Abstract
Ovigerous crabs of Erimacrus isenbeckii were collected from the northeastern coast of South Korea and their larvae were reared in the laboratory. Two zoeal stages were described and illustrated in detail. The first zoeal characteristics between the present material and Sasaki and Mihara's (1993: J Crust Biol. 13:511–522) were different especially in the setations of the endopods of the maxillipeds. The zoea of Erimacrus isenbeckii strongly resembled those of two Telmessus species in the Cheiragonidae, but the former could be distinguished from the latter by having spinulate carapace spines, a lateral process on the abdominal somite 3, and longer lateral spines on the telson fork.
Introduction
The family Cheiragonidae Ortmann, 1893 is a small family including only three species in the world: Erimacrus isenbeckii (Brandt, 1848), Telmessus cheiragonus (Tilesius, 1812) and Telmessus acutidens (Stimpson, 1858) (see Kim Citation1973; Ng et al. Citation2008). All of them are from the northern Pacific. These species had been previously regarded as atelecyclid crabs, but they were separated from atelecyclid crabs based mainly on the positions of gonophores in female crabs. The gonophores of the three species are exposed, whereas they are completely covered by the abdomens in atelecyclid female crabs (CitationTavares and Cleva 2010). The edible hair crab, Erimacrus isenbeckii, has been reported from the northeastern coast of Korea, the coast of Hokkaido of Japan, and the Bering Sea to Alaska (Kim Citation1973; Sakai Citation1976). It is commercially important as a fishery resource; however, its population has gradually declined at present (Abe Citation1992).
The larvae of the Cheiragonidae were reported for T. cheiragonus by Kurata (Citation1963) and Rice (Citation1980), T. acutidens by Kurata (Citation1963) and Ko (Citation2006), E. isenbeckii by Marukawa and Yasunari (Citation1931), Marukawa and Chong (Citation1933), Aikawa (Citation1937), Kurata (Citation1963) and Sasaki and Mihara (Citation1993). Marukawa and Yasunari (Citation1931), Marukawa and Chong (Citation1933) and Aikawa (Citation1937) described larvae of E. isenbeckii based on the plankton-caught materials. Kurata (Citation1963) described all larval stages of E. isenbeckii from laboratory-hatched materials. However, all these old descriptions were very brief and not fit for current comparative morphological studies as suggested Clark et al. (Citation1998). Later, Sasaki and Mihara (Citation1993) described the first zoeal stage of E. isenbeckii. Although the first zoeal stage was illustrated in detail, some characteristics were overlooked or were not described; moreover, their setations of the endopods of the maxillipeds were questionable. Therefore, the purpose of this paper is to describe the two zoeal stages of the species in detail and compare its morphology with the previously described zoeae from the same family.
Materials and methods
Two ovigerous crabs of Erimacrus isenbeckii were collected from Koseong off the northeastern part of South Korea, on 13 September 2000. They were transported to the East Sea Fisheries Research Institute and maintained at a constant temperature chamber at 12°C. The first-stage zoeae hatched on 17 April 2001 and were reared using the methods described by Ko (Citation1995). Larvae were fixed and preserved in 10% neutral formalin. Zoeal specimens were dissected using a Leitz zoom stereomicroscope and appendages were examined using a Leitz Laborlux S microscope. Appendages were mounted in polyvinyl lactophenol and allowed to clear for 24 hrs. Cover slips were sealed with clear nail varnish and drawings were made with the aid of a camera lucida. Setal counts and measurements were based on 10 specimens for each zoeal stage. The sequence of the zoeal description is based on the malacostracan somite plan and described from anterior to posterior. The setal armature of the appendages was described from the proximal to distal segments and in the order of endopod to exopod (Clark et al. Citation1998). The zoeal series and the spent females were deposited at Silla University, Korea. The two zoeal stages were described and fully illustrated. The long plumose natatory setae of the first and second maxillipeds and the telson fork were drawn truncated. A micrometer was used for the zoeal measurements: CL (carapace length) from the base of the rostral spine to the most posterior carapace margin and RDL (rostral and dorsal spine length) from the tip of rostral carapace spine to the tip of the dorsal carapace spine. The approximate measurement of the antennal exopod (for its ratio with the protopod) was taken from the base to the tip excluding the terminal setae.
Results
Five zoeal stages occurred before metamorphosis to the megalopa. The durations of the zoeal stages I to V at 12°i were 7, 11, 11, 13 and 15 days each, respectively. Metamorphosis to megalopa occurred 57 days after the first-stage zoeae hatched from eggs. The first and second zoeal stages were described and illustrated in detail, but the zoeal stages III to V could not be described because of the setal variation of the mouthpart appendages, the bad condition of the preservation, and the shortage in the number of specimens. Also, the moult to megalopa was not entirely successful because they were unable to extricate themselves from the zoeal exoskeleton and consequently this phase could not be properly described and illustrated.
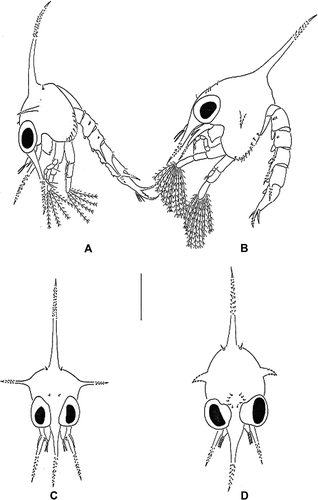
First zoea (A, 1C, 2A, 2C, 2E, 2G, 3A, 3C, 4A, 4B, 4E, 4G, 5A, 5C, 5E) Size. CL 1.291C, 2A, 2C, 2E, 2G, 3A, 3C, Carapace (A, C). Dorsal spine slightly curved, distally spinulate, longer than rostral spine; rostral spine distally spinulate, slightly longer than antennal protopod; lateral spines present, distally spinulate; pair of anterodorsal setae present; pair of posterodorsal setae present; each ventral margin denticulate, with 9 setae; eyes sessile.
Figure 1. Erimacrus isenbeckii (Brandt 1848). Lateral view of zoea: (A) first zoea, (B) second zoea. Anterior view of carapace: (C) first zoea, (D) second zoea. Scale bar is 1 mm.
Antennule (C). With four long (two stout and two thinner) terminal and two shorter subterminal aesthetascs.
Figure 2. Erimacrus isenbeckii (Brandt 1848). Chaetotaxy of ventral carapace margin: (A) first zoea, (B) second zoea; antennule: (C) first zoea, (D) second zoea; antenna: (E) first zoea, (F) second zoea; mandible: (G) first zoea, (H) second zoea. Scale bar is 0.5 mm.
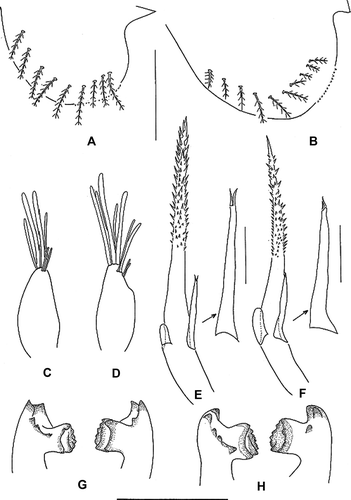
Antenna (E). Protopod shorter than rostral spine, distally spinulate; endopod bud present; exopod ca. 1/4 length of protopod, with two short terminal setae.
Mandibles (G). Asymmetrical; right molar with three teeth, left molar with tooth, confluent with incisor process.
Maxillule (A). Coxal endite with 13 terminal setae; basial endite with 12 terminal setae; endopod 2-segmented, proximal segment with seta, distal segment with six (two subterminal and four terminal) setae; exopod with one medial plumose and two proximal setae.
Figure 3. Erimacrus isenbeckii (Brandt 1848). Maxillule: (A) first zoea, (B) second zoea; maxilla: (C) first zoea, (D) second zoea. Scale bar is 0.1 mm.
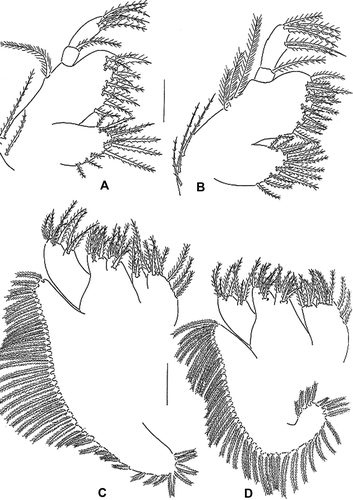
Maxilla (C). Coxal endite bilobed, with 8+4 setae; basial endite bilobed, with 7+7 setae; endopod bilobed, with 3+2+3 (9) setae; exopod (scaphognathite) margin with 43 plumose setae.
First maxilliped (A). Coxa with 1 seta; basis with 10 setae arranged 2+2+3+3; endopod 5-segmented, with 3, 3, 1, 2, 5 (one proximal and four terminal) setae, respectively; exopod 2-segmented, distal segment with four terminal plumose setae.
Figure 4. Erimacrus isenbeckii (Brandt 1848). First maxilliped: (A) first zoea, (C) second zoea; second maxilliped: (B) first zoea, (D) second zoea; third maxilliped: (E) first zoea, (F) second zoea; pereiopods: (G) first zoea, (H) second zoea. Scale bar is 0.5 mm.
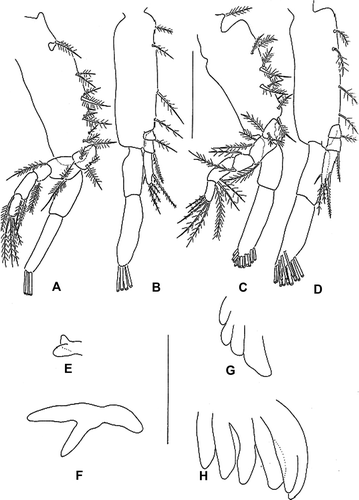
Second maxilliped (B). Coxa without seta; basis with four setae; endopod 3-segmented, with 1, 1, 5 (two subterminal and three terminal) setae, respectively; exopod 2-segmented, distal segment with four terminal plumose setae.
Third maxilliped (E). Developing as biramous.
Pereopods (G). Developing as buds.
Abdomen (A, 5C). Five somites; somite 2 with pair of lateral processes directed laterally; somite 3 with pair of lateral processes directed posteriorly; somites 3–5 with long posterolateral processes, which distally spinulate, posterolateral process of somite 4 longest; somites 2–5 with pair of posterodorsal setae; pleopod buds present.
Figure 5. Erimacrus isenbeckii (Brandt 1848). Dorsal view of abdomen: (A) first zoea, (B) second zoea; lateral view of abdomen: (C) first zoea, (D) second zoea; dorsal view of telson: (E) first zoea, (F) second zoea. Scale bar is 0.5 mm.
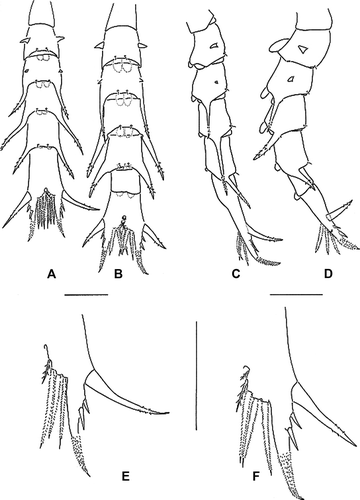
Telson (E). Each fork long, distally spinulate, with one lateral long (about equal in length of telson fork), two lateral smaller spines; posterior margin with three pairs of spinulate setae and pair of small setae.
Second zoea (B, 1D, 2B, 2D, 2F, 2H, 3B, 3D, 4C, 4D, 4F, 4H, 5B, 5D, 5F) Size. CL 1.561D, 2B, 2D, 2F, 2H, 3B, 3D, Carapace (B, 1D, 2B). Lateral spine relatively shorter than that of previous stage; five pairs of anterodorsal setae present; eyes stalked; each ventral margin with 10 setae; otherwise unchanged.
Antennule (D). Endopod bud rudimentary present; exopod with five long (three stout and two thinner) terminal, two thinner and two shorter subterminal aesthetascs; otherwise unchanged.
Antenna (F). Endopod bud larger than that of previous stage; otherwise unchanged.
Mandibles (H). Unchanged.
Maxillule (B). Coxal endite with 15 setae; basial endite with 16 setae; exopod with three plumose and three setae; otherwise unchanged.
Maxilla (D). Coxal endite bilobed, with 10+4 setae; basial endite bilobed, with 7+8 setae; exopod (scaphognathite) margin with 47 plumose setae; otherwise unchanged.
First maxilliped (C). Endopod 5-segmented, with 3, 3, 2, 2, 5 (one proximal and four terminal) setae, respectively; exopod distal segment with 12 terminal plumose setae; otherwise unchanged.
Second maxilliped (D). Exopod distal segment with 12 terminal plumose setae; otherwise unchanged.
Third maxilliped (F). Biramous; gill present.
Pereopods (H). Undifferentiated into segments; cheliped bilobed.
Abdomen (B, D): Six somites; pleopod buds more developing but without endopod buds; otherwise unchanged.
Telson (F). Posterior margin with three pairs of stout spinulate setae and two pairs of small setae; one lateral long spine slightly shorter than length of telson fork; otherwise unchanged.
Discussion
Although Aikawa (Citation1937) described the fifth zoeal and megalopal stages of Erimacrus isenbeckii from the materials of plankton sampling, it is not informative enough for this study. Also, Kurata (Citation1963) described all the larval stages of this species from eggs hatched in the laboratory; however, his description and figures are very brief and not adequate for a modern comparison (see the zoeal description of T. cheiragonus by Kurata (Citation1963) in ). The first zoeal stage was described later by Sasaki and Mihara (Citation1993). However, we found some important setal differences in the endopods of the maxillipeds (). In our study, the setations of the endopods of both maxillipeds were not 2, 2, 1, 2, 5 and 1, 1, 4 but 3, 3, 1, 2, 5 and 1, 1, 5. These setations were agreed well with those of T. acutidens within the same family (). In addition, some setae or buds were found on the carapace, the mouthpart appendages, and the pereopods in the present study ().
Table 1. A comparison of the first zoeal characteristics of Erimacrus isenbeckii described by Sasaki and Mihara (1993) with those of the present study.
Table 2. A comparison of the zoeal characteristics from three known species of the family Cheiragonidae.
As a result of our study, whether incomplete or complete, zoeal descriptions of all three species are available in the Cheiragonidae (). The common zoeal characteristics of the three species, which are almost consistent during zoeal development, can be summarized as follows: carapace with long dorsal, rostral, and short lateral spines; antennal exopod less than 1/3 length of protopod, with two setae; endopod of maxillule with 1, 2+4 setae; endopod of maxilla with 3+2+3 setae; basis of first maxilliped with 2+2+3+3 setation, proximal segment of endopod with three setae; basis of second maxilliped with 1+1+1+1 setation, endopod with 1, 1, 5 setation; telson fork with one long and two smaller lateral spines. Therefore, the Cheiragonidae seem to be homogeneous based on the zoeal characteristics.
Rice (Citation1980) suggested the derived zoeal characteristics as reductions or losses of the carapace spines, setations of the mouthpart appendages, lateral processes and posterolateral processes on the abdominal somites, and lateral spines on the telson. Based on his suggestion, E. isenbeckii may be the primitive taxon and T. acutidens the most derived taxon within the family because the zoea of E. isenbeckii has heavily spinulate carapace spines, more setae of the mouthpart appendages than those in zoeae of two Temessus species, additional processes on the abdominal somites, and a very long lateral spine on the telson ().
In the previous study of the cheiragonid zoeae (Ko Citation2006), only two diagnostic characteristics were used for identifying the zoea of E. isenbeckii, that is, (1) lateral processes on the abdominal somites 2 and 3 and (2) posterolateral process on the somite 4 spinulate and longer than the length of the somite 5. In addition, three diagnostic characteristics are found in this study: (1) heavily spinulate rostral and dorsal carapace spines, (2) the antennal exopod with two short equal sized setae, and (3) the telson fork with a very long (more or less the length of the telson fork) and two smaller lateral spines ().
Acknowledgements
The authors wish to thank Mrs S.J. Ok for preparing the figures. This study was supported by a grant from the Specific Program of National Fisheries Research and Development Institute, Korea.
References
- Abe , K. 1992 . Important crab resources inhabiting Hokkaido waters . Mar Behav Physiol. , 21 : 153 – 183 .
- Aikawa , H. 1937 . Further notes on brachyuran larvae . Rec Oceanogr Wks. , 9 : 87 – 162 .
- Clark , PF , Calazans , DK and Pohle , GW. 1998 . Accuracy and standardization of brachyuran larval descriptions . Invert Reprod Develop. , 33 : 127 – 144 .
- Kim , HS. 1973 . Anomura, Brachyura. Illustrated Encyclopedia of Fauna and Flora of Korea , Vol. 14 , 1 – 506 . Seoul : Sam Wha .
- Ko , HS. 1995 . Larval development of Benthopanope indica (De Man, 1887) (Decapoda: Brachyura: Pilumnidae) in the Laboratory . J Crust Biol. , 15 : 280 – 290 .
- Ko , HS. 2006 . Zoeal development of Telmessus acutidens (Crustacea: Decapoda: Brachyura: Atelecyclidae) reared in the laboratory . Korean J Zool. , 22 : 127 – 138 .
- Kurata , H. 1963 . Larvae of Decapoda Crustacea of Hokkaido. 1. Atelecyclidae (Atelecyclinae) . Bull Hokkaido Reg Fish Res Lab. , 27 : 13 – 24 .
- Marukawa H Chong H. 1933 [Oh-kurigani Erimacrus isenbeckii (Brandt), Larval stage ni tsuite] Rakusuikai-shi 28 1 11 [in Japanese]
- Marukawa H Yasunari K. 1931 [Kegani matawa Oh-kurigani Erimacrus isenbeckii (Brandt), Megalopa oyobi sono Chokugo-yosei ni tsuite] Suisan-Kenkyu-shi 26 1 11 [in Japanese]
- Ng , PKL , Guinot , D and Davie , PJF. 2008 . Systema Brachyurorum: Part 1. An annotated checklist of extant brachyuran crabs of the world . Raffles Bull Zool. , 17 ( 2008 ) : 1 – 286 .
- Rice , AL. 1980 . Crab zoeal morphology and its bearing on the classification of the Brachyura . Trans Zool Soc Lond. , 25 : 271 – 424 .
- Sakai , T. 1976 . Crabs of Japan and the adjacent seas , 1 – 773 . Japan : Kodansa .
- Sasaki , J and Mihara , Y. 1993 . Early larval stages of the hair crab Erimacrus isenbeckii (Brandt) (Brachyura: Atelecyclidae), with specieal reference to its hatching process . J Crust Biol. , 13 : 511 – 522 .
- Tavares , M and Cleva , R. 2010 . Trichopeltariidae (Crustacea, Decapoda, Brachyura), a new family and superfamily of eubrachyuran crabs with description of one new genus and five new species . Papéis Avulsos Zool. , 50 : 97 – 157 .