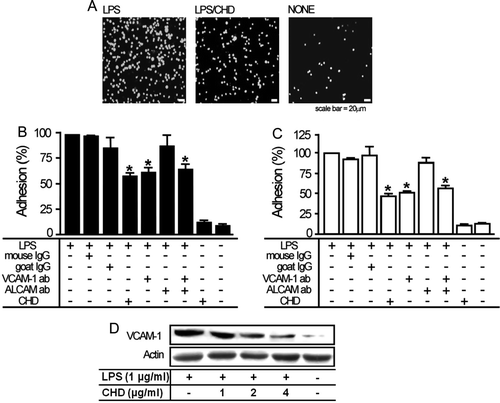
As shown in B and C, the inhibitory effect of CHD on LPS-induced THP-1 adhesion was not seen by pretreatment with antibodies against VCAM-1, but not ALCAM. These data indicate that adhesion of THP-1 leukocytes to endothelial cells was specifically mediated by VCAM-1. Based on our finding that CHD inhibited VCAM-1 expression (see ), we hypothesized that CHD-induced inhibition of leukocytic adhesion was mainly due to a decrease in VCAM-1 expression. To test this hypothesis, we first confirmed that LPS enhanced VCAM-1 expression (D), consistent with previous reports (Gupta et al. Citation2005). Importantly, chronic CHD treatment reduced LPS-induced upregulation of VCAM-1 expression (D), consistent with a model in which chronic CHD treatment regulates the adhesion of leukocytes to the endothelium by regulating VCAM-1 expression.
CHD reduces the transmigration of THP-1 cells through endothelial cells
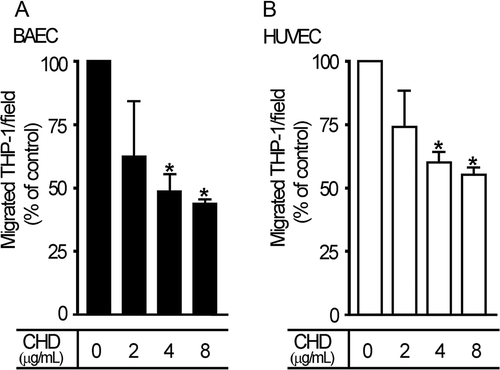
MCP-1 is known to induce transmigration of leukocytes and monocytes through the endothelium to the interstitial intima of blood vessels (Navab et al. Citation1991; Weiss et al. Citation1999). Chronic CHD treatment reduced MCP-1 expression, so we tested whether transmigration of THP-1 cells was affected by chronic CHD treatment, using serum to stimulate transmigration of THP-1 cells. The degree of THP-1 cell transmigration was decreased (by 50–70%) by pretreatment of endothelial cells with CHD (), lending further support to the anti-inflammatory effects of CHD in the vascular system.
Figure 4. CHD decreases serum-induced transmigration of THP-1 cells. Endothelial cells, added to the upper chamber of a Transwell apparatus and allowed to grow, were incubated with THP-1 cells, and transmigrating cells were subsequently identified and quantified as described in the Materials and methods. Migrant cells were counted in high-power fields. Degree of migration (% of control) is shown in bar graphs (mean±S.E., n=3, *P<0.05). A, BAECs; B, HUVECs.
Discussion
The combinatorial drug CHD was developed to improve the efficacy and reduce the side effects (such as constipation) of OT alone (Kim et al. Citation2002). A previous report has established that CHD mediates a variety of cellular functions, including cell migration, apoptosis and cell proliferation (Cho et al. Citation2004). In the current study, we found an additional function of CHD, namely regulation of the expression of various genes involved in inflammation. This new role for CHD, as well as previously established functions, is critical in controlling vascular remodeling, inflammation, wound healing and angiogenesis. These processes play crucial roles in vascular physiology and pathophysiology (Davies Citation1995; Stary et al. Citation1995), so CHD is predicted to be useful against a broad spectrum of cardiovascular conditions. In addition, our findings provide a molecular basis for the efficacy of CHD.
A clinical study has shown that CHD decreases stroke recurrence (Park et al. Citation2006). Our new finding that chronic CHD treatment blocks LPS-induced inflammatory responses in endothelial cells supports this clinical observation. The established vascular effects of CHD, such as cell migration, anti-apoptosis and cell proliferation, could be expected to contribute to the healing of lesions in damaged blood vessels. In addition to these actions, we found that CHD has effects on various steps in the vascular anti-inflammatory response, including inhibition of chemotaxis, leukocytic adhesion and transmigration. Such inflammatory processes represent the initial events in cerebral and myocardial infarctions, suggesting a mechanism by which CHD might improve arterial stiffness and decrease stroke recurrence (Park et al. Citation2006).
Using a diversity of experimental approaches, we showed that chronic CHD treatment reduced the expression of MCP-1 and VCAM-1, providing a likely mechanism for the vascular anti-inflammatory functions of this agent. The potentially crucial roles of MCP-1 and VCAM-1 in the efficacy of CHD suggest that monitoring changes in these target molecules might be a good model for studying the anti-inflammatory mechanisms of other natural compounds (Dattner Citation2003).
The cDNA microarray results showed that CHD regulates a broad spectrum of genes. We clustered tens of thousands of genes into several different groups based on their biological processes. Most differentially expressed genes are functionally unknown, so it is difficult to identify new functions for CHD using broad-based cDNA microarray screens. Accordingly, transcriptional profiling within pre-selected functional subgroups provides a better opportunity to establish new functional roles for an incompletely characterized agent, such as CHD. Our findings suggest that CHD exerts its functions by regulating the expression of various genes. Because of this mode of action, CHD efficacy is expected to develop more slowly relative to that of many other medically useful compounds.
In conclusion, the current study demonstrates the anti-atherogenic effects of CHD and suggests possible new applications for CHD in cardiovascular diseases, such as hypertension and atherosclerosis.
Acknowledgements
This work was supported by a Korea Science and Engineering Foundation (KOSEF) grant funded by the Korean Government (MOST) (M10527010002-07N2701-00210)
References
- Ardigo , D , Assimes , TL , Fortmann , SP , Go , AS , Hlatky , M , Hytopoulos , E , Iribarren , C , Tsao , PS and Tabibiazar , TR. 2007 . Quertermous; ADVANCE Investigators. Circulating chemokines accurately identify individuals with clinically significant atherosclerotic heart disease . Physiol Genomics. , 31 : 402 – 409 .
- Charo , IF and Taubman , MB. 2004 . Chemokines in the pathogenesis of vascular disease . Circ Res. , 95 : 858 – 866 .
- Cho , KH , Jung , WS , Park , SU , Moon , SK , Ko , CN , Ku , S , Chi , SG and Park , H. 2004 . Daio-Orengedokuto works as a cell-proliferating compound in endothelial cells . Can J Physiol Pharmacol. , 82 : 380 – 386 .
- Dattner , AM. 2003 . From medical herbalism to phytotherapy in dermatology: back to the future . Dermatol Ther. , 16 : 106 – 113 .
- Davies , PF. 1995 . Flow-mediated endothelial mechanotransduction . Physiol Rev. , 75 : 519 – 560 .
- Eriksson , EE , Werr , J , Guo , Y , Thoren , P and Lindbom , L. 2000 . Direct observations in vivo on the role of endothelial selectins and alpha(4) integrin in cytokine-induced leukocyte-endothelium interactions in the mouse aorta . Circ Res. , 86 : 526 – 533 .
- Eriksson , EE , Xie , X , Werr , J , Thoren , P and Lindbom , L. 2001 . Direct viewing of atherosclerosis in vivo: plaque invasion by leukocytes is initiated by the endothelial selectins . FASEB J. , 15 : 1149 – 1157 .
- Gupta , H , Dai , L , Datta , G , Garber , DW , Grenett , H , Li , Y , Mishra , V , Palqunachari , MN , Handattu , S , Gianturco , SH , Bradley , WA , Anantharamaiah , GM and White , CR. 2005 . Inhibition of lipopolysaccharide-induced inflammatory responses by an apolipoprotein AI mimetic peptide . Circ Res. , 97 : 236 – 243 .
- Ikeda , U. 2003 . Inflammation and coronary artery disease . Curr Vasc Pharmacol. , 1 : 65 – 70 .
- Jo , H , Sipos , K , Go , YM , Law , R , Rong , J and McDonald , JM. 1997 . Differential effect of shear stress on extracellular signal-regulated kinase and N-terminal Jun kinase in endothelial cells. Gi2- and Gbeta/gamma-dependent signaling pathways . J Biol Chem. , 272 : 1395 – 1401 .
- Karin , M , Lawrence , T and Nizet , V. 2006 . Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer . Cell. , 124 : 823 – 835 .
- Kim , YS , Jung , EA , Shin , JI , Chan , JC , Yang , HK , Kim , NJ , Cho , KH , Bae , HS , Moon , SK and Kim , DH. 2002 . Daio-Orengedokuto inhibits HMG-CoA reductase and pancreatic lipase . Biol Pharm Bull. , 25 : 1442 – 1445 .
- Mahalinagam , S and Karupiah , G. 1999 . Chemokines and chemokine recpetors in infectious diseases . Immunol Cell Biol. , 77 : 469 – 475 .
- Masedunskas , A , King , JA , Tan , F , Cochran , R , Stevens , T , Sviriov , D and Ofori-Acquah , SF. 2006 . Activated leukocyte cell adhesion molecule is a component of the endothelial junction involved in transendothelial monocyte migration . FEBS Lett. , 580 : 2637 – 2645 .
- Mi , H , Guo , N , Kejariwal , A and Thomas , PD. 2007 . PANTHER version 6: protein sequence and function evolution data with expanded representation of biological pathways . Nucleic Acids Res. , 35 : D247 – D252 .
- Navab M Imes SS Hama SY Hough GP Ross LA Bork RW Valente AJ Berliner JA Drinkwater DC Laks 1991 Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein J Clin Invest 88 2039 2046
- Park , H , Go , YM , St John , PL , Maland , MC , Lisanti , MP , Abrahamson , DR and Jo , H. 1998 . Plasma membrane cholesterol is a key molecule in shear stress-dependent activation of extracellular signal-regulated kinase . J Biol Chem. , 273 : 32304 – 32311 .
- Park , SG , Kang , YS , Ahn , YH , Lee , SH , Kim , KR , Kim , KW , Koh , GY , Ko , YG and Kim , SH. 2002 . Dose-dependent biphasic activity of tRNA synthetase-associating factor, p43, in angiogenesis . J Biol Chem. , 277 : 45243 – 45248 .
- Park , SU , Jung , WS , Moon , SK , Ko , CN , Cho , KH , Kim , YS and Bae , HS. 2006 . Chunghyul-dan (Qingxie-dan) improves arterial stiffness in patients with increased baPWV . Am J Chin Med. , 34 : 553 – 563 .
- Ramos , CL , Huo , Y , Jung , U , Ghosh , S , Manka , DR , Sarembock , IJ and Ley , K. 1999 . Direct demonstration of P-selectin- and VCAM-1-dependent mononuclear cell rolling in early atherosclerotic lesions of apolipoprotein E-deficient mice . Circ Res. , 84 : 1237 – 1244 .
- Schepers , A , Eefting , D , Bonta , PI , Grimbergen , JM , de Vries , MR , van Weel , V , de vries , CJ , Egashira , K , van Bockel , JH and Quax , PH. 2006 . Anti-MCP-1 gene therapy inhibits vascular smooth muscle cells proliferation and attenuates vein graft thickening both in vitro and in vivo . Arterioscler Thromb Vasc Biol. , 26 : 2063 – 2069 .
- Sertic J Xj V Bozina N Malencia B Kes P Reiner Z. 2007 Cytokines and growth factors in mostly atherosclerotic patients on hemodialysis determined by biochip array technology Clin Chem Lab Med 45 1347 1352
- Sheikine , Y and Hansson , GK. 2004 . Chemokines and atherosclerosis . Ann Med. , 36 : 98 – 118 .
- Shin , J , Kim , J , Ryu , B , Chi , S and Park , H. 2006 . Caveolin-1 is associated with VCAM-1 dependent adhesion of gastric cancer cells to endothelial cells . Cell Physiol Biochem. , 17 : 211 – 220 .
- Stary HC Chandler AB Dinsmore RE Fuster V Glagov S Insull W Jr Rosenfeld ME Schwartz CJ Wagner WD Wissler RW. 1995 A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis Circulation 92 : 1355 1374
- Suenaga T Shirakawa T Harada Tao C Yamasaki M Jomatsu H Matsumoto N Kida M Kawamoto Y Murakami Y. 1991 Mechanism of protective effects of ouren-gedoku-to and san'ou-syashin-to on the gastric mucosa Nippon Yakurigaku Zasshi 98 319 325
- Thomas PD Campbell MJ Kejariwal A Mi H Karlak B Daverman R Diemer Muruganujan A Narechania A. 2003 PANTHER: a library of protein families and subfamilies indexed by function Genome Res 13 2129 2141
- Weiss , JM , Nath , A , Major , EO and Berman , JW. 1999 . HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood-brain barrier and up-regulates CCR5 expression on human monocytes . J Immunol. , 163 : 2953 – 2959 .