Abstract
Nordihydroguaiaretic acid (NDGA), a well-known lipoxygenase inhibitor, exerts pleiotropic effects on various cellular events. It has also been known that NDGA suppresses immune response in lipopolysaccharide (LPS)-challenged immune cells. Although the immunosuppressive activity of NDGA was suggested to be related with its antioxidant activity, the precise mechanism for its diverse effects on immune system has not been clarified yet. Since transforming growth factor–β–activated kinase 1 (TAK1) is an essential intracellular signaling component in mediating the immune responses of macrophage and lymphocytes, we asked whether NDGA has specific effect on TAK1 signaling pathway. In this study, we examined the immunosuppressive activity of NDGA in murine macrophage cell line (RAW 264.7) as well as the changes in TAK1 after the NDGA challenge. Our results clearly showed that the LPS-induced iNOS, COX-2, and tumor necrosis factor α (TNF-α) expressions were suppressed and TAK1 was also significantly down-regulated in the presence of NDGA. However, the messenger ribonucleic acid (mRNA) level of TAK1 was not changed. Instead, the protein level was significantly decreased by the treatment of NDGA, and the specific proteasome inhibitor was able to block the decrease in TAK1 level. This result implied the involvement of protein degradation pathway in down-regulation of TAK1 by NDGA. Because NDGA also suppressed c-Jun NH2-terminal protein kinase (JNK) activation caused by LPS treatment in correlation with the decrease in TAK1 level, the immunosuppression exerted by NDGA seemed correlated with its down-regulating effect on TAK1 together with the blockage of NF-κB signaling.
Keywords:
Introduction
It is becoming increasingly apparent that inflammation lies at the basis of many diseases, such as rheumatoid arthritis, fibrosis, and various cancers. During the process of inflammation, neutrophils and monocytes are recruited to the site of tissue injury or infection and increase production of reactive oxygen species and inflammatory mediators (Balkwill & Mantovani Citation2001; Coussens & Werb Citation2002). Chronic inflammation or unregulated inflammation can lead to long-term pathogenesis, such as cancers, degenerative neural diseases, and hepatic fibrosis (Sunami et al. Citation2012; Wyss-Coray & Rodgers Citation2012). NF-κB is a key coordinator of innate immunity and inflammation (Bonizzi et al. Citation2004; Karin et al. Citation2004). NF-κB operates downstream of the sensing of microorganism or tissue damage by the toll-like receptor signaling pathway and also conveys the signal pathway of inflammatory cytokines, TNF-α, and IL-1β. Among the various upstream signal molecules modulating the NF-κB signaling, transforming growth factor (TGF)–β–activated kinase 1 (TAK1) is known to exert key roles in regulating NF-κB and AP-1 signaling in the inflammatory and stress response (Shim et al. Citation2005; Tang et al. Citation2008).
TAK1 was identified as a member of MAPKKK family and first reported as mediating a part of TGF-β signaling (Yamaguchi et al. Citation1995; Wang et al. Citation2001). TAK1 was reported to be involved in IL-1β and TNF-α signaling, which have occurred following the activation of NF-κB and JNK signaling (Ninomiya-Tsuji et al. Citation1999; Wang et al. Citation2001). More recently, TAK1 has been reported to play an important role in mesodermal induction of various animals as well (Ohkawara et al. Citation2004). Overexpression of inactivated form of TAK1 inhibits IL-1 and TNF pathway (Sakurai et al. Citation1999). Using the TAK1, small interfering RNA experiment for the TAK1 gene silencing defined that TAK1 is essential for both IL-1 and TNF-induced NF-κB activation in mammalian cells (Takaesu et al. Citation2003). Furthermore, the Drosophila homolog of TAK1 was identified as an essential molecule for host-defense signaling in Drosophila, indicating the involvement of TAK1 in the innate immunity as well (Vidal et al. Citation2001). Therefore, it can be expected that TAK1 is a good target for preventing inflammation and tissue destruction promoted by various antigens and proinflammatory mediators, such as TNF-α andIL-1β.
Several investigators reported that TAK1 inhibitors, 5Z-7-oxozeaenol and prodelphinidin B-4 3′-O-gallate, can inhibit TAK1 kinase activity as well as the activation of NF-κB induced by both TNF-α and IL-1 (Ninomiya-Tsuji et al. Citation2003). These results indicated the critical involvement of TAK1 in both NF-κB and AP-1 signaling per se.
NDGA, a well-known lipoxygenase inhibitor (Bokoch and Reed Citation1981), exerts many intriguing effects on various cellular events including growth factor signal transduction (Domin et al. Citation1994; Lee et al. Citation2003) and strongly suppresses the transport of newly synthesized secretory protein from the endoplasmic reticulum to the Golgi complex (Fujiwara et al. Citation2003). It also blocked cancer cell proliferation (Youngren et al. Citation2005) and the differentiation of myoblast (Ito et al. Citation2005). More recently, the clinical application and molecular mechanisms of NDGA were reviewed by Lü et al. (Citation2010).
In this study, we found that NDGA causes the dramatic decrease in the level of TAK1 protein without any significant changes in its mRNA level. The effect of NDGA on down-regulation of TAK1 level seemed unique since other structurally related compounds of NDGA had no effect on TAK1 level. In addition, we found that NDGA had an inhibitory effect on JNK activation upon exposure to LPS. JNK has also been shown as a regulator that mediates inflammatory response by stressors or inflammatory cytokines (Chang et al. Citation2007). Together, these results suggest NDGA as a novel and dual inhibitor for immune responses that are exerted through TAK1 and following NFκB and JNK pathway.
Materials and methods
Cells and reagents
RAW264.7 (murine macrophage cell line), ROS17/6.4, and MC3T3 cells were purchased from American Type Culture Collection (ATCC). Cells were maintained as indicated in ATCC cell maintenance manual, Dulbecco's Modified Eagle's Medium (DMEM) and other liquid media from Lonza (Australia), heat-inactivated fetal bovine serum (FBS) or qualified FBS, and antibiotics (100U/ml penicillin and 100U/ml streptomycin) from Invitrogen (CA, USA). Antibodies specific for TAK1 (4505), IκBα (9242), phospho-JNK (4668), JNK (9252), and GADPH (2118) form Cell Signaling Inc. and anti-actin (sc1616) from Santa Cruz Inc. Other general chemicals were purchased from Sigma-Aldrich Chem Comp. or Fluka Chem. The specific primers were purchased from Qiagen (QuantiTect primer sets, CA, USA). NF-κB and AP-1 promoter luciferase reporter plasmids were kindly provided by Dr. Shim, M. (NIH, USA).
Western blot analysis
The cultured cells were washed three times with phosphate-buffered saline and lysed in 0.5% Triton X-100 containing protease inhibitor cocktails (Sigma, MO, USA) by passing through the syringe needle (Guage26) for several times. Cell lysates were centrifuged at 12,000 x g for 5 minutes, and the resulting supernatant was used for protein assay. Protein concentrations were measured by BCA protein assay kit using bovine serum albumin as a standard. Electrophoresis was performed in a 10% polyacrylamide gel in the presence of 0.1% sodium dodecyl sulfate, and the proteins were blotted onto a polyvinylidene difluoride membrane (Invitrogen, CA, USA). The equal amount of protein in each lane was verified by Ponceau S staining. The membranes were incubated overnight at 4°C with an indicated primary antibody. After washing with Tris-buffered saline containing 0.05% Tween 20, pH8.0, the membrane was incubated with peroxidase conjugated secondary antibodies for 1 hour. The protein band on the membrane was detected using the ECL plus system (Amersham, NJ, USA).
Quantitative real-time polymerase chain reaction
Total RNA from RAW264.7 macrophages was isolated with the RNeasy kit according to the manufacturer's instructions (Qiagen, CA, USA). The reverse transcription reaction was carried out using oligo d(T) and Mulv reverse transcriptase with a following condition: 40°C for 60 minutes, 95°C 5 minutes, and 4°C rest of time. Quantitative real-time polymerase chain reaction (qRT-PCR) primers were purchased from Qiagen (CA. USA) as QuantiTect® Primer Assays. Applied Biosystems' (CA, USA) real-time PCR machine (Model 7500) and SYBR Green mix (Qiagen, CA, USA) were used for amplification of COX-2, iNOS, TNF-α, and action with following conditions: 40 cycle of 95°C for 10 seconds, 55°C for 33 seconds, and 72°C for 33 seconds. The fold changes in mRNA levels were calculated using the ΔΔCt method with β-actin as reference mRNA.
Transfection and luciferase assays
For transient transfection, cells were transfected using Fugene®HD reagent according to manufacturer's instructions (Roche Inc. NC, USA). After 16 hours following transfection, cells were pretreated with or without 10 µM NDGA for 1 hour and then stimulated with 100 ng/ml LPS for 3 hours. Then the cells were harvested and luciferase activity was measured according to the manufacturer's protocol in the Dual-Luciferase Reporter Assay System using GLOMA 20/20 luminometer (Promega, WI, USA). The fold induction of treated samples, relative to the untreated samples, was calculated from firefly luciferase levels that were normalized to Renilla luciferase activity.
Results
NDGA abrogates LPS-induced activation of proinflammatory gene expression and NF-κB reporter gene expression
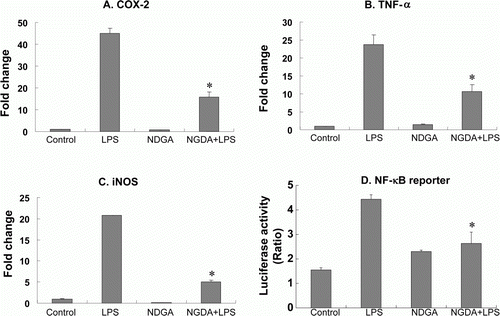
Because TAK1 is known to play a key role in immune responses, we asked whether NDGA affects the LPS-induced proinflammatory gene expression. NDGA is a well-known lipoxygenase inhibitor and also known as an antioxidant. It also has been shown to have some anti-cancer and anti-immune activities, but the precise mechanisms and effects were not clarified yet (Arteaga et al. Citation2005b). To elucidate the anti-inflammatory activity of NDGA, the expression levels of COX-2, iNOS, and TNF-α were measured using qRT-PCR (A–C). The present qPCR results showed that NDGA significantly abrogated the LPS-induced activation of COX-2, iNOS, and TNF-α gene expressions. NDGA proinflammatory genes were closely regulated through the combination of several pathways, including NF-κB and MAPKs signaling pathways. To examine whether the anti-inflammatory activity of NDGA were accompanied with the alteration in LPS-induced NF-κB activation, the NF-κB reporter assay was employed. As a result, NDGA suppressed LPS-induced luciferase gene expression (D). Because NDGA decreased various proinflammatory gene expressions together with NF-κB signaling, the anti-inflammatory activity of NDGA could be related with the inhibition of NF-κB signaling.
Figure 1. NDGA blocked LPS-induced proinflammatory gene expression. (A–C) The expression of each gene in RAW264.7 cells was accessed using real-time PCR and the specific primers. The cells were pretreated with 10 µM NDGA for 24 hours, then challenged by LPS and cultured for 6 hour before the cell harvest. (D) The cells were transfected with pNF-κB-LUC and pRL-LUC, then 16 hours after transfection the cells were treated with 10 µM NDGA for 1 hour and subsequently stimulated with 100 ng/ml LPS for 3 hours. Firefly luciferase was normalized for Renilla luciferase activity.
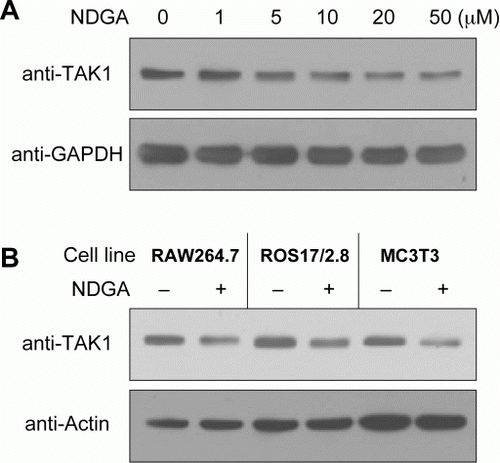
NDGA-induced downregulation of TAK1
Since TAK1 is known as one of important upstream regulator for NF-κB signaling, we asked whether NDGA affects NF-kB signaling through TAK1. At first, we examined the effect of NDGA on TAK1 protein level using western blot analysis following the NDGA treatment in RAW264.7 cells (A). Of interest, NDGA caused down-regulation of TAK1 protein level in a dose-dependent manner only after the prolonged incubation for 6 hours or more. Because most inhibitory activity of NDGA were observed within 1 hour in the previous study (Lee et al. Citation2003), this result suggested that different kind of activity of NDGA has been exerted here. Since the plateau of dose-response curve was observed starting at 10 µM of concentration, we used 10 µM NDGA to minimize the complication due to overdose of NDGA for the subsequent studies. This down-regulating effect of NDGA was also observed in other cell lines, including osteoblast and fibroblast (B). The NDGA effect on TAK1 level seems universal regardless of cell types because all the cell types that we examined responded in the same way.
Figure 2. NDGA down-regulates TAK1 protein level. (A) Dose-dependent decreases in TAK1 level following the challenge of NDGA. RAW264.7 cells were treated with the indicated concentration of NDGA for 6 hours, then harvested and subjected to the western blot analysis. (B) The decreases in TAK1 level were shown in various cell lines. Each cell line was treated with 10 µM NDGA for 6 hours and analyzed for TAK1 level.
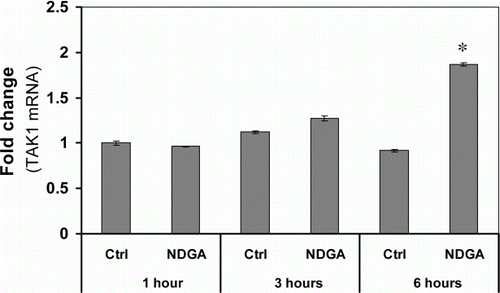
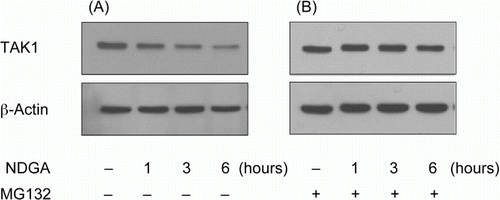
The changes in TAK1 mRNA and the effect of proteasome-specific inhibitor on NDGA-induced down-regulation of TAK1 protein
To examine whether the influence of NDGA on TAK1 is related with the transcriptional regulation of TAK1 gene, the changes in TAK1 mRNA level were assessed in different time points after NDGA treatment using qRT-PCR. RAW264.7 cells were pretreated with or without NDGA for the indicated time. As shown in , NDGA had no negative effect on the mRNA levels of TAK1. Instead, it significantly increased TAK1 mRNA following 6 hours of incubation with NDGA. This result strongly suggested that the decrease in TAK1 protein level did not stem from the transcriptional inhibition, but could be caused either by translational inhibition of TAK1 mRNA or by the stimulated degradation of TAK1. To investigate whether the altered degradation rate of TAK1 is related with the NDGA-induced down-regulation of TAK1, cells were treated with MG-132, a specific inhibitor of proteasome, and the TAK1 levels with or without NDGA were measured using western blot. Our western blot results showed that, in the presence of specific proteasome inhibitor, MG-132, NDGA had no effect on the protein level of TAK1 (), suggesting that NDGA reduces TAK1 levels through the enhanced degradation of TAK1 protein, not through the translational inhibition.
The NDGA-induced reduction of cellular TAK1 level resulted in the inhibition of JNK activation in the presence of LPS but did not relate with the inhibition of NF-κB signaling
To assess whether the decrease of TAK1 level by NDGA blocks the downstream pathway of TAK1, we examined the effect of NDGA on IκB kinase (IKK) and JNK signaling that are known to be activated through TAK1 activation. The maximum effects of LPS on phosphorylation of JNK and degradation of IκBα appeared at 25 minutes after LPS treatment (data not shown). We pretreated NDGA for the indicated times before LPS treatment and then after 25 minutes of LPS treatment, the cells were harvested and subjected to the western blot analysis. The maximum reduction of TAK1 level was observed in between 6 and 12 hours after NDGA treatment. At the same time period, LPS-induced JNK phosphorylation was greatly inhibited in the present of NDGA. However, the inhibition of IκBα degradation occurred at much earlier time under the same experimental conditions (). These results indicate that inhibition of JNK phosphorylation may be due to reduction of TAK1 protein levels by NDGA, while the effect on NF-κB signaling would not be caused by the same mechanism, TAK1 reduction. Accordingly, LPS-stimulated AP-1 reporter gene expression was significantly reduced by 12-hour treatment of NDGA.Footnote1
NDGA seems specific in down-regulating TAK1 level
In this study, we found that NDGA significantly decreased the endogenous TAK1 protein levels. To examine whether TAK1 down-regulation is a unique effect of NDGA, we tested other compounds that have similar molecular structure as NDGA ( and ). Other compounds structurally related with NDGA had no significant effect on TAK1 levels so far, suggesting that NDGA seems specific in reduction of TAK1 level.
Figure 5. The time-course of NDGA effect on TAK1 protein level and on the related signaling pathway. The changes in the degradation of IκBα and phosphorylation of JNK upon LPS treatment with the variation of NDGA pretreatment time in RAW264.7 cell. NDGA was pretreated for various times, followed by LPS treatment. The cells were treated with 100ng/ml LPS for 1 hour and harvested for the western blot analysis.
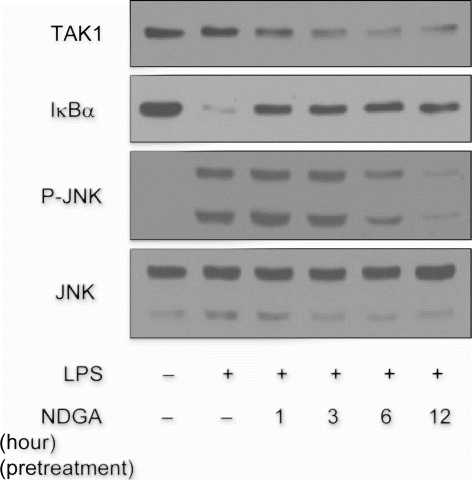
Figure 6. Effect of NDGA and its structurally related chemicals on the TAK1 protein level. RAW264.7 cells were treated with each compound (see for the molecular structure) at 10 µM concentration for 6 hours and subjected to the western blot analysis.
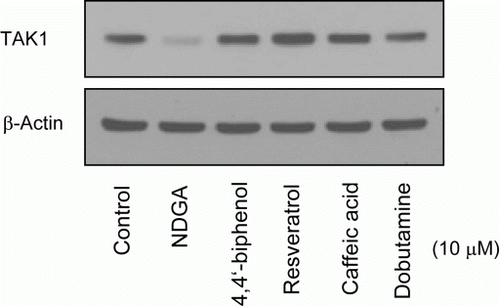
Table 1. The molecular structure of NDGA and structurally related compounds that were used in .
Discussion
NDGA extracted from the Larrea tridentata, L.divaricata, and L.mexicana (Creosote bush) has been used as a traditional medicine to relieve from ailments including gallbladder, kidney stones, and urinary tract infections (Arteaga et al. Citation2005a). NDGA has also been discussed for its potent anti-inflammatory activity, possibly due to its ability to inhibit the enzyme lipoxygenase in vitro (Bokoch and Reed Citation1981; Salari et al. Citation1984). In this study, qRT-PCR revealed that NDGA inhibited LPS-induced COX-2, TNF-α, and iNOS expression within 1 hour after treatment. NDGA also affected the NF-κB signaling as revealed in the reporter gene expression and western blot analysis within 1 hour of treatment (D and ). Accordingly, it has been reported that NDGA inhibited cytokine-induced NF-κB activation via its anti-oxidative activity (Brennan et al. Citation1998). Recent reviews indicated NDGA has extremely diverse and noble activities (Arteaga et al. Citation2005b; Chen Citation2009). Although some activity of NDGA was obviously related with its anti-oxidative and/or lipoxygenase inhibiting activity, most other diverse biological effects of NDGA are still under investigations searching for their precise action mechanisms (Lee et al. Citation2003; Lü et al. Citation2010).
In this study, we investigated the effect of NDGA on the TAK1 signaling pathway, which is important upstream signaling of NF-κB activation in the inflammatory response. As a result, NDGA treatment caused a dramatic decrease in the TAK1 protein level in a dose-dependent manner and with the unique delayed time schedule. Of interest, we observed that the decrease in TAK1 level by NDGA was insignificant at 1 hour after the treatment but became recognizable at 3 hours after the NDGA treatment and keep decreasing until 12 hours after the treatment. Previously Shi et al. (Citation2009) reported that Hsp90 regulates IL-1b signaling by stabilizing TAK1. Accordingly, NDGA could be the inhibitor of Hsp90. In this experiment, however, Hsp90-related signaling molecules, such as p-Erk, (Okawa et al. Citation2009) was not changed in NDGA-treated RAW264.7 cells. In addition, it has never been reported that NDGA was involved in protein stability.
Because multiple lines of evidence have demonstrated that TAK1 plays a critical role in NF-κB and MAPK activation induced by many cytokines and inflammatory products such as LPS (Ninomiya-Tsuji et al. Citation1999; Wang et al. Citation2001), we considered that the inhibition of LPS-induced expression of pro-inflammatory gene by NDGA might be the results of the down-regulation of TAK1 protein levels. Thus, we examined the inhibitory effects of NDGA on degradation of IκBα, which are further regulated by upstream kinase TAK1, with various time schedule. Western blotting results showed that the inhibition of IκBα degradation remarkably preceded the TAK1 diminution (). These data implied that the mechanism for the inhibition of IκB degradation was not related with the TAK1 disappearance. However, since there seems two distinct mechanisms for the regulation of NF-kB signaling upon T-cell recepton stimulation (Shambharkar et al. Citation2007), it would be necessary to use the CARMA1-deficient cells to examine whether NDGA inhibited NF-kB activation through the blockage of CARMA1-mediated pathway or through the CARMA1-independent pathway.
Meanwhile, another down-stream signaling pathway, JNK, was inhibited by NDGA in unison with the TAK1 down-regulation. The inhibition of LPS-induced phosphorylation of JNK began to show up at 3 hours after the treatment and then became clearer with the time lapse. The time schedule was in good correlation with the decline of TAK1 level caused by NDGA (). Thus NDGA seems to inhibit LPS-induced JNK activation through the reduction of TAK1 level.
NDGA decreased TAK1 protein levels in a dose-dependent manner. Studies with a specific proteasome inhibitor (MG132) abolished this effect, suggesting that NDGA-mediated reduction of TAK1 protein levels may be due to polyubiquitination and enhanced proteasomal degradation of TAK1. If the decrease in TAK1 protein level was caused by transcriptional regulation, there would be changes in mRNA level as well. qRT-PCR experiment using the TAK1 specific primer excluded the transcriptional regulation of TAK1 by NDGA. Because the protein level of TAK1 was not changed by NDGA in the presence of MG132, there would be no decrease in translational regulation either.
TAK1 was originally identified as a key regulator of MAPK activation in transforming growth factor family signaling pathway (Yamaguchi et al. Citation1995). And there were several reports suggesting TAK1 as a useful target for anti-inflammatory drug (Adhikari et al. Citation2007). Previously Ninomiya-Tsuji et al. (Citation2003) reported 5Z-7-oxozeaenol as a TAK1 specific inhibitor and they found it inhibits JNK/p38 MAPK and NF-kB signaling as well. More recently, prodelphinidin B-4 3′-O-gallate, a polyphenol compound from tea, was reported as a down-regulator of TAK1-NF-κB pathway (Hou et al. Citation2007). Comparing with NDGA, the time course of TAK1 and JNK inhibition was different and thus the different mechanism might be involved in the TAK1 inhibition by both previously reported TAK1 inhibitors.
In this study, the down-regulation of TAK1 by NDGA also appeared in various cell lines, such as osteoblastic cells. The possible mechanism for the inhibition of TAK1 by NDGA might be related with the cytoskeletal alteration induced by NDGA (Nakamura et al. Citation2003; Arasaki et al. Citation2007). The disruption of effective transfer system between the protein complexes would affect the protein stability if the ubiquitination is involved in the signal transduction. Ubiquitination of TAK1 is the key mechanism for its activation, thus the stability of TAK1 would be jeopardized if TAK1 was hindered from binding with other components of more stable complex. According to this study, since NDGA is a highly potent and unique inhibitor of TAK1, this compound will be a useful tool for the study on these signal transduction pathways. And this study also suggested another possible mechanism for the inhibitor of cellular signal transduction pathway. The time duration may explain why the results of therapeutic studies from the cell culture were so different from the animal studies, because the small molecule might need more prolonged time to show the long-term effect such as the stimulation of protein degradation.
We show that NDGA significantly reduces the TAK1 protein levels and inhibited NF-kB pathway as well in macrophage cell lines, thus NDGA might be a useful therapeutic agent for inflammation-related diseases.
Supplementary content: AP-1 Reporter
Download JPEG Image (145.1 KB)Acknowledgements
This study was supported by Gangwon-Alberta Research Cooperation Program and Natural Science Research Institute of Gangneung-Wonju National University. Dr. C.-H. Lee was supported by Gangneung-Wonju Research Year Program for Professor.
Notes
1. Supplementary material can be found by clicking on the Supplementary Content tab at http://dx.doi.org/10.1080/19768354.2012.745449
References
- Adhikari , A , Xu , M and Chen , ZJ. 2007 . Ubiquitin-mediated activation of TAK1 and IKK . Oncogene , 26 ( 22 ) : 3214 – 3226 . doi: 10.1038/sj.onc.1210413
- Arasaki , K , Tani , K , Yoshimori , T , Stephens , DJ and Tagaya , M. 2007 . Nordihydroguaiaretic acid affects multiple dynein-dynactin functions in interphase and mitotic cells . Mol Pharmacol , 71 ( 2 ) : 454 – 460 . doi: 10.1124/mol.106.029611
- Arteaga , S , Andrade-Cetto , A and Cardenas , R. 2005a . Larrea tridentata (creosote bush), an abundant plant of Mexican and US-American deserts and its metabolite nordihydroguaiaretic acid . J Ethnopharmacol , 98 ( 3 ) : 231 – 239 . doi: 10.1016/j.jep.2005.02.002
- Arteaga , S , Carmona , A , Luis , J , Andrade-Cetto , A and Cardenas , R. 2005b . Effect of Larrea tridentata (creosote bush) on. cholesterol gallstones and bile secretion in hamsters . J Pharm Pharmacol , 57 ( 9 ) : 1093 – 1099 . doi: 10.1211/jpp.57.9.0004
- Balkwill , F and Mantovani , A. 2001 . Inflammation and cancer: back to Virchow? . Lancet , 357 ( 9255 ) : 539 – 545 . doi: 10.1016/S0140-6736(00)04046-0
- Bokoch , GM and Reed , PW. 1981 . Evidence for inhibition of leukotriene A4 synthesis by 5,8,11,14-eicosatetraynoic acid in guinea pig polymorphonuclear leukocytes . J Biol Chem , 256 ( 9 ) : 4156 – 4159 .
- Bonizzi , G and Karin , M. 2004 . The two NF-kappa B activation pathways and their role in innate and adaptive immunity . Trends Immunol , 25 ( 6 ) : 280 – 288 . doi: 10.1016/j.it.2004.03.008
- Brennan , P and O'Neill , LA. 1998 . Inhibition of nuclear factor kappa B by direct modification in whole cells – mechanism of action of nordihydroguaiaritic acid, curcumin and thiol modifiers . Biochem Pharmacol , 55 ( 7 ) : 965 – 973 . doi: 10.1016/S0006-2952(97)00535-2
- Chang , SH , Choi , Y , Park , JA , Jung , DS , Shin , J , Yang , JH , Ko , SY , Kim , SW and Kim , JK. 2007 . Anti-inflammatory effects of BT-201, an n-butanol extract of Panax notoginseng, observed in vitro and in a collagen-induced arthritis model . Clin Nutr , 26 ( 6 ) : 785 – 791 . doi: 10.1016/j.clnu.2007.07.008
- Chen , Q. 2009 . Nordihydroguaiaretic acid analogues: their chemical synthesis and biological activities . Curr Top Med Chem , 9 ( 17 ) : 1636 – 1659 . doi: 10.2174/156802609789941915
- Coussens , LM and Werb , Z. 2002 . Inflammation and cancer . Nature , 420 ( 6917 ) : 860 – 867 . doi: 10.1038/nature01322
- Domin , J , Higgins , T and Rozengurt , E. 1994 . Preferential inhibition of platelet-derived growth factor-stimulated DNA synthesis and protein tyrosine phosphorylation by nordihydroguaiaretic acid . J Biol Chem , 269 ( 11 ) : 8260 – 8267 .
- Fujiwara , T , Misumi , Y and Ikehara , Y. 2003 . Direct interaction of the Golgi membrane with the endoplasmic reticulum membrane caused by nordihydroguaiaretic acid . Biochem Biophys Res Commun , 301 ( 4 ) : 927 – 933 . doi: 10.1016/S0006-291X(03)00069-X
- Hou , DX , Luo , D , Tanigawa , S , Hashimoto , F , Uto , T , Masuzaki , S , Fujii , M and Sakata , Y. 2007 . Prodelphinidin B-4 3′-O-gallate, a tea polyphenol, is involved in the inhibition of COX-2 and iNOS via the downregulation of TAK1-NF-kappaB pathway . Biochem Pharmacol , 74 ( 5 ) : 742 – 751 . doi: 10.1016/j.bcp.2007.06.006
- Ito , H , Ueda , H , Iwamoto , I , Inaguma , Y , Takizawa , T , Asano , T and Kato , K. 2005 . Nordihydroguaiaretic acid (NDGA) blocks the differentiation of C2C12 myoblast cells . J Cell Physiol , 202 ( 3 ) : 874 – 879 . doi: 10.1002/jcp.20177
- Karin , M , Yamamoto , Y and Wang , QM. 2004 . The IKK NF-kappa B system: a treasure trove for drug development . Nat Rev Drug Discov , 3 ( 1 ) : 17 – 26 . doi: 10.1038/nrd1279
- Lee , CH , Jang , YS , Her , SJ , Moon , YM , Baek , SJ and Eling , T. 2003 . Nordihydroguaiaretic acid, an antioxidant, inhibits transforming growth factor-beta activity through the inhibition of Smad signaling pathway . Exp Cell Res , 289 ( 2 ) : 335 – 341 . doi: 10.1016/S0014-4827(03)00282-9
- Lü , JM , Nurko , J , Weakley , SM , Jiang , J , Kougias , P , Lin , PH , Yao , Q and Chen , C. 2010 . Molecular mechanisms and clinical applications of nordihydroguaiaretic acid (NDGA) and its derivatives: an update . Med Sci Monit , 16 ( 5 ) : A93 – 100 .
- Nakamura , M , Nakazawa , J , Usui , T , Osada , H , Kono , Y and Takatsuki , A. 2003 . Nordihydroguaiaretic acid, of a new family of microtubule-stabilizing agents, shows effects differentiated from paclitaxel . Biosci Biotechnol Biochem , 67 ( 1 ) : 151 – 157 . doi: 10.1271/bbb.67.151
- Ninomiya-Tsuji , J , Kajino , T , Ono , K , Ohtomo , T , Matsumoto , M , Shiina , M , Mihara , M , Tsuchiya , M and Matsumoto , K. 2003 . A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase . J Biol Chem , 278 ( 20 ) : 18485 – 18490 . doi: 10.1074/jbc.M207453200
- Ninomiya-Tsuji , J , Kishimoto , K , Hiyama , A , Inoue , J , Cao , Z and Matsumoto , K. 1999 . The kinase TAK1 can activate the NIK-I kappa B as well as the MAP kinase cascade in the IL-1 signalling pathway . Nature , 398 ( 6724 ) : 252 – 256 . doi: 10.1038/18465
- Ohkawara , B , Shirakabe , K , Hyodo-Miura , J , Matsuo , R , Ueno , N , Matsumoto , K and Shibuya , H. 2004 . Role of the TAK1-NLK-STAT3 pathway in TGF-beta-mediated mesoderm induction . Genes Dev , 18 ( 4 ) : 381 – 386 . doi: 10.1101/gad.1166904
- Okawa , Y , Hideshima , T , Steed , P , Vallet , S , Hall , S , Huang , K , Rice , J , Barabasz , A , Foley , B Ikeda , H . 2009 . SNX-2112, a selective Hsp90 inhibitor, potently inhibits tumor cell growth, angiogenesis, and osteoclastogenesis in multiple myeloma and other hematologic tumors by abrogating signaling via Akt and ERK . Blood , 113 ( 4 ) : 846 – 855 . doi: 10.1182/blood-2008-04-151928
- Sakurai , H , Miyoshi , H , Toriumi , W and Sugita , T. 1999 . Functional interactions of transforming growth factor beta-activated kinase 1 with Ikappa B kinases to stimulate NF-kappa B activation . J Biol Chem , 274 ( 15 ) : 10641 – 10648 . doi: 10.1074/jbc.274.15.10641
- Salari , H , Braquet , P and Borgeat , P. 1984 . Comparative effects of indomethacin, acetylenic acids, 15-HETE, nordihydroguaiaretic acid and BW755C on the metabolism of arachidonic acid in human leukocytes and platelets . Prostaglandins Leukot Med , 13 ( 1 ) : 53 – 60 . doi: 10.1016/0262-1746(84)90102-1
- Shambharkar , PB , Blonska , M , Pappu , BP , Li , H , You , Y , Sakurai , H , Darnay , BG , Hara , H , Penninger , J and Lin , X. 2007 . Phosphorylation and ubiquitination of the IkappaB kinase complex by two distinct signaling pathways . EMBO J , 26 ( 7 ) : 1794 – 805 . doi: 10.1038/sj.emboj.7601622
- Shi , L , Zhang , Z , Fang , S , Xu , J , Liu , J , Shen , J , Fang , F , Luo , L and Yin , Z. 2009 . Heat shock protein 90 (Hsp90) regulates the stability of transforming growth factor beta-activated kinase 1 (TAK1) in interleukin-1beta-induced cell signaling . Mol Immunol , 46 ( 4 ) : 541 – 50 . doi: 10.1016/j.molimm.2008.07.019
- Shim , JH , Xiao , C , Paschal , AE , Bailey , ST , Rao , P , Hayden , MS , Lee , KY , Bussey , C , Steckel , M Tanaka , N . 2005 . TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo . Genes Dev , 19 ( 22 ) : 2668 – 2681 . doi: 10.1101/gad.1360605
- Sunami , Y , Leithauser , F , Gul , S , Fiedler , K , Guldiken , N , Espenlaub , S , Holzmann , KH , Hipp , N , Sindrilaru , A Luedde , T . 2012 . Hepatic activation of IKK/NFkappaB signaling induces liver fibrosis via macrophage-mediated chronic inflammation . Hepatology , 56 ( 3 ) : 1117 – 1128 . doi: 10.1002/hep.25711
- Takaesu , G , Surabhi , RM , Park , KJ , Ninomiya-Tsuji , J , Matsumoto , K and Gaynor , RB. 2003 . TAK1 is critical for IkappaB kinase-mediated activation of the NF-kappaB pathway . J Mol Biol , 326 ( 1 ) : 105 – 115 . doi: 10.1016/S0022-2836(02)01404-3
- Tang , M , Wei , X , Guo , Y , Breslin , P , Zhang , S , Zhang , S , Wei , W , Xia , Z , Diaz , M Akira , S . 2008 . TAK1 is required for the survival of hematopoietic cells and hepatocytes in mice . J Exp Med , 205 ( 7 ) : 1611 – 1619 . doi: 10.1084/jem.20080297
- Vidal , S , Khush , RS , Leulier , F , Tzou , P , Nakamura , M and Lemaitre , B. 2001 . Mutations in the drosophila dTAK1 gene reveal a conserved function for MAPKKKs in the control of rel/NF-kappaB-dependent innate immune responses . Genes Dev , 15 ( 15 ) : 1900 – 1912 . doi: 10.1101/gad.203301
- Wang , C , Deng , L , Hong , M , Akkaraju , GR , Inoue , J and Chen , ZJ. 2001 . TAK1 is a ubiquitin-dependent kinase of MKK and IKK . Nature , 412 ( 6844 ) : 346 – 351 . doi: 10.1038/35085597
- Wyss-Coray , T and Rogers , J. 2012 . Inflammation in Alzheimer disease: a brief review of the basic science and clinical literature . Cold Spring Harb Perspect Med , 2 ( 1 ) : a006346
- Yamaguchi , K , Shirakabe , K , Shibuya , H , Irie , K , Oishi , I , Ueno , N , Taniguchi , T , Nishida , E and Matsumoto , K. 1995 . Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction . Science , 270 ( 5244 ) : 2008 – 2011 . doi: 10.1126/science.270.5244.2008
- Youngren , JF , Gable , K , Penaranda , C , Maddux , BA , Zavodovskaya , M , Lobo , M , Campbell , M , Kerner , J and Goldfine , ID. 2005 . Nordihydroguaiaretic acid (NDGA) inhibits the IGF-1 and c-erbB2/HER2/neu receptors and suppresses growth in breast cancer cells . Breast Cancer Res Treat , 94 ( 1 ) : 37 – 46 . doi: 10.1007/s10549-005-6939-z