Abstract
Cecropins are basic antibacterial peptides that have potent activities against microorganisms. We have cloned and characterized a cecropin-like peptide of the lepidopteran insect Agrius convolvuli and analyzed its expression in Escherichia coli. The full-length cDNA of A. convolvuli cecropin D3 (AcCec) was 318 bp, containing a 5′ untranslated region (UTR) of 47 bp, a 3′ UTR of 82 bp with a poly (A) tail, and an open reading frame (ORF) of 189 bp encoding a polypeptide of 63 amino acids, including a 24 amino acid signal sequence and a 38 amino acid mature peptide (GenBank accession no. GQ888768). The mature peptide is highly similar to D-type cecropin. To understand the effect of C-terminal amidation, while overcoming the disadvantage of its lack in the prokaryote system, we added a lysine residue to AcCec (AcCec-K) and compared its antibacterial activity to the purified AcCec. The recombinant AcCec (rAcCec) and AcCec-K were expressed, respectively, in E. coli Rosetta cells using a pGEX-4T-1 expression vector, which contained the glutathione S-transferase (GST) gene for fusion partner, and the fusion proteins were induced by isopropyl-β-d-thiogalactopyranoside (IPTG). The recombinant proteins were purified by fast protein liquid chromatography (FPLC) using GSTrap FF and Resource RPC column. The result of the inhibition zone suggests that C-terminal lysine residue could increase the activity due to activated phosphorylation.
Introduction
Insects have developed efficient immune responses that protect them against the invasion of microbes, such as bacteria, fungi, and viruses, by producing a variety of potent and broad-spectra antimicrobial substances (Wang et al. Citation2007). Unlike vertebrates, there is no specific immunity that exists in insects, and this is, therefore, reflected in their different modes of action in the processes for recognizing microbial patterns and producing effector responses.
One of the most ubiquitously known mechanisms of insect innate immunity is the production of antimicrobial peptides (AMPs) that are produced by different types of cells and secretions, including the fat body, before being secreted into their body fluid (Cytryńska et al. Citation2007). These AMPs are also present in microorganisms, plants, and other animals, including mammals (Rieger & Barreda Citation2011), and are generally small basic proteins (typically between 3 and 14 kDa) containing 12–100 amino acid residues that are cationic and amphipathic, with a hydrophobic side facing a hydrophilic one (Sperstad et al. Citation2009). Thus, the cationic character of the AMPs, associated with their tendency to adopt an amphipathicity, facilitates their interaction and insertion into the anionic cell walls and phospholipid membranes of microorganisms, or disrupting their membrane integrity and function, ultimately leading to the lysis of microorganisms (Yang et al. Citation2002).
Cecropin-family peptides, the most potent of the induced peptides first isolated from Hyalophora cecropia pupae (Hultmark et al. Citation1980), have been purified mainly from lepidopteran (Yoe et al. Citation2006), dipteran (Liang et al. Citation2006), and coleopteran species (Saito et al. Citation2005), and their sequences have been reported. A cecropin was even found in pig intestine (Lee et al. Citation1989), showing an interesting link between the nonspecific immune systems in insects and mammals. Cecropin can be categorized into five types: cecropins A, B, C, D, and E (Hong et al. Citation2008). Their cDNA clones were isolated from the fat body of immunized Bombyx mori larvae (Cheng et al. Citation2006) and Agrius convolvuli (Kim et al. Citation2000). It was reported that cecropin genes were expressed at high levels in fat bodies and in hemocytes, at lower but significant levels in Malpighian tubules, and in midguts (Wang et al. Citation2007). Cecropins A, B, and D are major cecropin. Cecropin D shows homology to cecropins A and B, since they each are of about the same size, and contain a hydrophilic N-terminal region and a hydrophobic amidated C-terminus. However, cecropin D lacks most of the lysine residues at the N-terminus, and is, therefore, less basic than the two other forms and is released into hemolymph at a slower rate (Fink et al. Citation1989).
In this study, we report the full-length cDNA encoding A. convolvuli cecropin D3 (AcCec), which shows the highest homology with cecropin D-type. We have utilized a prokaryotic expression system for high-level production of heterologous proteins in E. coli, and successfully expressed the recombinant AcCec (rAcCec) as a fusion protein to GST. The pGEX expression system in E. coli Rosetta™ (DE3) Singles enhanced the solubility of the fusion protein, which was easily purified using GSTrap FF affinity column. Additionally, in order to overcome the disadvantage of lacking C-terminal amidation in the prokaryote system, we added a lysine residue (AcCec-K) and compared its antibacterial activity to the purified AcCec.
Materials and methods
Insects, tissue preparation, and plasmids
A. convolvuli larvae (fifth instar) were used as the resource of total RNA for cloning of the cecropin gene. Larvae were vaccinated with approximately 4×106 live, log-phase E. coli K12 (ATCC 12435). Fat bodies were dissected out, and immediately stored at −80°C for total RNA isolation. E. coli JM109 and pGEM-T Easy vector (Promega) were used as the cloning host strain and the cloning vector, respectively. Rosetta™ (DE3) Singles (Novagen) was used as the expression host strain, and pGEX-4T-1 vector (Amersham Biosciences) was used as the expression vector.
Total RNA isolation, cDNA production, and cloning of the full-length cDNA of AcCec by rapid amplification of cDNA ends (RACE)-PCR
The total RNA of A. convolvuli was isolated from fat bodies using Trizol (Invitrogen) according to the manufacturer's protocol and was dissolved in water treated with DEPC. cDNA was synthesized from 1 µg of total RNA using a SMART RACE cDNA Amplification Kit (Clontech). The full-length sequence of AcCec gene was revealed by RACE-PCR. 5′ RACE was carried out with the anti-sense primer (5′-TGGAATCCCTTCAAAGAA-3′), and 3′ RACE was carried out with sense primers (5′-TCAACCACCTCTGGCGAT-3′). Each RACE-PCR product was purified and cloned into pGEM-T Easy vector (Promega), and the nucleotide sequence was determined in both directions using an ABI-3700 automatic DNA sequencer (Applied Biosystems). The alignment of all the sequences was done using Clustal W multiple sequence alignment program (Thompson et al. Citation1994).
Construction of recombinant expression vector
The mature region of AcCec gene was amplified by reverse transcription-polymerase chain reaction (RT-PCR) using gene-specific primers containing BamHI and XhoI restriction enzyme sites (underlined). AcCec gene was amplified with AcCec BamHI primer (5′- CGTGGATCCTGGAATCCCTTCAAAGAA-3′), AcCec XhoI primer (5′-CTTCTCGAGTCAACCACCTCTGGCGAT-3′), and AcCec-K XhoI primer (5′-CTTCTCGAGTCATTTACCACCTCTGGCGAT-3′). AcCec-K XhoI primer was designed to amplify AcCec gene with an additional Lys residue at the C-terminus. The amplified AcCec gene fragments were first cloned into the pGEM-T Easy vector, and subcloned into pGEX-4T-1 expression vector between BamHI and XhoI restriction enzyme sites to produce two GST fused rAcCec, GST-AcCec, and GST-AcCec-K. The construct plasmids, pGEX-AcCec and pGEX-AcCec-K, were sequenced and transformed into Rosetta™ (DE3) Singles competent cell for recombinant cecropin expression.
Expression of recombinant cecropin in E. coli
Transformed colonies containing the appropriate plasmid pGEX-AcCec and pGEX-AcCec-K of Rosetta™ (DE3) Singles were picked and transferred to 5 ml Luria Bertani (LB) medium containing 100 µg ampicillin per ml. Primary culture was inoculated overnight at 37°C, and the overnight culture was diluted 1:100 (v/v) in LB containing 100 µg ampicillin per ml and incubated at 37°C. When the culture reached an OD600 of 0.3, the culture was then cooled on ice for 30 min, and expression of recombinant AcCec (rAcCec) was induced by the addition of 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 3 h at 25°C. After induction, the cells were centrifuged for 10 min at 5000 × g, and frozen at −70°C.
Purification of the rAcCec
Recombinant E. coli cells were resuspended in phosphate-buffered saline (PBS), pH 7.4 containing 1% (v/v) Triton X-100 and disrupted by sonication on ice for a few seconds. The total cell lysate was collected by centrifugation at 25,000 × g for 30 min at 4°C, and the supernatant of total cell lysate was centrifuged again at 25,000 × g for 15 min at 4°C. The subsequent supernatant was applied to the GSTrap FF column (GE Healthcare) that was equilibrated with PBS (pH 7.4) for purification of GST-AcCec and GST-AcCec-K. After purification, the purified GST-AcCec and GST-AcCec-K were treated with thrombin (1 unit/100 µg recombinant protein) for 1 h at 25°C to cleave AcCec and AcCec-K from GST-AcCec and GST-AcCec-K, respectively. Thrombin-treated GST-AcCec and GST-AcCec-K were loaded onto Resource RPC (GE Healthcare) column equilibrated with PBS (pH 7.4), and re-equilibrated with 0.05% trifluoroacetic acid (TFA) in Milli-Q. Recombinant cecropins, AcCec, and AcCec-K were eluted with a 0–100% acetonitrile gradient in 0.05% TFA.
Antimicrobial assay
Antimicrobial activities of rAcCec against Gram-positive and Gram-negative bacteria were measured by inhibition zone assay (Kim et al. Citation2011). Thin plates (1 mm) of 1% agarose containing 6 × 104 cells/ml were prepared, and wells of 3 mm in diameter were punched out of the plates. One microgram of samples in 0.05% TFA in Milli-Q (3 µl) were loaded into each well. After overnight incubation at 37°C, the diameters of inhibition zones were recorded.
Electrophoresis, western blot, and mass spectrometric analysis of rAcCec
Sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed on 15% acrylamide gel under reducing conditions and stained with Coomassie brilliant blue R-250 (Sigma) electrophoresis. The stained gel was excised with a razor and transferred to a sterile tube containing distilled water. Then, the gel was destained with methanol in order to remove remaining Coomassie brilliant blue and incubated with 200 mM ammonium bicarbonate. The gel piece was dehydrated with 100 µl of acetonitrile and dried under vacuum. The dried gel piece was then rehydrated with 20 µl of 50 mM ammonium bicarbonate containing 0.2 µg trypsin (Sigma). After 45 min, the solution was removed, and 30 µl of 50 mM ammonium bicarbonate was added. Digestion was performed overnight at 37°C. Matrix-assisted laser desorption/ionization (MALDI) mass spectrum was recorded on a MALDI-MS Voyager-DE STR (Applied Biosystems). Western blot was performed as previously described (Kim et al. Citation2011), using a rabbit polyclonal anti-cecropin B (Abcam). The protein concentration of each purification step was determined by bicinchoninic acid assay according to the manufacturer's specifications (Pierce).
Results and discussion
cDNA cloning of AcCec
The full-length cDNA of AcCec was isolated by combining sequences of 5′ and 3′ RACE-PCR. The complete cDNA sequence of AcCec gene was 318 bp. The cecropin cDNA consists of a 47 bp 5′ untranslated upstream region (5′-UTR) and a 189 bp open reading frame (ORF) that ended with termination codon (TGA). A hinge region that connected two helices was placed at the 60th sequence. The predicted molecular weight of AcCec from the deduced amino acid sequence was 3919.46 Da, and the theoretical isoelectric point (pI) from the deduced amino acid sequence was 10.67 (A). Cecropins are a family of 3–4 kDa linear amphipathic peptides, 35–40 residues in length, devoid of cysteine residues, and containing two α-helical segments (a strongly basic N-terminal domain and a long hydrophobic C-terminal helix) linked by a short hinge (Saito et al. Citation2005). Cloning of insect cecropin has been reported for lepidopteran (Jin et al. Citation2012), and type-D cecropins from A. convolvuli were first isolated and cloned (Kim et al. Citation2000), but we have found the other type of type-D cecropin from A. convolvuli, named as cecropin D3. We have studied Agrius cecropin D3 (AcCec), a D-type cecropin, which is comprised of 38 amino acids and has a molecular weight of about 4 kDa. We found that AcCec is a cationic protein possessing an overall positive charge; although its mature protein sequence includes negatively charged Asp and 3 Glu, it also includes positively charged Arg, Lys, and 5 His residues.
Figure 1. Nucleotide and deduced amino acid sequences of AcCec and alignment of deduced amino acids. (A) Nucleotide sequence of cDNA encoding AcCec and deduced amino acid sequences (GenBank accession no. GQ888768). The TGA is marked with an asterisk, and the putative polyadenylation signal (AATAAA) is underlined. (B) Alignment of the deduced amino acid sequence of AcCec with those of other lepidopteran insects. Identities are shown with asterisks and similarities with single dots. Ac CecD2: Agrius cecropin D2; Ac CecD1: Agrius cecropin D1; Ms Bac4: Manduca bactericidin 4; Ms bacB5: Manduca bactericidin B5; Hc CecD: Hyalophora cecropin D; Bm CecD: Bombyx cecropin D; Ar Hin2: Artogeia hinnavin2 (Yoe et al. Citation2006; Kim et al. Citation2000).

The deduced amino acid sequence of AcCec has high identity and similarity to other insect cecropins. AcCec has the highest identity to Agrius cecropin D2 (92.0%) and 88.9%, 87.1%, 80.3%, 64.5%, 56.5%, and 50.0% to Agrius cecropin D1, Manduca bactericidin 4, Manduca bactericidin B5, Hyalophora cecropin D, Bombix cecropin D, and Artogeia hinnavin 2, respectively (B). It was found that AcCec showed a high degree of homology with cecropins A and B, for they each are about the same size, and contain a hydrophilic N-terminal region and a hydrophobic amidated C-terminus. Nevertheless, its alignment showed that it belonged to D-type cecropin. While both cecropins A and B start with N-terminal Lys extending to Trp, AcCec lacked the Lys, and instead started with Trp and is, therefore, less basic than the two other forms (Fink et al. Citation1989), a distinctive characteristic in cecropin D. Additionally, basic Lys at residues 3 and 7 were replaced with neutral Asn and hydrophobic Leu, respectively. This is the first time since Manduca bactericidin, a cecropin D-like peptide with two consecutive Gly residues at the C-terminus was isolated. A database search showed that the homology between the mature protein amino acid sequences of AcCec and M. sexta cecropin is 94% (37 of 38 residues are identical), while H. cecropia, B. mori, and A. rapae are 78%, 62%, and 52% homologous, respectively. Furthermore, when the coding region comprised of 117 residues for M. sexta cecropin was compared, there was 87.1% homology.
Protein expression and purification of AcCec and AcCec-K
The DNA fragment encoding mature peptide of AcCec and AcCec-K was fused with glutathione S-transferase (GST) gene of pGEX-4T-1 and transformed E. coli Rosetta Singles to expression rAcCec and AcCec-K. Results from sequence analyses using E. coli codon usage analyzer obtained before protein overexpression revealed that mature AcCec contains four rare codons (data not shown), and consequently E. coli Rosetta Singles strain was used to avoid translational problems and to improve the yield and purity of AcCec. Fusion partners offer several advantages, including generic protein purification schemes, improved folding characteristics and protein solubility, limited proteolysis, and prevention of inclusion-body formation (LaVallie & McCoy Citation1995; Makrides Citation1996). In this study, GST was selected as a fusion partner to enhance the solubility of recombinant protein and was fused to the amplified products encoding mature AcCec.
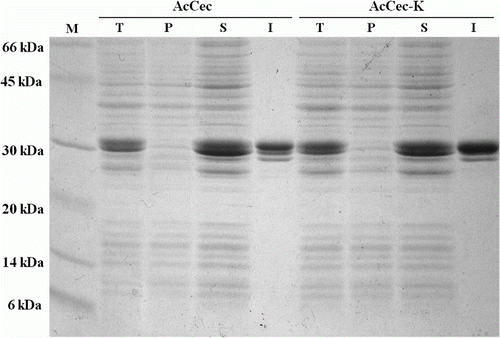
The recombinant protein was expressed with IPTG, and the bacterial cells were harvested by centrifuge. To purify the GST-fusion protein, we used GSTrap column. Total cell, soluble formed protein, pellet, and purified fusion protein were analyzed using SDS-PAGE. The result from SDS-PAGE was shown that AcCec and AcCec-K were expressed as soluble protein. Not only does GST provide a high expression level and favors intracellular accumulation of fusions in the soluble form, it also provides an effective and convenient isolation of fusions by chromatography on glutathione sepharose (Skosyrev et al. Citation2003). shows that GST-AcCec and GST-AcCec-K fusion proteins were expressed in water soluble form and isolated from total protein by GSTrap.
Figure 2. SDS-PAGE analyses of the expressed proteins. Lane M, protein molecular weight marker. Lane T, total protein of the transformed E. coli Rosetta. Lane P, pellet of disrupted bacterial cell. Lane S, soluble protein of disrupted bacterial cell. Lane I, isolated protein using GSTrap column chromatography.
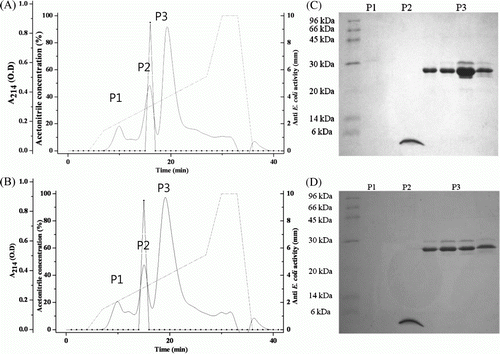
To obtain AcCec and AcCec-K, fusion proteins were digested with thrombin, and subsequently purified by Reource RPC column chromatography. The cleaved GST-fusion partner, AcCec, and AcCec-K were separated by Resource RPC with 10–90% acetonitrile in 0.05% TFA. The purified AcCec exhibiting antibacterial activity against E. coli JM109 was eluted at about 32% acetonitrile concentration gradient, and AcCec-K was eluted at about 34% acetonitrile concentration gradient (Figures 3A and B).
Figure 3. Purification of AcCec and AcCec-K using Resource RPC column. (A) Purification of AcCec (A buffer: 0.05% TFA in 10% acetonitrile; B buffer: 0.05% TFA in 90% acetonitrile). (B) Purification of AcCec-K (A buffer: 0.05% TFA in 10% acetonitrile; B buffer: 0.05% TFA in 90% acetonitrile). (C) SDS-PAGE analysis of purified AcCec. (D) SDS-PAGE analysis of purified AcCec-K.
Identification of AcCec and AcCec-K
To improve the resolution and the success rate of protein identification, we performed SDS-PAGE, western blot with anti-cecropin antibody, and MALDI-TOF-MS. An analysis of the Resource RPC fractions using SDS-PAGE revealed that AcCec and AcCec-K were purified to homogeneity, with a molecular mass of approximately 4 kDa (Figures 3C and D). The purified AcCec and AcCec-K were analyzed by SDS-PAGE and identified by western blot with anti-cecropin antibody. Both AcCec and AcCec-K were identified as cecropin and modifications of that with high confidence (A). The SDS-PAGE gels of purified recombinant cecropins with molecular weight of 4 kDa were excised and subjected to trypsin digestion and MALDI-TOF-MS. AcCec and AcCec-K were identified for three and four fragments in peptide sequence coverage of 85.0% and 90.24%, respectively ().
Figure 4. SDS-PAGE, western blotting analysis, and inhibition zone assay of recombinant cecropins. (A) SDS-PAGE and western blotting of recombinant cecropins with anti-cecropin antibody (Anti-Cec). Lane 1, AcCec; Lane 2, AcCec-K. (B) The antibacterial activity of AcCec and AcCec-K against E. coli K-12, E. coli JM109, and Bm, respectively.
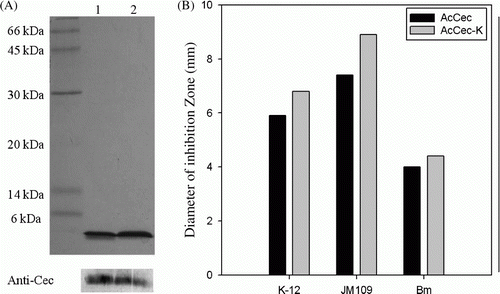
Table 1. Identification of purified AcCec and AcCec-K by MALDI-TOF-MS.
Antibacterial activities of AcCec and AcCec-K
To enhance the antibacterial activity, we have designed an additional AAA codon, encoding Lys residue located upstream of the termination codon for AcCec-K, thereby increasing net positive charge on the peptide and inducing protein phosphorylation in E. coli, then compared its activity to rAcCec. Several AMPs favor protein phosphorylation to increase their activities; of those it is chromacin that requires phosphorylation to activate the necessary protein enzyme, thus obtaining its full potency against Gram-negative bacteria (Andreu & Rivas Citation1998). The antimicrobial activities of AcCec and AcCec-K were tested against various bacterial strains, including Gram-negative E. coli and Gram-positive Bacillus megaterium (Bm), using inhibition zone assay. Both recombinant peptides were effective against all the bacteria tested, with increased activity of the AcCec-K for both Gram-negative and Gram-positive bacteria (B), suggesting that activated phosphorylation due to the C-terminal Lys residue addition indeed increased the activity.
The full-length cDNA encoding Agrius cecropin D3 was cloned and analyzed its sequence characterization. The rAcCec and AcCec-K were successfully produced as a soluble form using a prokaryotic system and found it could increase the antibacterial activity with additional Lys residue of C-terminus.
Recently, approximately 2 million people acquire nosocomial infections caused by multiresistant bacterial strains, such as Staphylococcus aureus, E. coli, Pseudomonas, Enterococcus, Legionella, and Aspergillus spps. Widespread overprescription, frequent misuse of antibiotic drugs, and massive use of preventive antibiotics in animal food have contributed to increasing rates of such infections (Bulet & Stocklin Citation2005). Therefore, our successful expression and purification of the rAcCec, and the innovative technique for mutagenesis to simultaneously enhance solubility, quantity, and activity of cecropin, would be expected to motivate efforts and studies to develop a new generation of natural antibiotics using AMPs from insects, especially for those with low amounts of hemolymph. The undetermined function of the N-terminal region of AcCec may provide information useful for developing analogs with enhanced potency in the future, and thus further study is necessary. Furthermore, searching for additional antibacterial components in A. convolvuli would also be of interest.
Acknowledgement
The present research was conducted by the research fund of Dankook University in 2010.
Additional information
Notes on contributors
Hong Sun An
These authors have the same contribution to this paperReferences
- Andreu , D and Rivas , L. 1998 . Animal antimicrobial peptides: an overview . Biopolymers. , 47 : 415 – 433 . doi: 10.1002/(SICI)1097-0282(1998)47:6%3C415::AID-BIP2%3E3.0.CO;2-D
- Bulet , P and Stocklin , R. 2005 . Insect antimicrobial peptides: structures, properties and gene regulation . Protein Pept Lett. , 12 : 3 – 11 . doi: 10.2174/0929866053406011
- Cheng , T , Zhao , P , Liu , C , Xu , P , Gao , Z , Xia , Q and Xiang , Z. 2006 . Structures, regulatory regions, and inductive expression patterns of antimicrobial peptide genes in the silkworm Bombyx mori . Genomics. , 87 : 356 – 365 . doi: 10.1016/j.ygeno.2005.11.018
- Cytryńska , M , Mak , P , Zdybicka-Barabas , A , Suder , P and Jakubowicz , T. 2007 . Purification and characterization of eight peptides from Galleria mellonella immune hemolymph . Peptides. , 28 : 533 – 546 . doi: 10.1016/j.peptides.2006.11.010
- Fink , J , Merrifield , RB , Boman , IA and Boman , HG. 1989 . The chemical synthesis of cecropin D and an analog with enhanced antibacterial activity . J Biol Chem. , 264 : 6260 – 6267 .
- Hong , SM , Kusakabe , T , Lee , JM , Tatsuke , T , Kawaguchi , Y , Kang , MW , Kang , SW and Kim , KA. 2008 . Structure and expression analysis of the cecropin-E gene from the silkworm, Bombyx mori . Biosci Biotech Bioch. , 72 : 1992 – 1998 . doi: 10.1271/bbb.80082
- Hultmark , D , Steiner , H , Rasmuson , T and Boman , HG. 1980 . Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia . Eur J Biochem. , 106 : 7 – 16 . doi: 10.1111/j.1432-1033.1980.tb05991.x
- Jin , F , Sun , Q , Xu , X , Li , L , Gao , G , Xu , Y , Yu , X and Ren , S. 2012 . cDNA cloning and characterization of the antibacterial peptide cecropin 1 from the diamondback moth, Plutella xylostella L . Protein Expres Purif. , 85 : 230 – 238 . doi: 10.1016/j.pep.2012.08.006
- Kim , CR , Lee , YH , Bang , IS , Kim , ES , Kang , CS , Yun , CY and Lee , IH. 2000 . cDNA cloning and antibacterial activities of cecropin D-like peptides from Agrius convolvuli . Arch Insect Biochem. , 45 : 149 – 155 . doi: 10.1002/1520-6327(200012)45:4%3C149::AID-ARCH2%3E3.0.CO;2-H
- Kim , JW , Park , SI , Yoe , J and Yoe , SM. 2011 . Cloning and overexpression of lysozyme from Spodoptera litura in prokaryotic system . Anim Cells Syst. , 15 : 29 – 36 . doi: 10.1080/19768354.2011.555127
- LaVallie , ER and McCoy , JM. 1995 . Gene fusion expression systems in Escherichia coli . Curr Opin Biotechnol. , 6 : 501 – 506 . doi: 10.1016/0958-1669(95)80083-2
- Lee , JY , Boman , A , Sun , C , Anderson , M , Jornvall , H , Mutt , V and Boman , HG. 1989 . Antibacterial peptides form pig intestine: isolation of mammalian cecropin . Proc Natl Acad Sci. , 86 : 9159 – 9162 . doi: 10.1073/pnas.86.23.9159
- Liang , Y , Wang , JX , Zhao , XF , Du , XJ and Xue , JF. 2006 . Molecular cloning and characterization of cecropin from the housefly (Musca domestica), and its expression in Escherichia coli . Dev Comp Immunol. , 30 : 249 – 257 . doi: 10.1016/j.dci.2005.04.005
- Makrides , SC. 1996 . Strategies for achieving high-level expression of genes in Escherichia coli . Microbiol Rev. , 60 : 512 – 538 .
- Rieger , AM and Barreda , DR. 2011 . Antimicrobial mechanisms of fish leukocytes . Dev Comp Immunol. , 35 : 1238 – 1245 . doi: 10.1016/j.dci.2011.03.009
- Saito , A , Ueda , K , Imamura , M , Atsumi , S , Tabunoki , H , Miura , N , Watanabe , A , Kitami , M and Sato , R. 2005 . Purification and cDNA cloning of a cecropin from the longicorn beetle, Acalolepta luxuriosa . Comp Biochem Phys B. , 142 : 317 – 323 . doi: 10.1016/j.cbpb.2005.08.001
- Skosyrev , VS , Kulesskiy , EA , Yakhnin , AV , Temirov , YV and Vinokurov , LM. 2003 . Expression of the recombinant antibacterial peptide sarcotoxin IA in Escherichia coli cells . Protein Expres Purif. , 28 : 350 – 356 . doi: 10.1016/S1046-5928(02)00697-6
- Sperstad , SV , Haug , T , Paulsen , V , Rode , TM , Strandskog , G , Solem , ST , Styrvold , OB and Stensvåg , K. 2009 . Characterization of crustins from the hemocytes of the spider crab, Hyas araneus, and the red king crab, Paralithodes camtschaticus . Dev Comp Immunol. , 33 : 583 – 591 . doi: 10.1016/j.dci.2008.10.010
- Thompson , JD , Higgins , DG and Gibson , TJ. 1994 . CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice . Nucleic Acids Res. , 22 : 4673 – 4680 . doi: 10.1093/nar/22.22.4673
- Wang , L , Li , Z , Du , C , Chen , W and Pang , Y. 2007 . Characterization and expression of a cecropin-like gene from Helicoverpa armigera . Comp Biochem Phys B. , 148 : 417 – 425 . doi: 10.1016/j.cbpb.2007.07.010
- Yang , D , Biragyn , A , Kwak , LW and Oppenheim , JJ. 2002 . Mammalian defensins in immunity: more than just microbicidal . Trends Immunol. , 23 : 291 – 296 . doi: 10.1016/S1471-4906(02)02246-9
- Yoe , SM , Kang , CS , Han , SS and Bang , IS. 2006 . Characterization and cDNA cloning of hinnavin II, a cecropin family antibacterial peptide from the cabbage butterfly, Artogeia rapae . Comp Biochem Phys B. , 144 : 199 – 205 . doi: 10.1016/j.cbpb.2006.02.010