Abstract
The decrease in sperm quality under heat stress causes a great loss in animal husbandry production. In order to reveal the mechanism underlying the sperm quality decrease caused by heat stress, we first established a mild heat-treated mouse model. Then, the sperm quality was identified. Further, the testicular proteome profile was mapped and compared with the control using 2D electrophoresis and mass spectrometry. Finally, the differential expressed proteins involved in the heat stress response were identified by real-time PCR and Western blotting. The results showed that heat stress caused a significant reduction in mouse sperm quality (P<0.05). Further, 52 protein spots on the 2D gel were found to differ between the heat-shocked tissues and the control. Of these spots, some repair proteins which might provide some explanation for the influence on sperm quality were found. We then focused on Bag-1, Hsp40, Hsp60 and Hsp70, which were found to be differently expressed after heat shock (P<0.05). Further analysis in this heat-shocked model suggests numerous potential mechanisms for heat shock-induced spermatogenic disorders.
Introduction
Temperature plays an important role in animal reproductive performance. It has been proven that heat stress has a negative impact on male fertility characterized by testicular weight loss and a period of infertility, followed by a gradual return to normality over a period of one to two spermatogenic cycles (Setchell et al. Citation1988; Okado-Matsumoto and Fridovich Citation2001; Naz and Rajesh Citation2004). Moreover, prevention measures controlling the heat stress are suggested. Spermatogenesis is the process by which stem cells develop into mature spermatozoa, characterized by three phases: spermatocytogenesis (mitosis), meiosis and spermiogenesis (Setchell et al. Citation1988; Maxwell and Johnson Citation1997; Holstein et al. Citation2003). Isolation and identification of the spermatogenesis-related proteins and further functional studies are very important for the elucidation of the molecular mechanism involved in meiosis and spermiogenesis. Previous research has shown that many testis-specific proteins are required for spermatogenesis, but the differential proteomic information involved in heat treatment of testicular tissue is still unclear.
In this study, we characterized cellular and molecular mechanisms involved in spermatogenesis following mild heat exposure of adult male mice. First, we have established an animal model which is different from the previous partial heating model in which only the testes were immersed into the warm water at 42°C (Zhu et al. Citation2006), using instead an intelligent temperature-controlled incubator. The temperatures were adjusted to 37°C for 12 h and 35°C for 12 h as one cycle to imitate the hot summer weather. Then, after observation of the bad effects of heat stress on sperm quality, we identified proteins with altered expression in mouse testes at different time intervals up to 5 days later using 2D electrophoresis and mass spectrometry (MS). Finally, the most probable proteins related to the response to heat stress were analyzed by real-time PCR and Western blotting.
Materials and methods
Animals
Animal experiments were done in accordance with the guidelines on animal care and use established by the Jilin University Animal Care and Use Committee. Adult male ICR mice (8 weeks old) bought from the Laboratory Animal Center, Jilin University, were maintained under a controlled environment with a 12 h/12 h light/dark cycle, and allowed access to standard laboratory chow and water ad libitum. Six of these mice were picked randomly as a control group to construct a reference map for the heat-induced differential expression analysis. The rest were maintained in the intelligent temperature-controlling incubator at 37°C. Then the mice were sacrificed after 1, 3, 5 or 7 heat exposure cycles (six animals per time phase).
Sperm quality and fertility status analysis
Virgin female mice pretreated by the intraperitoneal injection of pregnant mare serum gonadotropin (PMSG, 7.5 IU per mouse) and human chorionic gonadotropin (HCG, 7.5 IU per mouse) were paired with proven breeder males just prior to the end of the daily light cycle. The next morning, each female was examined for the presence of the ejaculatory plug in the vagina. The presence of the plug indicates that coitus has occurred, which would serve as an index of the fertility status of the sperm (WHO 1999; Watson 2000). The quality of the epididymal sperms was examined in the First Affiliated Hospital of Jilin University (Changchun).
Protein extraction
Mouse testes obtained at the above-noted time points were collected and extracted in a lysis buffer (8 M urea, 4% (w/v) CHAPS, 2% (w/v) DTT, 2% (v/v) IPG buffer, pH 3–10) in the presence of a 1% (v/w) protease inhibitor cocktail (Pierce, USA). The mixture was kept shaking at 4°C for 1 h and insoluble molecules were removed by centrifugation at 40,000×g, 4°C for 1 h. The protein concentration in each sample was determined by the Bradford method.
Two-dimensional electrophoresis
IPG strips (length, 18 cm; pH 3–10, nonlinear) loaded with 1.50 mg of protein extracted from testicular tissue were rehydrated. After isoelectric focusing, the IPG strips were equilibrated, and run in an Ettan DALT 12 electrophoresis system (GE Healthcare, USA). Then the gel was visualized by Coomassie staining as described previously (Wang et al. Citation2005). In this experiment, data were generated from three independently performed gels at each time-point.
Statistical analysis
The stained gels were scanned, and the spots were detected, quantified, and analyzed comparatively with ImageMasterTM2D Platinum Software (Version5.0, Amersham Bioscience) as described previously (Zhu et al. Citation2006). The amount of each protein spot was expressed as its volume, which was calculated as the volume above the spot border and situated at 75% of the spot height (measured from the peak of the spot). To reflect the quantitative variations in the protein spot volumes, we normalized the spot volumes as a percentage of the total volume of all the spots present in a gel. The values obtained from all experiments were pooled for the calculation of the mean and the standard derivations. Protein spots differentially changed across time-points were examined using one-way ANOVA and LSD multiple comparisons (SPSS 12.0 software, USA). A P value<0.05 is considered as a statistically significant difference.
Protein identification by mass spectrometry
The differential protein spots were excised and sent for mass spectrometry analysis (National Center of Biomedical Analysis, China). The identified proteins obtained from MS were further analyzed.
RNA extraction, reverse transcription (RT) and quantitative real-time PCR
Total RNA was extracted from pools of the mouse testes (three for each group) on post-heat-treatment days 0, 1, 3, 5 and 7 using Simply P Total RNA Extraction Kit (BioFlux). For the synthesis of the ?rst-strand cDNA, RNA was reverse transcribed by incubation with oligo(dT) primers, random hexamers and reverse transcriptase (TaKaRa), typically using 1g total RNA per reaction (RT-PCR kit from TaKaRa).
Quantitative PCR was performed in an Applied Biosystems Prism 7000 instrument using the SYBR® Prime-Script™ RT-PCR Kit II (TaKaRa) with oligonucleotide primers to detect Bag-1, Hsp40, Hsp60 and Hsp70 in the mouse testes and a housekeeping gene, β-actin, which was used to normalize the total mRNA level. Primer sequences were designed by TaKaRa and are shown in . All samples were performed in triplicate, and each mRNA quantification represents an average of at least three measurements.
Table 1. Primer sequences used in real time PCR
Western blotting
Samples containing 50 µg of protein from the normal and heat-treated mouse testes were electrophoresed on 12% SDS polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were blocked in phosphate-buffered saline (PBS) containing 5% nonfat milk powder for 1 h and then incubated overnight with rabbit anti-mouse Bag-1, Hsp40, Hsp60 and Hsp70 (1:100) or β-actin (1:500) polyclonal antibodies (Beijing Biosynthesis Biotechnology Co., Ltd). The membranes were washed three times (10 min each) with PBS, then incubated for 1 h with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG. Specific proteins were detected using a DAB kit. The protein expression level was analyzed using AlphaEaseFC software.
Results
Influence of heat stress on sperm quality
Our study found that when the male mice were exposed to high temperature (37°C), the sperm quality and the fertility status were decreased significantly, and became more and more affected with increasing exposure time, evidenced by the decrease in sperm concentration (P<0.01), sperm motility (P<0.05), percentage of the live sperms (P<0.05), and the percentage fertility (P<0.05), and also, the increase in the percentage of abnormal sperms (P<0.05) ().
Table 2. Sperm quality and fertility status of the normal (control) and heat shocked (37 °C) mice (mean±SD, 10 animals per group at each time point)
Identification of proteins expressed differentially after heat treatment
For the heat exposure and the deterioration of the sperm quality, we suspected that there would be many protein changes accompanying the physiological changes. So we constructed triplicate 2D maps of the mixed mouse testes samples (six mice per group) from four heat exposure time points (days 1, 3, 5 and 7; ). We identified 52 protein spots with significant differences in the expression levels relative to the untreated control groups during the 7 days post heat treatment (P<0.05). Also, after comparing with the 2D mouse reference map on line, we retrieved 21 spots from the 2DE database. One spot (ID number 2332) possibly contains two different proteins, according to the Mascot search results. We also identified several proteins as the same protein with different molecular weights and pIs in the 2D map, which were expressed differentially after heat treatment. These included 40 kDa, 60 kDa and 70 kDa heat shock proteins, as well as Bag-1. In total 37 proteins showed an expression peak between days 1 and 7. An overview of these proteins is presented in and , which include accession numbers, protein names, expression changes (fold change) and classfications of the protein spots in the control and heat-treated mouse testis gels.
Figure 1. Heat-shocked testes (7 d) and control 2DE maps. Left: a representative 2D map of the control testes. Right: a representative 2D map of the heat-shocked testes on day 7.
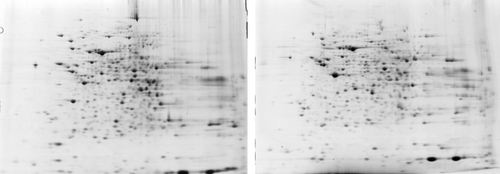
Table 3. Up-regulated genes in adult mouse testis on d 7 after heat shock
Table 4. Down-regulated genes in adult mouse testis on d 7 after heat shock
Differential expressions of Bag-1, Hsp40, Hsp60 and Hsp70 after heat shock
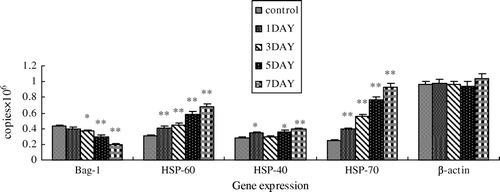
We further studied the mRNA and protein expressions of Bag-1, Hsp40, Hsp60 and Hsp70 in normal and heat-shocked mouse testes with real-time PCR and Western blotting. For the mRNA level, real-time PCR revealed that mice testes exerted significant down-regulation on Bag-1 mRNA expression (by 53.84%, P<0.05) and up-regulation of Hsp40, Hsp60, Hsp70 mRNA expression (by 38.39%, P<0.05; 125.28%, P<0.05; and 274.32%, P<0.05, respectively) relative to the control on day 7. The mRNA expression trends of the selected genes at different heat exposure levels (0, 1, 3, 5, 7 post-heated days) are shown in . For the protein level, as shown in , heat shock resulted in a significant decrease of Bag-1, and a significant increase of Hsp40, Hsp60 and Hsp70. The change in all of them became more and more serious with time. Bag-1 was significantly reduced by about 50% on day 7 compared with the control group (P<0.05). Meanwhile, Hsp40, Hsp60 and Hsp70 increased about 5.2-, 5.3- and 0.8-fold respectively (P<0.05). Comparing the mRNA and protein changes of the above genes, we found that the protein levels changed more than the mRNA level in the mouse testes, suggesting that the heat shock affects the related gene release more than their biosynthesis.
Figure 2. Changes of the protein expressions of Bag-1 and Hsps in mouse testes following heat treatment. Left: Western blotting analysis with anti-Bag-1, Hsp60, Hsp40, Hsp70, and β-actin polyclonal antibodies was performed on aliquots of total protein extracts prepared from normal mouse testes (0 d) and those after heat treatment (1, 3, 5 and 7 d). Right: Gray analysis of the Western blotting results shown in the left panel, normalized to β-actin. Error bars represent the standard deviation of mean values. Asterisks indicate a significant difference from the control (0 d) (*P<0.05; **P<0.01) .
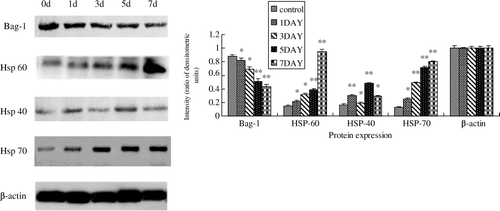
Figure 3. Analysis of the real-time PCR results. Changes of the mRNA levels of Bag-1, Hsp60, Hsp40 and Hsp70 in mouse testes of normal mouse testes (0 d) and those after heat treatment (1, 3, 5 and 7 d), normalized to β-actin. Error bars represent the standard deviation of mean values. Asterisks indicate a significant difference from the control (0 d) (*P<0.05; **P<0.01) .
Discussion
The physiological effect of heat stress on the male adult mammalian testis is documented in several species. Researchers have attributed this phenomenon to a reduction in the spermatid and spermatozoa numbers, caused by a failure of the completion of the spermatocyte cycle (Rockett et al. Citation2001; Giwercman et al. Citation2003). Results from our studies agreed with the preliminary findings with mice, rats (Setchell et al. Citation1998; Aktas and Kanter Citation2009) and some other species (Kowalowka et al. Citation2008) characterized by the reduced percentage of acrosomal integrity andlive sperms, sperm concentration and motility, as well as the percentage of fertility, and the increased rate of abnormal sperm (P<0.05). A dominant lethal breeding assay to determine definitively which kinds of proteins are changed by the heat shock had not been conducted and reported before in the mild heat-treated mouse model.
In order to reveal the protein expression and the mechanisms of heat toxicity, 2D electrophoresises of the heat shocked (37°C) and normal mice testes were employed in this study. From the data of the MALDI-TOF/TOF, 52 protein spots were expressed differentially between the heat-shocked testes and the control proteome map, in which 18 proteins were up-regulated on day 7 post-treatment. Surprisingly, compared with the up-regulated proteins, more are down-regulated, which means in an acute environment insult causes cellular activity to cease, with the exception of a few protective and regulatory genes that are required for initiating a defensive or reparative response (Muiño-Blanco et al. Citation2008).
An increasing number of reports have shown that Hsp70 plays a fundamental role in germ cell apoptosis in transgenic mice (Bravo et al. Citation2005). Heat shock and other stressful conditions that induce the heat-shock response can also lead to apoptosis or necrosis, in part determined by the intensity and duration of the stress (Barry et al. Citation1990; Georgopoulos and Welch Citation1993; Dix et al. Citation1997). Previous studies have shown that the pathways leading to apoptosis and the stress response are linked. We propose that the apoptosis induced by Hsps over-expression may have some relationship with the decrease in the sperm quality under heat stress. Although the mechanism through which heat shock factor 1 (Hsf1) is acting in this case is uncertain, the expression of Hsp70 is indeed mediated by Hsf1 in testicular cells, and this provides further evidence that the induction of Hsp70s is an important part of the apoptotic process (Jolly and Morimoto Citation2000). Concordant with the induction of Hsp70 overexpression, Hsp40, which has an essential co-chaperone activity with Hsp70 proteins to enhance the rate of ATPase activity and substrate release, is also up-regulated significantly. Hsp40 and Hsp70 could be regarded as a work-team providing critical protective functions. At the same time, Hsp60, which not only refolds and prevents aggregation of denatured proteins but also facilitates protein degradation by acting as a cofactor in proteolytic systems, is up-regulated as well. In this study, the 2DE analysis detected that Hsp70 is rapidly induced following heat shock. It is likely that a general protective response against heat shock would have shown a broader induction pattern across multiple cell types, which suggests that the expression of Hsp70 was specically related to the apoptosis observed in spermatocytes.
Results from the 2DE maps and MALDI-TOF/TOF also provided some information on the nature of the apoptotic events. The data showed that several proapoptosis proteins are down-regulated, which is perhaps an essential stress response mechanism to the heat exposure after the initial heat shock. Bag-1, classified as an anti-apoptotic protein that can be concordant with Hsp70 and heat shock cognate 70 (Hsc70) function (Nover Citation1991), was down-regulated 30.9%. Bag-1 appears to inhibit Hsp/Hsc70-mediated in vitro refolding of unfolded proteins (Georgopoulos and Welch Citation1993; Muiño-Blanco et al. Citation2008). Overexpression of Bag-1 has been found to protect certain cell lines from heat-induced cell death (Nover Citation1991). Our presumption of the series responses in the mouse testis is that the 37°C heat exposure causes an acute disruption of the majority of the cellular proteins’ conformation, while concomitantly inducing protective molecular chaperones such as Hsp/Hsc70. Bag-1 binds the ATPase domains of Hsp70 and Hsc70, modulating their chaperone activity and functioning as a competitive antagonist of the co-chaperone Hip (Takayama et al. Citation2003). Apparently the reduction in protein expression of Hsps cofactors (e.g. Bag-1) may serve to bring on the protective function. The modulatory activity of Bag-1 appears related to its molar ratio to Hsp/Hsc70 (Dix et al. Citation1996). Höhfeld and Jentsch (Citation1997) postulated that its anti-apoptotic function may be exerted through modulation of the chaperone activity of Hsp/Hsc70 on the specific protein folding and maturation pathways. Thus, reduction of Bag-1 following short-term 37°C heat exposure may give another appropriate explanation to the modulation of the chaperone activity: that Bag-1 mediates Hsp activity by combining less Hsp70 and that a cognate chaperone, in this case more improperly folded Hsp70, performs the protein refolding activity to help the testis cell recover from stress. The abnormally folded proteins may in turn induce apoptosis of the affected cells shortly thereafter (Georgopoulos and Welch Citation1993). The down-regulated proteins in adult mouse testis provide further evidence that the chaperonin-assisted protein regulation mechanism was disordered by testicular heat shock. The same as Bag-1, three of the down-regulation proteins were T-complex proteins of the cytosolic chaperonin complex. In contrast, Hsp60, which is a subunit of the mitochondrial chaperonin complex, is up-regulated. Hsp70s provide protein-folding intermediates for additional processing both in the cytosolic and mitochondrial chaperonins, so it is reasonable that further exploration of the significance of these effects on murine testis expression of HSPs and chaperonins should be undertaken (Dix et al. Citation1997).
Although from the results of the proteome profile we are far from understanding how the sperm quality decreases so greatly after heat stress in adult murine testis, we have taken some positive steps in identifying candidate pathways that may be related to the cellular observations. Further analysis in this heat-shock model should characterize the mechanisms of known and suspected toxicants. Pursuing 2DE and MALDI-TOF/TOF analysis for potential mechanisms for heat shock-induced spermatogenic disorder might be a potential way.
In summary, the combination of the negative impact on the sperm quality and the molecular biological testicular results following heat shock of the adult mouse has yielded an intuitively understandable combination of the observations that have begun to inform our mechanistic understanding of the heat disruption of spermatogenesis. Clearly it will be valuable to further analyze the functions of Hsp70s in the spermatogenic cell cycle and cell death. Definitely, the proteins and the protein networks identified as significant by 2DE and MALDI-TOF/TOF will provide important leads for pursuing a more complete understanding of male reproductive toxicity.
References
- Aktas , C and Kanter , M. 2009 . A morphological study on Leydig cells of scrotal hyperthermia applied rats in short-term . J Mol Histol. , 40 : 31 – 39 .
- Barry , MA , Behnke , CA and Eastman , A. 1990 . Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia . Biochem Pharmacol. , 40 : 2353 – 2362 .
- Bravo , MM , Aparicio , IM , Garcia-Herreros , M , Gil , MC , Peña , FJ and Garcia-Marin , LJ. 2005 . Changes in tyrosine phosphorylation associated with true capacitation and capacitation-like state in boar spermatozoa . Mol Reprod Dev. , 71 : 88 – 96 .
- Dix , DJ. 1997 . Hsp70 expression and function during gametogenesis . Cell Stress Chaperones. , 2 : 73 – 77 .
- Dix , DJ , Allen , JW , Collins , BW , Mori , C , Nakamura , N , Poorman-Allen , P , Goulding , EH and Eddy , EM. 1996 . Targeted gene disruption of Hsp70-2 results in failed meiosis, germ cell apoptosis, and male infertility . PNAS. , 93 : 3264 – 3268 .
- Dix , DJ , Allen , JW , Collins , BW , Poorman-Allen , P , Mori , C , Blizard , DR , Brown , PR , Goulding , EH , Strong , BD and Eddy , EM. 1997 . HSP70-2 is required for desynapsis of synaptonemal complexes during meiotic prophase in juvenile and adult mouse spermatocytes . Development. , 124 : 4595 – 4603 .
- Georgopoulos , C and Welch , WJ. 1993 . Role of the major heat shock proteins as molecular chaperones . Annu Rev Cell Biol. , 9 : 601 – 634 .
- Giwercman , A , Richthoff , J , Hjøllund , H , Bonde , JP , Jepson , K , Frohm , B and Spano , M. 2003 . Correlation between sperm motility and sperm chromatin structure assay parameters . Fertil Steril. , 80 : 1404 – 1412 .
- Höhfeld J Jentsch S. 1997 GrpE-like regulation of the Hsc70 chaperone by the anti-apoptotic protein BAG-1. EMBO J 16 6209 6216
- Holstein , AF , Schulze , W and Davidoff , M. 2003 . Understanding spermatogenesis is a prerequisite for treatment . Reprod Biol Endocrinol. , 1 : 107
- Jeong , YJ , Kim , MK , Song , HJ , Kang , EJ , Ock , SA , Kumar , BM , Balasubramanian , S and Rho , GJ. 2009 . Effect of alpha-tocopherol supplementation during boar semen cryopreservation on sperm characteristics and expression of apoptosis related genes . Cryobiology. , 58 : 181 – 189 .
- Jolly , C and Morimoto , RI. 2000 . Role of the heat shock response and molecular chaperones in oncogenesis and cell death . J Natl Cancer Inst. , 92 : 1564 – 1572 .
- Kowalowka , M , Wysocki , P , Fraser , L and Strzezek , J. 2008 . Extracellular superoxide dismutase of boar seminal plasma . Reprod Domest Anim. , 43 : 490 – 496 .
- Maxwell , WMC and Johnson , LA. 1997 . Chlortetracycline analysis of boar spermatozoa after incubation, flow cytometric sorting, cooling, or cryopreservation . Mol Reprod Dev. , 46 : 408 – 418 .
- Muiño-Blanco , T , Pérez-Pé , R and Cebrián-Pérez , JA. 2008 . Seminal plasma proteins and sperm resistance to stress . Reprod Domest Anim. , 4 : 18 – 31 .
- Naz , RK and Rajesh , PB. 2004 . Role of tyrosine phosphorylation in sperm capacitation/acrosome reaction (Review) . Reprod Biol Endocrinol. , 2 : 75
- Nover , L. 1991 . Heat shock response , Boca Raton, FL : CRC Press .
- Okado-Matsumoto , A and Fridovich , I. 2001 . Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu, Zn-SOD in mitochondria . J Biol Chem. , 276 : 38388 – 38393 .
- Rockett , JC , Mapp , FL , Garges , JB , Luft , JC , Mori , C and Dix , DJ. 2001 . Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice . Biol Reprod. , 65 : 229 – 239 .
- Setchell , BP , D'Occhio , MJ , Hall , MJ , Laurie , MS , Tucker , MJ and Zupp , JL. 1988 . Is embryonic mortality increased in normal female rats mated to subfertile males? . J Reprod Fertil. , 82 : 567 – 574 .
- Setchell , BP , Ekpe , G , Zupp , JL and Surani , MA. 1998 . Transient retardation in embryo growth in normal female mice made pregnant by males whose testes had been heated . Hum Reprod. , 13 : 342 – 347 .
- Takayama S Reed JC Homa S. 2003 Heat-shock proteins as regulators of apoptosis. Oncogene 22 9041 9047
- Wang L Zhu YF Guo XJ Huo R Ma X Lin M Zhou ZM Sha JH. 2005 A two-dimensional electrophoresis reference map of human ovary. J Mol Med 83 812 821
- Watson , PF. 2000 . The causes of reduced fertility with cryopreserved semen . Anim Reprod Sci. , 60 : 481 – 492 .
- WHO 1999 Laboratory manual for the examination of human semen and semen–cervical mucus interaction , 4th ed New York Cambridge University Press 55 62
- Zhu , Y , Cui , Y , Guo , X , Wang , L , Bi , Y , Hu , Y , Zhao , X , Liu , Q , Huo , R Lin , M . 2006 . Proteomic analysis of effect of hyperthermia on spermatogenesis in adult male mice . J Proteome Res. , 5 : 2217 – 2225 .