Abstract
The feeding habits of the sand shrimp Crangon hakodatei in the East Sea (Korea) were investigated through analysis and comparison of the stomach contents of 602 individuals according to season, shrimp size class, and prey diversity. The diet of C. hakodatei consisted of 17 prey categories mainly comprising crustaceans, molluscs, polychaetes, nematodes, algae, and fishes, with crustaceans dominating the diet. Molluscs, nematodes, and fishes were also important prey, whilst other categories including polychaetes and algae comprised small percentages of the diet. For small C. hakodatei individuals (<10 mm carapace length [CL]), amphipods and mysids comprised more than 67% of the prey in both relative abundance and frequency of occurrence. Large individuals (>10 mm CL) tended to be more dependent on amphipods than mysids. Amphipods and mysids together constituted the dominant prey, accounting for more than 50% of the diet in terms of both percent occurrence and relative abundance. The abundance and occurrence of prey showed a seasonal variation, with amphipods and mysids being the predominant prey in autumn (45%), winter (30%), and spring (40%). Amphipods were the dominant prey with regard to season, size class, sex, and area.
Introduction
The sand shrimp Crangon hakodatei Rathbun, 1902 is an abundant species in the East and Yellow seas (Hayashi & Kim Citation1999; Cha et al. Citation2001), occurring on sandy, muddy, or mixed substrata in sublittoral areas from 10 to 250 m depth. C. hakodatei is differentiated from other related species by the high blunt median dorsal carina on the third to fifth abdominal somites. Crangon nigricauda, reported from Korea by Kim and Park (Citation1972), was later reidentified as C. hakodatei by Kim (Citation1976). Crangon affinis, which was reported from northern China by Liu (Citation1955), is referred to as C. hakodatei by Hayashi and Kim (Citation1999) and Chen et al. (Citation2006).
As with other cardiean shrimps, Crangon spp. play an important role in the trophic processing of nekton (Chuhukalo & Shebanova Citation2008). They are commonly distributed on sandy, muddy, or mixed benthic substrata of littoral and sublittoral areas from cold to temperate regions of the northern hemisphere. The sand shrimp is one of the most abundant and important components of estuarine and coastal soft bottom habitats (where it is both prey and predator in the associated communities) and also has high commercial value as a protein source for human consumption (Price Citation1962; Tiews Citation1970; Boddeke Citation1989; Hayashi & Kim Citation1999; Oh et al. Citation2001; Hanamura & Matsuoka Citation2003).
A number of studies have investigated the feeding behavior of crangonid shrimp including C. affinis by Kosaka (Citation1970) and Hong and Oh (Citation1989); C. allmani by Allen (Citation1960); C. crangon by Lloyd and Yonge (Citation1947); Tiews (Citation1970), and Oh et al. (Citation2001); C. franciscorum and C. nigricauda by Wahle (Citation1985); C. septemspinosa by Price (Citation1962) and Wilcox and Jeffries (Citation1974); and C. uritai by Nakaya et al. (Citation2004). However, despite its ecological and economic importance, there is no information on the diet of C. hakodatei. The present study was therefore undertaken to describe the diet composition of this species. The results increase our understanding of the feeding strategies and patterns of C. hakodatei and also provide information on the relationships between this and other species and their effect in marine ecosystems. Such information will facilitate estimates of the energy flow from primary producers to higher trophic levels. Trophic interactions are central to establishing the ecological role of species in ecosystem communities, and stomach content analyses can enable descriptions of the composition of the diet of species and assignment of their trophic level (Lorman & Magnuson Citation1978; Momot et al. Citation1978). Estimation of prey abundance and frequency of occurrence, and the relative importance of food sources, can provide critical ecological information (Espinoza & Wehrtmann Citation2008) and information on the composition of various food types (Crisp Citation1963; Fagade & Olaniyan Citation1972).
The objective of this study was to investigate the diet and feeding behavior of C. hakodatei. The specific objectives were to (1) obtain data on the feeding ecology of C. hakodatei; (2) investigate seasonal variations in diet and size differentiation in its feeding habits; and (3) derive information on the relationship of this species to its prey and its prey preferences. All animals are selective with respect to their prey (Krebs Citation1989). These objectives were addressed using stomach content analysis and laboratory and field observations.
Materials and methods
Sampling
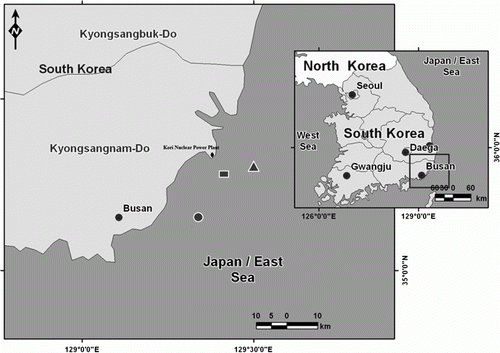
The C. hakodatei samples were obtained from trawls conducted in three areas (areas 1–3) from the Gijang coast, South Korea (). All individuals were collected randomly from depths of 30 to 60 m in each area using a small bottom trawl. The specimens of C. hakodatei were fixed for 24 hours in 4% neutralized formalin and stored in 70% alcohol. Samples were collected from area 3 during the period September 2010 to June 2011 and from areas 1 and 2 during the period from August 2010 to October 2010. The specimens from area 3 were grouped according to season: autumn (September–November), winter (December–February), and spring (March–May).
Laboratory analysis
The carapace length (CL; the shortest distance between the posterior margin of the orbit and the mid dorsal posterior edge of the carapace) of each C. hakodatei specimen was measured using Vernier calipers (accuracy 0.01 mm). The specimens were grouped into two size classes: small (<10 mm CL) and large (>10 mm CL). The sex of specimens was determined by binocular microscope examination of the endopod of the second pleopods and by the presence of the male appendage.
The relative degree of stomach fullness was assessed visually, and each foregut was assigned to one of five categories derived from the points method described by Wear and Haddon (Citation1987). The fullness categories and points were as follows: (1) full: 95%–100% full, 100 points; (2) semi full: <95% to >65% full, 75 points; (3) half full: <65% to >35% full, 50 points; (4) semi empty: <35% to >5% full, 25 points; and (5) empty: 5% or less full, 2.5 points. Prey items present in the stomach contents were identified to the lowest taxonomic level possible. Specimens with more than 2.5 points were excluded from the analyses, as were sand and mud. Prey was determined as being either present or absent and as a proportion of the number of points assigned for stomach fullness. Diet was examined for three months in areas 1 and 2 and for three seasons in area 3 (autumn, winter, and spring).
Each stomach was removed and opened on a microscope slide. The contents were first examined at low magnification using a dissecting microscope and then examined again at high magnification using a light microscope. Apparently, empty stomachs were also examined. Prey items from the stomach contents were identified using the following criteria: (1) Polychaetes: brownish or yellowish stout, tapering iridescent chaetae, stout dark brown jaws; (2) Fishes: whole bones or pieces of rib, vertebrae, muscle tissue, scales, or eggs; (3) Crustaceans: fragments of chitinous shell, parts of legs or claws, or parts of the telson; (4) Molluscs (gastropods/bivalves): parts of shell, part of the helical coil; and (5) Algae (diatoms): circular blue–green/gray items or items having radial iridescence. Sand and mud particles were found among food items in the stomach contents, as has been observed in studies of other marine animals. In menhaden deposit feeders, the diet consists of a combination of sediment grains (sand, mud), adsorbed material, and detritus comprising plant and algal material (Deegan et al. Citation1990). Land-derived organics have also been found (Conkright & Sackett Citation1986).
Data and statistical analysis
Many indices have been used to describe the importance of various preys in the diet of fish (Hynes Citation1950; Hyslop Citation1980). In the present study, percent frequency of occurrence (F) and relative abundance (A) of each prey type were estimated using the following formulae:
Trophic diversity (H′) in the diet (with respect to season and size class) was calculated according to the following equation (Shannon–Wiener index; Cody & Diamond Citation1975):
where S is the total number of species and p i is the frequency of the ith species.
Diet equality was calculated for the various size classes and seasons, using Pielou's (Citation1975) evenness index according to the following equation:
where H′ is the number derived from the Shannon diversity index, and H′ max is the maximum value of H′.
The data obtained were analyzed using the Kruskal–Wallis test to compare differences in stomach fullness among sizes and season, according to the equation of Conover (Citation1971) and Daniel (Citation1974):
This test indicates whether there is a difference, but provides no information on the cause of any difference.
To test whether the observed frequencies among feeding parameters differed from expected values, we used the χ2 (chi-squared) test. Statistical analyses were undertaken using SPSS Statistics version 19 and SAS version 9.1.3.
Results
Size composition
During the study, 602 individuals of C. hakodatei were collected and analyzed. Amongst these, the 304 individuals from areas 1 and 2 were used to assess the effect of area on feeding habits, and the 298 individuals from area 3 were used to assess the effect of seasonal variation on feeding habitats. Of all the stomachs that were examined from area 1 and 2, about 12% of stomachs from males and about 9% of those from females were found to be empty, while the proportion of empty stomach for males and females in area 3 comprised 10% and 11%, respectively. The length of specimens in the study ranged from 6.24 to 15.8 mm CL. No significant difference in the size distribution among areas was found (Kolmogorov–Smirnov 2-sample test, p=0.993), and there were no significant differences in size distribution among seasons (Kolmogorov–Smirnov 2-sample test; autumn and winter, p=0.808; autumn and spring, p=0.641; winter and spring, p=0.993).
Stomach fullness
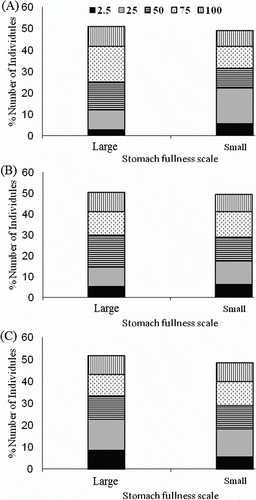
The relative degree of stomach fullness was assessed visually and each foregut was assigned to one of five categories (100, 75, 50, 25, and 2.5points). Of the stomachs examined among seasons (area 3), approximately 88% received scores greater than 2.5 points on the stomach fullness scale. There was no significant difference with respect to the stomach fullness proportion and season between males and females or for each size class (Kruskal–Wallis test, p>0.05) ().
Diet composition
Mysids and amphipods were the most important food items with respect to both area (areas 1 and 2) and season (area 3), based on the percent frequency of occurrence and relative abundance ( and ). The major components of the stomach contents of C. hakodatei were polychaetes, crustaceans (mysids, amphipods, isopods, decapods, and copepods), mollusc shells (primarily gastropods and bivalves), fish vertebrae, nematodes, and algae. These prey categories comprised 89.1% of the relative abundance and 92.79% of total occurrence for males and 85.9% of the relative abundance and 91.32% of the total occurrence in females. Mysids and amphipods were the dominate food items overall, both being present in each of the foreguts examined.
Figure 3. Relative importance of major stomach contents of Crangon hakodatei in overall diets for all combined samples irrespective of areas (Alg, algae; Amp, amphipods; Cum, cumaceans; Dec, decapods; Iso, isopods; Mol, molluscs; Mys, mysids; Pis, pisces; Pol, polychaets; Otc, other crustaceans; Oth, other species; Nem, nematodes).
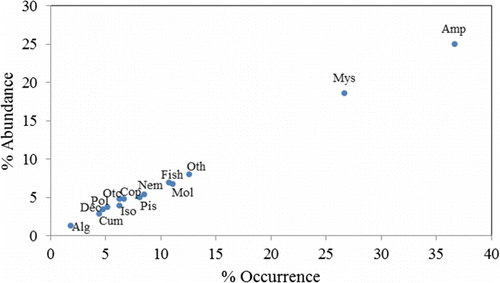
Table 1. Diet composition of Crangon hakodatei in the three seasonal groups and two size groups (%F, frequency of occurrence; %N, percentage abundance).
Diet differences by size class
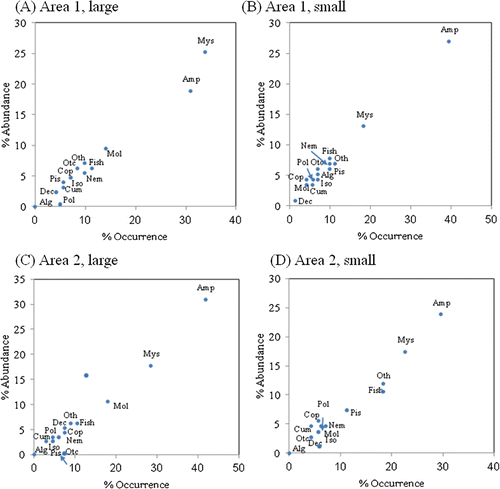
The diet composition for shrimp in the two size classes (small and large) in areas 1 and 2 is shown in . For those in the small size class, amphipods were clearly dominant in each area in terms of both relative abundance and frequency of occurrence; they comprised more than 39% and 30% of the prey items in areas 1 and 2, respectively. For shrimp in the large size class, mysids and amphipods were dominant prey in both areas. In both relative abundance and frequency of occurrence, mysids and amphipods comprised more than 65% of prey items in area 1 and more than 70% in area 2. No significant differences were found in the proportion of prey consumed by the two size classes in each area (χ2 test, df=13, p>0.05).
Figure 4. Relative importance of major stomach content items for areas 1 and 2 samples pooled by size classes: small (<10 mm CL) and large size (>10 mm CL) (Alg, algae; Amp, amphipods; Cum, cumaceans; Dec, decapods; Iso, isopods; Mol, molluscs; Mys, mysids; Pis, pisces; Pol, polychaets; Otc, other crustaceans; Oth, other species; Nem, nematodes).
Seasonal differences in diet
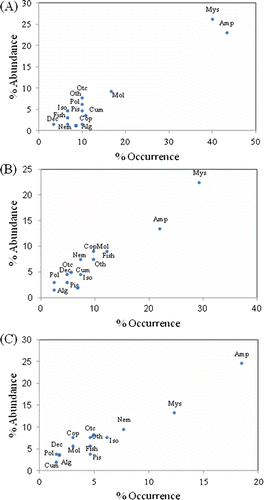
The relative abundance and frequency of occurrence of food items in stomach contents showed seasonal fluctuations (). Mysids and amphipods were the predominant prey in all three seasons, and based on relative abundance and frequency of occurrence, in combination accounted for 40% of the diet in autumn, 30% in winter, and 41% in spring. Mysids and amphipods were also the predominant prey items for each size class in all seasons. Each gender showed a mixed feeding strategy, with varying degrees of specialization and generalization on different prey types, but in terms of relative abundance and frequency of occurrence, the diets of males and females were similar, including among seasons: mysids and amphipods dominated the diet in autumn (45% males, 40% females), winter (41% males, 32% females), and spring (43% males, 39% females).
Figure 5. Relative importance of major stomach contents for Crangon hakodatei samples pooled by seasons (A) autumn, (B) winter and (C) spring (Alg, algae; Amp, amphipods; Cum, cumaceans; Dec, decapods; Iso, isopods; Mol, molluscs; Mys, mysids; Pis, pisces; Pol, polychaets; Otc, other crustaceans; Oth, other species; Nem, nematodes).
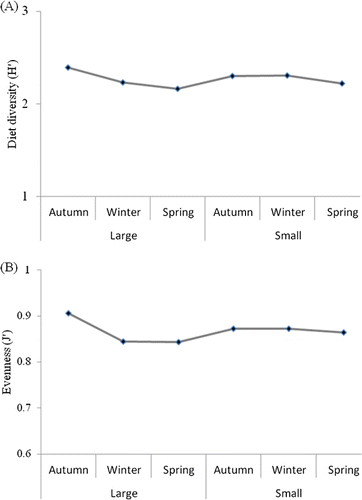
Trophic diversity and equality
Seasonally trophic diversity was generally low (A). For both size classes, diversity was highest in autumn. No appreciable differences were evident between size classes in any season. Trends were similar for diet evenness (B). The higher index values in autumn indicated that the prey items consumed were more evenly distributed, as demonstrated by the relative importance of the seasonal diet composition. In other seasons, the shrimps were more dependent on a small number of prey items.
Discussion
The most important prey items in the C. hakodatei diet were crustaceans, with other food classes not playing an important role. The dominance of crustaceans in the diet of several species of Crangon has been reported, including C. crangon (Pihl & Rosenberg Citation1984; Oh et al. Citation2001), C. franciscorum (Sitts & Knight Citation1979), and C. uritai (Hanamura & Matsuoka Citation2003).The diet of C. hakodatei consists of benthic organisms in three main categories: (1) organisms that, as a result of vertical migration, occur close to the benthos during part of the day (e.g. mysids, shrimps, fishes); (2) organisms that occur on or just beneath the surface of the substratum (e.g. amphipods, gastropods); and (3) organisms that live completely or partially buried, digging out small galleries in the substratum (e.g. bivalves, cumaceans, polychaetes). Although a diversity of prey was available to C. hakodatei, amphipods and mysids were the dominant food items with respect to area, season, size class, and sex. Amphipods are dominant in diverse habitats and play an important role in the food chain of marine ecosystems as the main food source for other predatory animals (Crawford Citation1937; Zhang Citation1974). Mysids are a ubiquitous component of zooplankton assemblages in a variety of aquatic environments and at times can comprise a significant proportion (up to 20%) of the total zooplankton biomass (Mauchline Citation1980; Price Citation1982; Webb et al. Citation1987; Grange & Allanson Citation1995; Jerling & Wooldridge Citation1995; Froneman et al. Citation2000).
The occurrence of major crustacean items in the shrimp stomachs was not related to size, sex, or area. Mysids and amphipods were found consistently in each shrimp size class, with mysids being the dominant food item. It has been reported that in silt and clay areas, meiofauna and macrofauna are important biological components (Pihl & Rosenberg Citation1984), while sand areas are dominated by calanoid copepods (Boddeke et al. Citation1986). The results of our investigation are similar to those reported in other studies of Crangonidae shrimp. Members of this family are almost exclusively carnivorous, including C. affinis by Kosaka (Citation1970) and Hong and Oh (Citation1989), C. allmani by Allen (Citation1960), C. crangon by Lloyd and Yonge (Citation1947), Tiews (Citation1970) and Oh et al. (Citation2001), C. franciscorum and C. nigricauda by Wahle (Citation1985), C. septemspinosa by Price (Citation1962) and Wilcox and Jeffries (Citation1974), and C. uritai by Nakaya et al. (Citation2004).
The most important factor affecting the diet of Crangon spp. is the spatial and temporal availability of prey (Wilcox & Jeffries Citation1974; Wahle Citation1985; Oh et al. Citation2001), with amphipods being the most important benthopelagic prey animals (Venables Citation1981; Moller & Rosenbreg Citation1982). Spatial differences in the diet of C. hakodatei are related to differences among habitats, particularly with respect to substrate type, which would determine the abundance and population structure of potential prey types in the community. Based on many studies it appears that Crangon species adopt an opportunistic strategy, which suggests in turn that the diet of C. hakodatei may shift depending on habitat type and the availability of potential prey.
In this study, the substantial differences found for the stomach fullness of sand shrimp among areas could not be related to the availability of food items, because the dominant and preferred trophic groups of macrobenthic organisms did not change markedly among seasons at either site. Stomach fullness is affected by both extrinsic (season) and intrinsic (sex) factors (Lloyd & Yonge Citation1947; Meredith Citation1952; Henderson & Holmes Citation1987). In this study, males had lower values for stomach fullness, food quality indices, and evenness than females. Both sexes consumed the same prey items, but showed different abundances and frequencies of occurrence of prey. These results indicate a slightly greater predatory ability for females. These differences could also be attributed to sexual dimorphism and to size differences between the sexes. There was no substantial positive relationship between C. hakodatei size and prey size. The trophic diversity indices varied little among areas but were higher for the larger size class. The highest values were observed in spring, probably reflecting a substantial increase in the availability of prey items in this season, while the lowest values occurred in autumn as a result of a reduction in the range of prey items. The results may also reflect changes in prey abundance associated with seasonal variations in primary production.
Additional information
Notes on contributors
Kyung-Jun Song
These authors are contributed equally to this workReferences
- Allen , JA. 1960 . On the biology of Crangon allmani Kinahan in Northumberland waters . J Mar Bio Assoc UK , 39 : 481 – 508 . doi: 10.1017/S0025315400013497
- Boddeke , R. 1989 . “ Management of the brown shrimp (Crangon crangon) stock in Dutch coastal waters ” . In Marine invertebrate fisheries: their assessment and management , Edited by: Caddy , JF . 35 – 62 . New York : Wiley Interscience Publication .
- Boddeke , R , Drissen , G , Doesburg , W and Ramaekers , G. 1986 . Food availability and predator presence in a coastal nursery area of the brown shrimp (Crangon crangon) . Ophelia , 26 : 77 – 90 . doi: 10.1080/00785326.1986.10421980
- Cha HK , Lee JU , Park CS , Baik CI , Hong SY , Park JH , Lee DW , Choi YM , Hwang K , Kim ZG , et al. 2001 . Shrimps of Korean waters . Busan : National Fisheries Research and Development Institute ; 1 – 188 (In Korean) .
- Chen S , Wang Q , Peng Y , Liu J. 2006 . Reducing environmental stress in the Yellow Sea large marine ecosystem . Third Meeting of the Regional Working Group for the Biodiversity Component UNDP/GEF/YS/RWG-B2/3 ; China .
- Chuhukalo , VI and Shebanova , MA. 2008 . Feeding habits of several mass shrimp species in the Sea of Okhotsk . Russ J Mar Bio , 34 ( 7 ) : 468 – 471 . doi: 10.1134/S1063074008070055
- Cody , ML and Diamond , JM. 1975 . Ecology and evolution of communities , Cambridge , MA : The Belknap Harvard University Press .
- Conkright , ME and Sackett , WM. 1986 . A stable carbon isotope evaluation of the contribution of terriginous carbon to the marine food web in Bayboro harbor, Tampa Bay, Florida . Mar Sci , 29 : 131 – 139 .
- Conover , WJ. 1971 . Practical nonparametric statistics , New York , NY : Willey .
- Crawford , GI. 1937 . A review of the amphipod genus Corophium, with notes on the British species . J Mar Bio Assoc UK , 21 : 589 – 630 . doi: 10.1017/S0025315400053753
- Crisp , DT. 1963 . A preliminary survey of brown trout (Salmo trutta L.) and bullheads (Cottus gobio L.) in high altitude becks . Salm Trout mag , 167 : 45 – 59 .
- Daniel , WW. 1974 . Biostatistics: a foundation for analysis in the health sciences , New York , NY : Willey .
- Deegan , LA , Peterson , BJ and Porther , R. 1990 . Stable isotopes and cellulase activity as evidence for detritus as a food source for juvenile gulf menhaden . Estuaries , 13 ( 1 ) : 14 – 19 . doi: 10.2307/1351427
- Espinoza , M and Wehrtmann , IS. 2008 . Stomach content analyses of the threadfin anglerfish Lophiodes spilurus (Lophiiformes: Lophiidae) associated with deepwater shrimp fisheries from the central Pacific of Costa Rica . J Trop Bio , 56 ( 4 ) : 1959 – 1970 .
- Fagade , SO and Olaniyan , CIO. 1972 . The biology of the West African shad Ethmalosa fimbriata (Bowditch) in the Lagos Lagoon, Nigeria . J Fish Bio , 4 : 519 – 533 . doi: 10.1111/j.1095-8649.1972.tb05699.x
- Froneman , PW , Pakhomov , EA and Treasure , A. 2000 . Trophodynamics of the hyperid amphipod, Themisto gaudichaudi, in the waters surrounding the Prince Edward Islands . Pol Bio , 23 : 429 – 436 . doi: 10.1007/s003000050464
- Grange , N and Allanson , BR. 1995 . The influence of freshwater inflow on the nature, amount and distribution of seston in estuaries of the Eastern Cape, South Africa . Est Coas Shelf Sci , 40 : 403 – 420 . doi: 10.1006/ecss.1995.0028
- Hanamura , Y and Matsuoka , M. 2003 . Feeding habits of the sand shrimp, Crangon uritai Hayashi & Kim, 1999, in the central Seto Inland Sea, Japan . Crustaceana , 76 ( 8 ) : 1017 – 1024 . doi: 10.1163/156854003771997873
- Hayashi , KI and Kim , JN. 1999 . Revision of the East Asian species of Crangon (Decapoda: Caridea: Crangonidae) . Crusta res , 28 : 62 – 103 .
- Henderson , PA and Holmes , RHA. 1987 . On the population biology of the common shrimp Crangoncrangon (L.) (Crustacea: Caridea) in the Severn Estuary and Bristol Channel . J Mar Bio Assoc UK , 67 : 825 – 847 . doi: 10.1017/S0025315400057076
- Hong , SY and Oh , CW. 1989 . Ecology of the sand shrimp, Crangon affinis, in the Nakdong River estuary, Korea . Bull Kor Fish Soc , 22 : 351 – 362 .
- Hynes , HBN. 1950 . The food of fresh-water sticklebacks (Gasterosteus aculeatus and Pygosteus pungitus), with a review of methods used in studies of the food of fishes . J Ani Eco , 19 : 36 – 58 . doi: 10.2307/1570
- Hyslop , EJ. 1980 . Stomach content analysis: a review of methods and their application . J Fish Bio , 17 : 411 – 429 . doi: 10.1111/j.1095-8649.1980.tb02775.x
- Jerling , HL and Wooldridge , TH. 1995 . Feeding of two mysid species on plankton in a temperate estuary . J Exp Mar Bio Eco , 188 : 243 – 259 . doi: 10.1016/0022-0981(95)00007-E
- Kim , HS. 1976 . A checklist of the Macrura (Crustacea, Decapoda) of Korea . Proc Coll Nat Sci, Seoul Nat'l Univ , 1 : 131 – 152 .
- Kim HS , Park KB. 1972 . Faunal studies on the Macrurans in Korea . In : Kim HS . Floral Studies on some Taxa of Plants and Faunal Studies on some Taxa of Animals in Korea . Seoul : Ministry of Science and Technology ; 185 – 222 (In Korean) .
- Kosaka , M. 1970 . On the ecology of the sand shrimp, Crangon affinis De Haan, as a prey of the demersal fishes in Sendai Bay . J Fac Mar Sci Tec, Tokai Univ , 42 : 59 – 80 .
- Krebs , CJ. 1989 . Ecological methodology , New York , NY : Harper and Row .
- Liu JY. 1955 . Economic shrimps and prawns of northern China . Beijing : Marine Biological Institute of Academy Science . (In Chinese) .
- Lloyd , A and Yonge , CM. 1947 . The biology of Crangon crangon L. in the Bristol Channel and Severn estuary . J Mar Bio Assoc UK , 26 : 626 – 661 . doi: 10.1017/S0025315400013837
- Lorman , JG and Magnuson , JJ. 1978 . The role of crayfishes in aquatic ecosystems . Fisheries , 3 : 8 – 10 . doi: 10.1577/1548-8446(1978)003%3C0008:ASOMOR%3E2.0.CO;2
- Mauchline , J. 1980 . The biology of mysids and euphausiids . Adv Mar Bio , 18 : 1 – 680 .
- Meredith , SS. 1952 . A study of Crangon vulgaris in the Liverpool Bay area . Proc Trans Liverpool Bio Soc , 58 : 75 – 109 .
- Moller , P and Rosenberg , R. 1982 . Production and abundance of the amphipod Corophium volutator on the west coast Sweden . Netherlands J Sea Res , 16 : 127 – 140 . doi: 10.1016/0077-7579(82)90024-2
- Momot , WT , Gowing , H and Jones , PD. 1978 . The dynamics of crayfish and their role in ecosystems . Amer Midland Naturalist , 99 : 10 – 35 . doi: 10.2307/2424930
- Nakaya , M , Takatsu , T , Nakagami , M , Joh , M and Takahashi , T. 2004 . Spatial distribution and feeding habits of the shrimp Crangon uritai, as a predator on larval and juvenile marbled sole Pleuronectes yokohamae . J Jap Soc Fish Sci , 70 ( 3 ) : 445 – 455 .
- Oh , CW , Hartnoll , RG and Nash , RDM. 2001 . Feeding ecology of the common shrimp Crangon crangon in Port Erin Bay, Isle of Man, Irish Sea . Mar Eco Pro Ser , 214 : 211 – 223 . doi: 10.3354/meps214211
- Pielou , EC. 1975 . Ecological diversity , New York , NY : Wiley .
- Pihl , L and Rosenberg , R. 1984 . Food selection and consumption of the shrimp Crangon crangon in some shallow marine areas in western Sweden . Mar Eco Pro Ser , 15 : 159 – 168 . doi: 10.3354/meps015159
- Price , KS. Jr . 1962 . Biology of the sand shrimp, Crangon septemspinosa, in the shore zone of the Delaware Bay region . Chesapeake Sci , 3 : 244 – 255 . doi: 10.2307/1350631
- Price , WW. 1982 . Key to adult shallow water Mysidacea of the Texas coast with notes on their ecology . Hydrobiologia , 93 : 9 – 22 . doi: 10.1007/BF00008094
- Sitts , RM and Knight , AW. 1979 . Predation by the estuarine shrimps Crangon franciscoru Stimpson and Palaemon macrodactylus Rathbun . Bio Bull , 156 : 356 – 368 . doi: 10.2307/1540923
- Tiews , K. 1970 . Synopsis of biological data on the common shrimp Crangon crangon (Linnaeus, 1758) . FAO Fish Syn , 91 : 1167 – 1224 .
- Venables , BJ. 1981 . Aspects of the population biology of a Venezuelan beach amphipod, Talorchestia margaritae (Talitridae), including estimates of biomass and daily production, and respiration rates . Crustaceana , 41 : 271 – 285 . doi: 10.1163/156854081X00859
- Wahle , RA. 1985 . The feeding ecology of Crangon franciscorum and Crangon nigricauda in San Francisco Bay, California . J Crusta Bio , 5 : 311 – 326 . doi: 10.2307/1547879
- Wear , RG and Haddon , M. 1987 . Natural diet of the crab Ovalipes catharus (Crustacea, Portunidae) around central and northern New Zealand . Mar Eco Pro Ser , 35 : 39 – 49 . doi: 10.3354/meps035039
- Webb , P , Perissinotto , R and Wooldridge , T. 1987 . Feeding of Mesopodopsis slabberi (Crustacea, Mysidaceae) on naturally occurring phytoplankton . Mar Eco Pro Ser , 38 : 115 – 123 . doi: 10.3354/meps038115
- Wilcox , JR and Jeffries , HP. 1974 . Feeding habitats of the sand shrimp, Crangon septemspinosa . Bio Bull , 146 : 424 – 434 . doi: 10.2307/1540416
- Zhang , WQ. 1974 . A new species of the genus Corophium (Crustacea, Amphipoda, Gammaridea) from the southern coast of Shantung Peninsula, North China . Studia Marina Sinica , 9 : 139 – 146 .