Abstract
The objectives of this study were to evaluate how chemical gradients and physical habitats influence trophic dynamics and chemical tolerance in relation to ecological health, based on biological integrity model in the absence of cascading pressures (cascading theory) as a control mechanism of aquatic ecosystems. We conducted physical, chemical, and biological surveys from 76 national streams and rivers of four major watersheds during 2004–2005 along with surveys of 80 reference streams. Maximum species richness lines (MSRLs) in reference sites vs. regular sampling sites indicated that the third- to fifth-order streams were evidently impaired in the metrics of trophic and tolerant components. These trophic modifications were linked with land-use patterns, which resulted in differences of nutrients (N, P), organic matter contents and the N:P ratios on trophic structures. Overall, these trophic factors influenced ecological health, based on multimetric index of biological integrity model in these systems, so that trophic compositions and tolerance were regulated by bottom-up hypothesis.
Introduction
Fundamental functions and classical food-chain complexity in aquatic ecosystems have been demonstrated by landmark paper of trophic dynamic concept (Lindeman Citation1942; Kozlovsky Citation1968). Food webs are limited representations of real ecosystems as they necessarily aggregate many species into trophic guilds, which are functional groups of species or community that have specific roles and status in a food-web network. The key functional feeding groups of omnivores and insectivores have been widely used as indicators of impaired water quality in North America, Europe, and Asia.
Functional trophic relationships are widely recognized as key factors influencing community structures and interaction within the community. Thus, spatial and temporal dynamics of top-trophic level organisms are closely associated with functional groups and guilds of each trophic levels, and three key hypotheses demonstrating the trophic dynamics of aquatic ecosystems have been widely suggested through quantitative (or mathematical) models of trophic dynamics (Briand & Cohen Citation1987; Banašek-Richter et al. Citation2009) and empirical models of various parameters (Ahlgren et al. Citation1988). Thus, the concept of cascading trophic interaction has been frequently used as a biological management tool or biomanipulation approach (Carpenter et al. Citation1985) to restore the impaired aquatic ecosystems. In the absence of top predators, however, bottom-up trophic interactions, which are basically controlled by ambient nutrient regimes or nutrient ratios (N:P), may be the alternative hypothesis, but still this hypothesis is needed to be tested.
Enrichments with nitrogen or phosphorus in stream and river ecosystems cause modification of biotic communities as well as biodiversity. The major sources of nutrients may be related with watershed characteristics (Jordan et al. Citation1997), land-use patterns of intensive agricultural activities and population-dense urban (Arheimer & Liden Citation2000), and point sources of industrial complex and wastewater disposal plants (WDPs, Wang et al. Citation2001). High-nutrient loadings and organic matter inputs may decrease the proportions of sensitive species (SS) and increase tolerant species (TS) (Frey et al. Citation2011). Also, nutrient enrichments and habitat degradations may increase the proportion of trophic guilds such as omnivore species (OS) and decrease the relative abundance of insectivores (An et al. Citation2004). Thus, changes in nutrient ratios of N:P can influence the biomass and the compositions of aquatic high-level organisms through the dynamics of low-level trophic structures by limiting factors (Carpenter et al. Citation1985). Under the circumstances, increased nutrients directly or indirectly influence the species compositions, trophic structures, and tolerance guilds, resulting in modifications of the food-chain and community structures.
Recently, multimetric bioassessment approaches, based on the Index of Biological Integrity (IBI), are widely used for the diagnosis of ecological health (US EPA Citation1993), and each metric indicates important ecological characteristics of biota such as trophic structures and food-chain linkages. Karr (Citation1981) developed a 12-metric IBI model to assess and manage ecological health of wadeable streams and rivers. This approach could not apply to other geographical regions, except North American and Canadian streams due to distinct regional differences of fish fauna and compositions. Many regional models, however, are available recently in North America (Karr Citation1981), Europe (Oberdorff & Hughes Citation1992), Japan (Koizumi & Matsumiya Citation1997), and Korea (An et al. Citation2004; Choi et al. Citation2011). These regional model developments made it possible for comparisons of ecological health to chemical water quality and trophic variables. The ecological health using the IBI model, thus, could be linked with functional feeding diversity of trophic levels for detecting the degradations of specific habitat and environments. This approach may be used as an efficient tool to detect functional modifications of the community structure and chemical impacts.
The aim of the present study was to elucidate how nutrient enrichments and land-use patterns are important in understanding the dynamics of trophic compositions and structures. We demonstrate some factors influencing the generalized patterns of trophic levels in the structure of real food-web networks.
Materials and methods
Sampling sites and watershed characteristics
National sampling was conducted from 76 streams and rivers () within four major Korean watersheds (34–42°N, 124–130°E) including Han River (HR; 514 km in total length, 26,219 km2 in watershed area), Geum River (GR; 414 km, 9886 km2), Nakdong River (NR; 525 km, 23,860 km2), and Yeongsan/Sumjin River (YR; 348 km, 8267 km2). Land-use patterns in the sampling streams varied depending on the types of watersheds and locations of point/nonpoint sources. HR is mainly surrounded by forest cover in the upstreams and croplands in the midstreams. In contrast, the downstreams of HR are largely surrounded by urban metropolitan capital city with a high population density, and thus are largely influenced by human activities, industrial complex, and wastewater treatment plants related with the urbanization. Watersheds GR and NR have similar longitudinal gradients of land-use along the main axis of the river, but the intensity of the human activity is much less compared to the HR watershed. In the meantime, the YR watershed is more surrounded by croplands, especially in regions of headwaters – midstreams and is influenced by urban sewages in the downstreams.
Chemical water quality analysis
Chemical parameters were analyzed in the ambient samples of stream waters during 2003–2005. Total nitrogen (TN) was measured using colorimetry after persulfate digestion (Crumpton et al. Citation1992). Ammonia nitrogen (NH4−N) and nitrate nitrogen (NO3−N) were measured by Standard Methods (APHA Citation1985). Total phosphorus (TP) was determined using the ascorbic acid method after persulfate oxidation (Prepas & Rigler Citation1982). The analysis of biological oxygen demand (BOD) and chemical oxygen demand (COD) were followed by Standard Methods (APHA Citation1985). Specific conductivity was measured at the time of sample collection using multiparameter YSI sonde probe (Model YSI 6600), and total dissolved solids (TDS) were estimated from the conductivity values divided by 640. Chlorophyll-a was used as algal biomass indicator (Creitz & Richards Citation1955). Assuming that chlorophyll-a constitutes, on average, 1.5% of the dry weight of organic matter (ash-free weight) of algae, estimate the algal biomass by multiplying the chlorophyll-a content by a factor of 67 (APHA Citation1985).
Biological integrity model and field samplings
In this study, multimetric index of biological integrity model (Mm-IBI model), based on the original 12-metric model of Karr (Citation1981), was employed for a diagnosis of stream ecosystem health in the four watersheds. The metric attributes of M1–M8 were selected from previous references (An et al. Citation2004; Choi et al. Citation2011). Value of each metric was compared to the values expected at 80 reference streams and rivers where human influences were minimal. Ratings of 5, 3, and 1 (Barbour et al. Citation1999) were assigned to each metric according to whether its value approximates, deviates from, or greatly deviates from the expected value in the 10-metric ratings. The three ratings were evaluated by the maximum species richness line (MSRL; Rankin & Yoder Citation1999). The sum of those ratings (5, 3, and 1) provided values of Mm-IBI model at each site. Detail information on biological integrity model and concept were well described through several references (Karr Citation1981; An et al. Citation2004; Choi et al. Citation2011). For the model applications, fishes were collected from all types of their habitats, including riffle, run, and pool area, according to the approach of wading method (Ohio EPA Citation1989) based on the catch per unit effort. The distance and time elapsed in the sampling were at least 200 m and 60 minutes, respectively. The sampling gears of casting net (4×4 mm) and hand net (2×2 mm) were used for the analysis of 76 streams and rivers. In addition, 80 reference streams/rivers were sampled to derive MSRLs against the stream order. In selecting the regional reference sites, we followed the approach of Hughes et al. (Citation1994), which used major eight steps and evaluated 14 variables such as road distance, riparian vegetation, flow, sedimentation, turbidity, human, livestock activity, and habitat structures.
Guild compositions and habitat analysis
Tolerance guild analysis was conducted using the categories of SS, intermediate species (IS), and TS, based on the approaches of US EPA (Citation1993) and Barbour et al. (Citation1999). To determine the guild classifications implying habitat conditions and ranges of guilds, we applied discriminant analysis using a statistical package of SPSS (Release 12.00, SPSS Inc., Chicago, IL, USA). Also, for the analysis of trophic compositions, we assigned each species to trophic groups, following the available literature (Kim Citation1997; Kim & Park Citation2002) and used our own data on stomach contents from subsamples of those species that were commonly frequent along the river. Species were assigned to one of the following trophic categories: insectivore species were classified as a group that typically eats terrestrial and aquatic insects, while OS were designated as a group whose diets are usually made of >25% plant material and >25% animal material.
Physical habitat conditions were calculated using a multimetric model of Qualitative Habitat Evaluation Index (QHEI). The original 10-metric model was based on the approach of Plafkin et al. (Citation1989) and 11-metric habitat models (M1–M11) were established to calculate the QHEI values from 76 streams and rivers (US EPA Citation1983). Values of QHEI were diagnosed by four criteria of >182 (excellent), 124–168 (good), 66–110 (fair), and <52 (poor).
Land-use patterns of sampling streams were categorized as two types of forest cover and urban land, using satellite image of 1:20,000 resolutions. Satellite image was obtained from National Geographic Information Institute, Korea. The land covers were estimated from the relative ratio of land-use type via calculation of square within each stream/river reach.
Results and discussions
Effects of land-use patterns on nutrient and organic matter contents
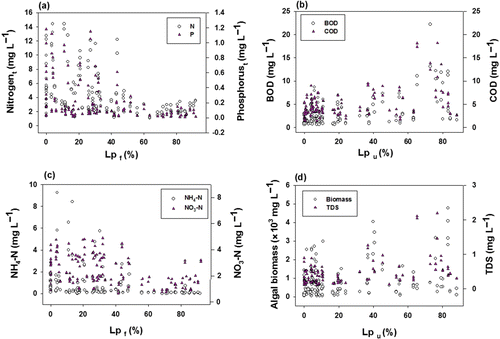
The concentrations of nutrients (N, P) and organic matters in the lotic ecosystems were closely associated with land-use pattern, which is expressed as a percent forest cover (Lpf) and urban (Lpu), respectively, in the watershed (). When the Lpf was <20%, TN averaged 5.89 mg L−1 and TP averaged 0.30 mg L−1 (a), but as the Lpf was >50%, TN averaged 2.42 mg L−1 and TP averaged 0.065 mg L−1. Thus, the maxima of TN and TP at the Lpf of 55–90% decreased by 6-fold and 18-fold, respectively, compared to the values at the Lpf of 0–30%. In contrast, concentrations of BOD and COD increased over the continuum of Lpu values. When the values of Lpu were >40%, BOD and COD were high (mean: 5.346 mg L−1, 6.758 mg L−1) and the maxima increased up to 18.2 mg L−1 (b). NH4−N and NO3−N on land-use pattern were similar to the TN, but the maximum NH4−N had abrupt decreases over the values of Lpf (c). Algal biomass as an estimation of phytoplankton productivity increased as the proportion of Lpu increases and showed a similar pattern with TDS over proportion of urban land-use (d). Also, the maximum values of algal biomass and TDS at a given Lpu increased over greater percentage of urban cover. The greater proportions of percentage of urban cover resulted in higher dissolved ionic contents and primary productivity even if these concentrations did not show typical linear functional relations with values of Lpu. This pattern was opposite to the forest cover, which had an inverse relation with percentage of urban cover. These results suggest that land-use pattern in the watershed determined the nutrients, dissolved ionic contents, and organic matters in the stream water, resulting in an influence on primary productivity (Mitchell et al. Citation2009).
Trophic compositions and tolerance on chemical gradients
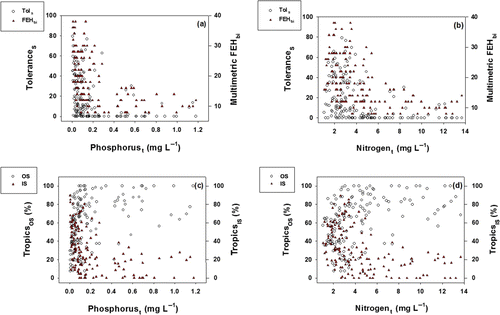
Distribution limits and ranges of SS were closely associated with gradients of phosphorus and nitrogen (). When the proportions of SS (Tols), as one of the tolerant guilds, had an inverse relation with TP in the lotic ecosystems (a). The inverse functions of Tols with TP were similar to the patterns of ecological health, based on the Mm-IBI model (FEHbi). These results indicate that values of Tols and FEHbi decreased over higher phosphorus levels, and this trend was especially evident over the TP of >0.7 mg L−1. The values of Tols also had an inverse relation with TN in the lotic ecosystem while the model values of FEHbi had an inverse relation with TN (b). The response of Tols and FEHbi values over the gradient of the nitrogen was similar to that of phosphorus (a and b). These outcomes suggest that tolerance ranges and ecological health of aquatic biota are largely determined by the trophic states such as nitrogen and phosphorus (Whittier & Hughes Citation1998). The proportions of trophic omnivores (Tros) and trophic insectivores (Tris) were directly influenced by phosphorus and nitrogen levels as shown in . The values of Tros decreased as the concentrations of phosphorus and nitrogen increased, whereas values of Tris increased as the concentrations of phosphorus and nitrogen decreased (c and d). The responses of Tris to the phosphorus levels were similar to the responses of Tols to phosphorus levels (a and c). Also, the responses of Tris to the nitrogen levels were similar to the responses of Tols to nitrogen levels (b and d).
Figure 3. Relations of tolerance guild, trophic guild, and multimetric-FEHbi to phosphorust (total phosphorus) and nitrogent (total nitrogen) contents. The FEHbi indicate an ecological health, based on fish assemblages as an index of biological integrity.
Abbreviations: Tols=the proportion of sensitive species in tolerance, Tropicsos=the proportion of omnivore species in the tropic state, TropicsIS=the proportion of insectivore species in the tropic state, OS=omnivore species, and IS=insectivore species.
Biological compositions on N:P ratios
For the analyses, we categorized the N:P mass ratios as three groups of <100, 100–200, and >300 and estimated the relative abundance (%) of each dominant species at a given N:P ratios along with taxa identifications of trophic guilds, tolerance guilds, and habitat guilds (). N:P ratios are widely used as a key indicator for diagnosis of trophic state and limiting nutrients in the ecosystems (Shannon & Brezonik Citation1972). The high N:P mass ratios of >17 indicate phosphorus limitation in the ecosystem and low N:P ratios of <4 indicate nitrogen limitation (Forsberg & Ryding Citation1980; Dodds Citation2006). This reference indicates that our streams largely have severe phosphorus limitation (Elwood et al. Citation1981), based on the N:P ratio calculations on the trophic components. When the N:P ratios were <100, the dominant taxa with 42.2% of the total was Zacco platypus, which was an OS in the trophic levels as well as TS in tolerance guilds. This phenomenon indicates that low N:P ratios resulted in high omnivore and tolerant abundance and supports the fact (US EPA Citation1993) that the proportion of OS increases as chemical water quality degradated and also the proportion of TS increases. In contrast, when the N:P ratios were >300, dominant taxa with >5% were all insectivore species and were composed of sensitive and IS. In the meantime, when the N:P ratios were between 100 and 200, dominant taxa with >5% indicated a mixing community of TS, SS, and IS.
Table 1. The relation of N:P mass ratios on each dominant species composition (>1% of the total) based on relative abundance along with trophic guilds, tolerance guilds, and habitat guilds.
Mm-IBI model and their relations to other variables
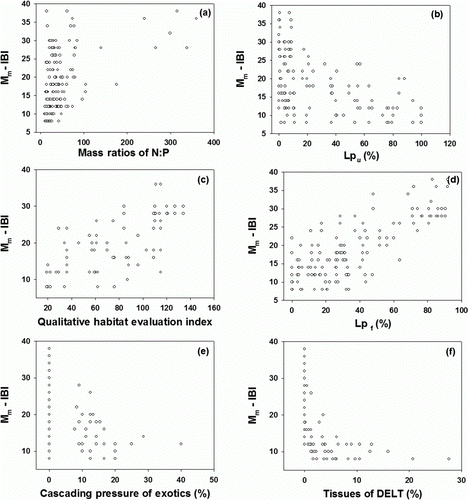
Multi-metric model values of IBI (Mm-IBI) were calculated using 8-metric biological integrity model, based on the IBI. The model values of IBI (Mm-IBI) were influenced by N:P ratios and physical habitat health, based on the QHEI, which are closely associated with land-use patterns. When N:P ratios were <50, values of Mm-IBI averaged 21.2 and the minimum and maximum were 8 and 38, respectively (a). In contrast, when N:P ratios were >300, values of Mm-IBI averaged 34 and the minimum and maximum were 28.5 and 38, respectively. The values of Mm-IBI were directly determined by land-use patterns; values decreased with higher Lpu and increased with higher Lpf in the stream ecosystem (b and d). But the Mm-IBI had stronger relations with the parameter of Lpf than the Lpu in the streams. Also, the values of Mm-IBI were influenced by physical habitat health, based on QHEI model. In addition, cascading pressures of exotics and morphological abnormality expressed as percentage of DELT also influenced the ecosystem health, based on the model values of Mm-IBI. The values of Mm-IBI decreased under the conditions of exotics of >20 and >10% DELT abnormalities (e and f).
Mm-IBI model and habitat health
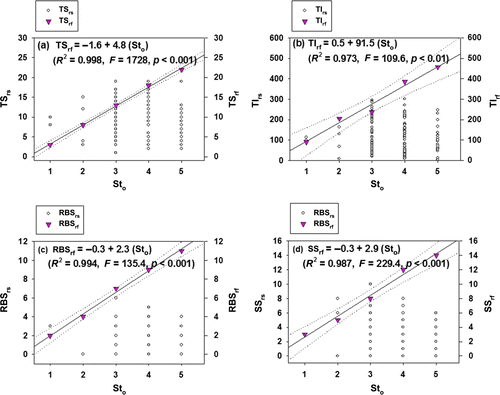
In reference systems, MSRL of total number of species (TSrf) had a significant linear function (slope=4.8, F=1728, p<0.001) with the magnitude of stream order (Sto), and the variation was accounted by 99.8% (a). Such functional relations are well supported by previous MSRL studies against the stream order or basin area (Barbour et al. Citation1999). Similarly, total number of individuals (TIrf), riffle-benthic species (RBSrf), and SS (SSrf), based on the MSRL of references, were significant (p values <0.01, R 2 values >0.97) in the regression equations (b–d). This outcome indicates that species compositions such as RBSrf and SS in the reference systems increase in larger size-streams and rivers (US EPA Citation1993; Choi et al. Citation2011). However, the comparisons of MSRL, based on TI, RBS, and SS in the reference sites (rf) vs. regular sampling sites (rs) indicated that fourth- to fifth-order streams were evidently impaired in the metrics of trophic guilds and tolerant guilds. Especially, TIrs and total number of RBSrs in fifth-order streams sampled during the study were lower, compared to the values expected in the reference streams. Thus, larger streams (high-order streams) located in downstream regions were largely impacted by the ecological health, compared to the references streams (An et al. Citation2004). These circumstances are frequently reported in urban streams, which are directly impacted by discharge waters from WDPs or/and industrial complex (Wang et al. Citation2001).
Figure 5. The maximum species richness lines (MSRLs) of some Mm-IBI metrics on stream orders (Sto). (a) Total number of native species (TS); (b) total number of native individuals (TI); (c) number of riffle-benthic species (RBS); and (d) number of sensitive species (SS) in 76 regular sampling sites (rs) and 80 reference sites (rf).
Mm-IBI vs. trophic and tolerance guilds
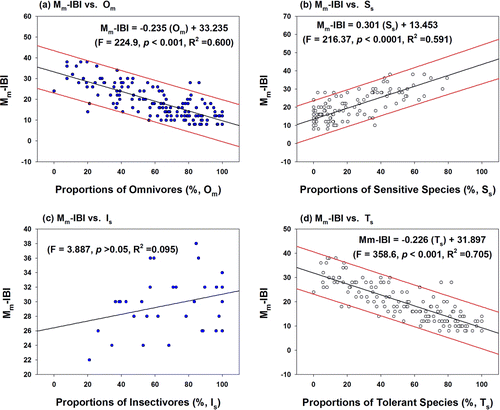
Biological stream health based on the Mm-IBI model has typical functional relations with trophic components of omnivores, but not with trophic components of insectivores (). Regression analysis of the Mm-IBI against the proportions of omnivores showed that the variation of omnivores accounted (F=224.9, p<0.001) for 60% of the Mm-IBI variation (a). This result indicates that the greater proportion of omnivores in the trophic composition resulted in poor ecological health in the ecosystem, and the food trophic are closely associated with biological integrity (Karr Citation1981; US EPA Citation1993; Choi et al. Citation2011). Conversely, the Mm-IBI had a positive linear function (F=216.37, p<0.0001, R 2=0.591) with the proportion of SS (b) and a negative linear function (F=358.6, p<0.001, R 2=0.705) with the proportion of TS (d). The greater proportions of SS, which declined as the water quality degradates, resulted in better stream health as the previous studies (Karr et al. Citation1986; Barbour et al. Citation1999; An et al. Citation2004). In the meantime, the Mm-IBI had no significant statistical functions (F=3.887, p>0.05, R 2=0.095) with the proportion of insectivores (c). These outcomes suggest that biological stream health of Mm-IBI is largely determined by the trophic compositions of omnivores and tolerance on water chemistry (US EPA Citation1993; Barbour et al. Citation1999).
Conclusions
The surveys of 76 national streams and 80 reference streams indicated that the concentrations of nutrients, dissolved solids, organic matters, and algal biomass were determined by the land-use pattern in the aquatic ecosystems. Water quality gradients such as nitrogen and phosphorus resulted in heterogeneities on the distribution limits and ranges in trophic compositions. Mass ratios of N:P in most observations indicated a phosphorus limitation and were used as a key indicator for a composition of trophic community. The values Mm-IBI were influenced by nutrient regimes, N:P ratios, physical habitat health, based on QHEI model, and the land-use patterns of the watersheds. In reference systems, MSRLs were linear functional relations with stream orders, but 76 sampling streams were largely impacted in the fourth to fifth-order streams. This phenomenon was evident in abundance and several metrics of RBS, Tols and Ss. Thus, biological stream health, based on the Mm-IBI model was typically related to trophic components according to simple linear regression models. In conclusion, the modifications of ecological trophic compositions were closely associated with chemical gradients, habitat quality, and biological tolerance, which determined the stream ecosystem health.
Acknowledgements
This study was financially supported by the research fund of Chungnam National University in 2011.
References
- Ahlgren , I , Frish , T and Kamp-Nielsen , L. 1988 . Empirical and theoretical models of phosphorus loading, retention and concentration vs. trophic state . Hydrobiologia. , 170 : 285 – 303 . doi: 10.1007/BF00024910
- An , KG , Kim , DS , Kong , DS and Kim , SD. 2004 . Integrative assessments of a temperate stream based on a multimetric determination of biological integrity, physical habitat evaluations, and toxicity tests . Bull Environ Contam Toxicol. , 73 : 471 – 478 . doi: 10.1007/s00128-004-0453-6
- APHA . 1985 . Standard methods for the examination of water and waste water , 16th ed . New York : American Public Health Association .
- Arheimer , B and Liden , R. 2000 . Nitrogen and phosphorus concentrations from agricultural catchments-influence of spatial and temporal variables . J Hydrol. , 227 : 140 – 159 . doi: 10.1016/S0022-1694(99)00177-8
- Banašek-Richter , C , Bersier , L , Cattin , M , Baltensperger , R , Gabriel , J , Merz , Y , Ulanvowicz , RE , Tavares , AF , Williams , DD Ruiter , PC . 2009 . Complexity in quantitative food webs . Ecology. , 90 : 1470 – 1477 . doi: 10.1890/08-2207.1
- Barbour MT , Gerritsen J , Snyder BD , Stribling JB. 1999 . Rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates and fish , 2nd ed. EPA 841–B-99-002 . Washington , DC : US EPA; Office of Water .
- Briand , F and Cohen , JE. 1987 . Environmental correlates of food chain length . Science. , 4829 : 956 – 960 . doi: 10.1126/science.3672136
- Carpenter , SR , Kitchell , JF and Hodgson , JR. 1985 . Cascading trophic interactions and lake productivity . BioScience. , 35 : 634 – 639 . doi: 10.2307/1309989
- Choi , JW , Kumar , HK , Han , JH and An , KG. 2011 . The development of a regional multimetric fish model based on biological integrity in lotic ecosystems and some factors influencing the stream health . Water, Air, & Soil Poll. , 217 : 3 – 24 . doi: 10.1007/s11270-010-0563-1
- Creitz , GI and Richards , FA. 1955 . The estimation and characterization of plankton populations by pigment analysis. III. A note on the use of “Millipore” membrane filters in the estimation of plankton pigments . J Mar Res. , 14 : 211 – 216 .
- Crumpton , WG , Isenhart , TM and Mitchell , PD. 1992 . Nitrate and organic N analyses with second-derivative spectroscopy . Limnol Oceanogr. , 37 : 907 – 913 . doi: 10.4319/lo.1992.37.4.0907
- Dodds , WK. 2006 . Eutrophication and trophic state in rivers and streams . Limnol Oceanogr. , 51 : 671 – 680 . doi: 10.4319/lo.2006.51.1_part_2.0671
- Elwood , JW , Newbold , JD , Trirnble , AF and Stark , RW. 1981 . The limiting role of phosphorus in a woodland stream ecosystem: effects of P enrichment on leaf decomposition and primary producers . Ecology. , 62 : 146 – 158 . doi: 10.2307/1936678
- Forsberg , C and Ryding , SO. 1980 . Eutrophication parameters and trophic state indices in 30 Swedish wastelakes . Arch Hydrobiol. , 89 : 189 – 207 .
- Frey JW , Bell AH , Hambrook Berkman JA , Lorenz DL. 2011 . Assessment of nutrient enrichment by use of algal-, invertebrate-, and fish-community attributes in wadeable streams in ecoregions surrounding the Great Lakes: U.S . Geological Survey Scientific Investigations Report 2011–5009 .
- Hughes , RM , Heiskary , SA , Mathews , WJ and Yoder , CO. 1994 . “ Use of ecoregions in biological monitoring ” . In Biological monitoring of aquatic systems , Edited by: Loeb , SL and Spacie , A . 125 – 151 . Chelsea : Lewis .
- Jordan , TE , Correll , DL and Weller , DE. 1997 . Relating nutrient discharges from watersheds to land use and streamflow variability . Water Resour Res. , 33 : 2579 – 2590 . doi: 10.1029/97WR02005
- Karr , JR. 1981 . Assessment of biotic integrity using fish communities . Fisheries. , 6 : 21 – 27 . doi: 10.1577/1548-8446(1981)006%3C0021:AOBIUF%3E2.0.CO;2
- Karr , JR , Fausch , KD , Angermeir , PL , Yant , PR and Schlosser , IJ. 1986 . Assessing biological integrity in running waters: a method and its rationale , Champaign , IL : Illinois Natural History Survey Special Publication 5 .
- Kim IS. 1997 . Illustrated encyclopedia of fauna and flora of Korea . 37 . Seoul : Ministry of Education (Freshwater fishes) .
- Kim , IS and Park , JY. 2002 . Freshwater fishes of Korea , Seoul : KyoHak .
- Koizumi , N and Matsumiya , Y. 1997 . Assessment of stream fish habitat based on Index of Biotic Integrity . Bull Jpn Soc Oceanogr. , 61 : 144 – 156 .
- Kozlovsky , DG. 1968 . A critical evaluation of the trophic level concept. I. Ecological efficiencies . Ecology. , 49 : 48 – 60 . doi: 10.2307/1933560
- Lindeman , RL. 1942 . The trophic-dynamic aspect of ecology . Ecology. , 23 : 399 – 417 . doi: 10.2307/1930126
- Mitchell , A , Reghenzani , J , Faithful , J , Furnas , M and Brodie , J. 2009 . Relationships between land use and nutrient concentrations in streams draining a ‘wet-tropics’ catchment in northern Australia . Mar Freshwater Res. , 60 : 1097 – 1108 . doi: 10.1071/MF08330
- Oberdorff , T and Hughes , RM. 1992 . Modification of an index of biotic integrity based on fish assemblages to characterize rivers of the Seine Basin, France . Hydrobiologia. , 228 : 117 – 130 . doi: 10.1007/BF00006200
- Ohio EPA . 1989 . Biological criteria for the protection of aquatic life. Vol. III . Standardized biological field sampling and laboratory method for assessing fish and macroinvertebrate communities . Columbus : Ohio EPA Division of Water Quality Monitoring and Assessment, Surface Water Section .
- Plafkin JL , Barbour MT , Porter KD , Gross SK , Hughes RM. 1989 . Rapid assessment protocols for use in streams and rivers: benthic macroinvertebrates and fish . EPA/ 444/4-89-001 . Washington , DC : US Environmental Protection Agency Office of Water Regulations & Standards.
- Prepas , EE and Rigler , FA. 1982 . Improvements in qualifying the phosphorus concentration in lake water . Can J Fish Aquat Sci. , 39 : 822 – 829 . doi: 10.1139/f82-112
- Rankin , ET and Yoder , CO. 1999 . “ Adjustments to the index of biotic integrity: a summary of Ohio experiences and some suggested modifications ” . In Assessing the sustainability and biological integrity of water resources using fish communities , Edited by: Simon , TP . Boca Raton : CRC .
- Shannon , EE and Brezonik , PL. 1972 . Relations between lake trophic state and nitrogen and phosphorus loading rates . Environ Sci Technol. , 6 : 719 – 725 . doi: 10.1021/es60067a005
- US EPA . 1983 . Technical support manual: waterbody surveys and assessment for conducting use attainability analyses . Washington , DC : US EPA Office of Water Regulations and Standards .
- US EPA . 1993 . Fish field and laboratory methods for evaluating the biological integrity of surface waters . EPA 600-R-92-111 . Environmental Monitoring Systems Laboratory – Cincinnati office of Modeling, Monitoring Systems, & Quality Assurance Office of Research Development, U.S. Environmental protection Agency , USA .
- Wang , L , Lyons , J , Kanehl , P and Bannerman , R. 2001 . Impacts of urbanization on stream habitat and fish across multiple spatial scales . Environ Manage. , 28 : 255 – 266 . doi: 10.1007/s0026702409
- Whittier , TR and Hughes , RM. 1998 . Evaluation of fish species tolerances to environmental stressors in lakes in the Northeastern United States . N Am J Fish Manage , 18 : 236 – 252 . doi: 10.1577/1548-8675(1998)018%3C0236:EOFSTT%3E2.0.CO;2