Abstract
A variety of fish species undergo an ontogenetic change in prey selectivity, and several potentially interacting factors, including nutrient requirement, microhabitat change, and foraging ability, may account for the occurrence of the shift. Here we examine the foraging ecology and ontogenetic diet shift of a micro-carnivorous goby, Pterogobius elapoides (serpentine goby), dominant component of fish assemblage in shallow rocky areas off the coast in Korea and Japan. Although most other gobies are primarily benthic carnivores, P. elapoides is a semipelagic fish; however, little is known about how those species change their foraging tactics with growth. In our diet analyses, the most common diet was pelagic copepods and benthic amphipods, and diet shift was observed from pelagic to benthic with growth. The ontogenetic diet shift seems to be the result of the preference for energetically more profitable prey in larger-size classes as well as the results of different prey availability due to among-habitat variation in diet. However, differential food preference does not appear to affect individual scope for searching food. Several factors such as predation pressures and interspecific resource partitioning might contribute to the changes in diet observed among size classes, which were included in our ongoing tests.
Introduction
A variety of fish species undergo an ontogenetic change in prey selectivity, that is, from planktivory to piscivory (Schmitt and Holbrook Citation1984; Holbrook et al. Citation1985; Shibuno et al. Citation1994; Lockett and Suthers Citation1998; Graham et al. Citation2007; Schellelens et al. Citation2010; Baeck et al. Citation2011). Many potentially interrelating factors may account for the occurrence of these shifts (McCormick Citation1998). Growth-related morphological differences can lead to differential exploitation of a food resource and, in turn, changes in microhabitat use, being a function of changing in nutrient requirement (MacNeill and Brandt Citation1990; Luczkovich et al. Citation1995; Peterson and McIntyre Citation1998). Likewise, foraging schedule and ability may change with ontogeny, which can be compromised with availability and density of prey organisms (Clements and Choat Citation1993; Lukoschek and McCormick Citation2001). A thoughtful consideration of how a species exploits its food resource and how that changes with growth is prerequisite to any examination for the pattern and dynamics of species assemblage on temporal and spatial scales (McCormick Citation1998).
The Gobiid species (gobies), one of the largest families of fish with more than 200 genera, are commonly distributed in temperate, subtropical, and tropical regions (Nelson Citation2006). Gobies are primarily fish of shallow oceanic habitats including tide pools, coral reefs, and sea-grass fields; they are also very abundant in brackish water, and a small number of gobiid species are also entirely adapted to freshwater system (Nelson Citation2006). Although few are valuable as food for humans, they are generally of great significance as prey and predator species for commercially important organisms. While the occurrence of ontogenetic change in prey selectivity has been reported for gobies in several studies (Gibson Citation1970; Vass et al. Citation1975; Grossman Citation1980; Grossman et al. Citation1980; Huh and Kwak Citation1998a, Citation1998b, Citation1999), little is known about how those species change their foraging tactics with growth.
In this study, we examine the foraging ecology of a goby, Pterogobius elapoides (serpentine goby; ), a common component of fish assemblage in shallow rocky areas off the coast in Korea and Japan (Masuda et al. Citation1984; Kim et al. Citation1986). While most gobies are benthic carnivores, consuming mainly crustaceans and polychaetes (Gibson and Ezzi Citation1978; Grossman et al. Citation1980; Behrents Citation1989; Kikuchi and Yamashita Citation1992; Aarnio and Bonsdorff Citation1993; Humphries and Potter Citation1993; Swenson and McCray Citation1996), P. elapoides is a semipelagic fish, seldom settling on bottom structure (Dŏtu and Tsutsumi Citation1959). However, temporal (seasonal) space defense is observed in this species sometimes, but not often, for their benthic feeding territories (personal observation). P. elapoides is thus expected to undergo distinct pattern of ontogenetic shifts in foraging ecology from other gobies, even though it has never been thoroughly investigated.
Figure 1. The serpentine goby, Pterogobius elapoides, hovering to search prey on the bottom in the ocean around Kurahashi Island, Japan.

In the present study, we investigated for the first time whether diet changes with ontogeny and explored the mechanisms by which partitioning of the food resource occurs among different size classes in P. elapoides. First, the stomach of individuals from different size classes was dissected open, and the contents inside were examined to find differences, if any, in diet taxa. Second, information on prey species availability, size-related feeding mechanics, and microhabitat selectivity was used to find the potential factors accounting for the occurrence of the shift.
Materials and methods
Locations and study species
Collections for estimation and observation were carried out on shallow rocky areas off the coast of Kurahashi Island (Honmura Bay, the Seto Inland Sea; 34°5′14′′N, 132°30′0′′E) in Japan. P. elapoides is one of the dominant inhabitants in our study sites. Many individuals cruise around boulders and conceal themselves in cracks beneath boulders or inside of brown algae patches to avoid predation (S.-H. Choi, personal observation). P. elapoides is relatively smaller in size than that of other Pterogobius species. This species exhibit pale brown with six or seven transverse black bands on their body side continuing behind the dorsal and anal fins, which make each individual visually conspicuous (). This species reaches sexual maturity at around 70–80 mm in standard length (hereafter SL; Dŏtu and Tsutsumi Citation1959). The breeding season of the studied population starts in November and continues well into December. The male builds a nest underneath a boulder and provides exclusive paternal care for eggs, which includes fanning and defending the brood until hatching (Dŏtu and Tsutsumi Citation1959). Adult individuals generally die at the completion of the breeding season.
Diet analysis
A total of 34 individuals were collected in the study site using a gill net during the daytime (dates were chosen based on the weather condition; 16–17 May, 22–23 June, 17 July, and 6 August in 2000). The captured fishes were preserved in 10% buffered formalin solution and were measured to the nearest 0.01 mm by digital calipers. Each individual was allocated into three different size classes: small (50–60 mm SL), medium (61–70 mm SL), and large (> 70 mm SL). The stomach of each individual was dissected open, and the contents inside were examined under a binocular dissecting microscope. Prey were removed from the stomach and identified to the lowest taxon feasible. The greatest length or carapace length of all of the individuals was measured to the nearest 0.01 mm using an ocular micrometer. Prey volume was estimated by measuring the length and width of each item and calculating its cylindrical or spherical volume, depending on its shape. For each sample, the percent composition was calculated in number and in volume.
Invertebrate fauna
To obtain fauna information of planktonic, algal and benthic invertebrates in the study site, different methods were used for sampling from May to July 2000. Planktonic invertebrates were sampled by quickly sweeping a portable handle-fitted plankton net (0.1 mm mesh) in the upper 30–50 cm of the sediment. Algal invertebrates were collected (eight replicates) by taking (into the 0.1 mm mesh bag) parts of algae where fish were feeding. Benthic invertebrates were sampled from substrates taken in the sandy and muddy bottom between boulders (eight replicates) using a metal core (100×100 mm). All the samples collected were preserved in 5% formalin solution prior to examination. Each invertebrate were identified and counted, and 50 intact individuals randomly chosen per taxon were measured for their maximum length in the laboratory. The total counts were adjusted to the number per cubic meter for planktonic invertebrates or per square meter for algal and benthic invertebrates.
Foraging behaviors and microhabitat preferences
Foraging behaviors and microhabitat preference (including home ranges) for each of the three size classes were examined using scan field observations with scuba diving and snorkeling at a chosen time depending on the weather condition between 08:00 and 17:00 for 30–60 min per dive from May to August in 2000. The observation was made in depth ranging from 1 to 6 m on rocky terrain with cracks and crevices irregularly formed and overgrown brown algae. A total of 17 individuals (53–78 mm SL) were captured from the study site using encircling monofilament nylon net (15×1 m; 5 mm square mesh). The collected individuals were marked by color paint injection (blue acrylic paint) over the skin (see Thresher and Gronell Citation1978 for the method). After measuring their SL using a scale bar to the nearest 1 mm, each individual was allocated into three size classes (see Diet analysis section) and was released. Seventeen individuals were tracked down for observation; small- (N=6), medium- (N=6), and large-size class individuals (N=5) were observed for 21, 17, and 27 h, respectively.
Results
Ontogenetic shift in diet composition
The stomach of small-size class P. elapoides contained pelagic copepods, amphipods, and branchiopods. More than 90% of the stomach contents (in volume) were pelagic copepods, including Palacalanus spp. (Calanoida), Euterpina spp. (Harpacticoda), Oithona spp. (Cyclopoida), and Calanus spp. (Calanoida; a). Among them, Palacalanus species were exclusively predominant, covering 76.5% in the total volume (a), as expected from the fact that genus Palacalanus is the most abundant species found in the water column (). Although other copepods, including Oithona spp. and Nauplius spp., were also abundant in water column (), they were rarely found in the stomach contents of this size class.
Figure 2. Comparison of numeric (shaded bar) and volumetric (empty bar) occurrence (presented as relative frequencies;%±standard deviation) of major dietary items found in the stomach contents among three size classes investigated.
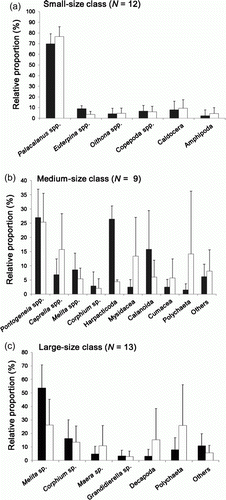
Table 1. Individual numbers, numeric occurrence (%; in bracket), and mean sizes (mm±standard deviation) of the taxa collected from water column, alga, and substratum in the study site.
Individuals of medium-size class are likely to consume more diverse items of prey than do smaller ones (b). Copepods, including Harpacticoda, Mysidacea, and Calanoida, were no more exclusively dominant food items for medium-size class (44.68% in number, 23.66% in volume), while various amphipod species (algal amphipods such as Pontongeneia spp. and Caprella spp., and benthic amphipods such as Melita spp., Corphium sp., and Cumacea) were more frequently found (47.68% in number, 54.11% in volume; b). Big organisms, such as benthic annelid worms (Polychaeta), were also included in the items. In the medium-size fishes, Harpacticoid is one of the predominant item in number (26.38±4.68%) but was only a minor component volumetrically (4.33±0.74%), probably due to its relatively small sizes (b). On the contrary, Caprella spp., Mysidacea and Polychaeta showed low proportions in numbers but not volumetrically (b).
The stomach contents of the large-size class comprised only of benthic invertebrates, including benthic amphipods, decapods, and Polychaetes (c). Benthic amphipods, including Melita spp., Corphium sp., Maera sp., and Grandidierella sp., were the dominant ones in numbers, while pelagic copepods and algal amphipods were not found in the stomach items of this size class (c). Decapoda and Polychaeta showed high values in volume but not in number (c). Although Harpacticoids were the most abundant invertebrate in the substratum (), they were rarely found in the stomach of large-size fishes. The large fish consumed proportionally more benthic amphipods in their diet than did the medium-sized fish (Mann–Whitney U-test, U=108.0, p < 0.001).
Ontogenetic shift in foraging behaviors
Pterogobius elapoides show three distinct foraging behaviors to catch prey in three types of environments: capturing in mid-water (CM), sucking algal invertebrates (SA), and picking benthic invertebrates (PB). The three size classes significantly differ in occurrence of three different foraging behaviors (χ2=61.51, df = 1, p < 0.001; ). CM and SA were observed in small-size fishes (). For CM, small-size fishes generally hover in water column around rocks where they rapidly feed on pelagic prey. When potential prey available for SA are found on the surface of brown algae, individuals halt around the algae for 3–4 seconds and feed on the prey with powerful suction. In this size class, CM was more frequently observed than SA (Wilcoxon singed rank test, z = –4.15, p < 0.001; ).
Table 2. Ontogenetic changes in using different types of foraging behavior by serpentine gobies, that is, capturing in mid-water (CM), sucking algal invertebrate (SA), and picking benthic invertebrates (PB).
All types of foraging behaviors were observed in the fishes of medium-size class (). The frequency of the CM was significantly decreased compared to small-size class (z = –4.97, p < 0.001), while the frequency of SA was increased relative to the small fish (z = –4.36, p < 0.001). For PB, individuals search for prey on the substrate surface, and they always stop hovering for a few seconds when they found prey on the substrate. After taking the prey, the fish generally churn the prey with sediment and expel inedible materials out of opercula and mouth. SA seems to be the dominant foraging pattern in medium-size class (Kruskal-Wallis H = 262.10, df = 2, p<0.001).
Capturing in mid-water (CM) and SA were not observed at all among the fishes of large-size class (). The frequencies of feeding were the lowest (9.64±7.77) when compared with small-size class (27.88±21.05) and medium-size class (16.88±8.53; Kruskal-Wallis test H = 104.15, df = 2, p<0.001).
Microhabitat preferences
Home ranges did not appear to change with growth as this species primarily prefer to stay in areas with boulders and sand bottoms around the reefs. However, individuals in different size classes showed clear difference in space use, suggesting that P. elapoides undergo vertical microhabitat shifts from water columns to the bottom with growth. The individuals in small-size class were usually found around reefs in the middle water column. The individuals in medium-size class always swam around reefs with patches of brown algae. The individuals in large-size class generally hovered around reefs in 5–10 cm upper from the bottom.
Discussion
The most common prey, by number and volumetrically, in P. elapoides’ diet were pelagic copepods and benthic amphipods. There was an ontogenetic diet shift from pelagic to benthic prey, as well as among-habitat variation in diet as a result of different prey availability. P. elapoides has been known as omnivorous fish because copepod, organic deposit, and algae particles were found from the stomach contents in a previous study (Dŏtu and Tsutsumi Citation1959), which did not consider the feeding microhabitats and prey availability. Algae particles were not found as stomach contents in our study. Our diet analyses consequently show that P. elapoides is microcarnivore, as is the case for many other gobies (Blaber and Whitfield Citation1977; Gibson and Ezzi Citation1978; Grossman et al. Citation1980; Kikuchi and Yamashita Citation1992; Onadeko Citation1992; Aarnio and Bonsdorff Citation1993; Humphries and Potter Citation1993; Swenson and McCray Citation1996).
The ontogenetic diet shift was the result of the preference for energetically more profitable prey in P. elapoides in larger-size classes, as shown in many other microcarnivores (Grossman Citation1980; Schmitt and Holbrook Citation1984; MacNeill and Brandt Citation1990; Gill and Hart Citation1994; Luczkovich et al. Citation1995; Peterson and McIntyre Citation1998). Pelagic copepods, in particular Calanoida, are generally much smaller than amphipods and polychaetes that are consumed by the individuals from medium- and large-size classes. Pelagic copepods may energetically be less profitable, but are valuable for the individuals in the small-size class with reduced handling and foraging efforts and with relatively high abundance. By contrast, the increased searching and capturing efforts for larger individuals can be compensated by exploitation of differing prey sources and the increased caloric profitability (Ellison et al. Citation1979). This is in perfect agreement with optimal foraging theory of Estabrook and Dunham (Citation1976) predicting that ontogenetic diet shift of an individual is a stepwise development to maximize its net energy gain.
Foraging techniques is an essential determinant of ontogenetic difference in the diets of P. elapoides. CM is the foraging pattern appropriate for taking small copepods and most frequently observed in small-size class, while large-size individuals generally pick up relatively large benthic invertebrates. The exploitation of differing prey sources is directly constrained by morphological capacity of feeding apparatus (Schmitt and Holbrook Citation1984; Stoner and Livingston Citation1984; MacNeill and Brandt Citation1990; Peterson and McIntyre Citation1998). In the study of a microcarnivorous fish, Cheilodactylus spectabilis, the increase in size of the buccal cavity, hyoid complex, and associated musculature lead directly to an increase in the suction primarily used to capture prey (McCormick Citation1998). In P. elapoides, the increasing size of the feeding apparatus with growth (Kendall's rank correlation test; gape width, τ = 0.874, p < 0.001; snout length, τ = 0.890, p < 0.001; S.-H. Choi and H.Y. Suk, unpublished data) may contribute to a feeding on larger prey. However, the present data do not provide any direct evidence for increasing efficiency of handling procedure with the growth in feeding apparatus.
Growth-related preference for different food resources might require individuals to change their microhabitats, as also shown in many other studies (Werner and Hall Citation1988; Clements and Coat Citation1993; Lukoschek and McCormick Citation2001). Vertical up-shifting in microhabitat was observed in the present study. Water column is the perfect place for small individuals to feed pelagic organisms with occupying exposed perches within rocky reef habitats. Although the medium-sized fish consume prey in the water column, they seem to prefer to find algal invertebrates, as they spend much time for foraging and searching around algae patches on the reefs. The large-sized fish only feeds on prey found on sediments in the sand bottom. However, ontogenetic shift in food preferences does not appear to affect individual scope for searching food, as the home ranges of individuals from different size classes (collected in the same site) were totally overlapped, and the sizes of the home ranges were not different from the individuals in different size classes (actually decreased with growth).
In conclusion, ontogenetic diet shift in P. elapoides is influenced by several factors, including body size and the availability of prey, and results in differential microhabitat utilization. A number of factors have not been assessed, and they might contribute to the changes in diet observed among size classes, even though several more factors are included in our ongoing tests and upcoming publications. For instances, exposure of individuals to predation has been shown to be an important determinant of microhabitat utilization in many fish species (e.g., Schmitt and Holbrook Citation1984; Werner and Hall Citation1988) and may influence the patterns documented in our study. Intraspecific territorial interaction and the resultant resource partitioning may also be a factor determining the home range size and evolutionary processes related to feeding strategies.
Acknowledgements
We heartily appreciate Kenji Gushima for thoughtful comments throughout our research. This study was supported by the grant from Yeungnam University Research Grant in 2009 (209-A-356-027). We also thank Sihn-Ae Lee and anonymous reviewers for providing valuable comments on the manuscript.
References
- Aarnio , K and Bonsdorff , E. 1993 . Seasonal variation in abundance and diet of the sand goby Pomatoschistus minutus (Pallas) in a northern Baltic archipelago . Ophelia , 37 : 19 – 30 .
- Baeck , GW , Park , JM and Hashimoto , H. 2011 . Feeding ecology of three tonguefishes, genus Cynoglossus (Cynoglossidae) in the Seto Inland Sea, Japan . Anim Cells Syst , 15 : 325 – 336 .
- Behrents , KC. 1989 . The foraging ecology of two sympatric gobiid fishes: importance of behavior in prey type selection . Environ Biol Fish , 26 : 105 – 118 .
- Blaber , SJM and Whitfield , AK. 1977 . The biology of the burrowing goby Croila mossambica Smith (Teleostei, Gobiidae) . Environ Biol Fish , 1 : 197 – 204 .
- Clements , KD and Choat , JH. 1993 . Influence of season, ontogeny and tide on the diet of the temperate marine herbivorous fish Odax pullus (Odacidae) . Mar Biol , 117 : 213 – 362 .
- Dŏtu Y , Tsutsumi T. 1959 . The reproduction behaviour in the gobiid fish, Pterogibius elapoides (Gunther) . Bull Fac Fish. Nakasaki Univ . 8 : 186 – 190 . ( In Japanese )
- Ellison , JP , Terry , C and Stephens , JS. 1979 . Food resource utilization among five species of embiotocids at King Harbor, California, with preliminary estimates of caloric intake . Mar Biol , 52 : 161 – 169 .
- Estabrook , F and Dunham , AE. 1976 . Optimal diet as a function of absolute abundance, relative abundance, and relative value of available prey . Am Nat , 110 : 401 – 413 .
- Gibson , RN. 1970 . Observation on the biology of the giant goby Gobius cobitis Pallas . J Fish Biol , 2 : 281 – 288 .
- Gibson , RN and Ezzi , IA. 1978 . The biology of a Scottish population of Fries’ goby (Lesueurigobius friesii) . J Fish Biol , 12 : 371 – 389 .
- Gill , AB and Hart , PJB. 1994 . Feeding behaviour and prey choice of the threespine stickleback: the interacting effects of prey size, fish size and stomach fullness . Anim Behav , 47 : 921 – 932 .
- Graham , BS , Grubbs , D , Holland , K and Popp , BN. 2007 . A rapid ontogenetic shift in the diet of juvenile yellowfin tuna from Hawaii . Mar Biol , 150 : 647 – 658 .
- Grossman , GD. 1980 . Ecological aspects of ontogenetic shifts in prey size utilization in the bay goby (Pisces: Gobiidae) . Oecologia , 47 : 233 – 238 .
- Grossman , GD , Coffin , R and Moyle , PB. 1980 . Feeding ecology of the bay goby (Pisces: Gobiidae): effects of behavioral, ontogenetic, and temporal variation on diet . J Exp Mar Biol Ecol , 44 : 47 – 59 .
- Holbrook SJ , Schmitt RJ , Coyer JA. 1985 . Age-related dietary patterns of sympatric adult surfperch . Copeia . 1985 : 986 – 994 .
- Huh SH , Kwak SN. 1998a . Feeding habits of Acentrogobius pflaumii in the eelgrass (Zostera marina) bed in Kwangyang Bay . Kor J Ichthyol . 10 : 24 – 31 . ( In Korean )
- Huh SH , Kwak SN. 1998b . Feeding habits of Favonigobius gymnauchen in the eelgrass (Zostera marina) bed in Kwangyang Bay . J Kor Fish Soc . 31 : 372 – 379 . ( In Korean )
- Huh SH , Kwak SN. 1999 . Feeding habits of Acanthogobius flavimanus in the eelgrass (Zostera marina) bed in Kwangyang Bay . J Kor Fish Soc . 32 : 10 – 17 . ( In Korean )
- Humphries , P and Potter , IC. 1993 . Relationship between the habitat and diet of three species of atherinids and three species of gobies in a temperate Australian estuary . Mar Biol , 116 : 193 – 204 .
- Kikuchi , T and Yamashita , Y. 1992 . Seasonal occurrence of gobiid fish and their food habitats in a small mud flat in Amakusa . Publ Amakusa Mar Biol Lab , 11 : 73 – 93 .
- Kim IS , Kim YO , Lee YJ. 1986 . Synopsis of the family Gobiidae (Pisces, Perciformes) from Korea . Bull Kor Fish Soc . 19 : 387 – 408 . ( In Korean )
- Lockett , MM and Suthers , IM. 1998 . Ontogenetic diet shift and feeding activity in the temperate reef fish Cheilodactylus fuscus . Proc Linn Soc NSW , 120 : 105 – 116 .
- Luczkovich , JJ , Norton , SF and Gilmore , RG. 1995 . The influence of oral anatomy on prey selection during the ontogeny of two Percoid fishes, Lagodon rhomboids and Centropomus undecimalis . Environ Biol Fish , 44 : 79 – 95 .
- Lukoschek , V and McCormick , MI. 2001 . Ontogeny of diet changes in a tropical benthic carnivorous fish, Parupeneus barberinus (Mullidae): relationship between foraging behaviour, habitat use, jaw size, and prey selection . Mar Biol , 138 : 1099 – 1113 .
- MacNeill DB , Brandt SD. 1990 . Ontogenetic shifts in gill-raker morphology and predicted prey capture efficiency of the alewife, Alosa pseudoharengus . Copeia . 1990 : 164 – 171 .
- Masuda , H , Amaoka , K , Araga , C , Uyeno , T and Yoshino , T. 1984 . The fishes of the Japanese Archipelago , Tokyo : Tokai University Press .
- McCormick , MI. 1998 . Ontogeny of diet shifts by a microcarnivorous fish, Cheilodactylus spectabilis: relationship between feeding mechanics, microhabitat selection and growth . Mar Biol , 132 : 9 – 20 .
- Nelson JS. 2006 . Fishes of the world , 4th ed . New York : John Wiley and Sons, Inc . 601 p.
- Onadeko , CA. 1992 . The food and feeding habits of the sleeper goby, Batanga lebretonis (Steindachner) (Pisces: Teolestei: Eleotrididae) in the Lagos Lagoon, Nigeria . J Afr Zool , 106 : 176 – 189 .
- Peterson , CC and McIntyre , P. 1998 . Ontogenetic diet shifts in Roeboides affinis with morphological comparisons . Environ Biol Fishes , 53 : 105 – 110 .
- Schellelens , T , de Roos , AM and Persson , L. 2010 . Ontogenetic diet shifts result in niche partitioning between two consumer species irrespective of competitive abilities . Amer Nat , 176 : 625 – 637 .
- Schmitt , RJ and Holbrook , SJ. 1984 . Ontogeny of prey selection by black surfperch Embiotoca jacksoni (Pisces: Embiotocidae): the roles of fish morphology, foraging behavior, and patch selection . Mar Eco Prog Ser , 18 : 225 – 239 .
- Shibuno , T , Hashimoto , H and Gushima , K. 1994 . Changes with growth in feeding habits and gravel turning behavior of the wrasse, Coris gaimard . Jap J Ichthyol , 41 : 301 – 306 .
- Stoner AW , Livingston RJ. 1984 . Ontogenetic patterns in diet and feeding morphology in sympatric sparid fishes from seagrass meadows . Copeia . 1984 : 174 – 187 .
- Swenson , RO and McCray , AT. 1996 . Feeding ecology of the tidewater goby. Trans . Amer Fish Soc , 125 : 956 – 970 .
- Thresher RE , Gronell AM. 1978 . Subcutaneous tagging of small reef fishes . Copeia . 1978 : 352 – 353 .
- Vass , KF , Vlasbalau , AG and DeKoeijer , P. 1975 . Studies on the black goby (Gobius niger Gobiidae, Pisces) in the Veerse Meer SW Netherlands . Neth J Sea Res , 9 : 56 – 68 .
- Werner , EE and Hall , DJ. 1988 . Competition and habitat shift in tow sunfish (Centrarchidae) . Ecology , 69 : 1352 – 1366 .