Abstract
Nur77 is a member of the nuclear receptor 4A (NR4A) subgroup, which has been implicated in energy metabolism. Although Nur77 is found in adipose tissue, where TR4 plays a key role in lipid homeostasis, the role of Nur77 in adipogenesis is still controversial. Although the Nur77 responsive element (AAAGGTCA) is partially overlapped with TR4-binding sites (AGGTCA n AGGTCA: n=0–6), the regulatory role of Nur77 in TR4 function associated with adipocyte biology remains unclear. Here, we found that Nur77 inhibits adipogenesis and TR4 transcriptional activity. Treatment with a Nur77 agonist, 1,1-bis(3′-indolyl)-1-(p-anisyl)-methane, during 3T3-L1 adipocyte differentiation reduced adipogenesis. In reporter gene analysis, Nur77 specifically suppressed TR4 transcription activity but had little effect on PPARγ transcription activity. Consistently, Nur77 also suppressed TR4-induced promoter activity of the TR4 target gene PEPCK, which is known to be important for glyceroneogenesis in adipose tissue. Furthermore, Nur77 suppressed TR4 binding to TR4 response elements without direct interaction with TR4, suggesting that Nur77 may inhibit TR4 transcription activity via binding competition for TR4-binding sites. Furthermore, DIM-C-pPhOCH3 substantially suppressed TR4-induced PEPCK expression in 3T3-L1 adipocytes. Together, our data demonstrate that Nur77 plays an inhibitory role in TR4-induced PEPCK expression in 3T3-L1 adipocytes.
Keywords:
Introduction
Adipose tissue stores excess nutrients in the form of triacylglycerides (TGs) and mobilizes free fatty acids by lipolysis during periods of energy deprivation. Alteration of the metabolic functions of adipose tissue is frequently associated with insulin resistance and type 2 diabetes (Rosen and MacDougald Citation2006).
TR4 (NR2C2), a member of the nuclear receptor (NR) superfamily, regulates the expression of target genes via binding to TR4 response elements (TR4REs) consisting of various direct repeats of AGGTCA-like motifs (DR 0–DR 6); it has the highest affinity for the DR1 element (Lee et al. Citation1997, Citation1999; Kim et al. Citation2003). TR4 functions dominantly as a homodimer via direct binding to DRs. In addition, TR4 is also able to bind to a single AGGTCA-like sequence as a monomer (Lee et al. Citation2001). Like many other NRs, TR4 is known to participate in other NR signaling pathways through binding competition for coactivators or DNA-binding sites located in the promoter of target genes or through heterodimer formation via protein-protein interactions (Lee et al. Citation1998; Shyr et al. Citation2002). Thus, it is highly possible that TR4 fine-tunes energy homeostasis in insulin-sensitive tissues such as liver, adipose, and skeletal muscle via cross-talk with other NRs. Recently, we and Kang et al. found that fat mass is significantly reduced in TR4-deficient mice (Kang et al. Citation2011; Kim et al. Citation2011). Consistent with the phenotype of TR4-deficient mice, we also found that knockdown of TR4 in 3T3-L1 adipocytes results in decreased intracellular TG accumulation via downregulation of the FATP1 gene (submitted).
Nur77, a member of the NR4A subfamily of NRs, controls target gene expression via binding to the NGFI-B response element (NBRE: AAAGGTCA) as a monomer (Philips et al. Citation1997a; Wilson et al. Citation1991). Nur77 is considered to play a role in adipogenesis, as the Nur77 gene is dramatically induced during adipocyte differentiation. However, conflicting results have been reported concerning the role of Nur77 in adipogenesis. A recent study suggested that Nur77 plays an inhibitory role in adipogenesis despite its sharp induction in the early phase of differentiation (Chao et al. Citation2008). In contrast, Fumoto et al. reported that Nur77 stimulates adipocyte differentiation (Fumoto et al. Citation2007). Considering these conflicting reports regarding the role of Nur77 in adipocyte differentiation, it is highly possible that involvement of Nur77 in adipogenesis could be affected by complicated metabolic milieus which control the activities of NRs important to adipocyte biology. Interestingly, Nur77 is also known to functionally and/or physically interact with other transcription factors via binding competition for coregulators or DNA-binding sites (Philips et al. Citation1997b; Hong et al. Citation2004; Song et al. Citation2004). Since TR4 has broad binding affinities for various DRs and monomeric AGGTCA-like sequences, we are interested in addressing the cross-talk between TR4 and Nur77 in the regulation of energy metabolism in adipocytes. Here, we demonstrate that Nur77 specifically inhibits TR4 transcriptional activity through inhibition of TR4 binding to TR4REs, resulting in the suppression of TR4-induced PEPCK expression in 3T3-L1 adipocytes.
Materials and methods
Plasmids
Plasmids pSG5-PPARγ, pCMX-RXRα, pCMX-TR4, TR4RE-Luc, PPRE-Luc, and pG5-Luc as well as fusion vectors Gal4-ER and VP-16 have been described previously (Shyr et al. Citation2002; Song et al. Citation2006; Kim and Kim Citation2011). Plasmid pcDNA3-HA-Nur77 was a gift from Dr. Keesook Lee (Chonnam Natl. Univ., Gwangju). The mouse PEPCK 5′ promoter region consisting of −472 to +79 bp was amplified by PCR from NIH-3T3 genomic DNA and then cloned into pGL3-luciferase (Promega) to generate pGL3-mPEPCK. Three copies of synthesized consensus DR1 (cDR1) were cloned into pGL3-TK-Luc to create pGL3-TK-cDR1.
Cell culture, differentiation, and Oil Red O staining
NIH-3T3, 3T3-L1, HEK293T, and CV-1 cells were maintained in DMEM containing 10% bovine calf serum or FBS. 3T3-L1 cells stably transfected with pcDNA3 or pcDNA3-TR4 were maintained in DMEM containing 800 µg/ml of Geneticin (Gibco). Adipocyte differentiation and Oil Red O staining were performed as described previously (Wang et al. Citation2010).
Transient transfection and luciferase assay
Transfections were performed using SuperFect reagent (Qiagen) according to the manufacturer's instructions, and luciferase activities were measured in a luciferase reporter assay system (Berthold). Relative luciferase activity (fold) was expressed based on induction relative to the transfection of empty vector (set as 1-fold) without agonist; the results are expressed as the mean±standard deviation (SD) of three separate experiments.
Reverse transcription PCR (RT-PCR)
mRNA levels of TR4, Nur77, PEPCK, and 36B4 were determined by RT-PCR as described previously (Park et al. Citation2008). Relative levels of PEPCK, TR4, and Nur77 mRNA were normalized to an internal control (36B4). Primer sequences for the following genes were: TR4 (sense; 5′-CAGCAGTTCATCCTAACCAGCCC-3′) and (antisense; 5′-CTGCTCCGACAGCTGTAGGTC-3′), Nur77 (sense; 5′-CCACCTCTCCGAACCGTGACA-3′) and (antisense; 5′-GAGAAGATTGGTAGGGGAGGC-3′, mPEPCK (sense; 5′-TCAACACCGACCTCCCTTAC-3′) and (antisense; 5′-CCCTAGCCTGTTCTCTGTGC-3′), 36B4 (sense; 5′-AGATGCAGCAGATCCGCAT-3′) and (antisense; 5′-ATATGAGGCAGCAGTTTCTCCAG-3′).
Glutathione-S-transferase (GST) pull-down assay and electrophoretic mobility shift assay (EMSA)
GST pull-down assay and EMSA were performed as described previously (Shyr et al. Citation2002). The following oligonucleotides were used in EMSA: cDR1 (5′-GATCTCTCTAGGTCAAAGGTCAATTTC-3′) and PEPCK-TR4RE (5′-CCTTCTCATGACCTTTGGCCGTGGGAGTA-3′).
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was performed in NIH-3T3 cells as previously described (Park et al. Citation2008). Samples were immunoprecipitated with normal IgG (Santa Cruz) or anti-TR4 antibody (Santa Cruz). Primer sequences used for amplification of the region encompassing TR4RE within the PEPCK 5′ promoter were: TR4RE-sense 5′-AGGTAACACACCCCAGCTAAC-3′, TR4RE-antisense 5′-GGCTCTTGCCTTAATTGTCAG-3′.
Results
Nur77 agonist inhibits adipogenesis of 3T3-L1 preadipocytes
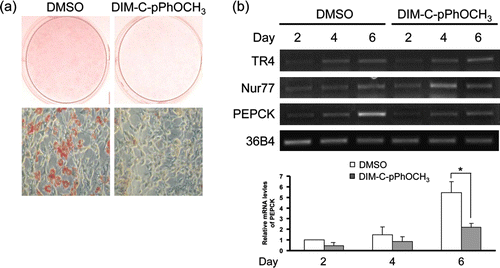
We first determined whether DIM-C-pPhOCH3, a Nur77 agonist, could affect adipogenesis of 3T3-L1 preadipocytes using Oil Red O staining. Two-day post-confluent 3T3-L1 cells (designated day 0) were differentiated by addition of standard adipogenic stimuli. Twenty micromolar DIM-C-pPhOCH3 was also added on day 0 and continuously treated to 3T3-L1 cells throughout the course of differentiation. As shown in a, treatment with DIM-C-pPhOCH3 significantly reduced TG accumulation in day 7 3T3-L1 adipocytes as compared with 3T3-L1 adipocytes treated with vehicle. Next, we measured the levels of TR4 mRNA during adipogenesis by RT-PCR to determine whether DIM-C-pPhOCH3 affects expression of TR4 during adipocyte differentiation. Consistent with a previous report (Margolis et al. Citation2005), the TR4 mRNA level was lowest on day 2 and then progressively increased until day 6 (b). In addition, DIM-C-pPhOCH3 did not significantly affect TR4 expression in 3T3-L1 adipocytes.
Figure 1. Effect of Nur77 agonist on adipocyte differentiation. (a) At 2 days post-confluence, 3T3-L1 cells were differentiated by standard protocol in the absence or presence of 20 µM DIM-C-pPhOCH3 and then stained with Oil Red O on day 7 to determine the effect of DIM-C-pPhOCH3 on adipogenesis. (b) 3T3-L1 cells were harvested on indicated days after the induction of differentiation and the mRNA levels of TR4, Nur77 and PEPCK were analyzed by RT-PCR analysis. The data shown are representative of three individual experiments (a and b). Relative mRNA levels of the PEPCK genes are expressed as mean±S.D. of three individual experiments (*P < 0.05).
Nur77 selectively inhibits TR4 transcriptional activity
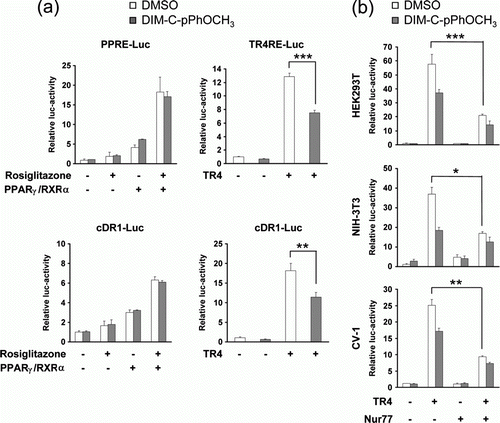
Since TR4 and PPARγ are known to play essential roles in lipid metabolism and have binding affinities for DRs containing AGGTCA-like motifs, we investigated whether Nur77 could regulate the transcriptional activities of TR4 and PPARγ using reporter genes containing TR4 or PPARγ response element (TR4RE-Luc or PPRE-Luc). Addition of DIM-C-pPhOCH3 resulted in inhibition of TR4 transactivation in HEK293T cells, whereas DIM-C-pPhOCH3 did not affect the transcriptional activity of PPARγ (a). To rule out any effect that sequence difference between these reporter genes may have, we tested the effect of DIM-C-pPhOCH3 on TR4 and PPARγ activities using a reporter gene fused with three copies of consensus DR1 (cDR1-Luc). As observed in the above data, DIM-C-pPhOCH3 inhibited only TR4 transcriptional activity, suggesting that Nur77 may have a negative effect on adipogenesis via specific modulation of TR4 activity. To evaluate whether DIM-C-pPhOCH3 inhibits TR4 activity in a cell-independent manner, we also performed reporter gene assays using cDR1-Luc in HEK293T, NIH-3T3, or CV-1 cells. The addition of DIM-C-pPhOCH3 reduced TR4 transcriptional activity about 32 to 50% depending on the cell line (b). Co-transfection of Nur77 with TR4 into these cells strongly suppressed TR4 activity. Further, addition of DIM-C-pPhOCH3 together with Nur77 to TR4-transfected cells further inhibited TR4 activity as compared with DIM-C-pPhOCH3 alone.
Figure 2. Nur77 specifically inhibits TR4 transcriptional activity. (a) Effect of Nur77 agonist on transcriptional activities of TR4 and PPARγ. The indicated reporter genes (300 ng of each) were co-transfected with expression plasmids for PPARγ, RXRα and TR4 (100 ng of each) into HEK293T cells. Transfected cells were incubated for 24 hr in the absence or presence of appropriate ligands (100 µM Rosiglitazone or/and 20 µM DIM-C-pPhOCH3) and then harvested to measure luciferase activity (**P < 0.01, ***P < 0.001). (b) The effect of Nur77 on TR4 transcriptional activity in different cells. Reporter gene (cDR1-Luc 100 ng) was co-transfected with the indicated combinations of TR4 or Nur77 expression plasmid (100 ng of each) into different cells as indicated. Transfected cells were incubated for 24 hr in the absence or presence of 20 µM DIM-C-pPhOCH3 and then luciferase activity was analyzed (*P < 0.05, **P < 0.01, ***P < 0.001).
Nur77 inhibits TR4 binding to TR4 responsive elements
To determine whether Nur77 inhibition of TR4 transactivation occurs through physical association of Nur77 with TR4, we first performed a GST pull-down assay. As shown in a, GST-TR4 fusion protein could interact with 35S-labeled estrogen receptor (ER), which has been reported to form a heterodimer with TR4 (Shyr et al. Citation2002). However, we were unable to observe any interaction between 35S-labeled Nur77 and GST-TR4 fusion protein. Next, we used a mammalian two-hybrid assay to test whether Nur77 could affect the interaction between TR4 and ER. Consistent with a previous report (Shyr et al. Citation2002), significant induction was observed when VP16-TR4 was co-transfected with GAL4-ER into HEK293T cells (b). However, interaction between TR4 and ER was not affected by the addition of Nur77, indicating that TR4 does not form a heterodimer with Nur77. It is also possible that Nur77 may inhibit TR4 transactivation via suppression of TR4 binding to TR4REs. To investigate this possibility, we performed an EMSA using 32P-labeled cDR1 to determine the effect of Nur77 on TR4 binding to cDR1. As expected, in vitro translated TR4, but not Nur77 protein, formed a specific complex with 32P-labeled cDR1 (c, left). However, the TR4/cDR1 complex was reduced by the addition of Nur77 in a dose-dependent manner, suggesting that despite very low affinity of Nur77 for cDR1, Nur77 may inhibit TR4 binding to cDR1. Furthermore, the negative effect of Nur77 on TR4 binding to cDR1 was abolished by increasing amounts of TR4. When we used PEPCK-TR4RE as a probe, Nur77 similarly inhibited the formation of TR4/PEPCK-TR4RE complex, and this suppression was also inhibited by increasing amounts of TR4 (c, right). The binding of Nur77 to cDR1 and PEPCK-TR4RE may not be observed due to different expression levels of in vitro translated TR4 and Nur77. To test this possibility, we examined the protein levels of coupled in vitro transcription and translated Nur77 and TR4 with [35S]-Met by SDS-PAGE. The Nur77 protein level was similar to or higher than that of TR4 (d). To further confirm Nur77 inhibition of TR4 binding to PEPCK-TR4RE, we performed a ChIP assay in NIH-3T3 cells transiently transfected with TR4 and/or Nur77 expression plasmids. PCR was performed to amplify the -529 to -245 bp region encompassing PEPCK-TR4RE from protein-DNA complexes immunoprecipitated with normal serum IgG or anti-TR4 antibody. As expected, no PCR product was observed using DNA immunoprecipitated with normal serum IgG (e). In contrast, we observed specific binding of TR4 to the PEPCK promoter region containing PEPCK-TR4RE, even though the PCR product band amplified from control cells was weaker than that of NIH-3T3 cells transfected with TR4. However, when Nur77 was co-transfected with TR4 into NIH-3T3 cells, we were not able to observe any amplified PCR band.
Figure 3. Nur77 inhibits TR4 biding to TR4 responsive elements. (a) GST pull-down assay was performed by incubation of 35S-labeled Nur77 or ER with GST or GST-TR4 bound glutathione-Sepharose beads. (b) The pG5-Luc (300 ng) was co-transfected with Gal4-ER, VP16-TR4 (100 ng of each) and Nur77 expression plasmid (100 ng) into HEK293T cells as indicated and luciferase activity was analyzed. (c) EMSAs were performed using 32P-labeled probes (cDR1 or PEPCK-TR4RE) with increasing amounts of in vitro translated TR4 or Nur77 as indicated. (d) After expression with [35S]-Met in a coupled-transcription and translation system (25 µl reaction), 3 µl in vitro translated TR4 and Nur77 were subjected to 10% SDS-PAGE for analysis of relative expression between samples. (e) Nur77 inhibition of TR4 binding to TR4RE within the mouse PEPCK 5′ promoter. NIH-3T3 cells were transiently transfected with TR4 and Nur77 expression plasmid (15 µg each) as indicated. Sixteen hr later, cells were harvested for ChIP assay. ChIP assay was performed using DNA-protein complex pulled-down by normal IgG, or anti-TR4 antibody as indicated.
![Figure 3. Nur77 inhibits TR4 biding to TR4 responsive elements. (a) GST pull-down assay was performed by incubation of 35S-labeled Nur77 or ER with GST or GST-TR4 bound glutathione-Sepharose beads. (b) The pG5-Luc (300 ng) was co-transfected with Gal4-ER, VP16-TR4 (100 ng of each) and Nur77 expression plasmid (100 ng) into HEK293T cells as indicated and luciferase activity was analyzed. (c) EMSAs were performed using 32P-labeled probes (cDR1 or PEPCK-TR4RE) with increasing amounts of in vitro translated TR4 or Nur77 as indicated. (d) After expression with [35S]-Met in a coupled-transcription and translation system (25 µl reaction), 3 µl in vitro translated TR4 and Nur77 were subjected to 10% SDS-PAGE for analysis of relative expression between samples. (e) Nur77 inhibition of TR4 binding to TR4RE within the mouse PEPCK 5′ promoter. NIH-3T3 cells were transiently transfected with TR4 and Nur77 expression plasmid (15 µg each) as indicated. Sixteen hr later, cells were harvested for ChIP assay. ChIP assay was performed using DNA-protein complex pulled-down by normal IgG, or anti-TR4 antibody as indicated.](/cms/asset/fd66998b-d344-4f0f-a1c6-59f74c064b1b/tacs_a_603748_o_f0003g.gif)
Nur77 suppresses TR4-induced PEPCK gene expression in 3T3-L1 adipocytes
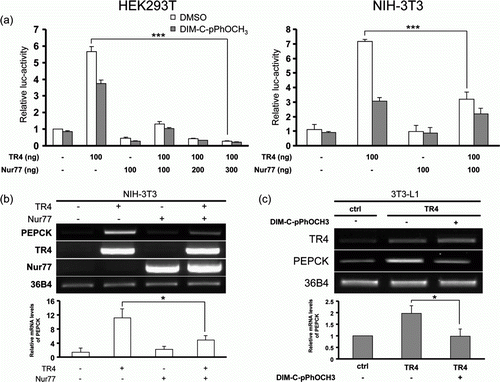
Since Nur77 inhibited TR4 binding to PEPCK-TR4RE, we next determined if Nur77 could inhibit TR4 transactivation of the PEPCK promoter using a luciferase reporter linked to the PEPCK 5′ promoter spanning −472 to +79 bp (pGL3-mPEPCK). As shown in a, TR4 induced PEPCK promoter activity about 5.6-fold in HEK293T cells compared with control cells transfected with empty plasmid. Interestingly, when DIM-C-pPhOCH3 was treated to TR4-transfected HEK293T cells, TR4 transcriptional activity was reduced about 33%. DIM-C-pPhOCH3-mediated suppression of TR4 activity could be attributed to the activation of endogenous Nur77. Furthermore, Nur77 dramatically inhibited TR4 transcriptional activity when co-transfected with TR4 into HEK293T cells. This effect of Nur77 on TR4 transactivation of the PEPCK promoter was further pronounced by increasing amounts of Nur77. We also obtained a similar result when we performed the same reporter gene assay in NIH-3T3 cells. Next, we evaluated the effect of Nur77 on TR4 induction of the PEPCK gene by transient transfection of TR4 with or without Nur77 into NIH-3T3 cells. As expected, RT-PCR analysis showed that TR4 induced mRNA expression of the PEPCK gene about 11-fold as compared with empty vector-transfected cells (b). Interestingly, addition of Nur77 did not reduce the basal level of PEPCK mRNA expression. However, when TR4 was co-transfected with Nur77, the induction effect of TR4 on PEPCK gene expression was reduced to 56% of that in TR4-transfected cells. To further confirm the negative effect of Nur77 on TR4-induced PEPCK expression in adipocytes, we investigated the effect of DIM-C-pPhOCH3 on TR4-induced PEPCK expression in 3T3-L1 cells stably overexpressing TR4. As shown in d, RT-PCR analysis showed that TR4 overexpression resulted in a significant increase in PEPCK expression compared with control 3T3-L1 adipocytes stably transfected with empty vector. However, treatment with DIM-C-pPhOCH3 reduced TR4-induced PEPCK gene expression in 3T3-L1 adipocytes. Together, these data indicate that Nur77 plays a role as a functional modulator of TR4 transcriptional activity in 3T3-L1 adipocytes.
Figure 4. Nur77 suppresses PEPCK gene expression via inhibition of TR4 transcriptional activity. (a) HEK293T or NIH-3T3 cells were transfected with pGL3-mPEPCK-Luc (300 ng) and pCMX-TR4 (100 ng) with different amounts of Nur77 expression plasmid as indicated. Cells were treated with or without 20 µM DIM-C-pPhOCH3 for 24 hr and harvested for luciferase assays (***P < 0.001). (b) Expression plasmids for TR4 and Nur77 (6 µg of each) were transiently transfected into NIH-3T3 cells as indicated. Twenty-four hr later, cells were harvested for RT-PCR analysis. (c) DIM-C-pPhOCH3 inhibits TR4-induced PEPCK expression in 3T3-L1 adipocytes. At 2 days post-confluence, 3T3-L1 adipocytes stably overexpressing TR4 were differentiated by standard protocol in the absence or presence of 20 µM DIM-C-pPhOCH3 and harvested on day 4 for RT-PCR analysis. The data shown are representative of three individual experiments and relative mRNA levels of each gene are expressed as mean±S.D. of three individual experiments (b, c) (*P < 0.05).
Discussion
TR4 is expressed in metabolic tissues such as adipose tissue, liver, and skeletal muscle and has been identified as an important regulator of energy homeostasis (Kim et al. Citation2003 Kim et al. Citation2011; Liu et al. Citation2007; Kang et al. Citation2011). TR4 regulates gluconeogenesis through hepatic induction of the PEPCK gene (Liu et al. Citation2007). In addition, TR4-deficient mice show reduced TG accumulation in adipose tissue (Kang et al. Citation2011; Kim et al. Citation2011).
Early studies have reported that several NRs regulate the expression of PEPCK in the liver (Chakravarty et al. Citation2005). Thus, differential activation of NRs under particular metabolic cues might be critical for determining the spatial and temporal control of metabolic genes such as PEPCK. Here, we demonstrated that Nur77 negatively regulated TR4 transcriptional activity, resulting in decreased TR4-induced PEPCK expression in 3T3-L1 adipocytes. Nur77 is acutely induced during early adipogenesis of 3T3-L1 preadipocytes but subsequently declined to the basal level within 24 hr (Fumoto et al. Citation2007; Chao et al. Citation2008). Although the level of Nur77 mRNA in the late phase of differentiation is much lower than its surge after adipogenic activation, Nur77 expression is progressively increased during adipocyte differentiation (Chao et al. Citation2008). Conflicting reports on the role of Nur77 in adipogenesis have been reported. Fumoto et al. reported that Nur77 is induced in the very early phase of adipogenesis by treatment with a differentiation cocktail and promotes adipogenesis by triggering clonal expansion (Fumoto et al. Citation2007). In contrast, a recent report showed that NR4A NRs, including Nur77, inhibit adipogenesis in different cell lines (Chao et al. Citation2008). It has been reported that Nur77 regulates target gene expression via binding to AAAGGTCA-like motifs as a monomer (Wilson et al. Citation1991; Philips et al. Citation1997a). TR4 usually regulates target gene expression via binding to DRs as a TR4 homodimer or heterodimer with TR2 (Lee et al. Citation1998). Interestingly, a previous report showed that TR4 is also able to bind to monomeric AAAGGTCA-like sequences (Lee et al. Citation2001), strongly suggesting that these two NRs might functionally cross-talk with each other to fine-tune adipocyte biology. As expected, Nur77 inhibited TR4 transcriptional activity at least in part by suppressing TR4 binding to TR4REs. However, we were not able to observe Nur77 binding to this TR4RE. Since TR4 could form a heterodimer with ER, leading to a loss of TR4 binding affinity for TR4RE, it is possible that Nur77 inhibition of TR4 activity could occur via heterodimerization with TR4. However, TR4 showed no interaction with Nur77 in this study. Interestingly, both Nur77 and TR4 have been shown to interact with several common coregulators such as SRC-1 (Wansa et al. Citation2002; Zhou et al. Citation2011). Various NRs frequently participate in other NR signaling pathways through competitive binding for coactivators and several coactivators are known to facilitate NR DNA binding activity (Yan et al. Citation1998; Nakajima et al. Citation2004). Thus, it is also plausible that Nur77 may compete with TR4 for limited amounts of the endogenous coactivator which is important for the stable binding of TR4 to TR4REs located in its target gene promoters. When Nur77 is highly expressed or activated in adipocytes, Nur77 may occupy most of this coactivator, which is required for the support of a stable TR4-TR4RE complex, resulting in a decrease of TR4-TR4RE interaction. However, to fully understand the repressive behavior of Nur77 on TR4 activity, further studies will be needed. It is also possible that an excessive amount of Nur77 with low affinity for TR4RE is able to out-compete TR4 for TR4RE. Indeed, when TR4 was increased, Nur77 suppression of TR4 binding to TR4RE located in the PEPCK promoter region was abolished. Consistently, when Nur77 was added together with TR4 to NIH-3T3 cells, Nur77 dramatically reduced TR4 induction of PEPCK expression. In addition, DIM-C-pPhOCH3, a Nur77 agonist, suppressed TR4-induced expression of the PEPCK gene in 3T3-L1 adipocytes stably overexpressing TR4. PEPCK is known to be critical for glyceroneogenesis in adipocytes (Forest et al. Citation2003). Thus, Nur77 may have a negative effect on lipid homeostasis in adiopocytes by inhibiting TR4 transcriptional activity.
In summary, our data demonstrate that Nur77 negatively modulates TR4 transcriptional activity via inhibition of TR4 binding affinity for TR4RE, resulting in decreased expression of PEPCK in 3T3-L1 adipocytes. Our findings imply that TR4 is a key modulator of the NR network important for lipid homeostasis, and thus our study will help others to understand the transcriptional network involved in obesity-related diseases.
Acknowledgements
This work was supported by a National Research Foundation of Korea Grant funded by the Korean Government (KRF-2006-312-C00392). We thank Dr. Keesook Lee for kindly providing plasmid.
References
- Chakravarty , K , Cassuto , H , Reshef , L and Hanson , RW. 2005 . Factors that control the tissue-specific transcription of the gene for phosphoenolpyruvate carboxykinase-C . Crit Rev Biochem Mol Biol. , 40 ( 3 ) : 129 – 154 .
- Chao , LC , Bensinger , SJ , Villanueva , CJ , Wroblewski , K and Tontonoz , P. 2008 . Inhibition of adipocyte differentiation by Nur77, Nurr1, and Nor1 . Mol Endocrinol. , 22 ( 12 ) : 2596 – 2608 .
- Forest , C , Tordjman , J , Glorian , M , Duplus , E , Chauvet , G , Quette , J , Beale , EG and Antoine , B. 2003 . Fatty acid recycling in adipocytes: a role for glyceroneogenesis and phosphoenolpyruvate carboxykinase . Biochem Soc Trans. , 31 ( Pt 6 ) : 1125 – 1129 .
- Fumoto , T , Yamaguchi , T , Hirose , F and Osumi , T. 2007 . Orphan nuclear receptor Nur77 accelerates the initial phase of adipocyte differentiation in 3T3-L1 cells by promoting mitotic clonal expansion . J Biochem. , 141 ( 2 ) : 181 – 192 .
- Hong , CY , Park , JH , Ahn , RS , Im , SY , Choi , HS , Soh , J , Mellon , SH and Lee , K. 2004 . Molecular mechanism of suppression of testicular steroidogenesis by proinflammatory cytokine tumor necrosis factor alpha . Mol Cell Biol. , 24 ( 7 ) : 2593 – 2604 .
- Kang , HS , Okamoto , K , Kim , YS , Takeda , Y , Bortner , CD , Dang , H , Wada , T , Xie , W , Yang , XP , Liao , G and Jetten , AM. 2011 . Nuclear orphan receptor TAK1/TR4-deficient mice are protected against obesity-linked inflammation, hepatic steatosis, and insulin resistance . Diabetes. , 60 ( 1 ) : 177 – 188 .
- Kim , SJ and Kim , E. 2011 . Stearoyl-CoA desaturase induces lipogenic gene expression in prostate cancer cells and inhibits ceramide-induced cell death . Anim Cells Syst. , 15 ( 1 ) : 1 – 8 .
- Kim , ES , Xie , S , Yeh , SD , Lee , YF , Collins , LL , Hu , YC , Shyr , CR , Mu , XM , Liu , NC , Chen , YT , Wang , PH and Chang , C. 2003 . Disruption of TR4 orphan nuclear receptor reduces the expression of liver apolipoprotein E/C-I/C-II gene cluster . J Biol Chem. , 278 ( 47 ) : 46919 – 46926 .
- Kim E , Liu NC , Yu IC , Lin HY , Lee YF , Sparks JD , Chen LM , Chang C. 2011 . Metformin inhibits nuclear receptor TR4-mediated hepatic stearoyl-coenzyme A desaturase 1 gene expression with altered insulin sensitivity . Diabetes . doi: 10.2337/db2310–0393
- Lee , YF , Pan , HJ , Burbach , JP , Morkin , E and Chang , C. 1997 . Identification of direct repeat 4 as a positive regulatory element for the human TR4 orphan receptor. A modulator for the thyroid hormone target genes . J Biol Chem. , 272 ( 18 ) : 12215 – 12220 .
- Lee , CH , Chinpaisal , C and Wei , LN. 1998 . A novel nuclear receptor heterodimerization pathway mediated by orphan receptors TR2 and TR4 . J Biol Chem. , 273 ( 39 ) : 25209 – 25215 .
- Lee , YF , Young , WJ , Lin , WJ , Shyr , CR and Chang , C. 1999 . Differential regulation of direct repeat 3 vitamin D3 and direct repeat 4 thyroid hormone signaling pathways by the human TR4 orphan receptor . J Biol Chem. , 274 ( 23 ) : 16198 – 16205 .
- Lee , HJ , Lee , YF and Chang , C. 2001 . TR4 orphan receptor represses the human steroid 21-hydroxylase gene expression through the monomeric AGGTCA motif . Biochem Biophys Res Commun. , 285 ( 5 ) : 1361 – 1368 .
- Liu , NC , Lin , WJ , Kim , E , Collins , LL , Lin , HY , Yu , IC , Sparks , JD , Chen , LM , Lee , YF and Chang , C. 2007 . Loss of TR4 orphan nuclear receptor reduces phosphoenolpyruvate carboxykinase-mediated gluconeogenesis . Diabetes. , 56 ( 12 ) : 2901 – 2909 .
- Margolis , RN , Evans , RM and O'Malley , BW. 2005 . The Nuclear Receptor Signaling Atlas: development of a functional atlas of nuclear receptors . Mol Endocrinol. , 19 ( 10 ) : 2433 – 2436 .
- Nakajima , T , Fujino , S , Nakanishi , G , Kim , YS and Jetten , AM. 2004 . TIP27: a novel repressor of the nuclear orphan receptor TAK1/TR4 . Nucleic Acids Res. , 32 ( 14 ) : 4194 – 4204 .
- Park , SS , Choi , HJ , Kim , SJ , Kim , OJ , Chae , KS and Kim , ES. 2008 . FXRalpha down-regulates LXRalpha signaling at the CETP promoter via a common element . Mol Cells , 26 ( 4 ) : 409 – 414 .
- Philips , A , Lesage , S , Gingras , R , Maira , MH , Gauthier , Y , Hugo , P and Drouin , J. 1997a . Novel dimeric Nur77 signaling mechanism in endocrine and lymphoid cells . Mol Cell Biol. , 17 ( 10 ) : 5946 – 5951 .
- Philips , A , Maira , M , Mullick , A , Chamberland , M , Lesage , S , Hugo , P and Drouin , J. 1997b . Antagonism between Nur77 and glucocorticoid receptor for control of transcription . Mol Cell Biol. , 17 ( 10 ) : 5952 – 5959 .
- Rosen , ED and MacDougald , OA. 2006 . Adipocyte differentiation from the inside out . Nat Rev Mol Cell Biol. , 7 ( 12 ) : 885 – 896 .
- Shyr , CR , Hu , YC , Kim , ES and Chang , C. 2002 . Modulation of estrogen receptor-mediated transactivation by orphan receptor TR4 in MCF-7 cells . J Biol Chem. , 277 ( 17 ) : 14622 – 14628 .
- Song , KH , Park , YY , Park , KC , Hong , CY , Park , JH , Shong , M , Lee , K and Choi , HS. 2004 . The atypical orphan nuclear receptor DAX-1 interacts with orphan nuclear receptor Nur77 and represses its transactivation . Mol Endocrinol. , 18 ( 8 ) : 1929 – 1940 .
- Song , KH , Park , YY , Kee , HJ , Hong , CY , Lee , YS , Ahn , SW , Kim , HJ , Lee , K , Kook , H , Lee , IK and Choi , HS. 2006 . Orphan nuclear receptor Nur77 induces zinc finger protein GIOT-1 gene expression, and GIOT-1 acts as a novel corepressor of orphan nuclear receptor SF-1 via recruitment of HDAC2 . J Biol Chem. , 281 ( 23 ) : 15605 – 15614 .
- Wang Y , Van Oort MM , Yao M , Van der Horst DJ , Rodenburg KW. 2010 . Insulin and chromium picolinate induce translocation of CD36 to the plasma membrane through different signaling pathways in 3T3-L1 adipocytes, and with a differential functionality of the CD36 . Biol Trace Elem Res . doi: 10.1007/s12011–12010–18809–12018
- Wansa , KD , Harris , JM and Muscat , GE. 2002 . The activation function-1 domain of Nur77/NR4A1 mediates trans-activation, cell specificity, and coactivator recruitment . J Biol Chem. , 277 ( 36 ) : 33001 – 33011 .
- Wilson , TE , Fahrner , TJ , Johnston , M and Milbrandt , J. 1991 . Identification of the DNA binding site for NGFI-B by genetic selection in yeast . Science. , 252 ( 5010 ) : 1296 – 1300 .
- Yan , ZH , Karam , WG , Staudinger , JL , Medvedev , A , Ghanayem , BI and Jetten , AM. 1998 . Regulation of peroxisome proliferator-activated receptor alpha-induced transactivation by the nuclear orphan receptor TAK1/TR4 . J Biol Chem. , 273 ( 18 ) : 10948 – 10957 .
- Zhou , XE , Suino-Powell , KM , Xu , Y , Chan , CW , Tanabe , O , Kruse , SW , Reynolds , R , Engel , JD and Xu , HE. 2011 . The orphan nuclear receptor TR4 is a vitamin A-activated nuclear receptor . J Biol Chem. , 286 ( 4 ) : 2877 – 2885 .