Abstract
The purpose of this study was to examine whether Phellodendri Cortex extract (PCE) could improve learning and memory impairments caused by lipopolysaccharide (LPS)-induced inflammation in the rat brain. The effect of PCE on modulating pro-inflammatory mediators in the hippocampus and its underlying mechanism were investigated. Injection of LPS into the lateral ventricle caused acute regional inflammation and subsequent deficits in spatial learning ability in the rats. Daily administration of PCE (50, 100, and 200 mg/kg, i.p.) for 21 days markedly improved the LPS-induced learning and memory disabilities in the Morris water maze and passive avoidance test. PCE administration significantly decreased the expression of pro-inflammatory mediators such as tumor necrosis factor-α, interleukin-1β, and cyclooxygenase-2 mRNA in the hippocampus, as assessed by RT-PCR analysis and immunohistochemistry. Together, these findings suggest that PCE significantly attenuated LPS-induced spatial cognitive impairment through inhibiting the expression of pro-inflammatory mediators in the rat brain. These results suggested that PCE may be effective in preventing or slowing the development of neurological disorders, including Alzheimer's disease, by improving cognitive and memory function because of its anti-inflammation activity in the brain.
Introduction
Inflammation has been implicated as a common cause in various neurodegenerative diseases, such as Alzheimer's disease (AD), Parkinson's disease, and ischemic stroke (Mrak Citation2009). Much evidence suggests that neuroinflammation and neurodegenerative disorders with sustained increase in various pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), are closely correlated with the cognitive dysfunction primarily associated with the progression of AD pathogenesis (Schwab and McGeer Citation2008; Mrak Citation2009).
Lipopolysaccharide (LPS), a non-infectious component of the outer membranes of Gram-negative bacteria, when administered by intraventricular microinjection or chronic infusion, induces neuroinflammation and impairment of memory function (Kim et al. Citation2000; Kitazawa et al. Citation2005). The memory dysfunction caused by LPS-induced inflammation has been hypothesized to play an important role in the pathogenesis of the degenerative changes and cognitive impairments (Cunningham et al. Citation2009). The pathogenesis of various neurodegenerative changes, and cognitive and memory impairments, closely associated with AD or senile CNS dysfunction (Lukiw and Bazan Citation2000). In fact, it is well established that LPS-induced inflammation in the hippocampus produces severe deficits in learning and memory in a variety of behavioral tasks (Frank-Cannon et al. Citation2009). The hippocampal neurons that are known to play an important role in learning and memory functions are particularly vulnerable to a neuronal damage such as that from LPS-induced inflammation, which results in serious deficits in spatial memory tasks (Hwang et al. Citation2011) and synaptic plasticity (Min et al. Citation2009).
Thus, many studies have suggested that chronic use of non-steroidal anti-inflammatory drugs (NSAIDs) can prevent cognitive decline in individuals diagnosed with developing AD dementia or in elderly people (Szekely et al. Citation2008). However, the long-term treatments using NSAIDs can cause gastrointestinal side effects and even occasional liver and kidney toxicities (Knight et al. Citation1996; Warner et al. Citation1999; Graupera et al. Citation2003). These side effects have encouraged the development of new NSAIDs that are safer for long-term treatments (Kelloff et al. Citation2000). Recent studies have also suggested the use of herbal medicines or natural products for treating Alzheimer's-type, dementia-related disorders exhibiting cognitive memory impairment and neuroinflammation (Ho et al. Citation2011).
In Korean traditional medicine, Phellodendri Cortex extract (PCE) has been widely used to prevent bacterial infection of the respiratory system and to treat various chronic inflammatory diseases (Lu et al. Citation2011). PCE has multiple pharmacological activities and produces a variety of biological effects in the central nervous system (Zhang et al. Citation2009). Some studies have demonstrated that PCE alleviated LPS-induced acute airway inflammation through decreasing the infiltration of inflammatory cells and releasing inflammatory mediators (Mao et al. Citation2010). It also attenuated LPS-induced inflammatory responses and inhibited the expression of TNF-α, IL-1β, and nitric oxide mRNAs in BV2 mouse microglia cells (Park et al. Citation2007). These studies suggest that PCE could be useful for suppressing inflammation in neurodegenerative diseases. However, the underlying mechanism of pharmacological effect of PCE on improving LPS-induced cognitive deficits and spatial learning disability remains poorly understood.
The aim of the present study was to evaluate the anti-inflammatory effects of PCE in improving learning and memory function in rats exposed to LPS-induced inflammation, as measured by performance on the passive avoidance test (PAT) and the Morris water maze (MWM) tests. We also examined how these effects were related to the molecular modulation of inflammation in terms of the neural mechanism underlying the memory-enhancing activity of PCE.
Materials and methods
Animals
Adult male Sprague–Dawley (SD) rats weighing 260–280 g were obtained from Samtako Animal Co. (Seoul, Korea). The rats were housed up to five per polycarbonate cage in a limited-access rodent facility. The room controls were set to maintain the temperature at 22±2°C and the relative humidity at 55±15%. Cages were lit by artificial light for 12 h each day. Sterilized drinking water and standard chow diet were supplied ad libitum to each cage during the experiments. All animal experiments began at least seven days after the animals arrived.
Lesion generation and LPS administration
Learning and memory deficits in the rats were induced by bilateral intracranial injection (right and left side) of LPS, according to the procedures described previously (Guo et al. Citation2010; Lim et al. Citation2008). Fifty micrograms of LPS dissolved in 10-µl cerebrospinal fluid (CSF) (Sigma-Aldrich Co., St. Louis, MO, USA) was microinjected into the lateral ventricle of the rat brains in all lesion groups. Sham animals as a vehicle control received microinjection of artificial CSF instead of LPS (Hwang et al. Citation2011). Artificial CSF consists of 140 mM NaCl, 3.0 Mm KCl, 2.5 mM CaCl2, and 1.2 mM Na2HPO4, and maintained at pH 7.4. The lateral ventricle in the stereotaxic coordinate was designated according to the Paxinos and Watson brain atlas (AP: −0.2, L:±0.3, DV: −6.2 referenced to the bregma; Paxinos and Watson Citation1986). Artificial CSF or LPS solution was injected for 5 min at a flow rate of 2 µl/min using 22-gauge Hamilton syringe and microinjection pump (Pump 22; Harvard Apparatus Inc., Holliston, MA, USA). LPS (Escherichia coli; 055:B5) and ibuprofen were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Administration of PCE was started 24 h after the lesion generation.
Experimental groups
Rats were randomly divided into seven groups of seven individuals as follows: CSF-injected sham group being regarded as normal (CON group, n=7), CSF-injected plus 200 mg/kg PCE-treated group (PCE group, n=7), LPS-injected plus saline-treated group (LPS group as a negative control, n=7), LPS-injected plus 50 mg/kg PCE-treated group (PCE50 + LPS group, n=7), LPS-injected plus 100 mg/kg PCE-treated group (PCE100 + LPS group, n=7), LPS-injected plus 200 mg/kg PCE-treated group (PCE200 + LPS group, n=7), and LPS-injected plus 40 mg/kg ibuprofen-treated (IBU + LPS group as a positive control, n=7). PCE extract was manufactured in the oriental pharmacy in Kyung Hee Hospital of Oriental Medicine, Kyung Hee University (Seoul, Korea), according to the protocol of Lee et al. (Citation2004). IBU, a centrally acting cholinesterase inhibitor, was used as a positive control. The rats were intraperitoneally administrated with PCE and IBU for 21 days, and PCE and IBU were dissolved in 0.9% physiological saline before use. The experimental schedule of all drug administration and behavioral tests is shown in .
Open field test
Prior to water maze testing, the rats were individually housed in a rectangular container that was made of black polyethylene (45×45×35 cm) to provide best contrast to the white rats in a dimly lit room equipped with a video camera above the center of the room, and their locomotor activities (animal's movements) were then measured. The locomotor activity indicated by the speed and the distance of movements was monitored by a computerized video-tracking system using S-MART program (PanLab Co., Barcelona, Spain). After 5 min of adaptation, the distance they traveled in the container was recorded for another 5 min. The locomotor activity was expressed in meters. The number of rearing events of the rats was also recorded to analyze locomotor activity in the open field test.
Passive avoidance test
The test was performed according to the step-through method described previously (Lee et al. Citation2011). The Gemini Avoidance System (SD Instruments., San Diego, CA, USA) was used for this test. The step-through passive avoidance apparatus consists of a tilting floor acrylic box divided into two compartments, a lightened compartment connected to darkened one, by an automatic guillotine door and a control unit generating electric shock (Behbood Pardaz Co., Ghaem, Iran). Electric shock can be delivered to the grid floors, made of stainless steel rods (3 mm diameter) spaced 1 cm apart, in both compartments. First, the rats were taken trials to acquisition test in the apparatus. In the training session, a rat was placed in a lightened compartment of the passive avoidance apparatus facing away from the entrance to the dark compartment, and then the guillotine door was opened. Because of intrinsic preference to the dark environment, the rat immediately entered the dark compartment and the door was closed. During acquisition test, the latency time before entry into the dark compartment was recorded for each rat. After 30 min, the rat was placed in the lightened compartment once again. After entering the dark compartment, the guillotine door was closed and subsequently a mild electrical shock (0.5 mA) was applied for 3 s. The retention test was started 24 h after the acquisition trial for training. The rat was again placed in the lightened compartment and the guillotine door was opened. In the retention test, the rat was placed in the passive avoidance apparatus as previously described and the time required for the rat to enter the dark compartment was measured for a maximum period of 3 min in the same method with the acquisition test. The rat did not enter the dark compartment within this period received a latency time of 180 s (Mohamed et al. Citation2000).
MWM test
MWM apparatus
The MWM test was performed in a small circular pool (2.0 m in diameter and 0.35 m deep) made of polypropylene and internally painted white. The pool was half-filled with water to 30 cm in depth. The water in the pool was made opaque by adding 1 kg skim milk powder and continuously maintained at 22±2°C. The pool was divided into four quadrants of equal area. During the MWM test, an escape platform (15 cm in diameter) was located in one of four sections of the pool being hidden 1.5 cm below the water surface and apart approximately 50 cm from the sidewalls. Several visual cues were placed around the pool in plain sight of the animals. A digital camera was mounted to the ceiling straight above the center of the pool and was connected to a computerized recording system equipped with a tracking program (S-MART: PanLab Co., Barcelona, Spain), which permitted on- and off-line automated tracking of the paths taken by the rats.
Hidden platform trial for acquisition test
The MWM test was initiated on the 14th day after injections of PCE extract and LPS. The MWM test was basically performed according to the MWM apparatus and the method described previously (Lee et al. Citation2011). The animals received three trials per day. The rats were trained to find the hidden platform, which remained in a fixed location throughout the test. The trials lasted for a maximum of 180 s, and the escape latency was expressed by the swimming time (or swim distance) to find the submerged platform in the pool. The animals were tested in three trials per day for five days, and they received a 60-s probe trial on the sixth day. Finding the platform was defined as staying on it for at least 4 s before the acquisition time of 180 s ended. When the rat failed to find the platform in the limited time in the first trial of hidden platform test, the rat should be placed on the platform for 20 s and assigned a latency of 180 s. Between one trial and the next, the water in the pool was stirred to remove olfactory traces of previous swim patterns. The entire schedule proceeded for six days and each animal had three trials for training per day with 30–40 min inter-trial interval.
Probe trial for retention test
For the probe trial, a rat was placed in the quadrant located diagonally from the target quadrant and allowed to swim to the quadrant from which the escape platform had been removed for a maximum of 60 s. The probe trial was expressed by the ratio of the time spent (or the distance) for searching the platform in the target quadrant to total duration spent for swimming in the pool.
Immunohistochemistry for proinflammatory markers
For immunohistochemical studies, the animals were deeply anesthetized with sodium pentobarbital (80 mg/kg, by intraperitoneal injection) and perfused through the ascending aorta with normal saline (0.9%) followed by 300 ml (per rat) of 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). The brains were removed in a randomized order, post-fixed over-night, and cryoprotected with 20% sucrose in 0.1 M PBS at 4°C. Coronal sections with 30 µm thickness were cut through the hippocampus using a cryostat (Leica CM1850; Leica Microsystems Ltd., Nussloch, Germany). The sections were obtained according to the rat atlas of Paxinos and Watson (hippocampus; between bregma −2.6 and −3.6) (Paxinos and Watson Citation1986). The sections were immunostained for TNF-α, IL-1β, and cyclooxygenase-2 (COX-2) expression using the avidin–biotin–peroxidase complex (ABC) method. The sections were incubated with primary rabbit anti-TNF-α antibody (1:200 dilution; Novus Biochemicals LLC., Littleton, CO, USA), rabbit anti-IL-1β antibody (1:200 dilution; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), and goat anti-COX-2 antibody (1:200 dilution; Cambridge Research Biochemicals Co., Bellingham, UK) in PBST (PBS plus 0.3% Triton X-100) for 72 h at 4°C, respectively. The sections were incubated for 120 min at room temperature with biotinylated anti-rabbit goat IgG (for the anti-TNF-α antibody and anti-IL-1β antibody) and biotinylated anti-goat rabbit IgG (for the anti-COX-2 antibody). The secondary antibodies were obtained from Vector Laboratories Co. (Burlingame, CA, USA) and diluted 1:200 in PBST containing 2% normal serum. To visualize immunoreactivity, the sections were incubated for 90 min in ABC reagent (Vectastain Elite ABC kit; Vector Labs. Co., Burlingame, CA, USA), and incubated in a solution containing 3,3′-diaminobenzidine (DAB; Sigma-Aldrich Co., St. Louis, MO, USA) and 0.01% H2O2 for 1 min. Images were captured using an AxioVision 3.0 imaging system (Carl Zeiss, Inc., Oberkochen, Germany) and processed using Adobe Photoshop (Adobe Systems, Inc., San Jose, CA, USA). The sections were viewed at 200× magnification, and the numbers of cells within 100×100-um2 grids were counted by observers blinded to the experimental groups. Counting immunopositive cells was performed in at least three different hippocampal sections per rat brain. Stained sections were randomly chosen from equal levels of serial sections along the rostral–caudal axis. The TNF-α-, IL-1β-, and COX-2-immunopositive cells were only counted when their densities reached a defined darkness above the background level (Guo et al. Citation2010). Distinct brown spots for TNF-α, IL-1β, and COX-2 were observed in the cytoplasms and in the membranes of the cone-shaped cells of the hippocampus. The cells were anatomically localized according to the stereotactic rat brain atlas of Paxinos and Watson (Citation1986). The brightness and contrast between images were not adjusted to exclude any possibility of subjective selection of immunoreactive cells.
Total RNA preparation and RT-PCR analysis
The expression levels of TNF-α, IL-1β, and COX-2 mRNAs were determined by the reverse transcription-polymerase chain reaction (RT-PCR). The brain hippocampus was isolated from three rats per group. The total RNAs were prepared from the brain tissues using a TRIzol® reagent (Invitrogen Co., Carlsbad, CA, USA) according to the supplier's instruction. Complementary DNA was first synthesized from total RNA using a reverse transcriptase (Takara Co., Shiga, Japan). PCR was performed using a PTC-100 programmable thermal controller (MJ Research, Inc., Watertown, MA, USA). All primers were designed using published mRNA sequences of those cytokines and a primer designing software, Primer 3, offered by the Whitehead Institute for Biomedical Research (Cambridge, MA, USA; www.genome.wi.mit.edu) on the website. The PCR products were separated on 1.2% agarose gels and stained with ethidium bromide. The density of each band was quantified using an image-analyzing system (i-MaxTM, CoreBio System Co., Seoul, Korea). The expression levels were compared each other by calculating the relative density of target band, such as TNF-α, IL-1β, and COX-2, to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Statistical analysis
All measurements were performed by an independent investigator blinded to the experimental conditions. Results in figures are expressed as mean±standard error of means. Differences within or between normally distributed data were analyzed by analysis of variance using SPSS (Version 13.0; SPSS, Inc., Chicago, IL, USA) followed by Tukey's post hoc test. Statistical significance was set at p<0.05.
Results
Effect of PCE on LPS-induced body weight loss
Rats exposed to LPS infusion begin to lose their body weights on the first day of LPS infusion, and this LPS-induced initial reduction of body weight is sustained for three days without restoring to normal level or even exacerbated in some cases (Gaab et al. Citation2005). The LPS-injected rats gradually gained less body weights over the treatment period than did the sham control rats in the CON group (a). Analysis of body weight values revealed no significant differences among the seven groups on the first day prior to starting LPS infusion. However, there was a significant reduction in body weight gain during 21 days of the treatment in the LPS group, as compared to the CON group (p<0.001). Interestingly, body weights of the 100 mg/kg PCE-treated rats and 200 mg/kg PCE-treated rats showed significant inhibition of reductions of body weight gain during 21 days of the treatment, as compared to the LPS group (p<0.05 and p<0.01, respectively). It also indicated that the recovery of body weights in the PCE100 + LPS and PCE200 + LPS groups was closely associated with those in the IBU + LPS group.
Figure 2. Effects of PCE on body weight gain on the first day prior to LPS injection and on the 21th day after LPS injection, and the activity counts of locomotor activity and the total numbers of rearings in the open field test on the 21th day. PCE, Phellodendri Cortex extract; LPS, lipopolysaccharide; IBU, ibuprofen. * p<0.01, *** p<0.001 vs. CON group; # p<0.05 and ## p<0.01, ### p<0.001 vs. LPS group.
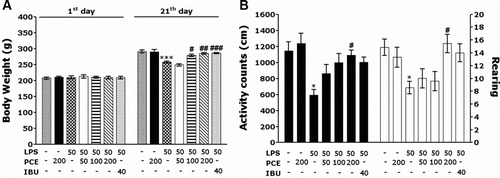
Effect of PCE on LPS-induced locomotor disability in the open field test
Open field activity was used to evaluate locomotor activity and exploratory behavior in the rats that received an LPS injection into the lateral ventricle (b). These results indicated that the rats with LPS infusion significantly reduced their locomotor activities and total numbers of rearing, as compared with those in the CON group (p<0.05). It suggested that the rats, treated with LPS, subsequently produced impairments of motor and exploration activities which have been closely associated with motor function behavior in the open field test. However, the PCE-treated rats (200 mg/kg) significantly increased their locomotor activity and rearing, as compared to those in the LPS group (p<0.05). Therefore, the PCE-treated rats showed remarkable enhancements of the active responses via stimulating motivation required for exploring for 21 days, ultimately indicating that the treatment of PCE significantly restored locomotor activity and exploratory behavior and accordingly alleviated LPS-induced impairment of motor function behavior in rats.
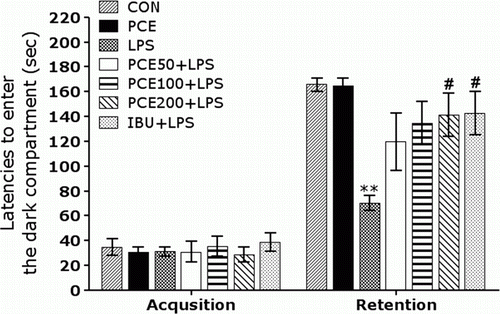
Effect of PCE on LPS-induced step-through latency deficit in the PAT
To determine whether PCE promotes the recovery of memory dysfunction, PCE was administrated in the rats with LPS-induced impairment of memory, and their memory and cognitive functions were examined in the PAT (). It was verified that the rats in all groups had no physiological defects (i.e. motor function) or intrinsic cognitive impairments through acquisition trials without electric challenge. In the time for acquisition trials, indicated by the latencies for entering the dark compartment, there were no significant differences among all groups. After acquisition trials, the effect of PCE on the retention latency was examined 24 hours after applying electric shock in the dark box in the PAT. In the retention, it was shown that the rats in the PCE200 + LPS group had significantly increased latencies to enter the dark compartment for retention as compared to those in the LPS group (p<0.05). This study indicated that the LPS infusion severely impaired long-term memory, and the treatment of PCE significantly attenuated LPS-induced memory deficit in the PAT. It also indicated that the restoration of memory function in the PCE200 + LPS group was almost close to that in the IBU + LPS group.
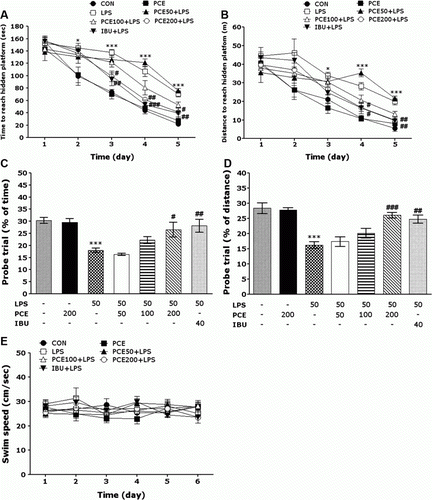
Effect of PCE on LPS-induced spatial memory impairment in the water maze test
The effect of the PCE treatment on swimming to reach the submerged platform in the MWM test is shown in . In the present study, a spatial learning and memory deficit rat model was established by infusion of LPS into the lateral ventricle, by which the escape latency and the searching distance were significantly increased as compared with those in the CON group. Analysis of escape latency revealed that the rats in the PCE200 + LPS group significantly reduced swimming latency as compared with those in the LPS group (PCE200 + LPS group: p<0.05 on day 3, and 5, p<0.01 on day 4; a). Swimming distance traveled in each group was closely associated with escape latency in this task. Analysis of searching distance values revealed that the rats in the PCE200 + LPS group significantly reduced swimming distance as compared with those in the LPS group (PCE200 + LPS group: p<0.05 on day 4, p<0.01 on day 5; b). The PCE treatment of 200 mg/kg improved spatial learning ability and memory function in the rats with chronic inflammation in their brain by LPS infusion, as evidenced by decreases in escape latency and searching distance throughout the training period. To investigate the effect on the spatial memory, the performance in the probe trial on day 6 was examined by analyzing the percentages of time and distance required for swimming to the expected position of the platform, respectively (c,d). The swimming times and distances are reduced in the rat that directly swims to the target quadrant where the escape platform had been removed without confusion. The rats with LPS infusion showed severe impairment of spatial performance in the MWM (p<0.001). The rats in the 200 mg/kg PCE-treated group spent more time (p<0.05) and distance (p<0.001) around the platform area than did those in the LPS group. The mean swimming speeds, as calculated by dividing the total swim distance by latency (e), of the rats in the LPS group were not significantly different from those in other groups. On the basis of these results, it was suggested that 200 mg/kg PCE-treated rats exhibited better improvement of acquisition in the hidden platform trial, and this memory improvement resulted in more rapid escape from the water than did the LPS-treated rats. Administration of PCE significantly attenuated LPS-induced deficits of learning and memory demonstrated in the MWM test. Thus, PCE-treated rats showed a significant amelioration in the memory retention test because they spent more time and distance in the quadrant where the platform was formerly located and swam over the location of platform more frequently. It also indicated that swimming latency of the LPS-injected rats receiving 200 mg/kg PCE was closely associated with those of rats receiving 40 mg/kg IBU.
Figure 4. Effects of PCE on latency of escaping from water (a) and swimming distance (b) during acquisition trials using a submerged platform, the percentages of time (c) and distance (d) in a probe trial without a platform, and swimming speed (e) in the Morris water maze test. * p<0.05 and *** p<0.001 vs. CON group; # p<0.05, ## p<0.01 and ### p<0.001 vs. LPS group.
Effects of PCE on LPS-induced immunohistochemical changes of inflammatory mediators
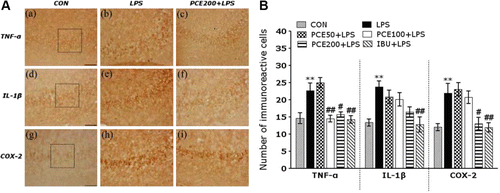
Following the behavioral tasks, brain tissue samples from the subjects were analyzed using immunohistochemistry to investigate the effect of PCE administration on the expression of pro-inflammatory markers formerly activated by LPS-induced inflammation in the rat brains (A). The brain immunohistochemistry in the LPS group showed a significant increase in TNF-α, IL-1β, and COX-2 expression in the hippocampus, as compared to those in the CON group (p<0.01). TNF-α immunoreactive cells significantly decreased in the hippocampal regions in the PCE100 + LPS (p<0.01) and PCE200 + LPS groups (p<0.05), as compared to those in the LPS group (B). Despite appearance of IL-1β-immunoreactive cells in the hippocampal regions in the PCE200 + LPS group, there was no statistical significance as compared to the LPS group (B). Similarly, the COX-2-immunoreactive cells were significantly increased in the hippocampal region in the PCE200 + LPS group (p<0.05), as compared to those in the LPS group (B). The increases in the pro-inflammatory mediators such as TNF-α, IL-1β, and COX-2 by LPS-induced inflammation were significantly inhibited by PCE administration, and their restorations were almost close to those in the IBU + LPS group. These results clearly indicated that the LPS-induced increase in the expression of TNF-α and COX-2 in the rat hippocampus was significantly attenuated by the treatment of PCE.
Figure 5. Effects of PCE on the mean number of tumor necrosis factor-α (TNF-α)-, interleukin-1β (IL-1β)-, and cyclooxygenase-2 (COX-2)-stained hippocampal areas after the Morris water maze test. Representative photographs and the relative percentage values are indicated in (A) and (B), respectively. (a) TNF-α expression in the CON group, (b) TNF-α expression in the LPS group, (c) TNF-α expression in the PCE200 + LPS group, (d) IL-1β expression in the CON group, (e) IL-1β expression in the LPS group, (f) IL-1β expression in the PCE200 + LPS group, (g) COX-2 expression in the CON group, (h) COX-2 expression in the LPS group, (i) COX-2 expression in the PCE200 + LPS group. Sections were cut coronally at 30 µm. Scale bar indicates 50 µm. ** p<0.01 vs. CON group; # p<0.05 and ## p<0.01 vs. LPS group.
Effect of PCE on LPS-induced expression of TNF-α, IL-1 β, and COX-2 mRNAs in the hippocampus
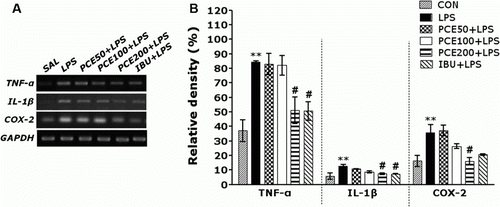
The effect of PCE administration on LPS-induced expression of TNF-α, IL-1β, and COX-2 mRNAs in the rat hippocampus was investigated using RT-PCR analysis (). Hippocampal expression of TNF-α, IL-1β, and COX-2 mRNA in the LPS group was significantly increased as compared to that in the CON group (p<0.01). In case of PCE administration of 200 mg/kg, the increased expression of TNF-α, IL-1β, and COX-2 mRNA in the LPS group was significantly restored (p<0.05). The restored levels of these inflammatory mediators were almost close to those in the IBU + LPS group.
Figure 6. Effects of PCE on the expression of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and cyclooxygenase-2 (COX-2) mRNAs in rats with LPS-induced hippocampal impairment. PCR bands on agarose gel and their relative intensities are indicated in (a) and (b), respectively. The expression levels of TNF-α, IL-1β, and COX-2 mRNAs were normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA as an internal control. ** p<0.01 vs. CON group; # p<0.05 vs. LPS group.
Discussion
In the Korean traditional medicine, PCE has been widely used to treat chronic inflammatory diseases and prevent bacterial infection of the respiratory system and gastrointestinal tract (Yang et al. Citation2010; Lu et al. Citation2011). Recently, several studies have shown that PCE had a protective role against ischemia-reperfusion-induced brain injury through the reduction of neutrophil infiltration into the brain (Hwang et al. Citation2002b). It also alleviated LPS-stimulated inflammatory responses, including LPS-induced expression of TNF-α, IL-1β, and iNOS through the negative modulation of nuclear factor-kappaB (NF-κB) signaling in the microglial cells (Park et al. Citation2007). Thus, these studies suggest a new medicinal role of PCE on suppressing inflammatory response in the central nervous system, which can lead to developing a novel therapeutics using PCE for treating neurodegenerative diseases in humans.
Our results clearly demonstrated that the administration of PCE significantly improved spatial learning and memory impairments induced by LPS infusions into the bilateral ventricles, as evidenced by the behavior in the PAT and MWM tests. It also attenuated the increase in pro-inflammatory markers such as TNF-α, IL-1β, and COX-2 in the hippocampus, as assessed by RT-PCR analysis and immunohistochemistry.
We found that the administration of PCE after LPS infusion into the lateral ventricle markedly restored body weight gain and motor activity, suggesting that PCE could inhibit the physiological changes and the sickness behavioral symptoms caused by LPS-induced neuroinflammation. It can be suggested that PCE enhanced resistance against stress and various psychosomatic disorders and exerted various benefits, strengthening the immune system.
To identify the effects of PCE on two different types of memory, cognitive memory and spatial learning, we used the PAT and MWM, respectively. Our results demonstrated that the administration of PCE significantly increased the step-through latency in the memory retention trial, which had been shortened by LPS infusion, in the PAT. During the trial sessions in the MWM, administration of PCE resulted in a significant reduction in the escape latency, enhanced cognitive performance, and ameliorated of memory deficits, associated with LPS injection. These results showed that LPS infusion into the lateral ventricle of rat brains retarded escape latency in the MWM test, indicating deficits in spatial learning ability and reference memory. The scoring of the escape latency from water in the spatial probe tests in the MWM test is primarily considered to reflect long-term spatial memory ability (Lee et al. Citation2011). In this study, administration of PCE shortened the escape latency without affecting swimming velocity and extended the time spent swimming in the place where the platform was previously located. This indicated that the administration of PCE significantly improved the long-term memory deficit in rats that had suffered LPS-induced spatial memory impairment. From the results of the PAT and MWM tests, we concluded that a chronic inflammatory response played an important role in learning acquisition and synaptic plasticity (Collister and Albensi Citation2005).
Our results also showed that LPS infusion into the lateral ventricle significantly increased the expression of TNF-α and IL-1β in the hippocampus, ultimately leading to a chronic neuroinflammatory response in the brain. Thus, LPS-stimulated sustained increases in the expression of pro-inflammatory cytokines have been directly linked to neurodegenerative disorders associated with decreased working memory (Frank-Cannon et al. Citation2009). The expression of TNF-α and IL-1β mRNAs is dynamically regulated by various immune cells in the inflammatory response of the hippocampus (Kim and Joh Citation2006). Administration of PCE continuously decreased the LPS-induced expression of TNF-α and IL-1β mRNAs, which eventually resulted in recovery from the chronic inflammation and persistent brain dysfunction (Ringheim and Conant Citation2004). According to the inflammation hypothesis, the memory impairment in patients with senile dementia is due to selective and irreversible dysfunction and chronic inflammation in the brain (Hoozemans et al. Citation2001). Thus, we propose that the anti-inflammatory effects of PCE significantly reversed the impairment in memory retention and the increased expression of pro-inflammatory cytokines such as TNF-α and IL-1β.
Also, much experimental evidence has suggested that the inducible gene encoding COX-2 is a key element that modulates the generation of proinflammatory mediators including various prostagladins (Fehér et al. Citation2010). It is known that the expression of COX-2 and the synthesis of prostaglandin E2, one of its products, are increased in the hippocampus of patients with AD (Fujimi et al. Citation2007), which may be related to the pathogenesis of the degenerative changes and cognitive impairments (Hwang et al. Citation2002a). These results suggest that inflammatory responses to LPS infusion significantly stimulated the expression of COX-2 protein or mRNA in the hippocampus through modulating the NF-κB pathway (Gong et al. Citation2011). The increase in COX-2 expression by NF-κB activation can accelerate inflammatory responses and subsequently contribute to learning and memory deficits. Thus, treatment with long-lasting COX-2 inhibitors during the initial stages of AD, before clinical symptoms of dementia appear, may suppress inflammatory responses and the synthesis of pro-inflammatory mediators in the brain (Kumar et al. Citation2006). In the present study, PCE significantly decreased LPS-stimulated behavioral changes and memory disturbances through inhibition of COX-2 mRNA expression.
In summary, we demonstrated that PCE significantly improved spatial learning and memory abilities in the rats with LPS-induced brain dysfunction, as evidenced by performance in the PAT and the MWM tests. PCE also suppressed LPS-simulated mRNA expression of pro-inflammatory mediators such as TNF-α, IL-1β, and COX-2 in the hippocampus. Thus, PCE may be useful as an alternative medicine for treating or retarding the development of neurodegenerative diseases including AD.
Acknowledgements
This work was supported by a grant from the Kyung Hee University in 2011 (KHU-20110087).
References
- Collister , KA and Albensi , BC. 2005 . Potential therapeutic targets in the NF-kappaB pathway for Alzheimer's disease . Drug News Perspect , 18 : 623 – 629 .
- Cunningham , C , Campion , S , Lunnon , K , Murray , CL , Woods , JF , Deacon , RM , Rawlins , JN and Perry , VH. 2009 . Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease . Biol Psych , 65 : 304 – 312 .
- Fehér , A , Juhász , A , Rimanóczy , A , Kálmán , J and Janka , Z. 2010 . Association study of interferon-γ, cytosolic phospholipase A2, and cyclooxygenase-2 gene polymorphisms in Alzheimer disease . Am J Geriatr Psych , 18 : 983 – 987 .
- Frank-Cannon , TC , Alto , LT , McAlpine , FE and Tansey , MG. 2009 . Does neuroinflammation fan the flame in neurodegenerative diseases? . Mol Neurodegener , 16 : 47 – 59 .
- Fujimi , K , Noda , K , Sasaki , K , Wakisaka , Y , Tanizaki , Y , Iida , M , Kiyohara , Y , Kanba , S and Iwaki , T. 2007 . Altered expression of COX-2 in subdivisions of the hippocampus during aging and in Alzheimer's disease: the Hisayama Study . Dement Geriatr Cogn Disord , 23 : 423 – 431 .
- Gaab , J , Rohleder , N , Heitz , V , Engert , V , Schad , T , Schürmeyer , TH and Ehlert , U. 2005 . Stress-induced changes in LPS-induced pro-inflammatory cytokine production in chronic fatigue syndrome . Psychoneuroendocrinology , 30 : 188 – 198 .
- Gong , QH , Pan , LL , Liu , XH , Wang , Q , Huang , H. and Zhu , YZ. 2011 . S-propargyl-cysteine (ZYZ-802), a sulphur-containing amino acid, attenuates beta-amyloid-induced cognitive deficits and pro-inflammatory response: involvement of ERK1/2 and NF-κB pathway in rats . Amino Acids , 40 : 601 – 610 .
- Graupera , M , García-Pagán , JC , Abraldes , JG , Peralta , C , Bragulat , M , Corominola , H , Bosch , J and Rodés , J. 2003 . Cyclooxygenase-derived products modulate the increased intrahepatic resistance of cirrhotic rat livers . Hepatology , 37 : 172 – 181 .
- Guo , J , Li , F , Wu , Q , Gong , Q , Lu , Y and Shi , J. 2010 . Protective effects of icariin on brain dysfunction induced by lipopolysaccharide in rats . Phytomedicine , 17 : 950 – 955 .
- Ho , YS , So , KF and Chang , RC. 2011 . Drug discovery from Chinese medicine against neurodegeneration in Alzheimer's and vascular dementia . Chin Med , 6 : 1 – 6 .
- Hoozemans JJ , Rozemuller AJ , Veerhuis R , Eikelenboom P. 2001 . Immunological aspects of alzheimer's disease: therapeutic implications . BioDrugs . 15 : 325 – 337 . Review .
- Hwang , DY , Chae , KR , Kang , TS , Hwang , JH , Lim , CH , Kang , HK , Goo , JS , Lee , MR , Lim , HJ Min , SH . 2002a . Alterations in behavior, amyloid beta-42, caspase-3, and COX-2 in mutant PS2 transgenic mouse model of Alzheimer's disease . FASEB J , 16 : 805 – 813 .
- Hwang , YK , Ma , J , Choi , BR , Cui , CA , Jeon , WK , Kim , H , Kim , HY , Han , SH and Han , JS. 2011 . Effects of Scutellaria baicalensis on chronic cerebral hypoperfusion-induced memory impairments and chronic lipopolysaccharide infusion-induced memory impairments . J Ethnopharmacol , 1 : 681 – 689 .
- Hwang , YS , Shin , CY , Huh , Y and Ryu , JH. 2002b . Hwangryun-Hae-Dok-tang (Huanglian-Jie-Du-Tang) extract and its constituents reduce ischemia-reperfusion brain injury and neutrophil infiltration in rats . Life Sci , 71 : 2105 – 2117 .
- Kelloff , GJ , Crowell , JA , Steele , VE , Lubet , RA , Malone , WA , Boone , CW , Kopelovich , L , Hawk , ET , Lieberman , R Lawrence , JA . 2000 . Progress in cancer chemoprevention: development of diet-derived chemopreventive agents . J Nutr , 130 : 467S – 471S .
- Kim YS , Joh TH . 2006 . Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson's disease . Exp Mol Med . 38 : 333 – 347 . Review .
- Kim , WG , Mohney , RP , Wilson , B , Jeohn , GH , Liu , B and Hong , JS. 2000 . Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia . J Neurosci , 15 : 6309 – 6316 .
- Kitazawa , M , Oddo , S , Yamasaki , TR , Green , KN and LaFerla , FM. 2005 . Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease . J Neurosci , 28 : 8843 – 8853 .
- Knight , EV , Kimball , JP , Keenan , CM , Smith , IL , Wong , FA , Barrett , DS , Dempster , AM , Lieuallen , WG , Panigrahi , D Powers , WJ . 1996 . Preclinical toxicity evaluation of tepoxalin, a dual inhibitor of cyclooxygenase and 5-lipoxygenase, in Sprague-Dawley rats and beagle dogs . Fundam Appl Toxicol , 33 : 38 – 48 .
- Kumar , A , Seghal , N , Padi , SV and Naidu , PS. 2006 . Differential effects of cyclooxygenase inhibitors on intracerebroventricular colchicine-induced dysfunction and oxidative stress in rats . Eur J Pharmacol , 551 : 58 – 66 .
- Lee , B , Shim , I , Lee , H and Hahm , DH. 2011 . Rehmannia glutinosa ameliorates scopolamine-induced learning and memory impairment in rats . J Microbiol Biotechnol , 21 : 874 – 883 .
- Lee , B , Chae , YB , Kwon , YK , Yang , CH , Kim , MR , Kim , KJ , Hahm , DH , Lee , HJ and Shim , I. 2004 . Inhibitory action of Cortex Phellodendris on nicotine-induced behavioral sensitization . Kor J Ori Physiol & Pathol , 18 : 767 – 773 .
- Lim , JS , Kim , H , Choi , Y , Kwon , H , Shin , KS , Joung , I , Shin , M and Kwon , YK. 2008 . Neuroprotective effects of berberine in neurodegeneration model rats induced by ibotenic acid . Anim cell Syst , 12 : 203 – 209 .
- Lu , J , Wang , JS and Kong , LY. 2011 . Anti-inflammatory effects of Huang-Lian-Jie-Du decoction, its two fractions and four typical compounds . J Ethnopharmacol , 134 : 911 – 918 .
- Lukiw , WJ and Bazan , NG. 2000 . Neuroinflammatory signaling upregulation in Alzheimer's disease . Neurochem Res , 25 : 1173 – 1184 .
- Mao , YF , Li , YQ , Zong , L , You , XM , Lin , FQ and Jiang , L. 2010 . Methanol extract of Phellodendri cortex alleviates lipopolysaccharide-induced acute airway inflammation in mice . Immunopharmacol Immunotoxicol , 32 : 110 – 115 .
- Min , SS , Quan , HY , Ma , J , Han , JS , Jeon , BH and Seol , GH. 2009 . Chronic brain inflammation impairs two forms of long-term potentiation in the rat hippocampal CA1 area . Neurosci Lett , 29 : 20 – 24 .
- Mohamed , AF , Matsumoto , K , Tabata , K , Takayama , H , Kitajima , M and Watanabe , H. 2000 . Effects of Uncaria tomentosa total alkaloid and its components on experimental amnesia in mice: elucidation using the passive avoidance test . J Pharm Pharmacol , 52 : 1553 – 1561 .
- Mrak RE . 2009 . Neuropathology and the neuroinflammation idea . J Alzheimers Dis . 18 : 473 – 481 . Review .
- Paxinos , G and Watson , C. 1986 . The rat brain in stereotaxic coordinates , New York : Academic Press .
- Park , YK , Chung , YS , Kim , YS , Kwon , OY and Joh , TH. 2007 . Inhibition of gene expression and production of iNOS and TNF-alpha in LPS-stimulated microglia by methanol extract of Phellodendri cortex . Int Immunopharmacol , 7 : 955 – 962 .
- Ringheim GE , Conant K . 2004 . Neurodegenerative disease and the neuroimmune axis (Alzheimer's and Parkinson's disease, and viral infections . J Neuroimmunol . 147 : 43 – 49 . Review .
- Schwab , C and McGeer , PL. 2008 . Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders . J Alzheimers Dis , 13 : 359 – 369 .
- Szekely , CA , Breitner , JC , Fitzpatrick , AL , Rea , TD , Psaty , BM , Kuller , LH and Zandi , PP. 2008 . NSAID use and dementia risk in the cardiovascular health study: role of ApoE and NSAID type . Neurology , 1 : 17 – 24 .
- Warner , TD , Giuliano , F , Vojnovic , I , Bukasa , A , Mitchell , JA and Vane , JR. 1999 . Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis . Proc Natl Acad Sci USA , 96 : 7563 – 7568 .
- Yang , Q , Zhang , F , Gao , SH , Sun , LN and Chen , WS. 2010 . Determination of bioactive compounds in Cortex Phellodendri by high-performance liquid chromatography . J AOAC Int , 93 : 855 – 861 .
- Zhang , J , Yang , JQ , He , BC , Zhou , QX , Yu , HR , Tang , Y and Liu , BZ. 2009 . Berberine and total base from rhizoma coptis chinensis attenuate brain injury in an aluminum-induced rat model of neurodegenerative disease . Saudi Med J , 30 : 760 – 766 .