Abstract
Human chromosome maintenance 1 (CRM1) was originally cloned based on homology to a yeast gene. CRM1, which belongs to the family of importin β-related nuclear transport receptors, directly and specifically associates with nuclear export signals (NESs) and mediates nuclear export of proteins containing leucine-rich NESs. We present evidence that CRM1 associates with a 22-kDa 14-3-3 scaffolding protein that is a principal structural and regulatory component of Human embryonic kidney (HEK 293) cells. We found a potential 14-3-3-binding motif (1049KHKRQMSVPG1058) in the CRM1 C-terminal domain that depended on serine 1055 phosphorylation by Protein Kinase A (PKA). We demonstrated that CRM1 is a PKA substrate using an in vitro assay. Using a pull-down approach and co-immunoprecipitation, we found that CRM1 interacted with the 14-3-3 motif in vivo and in vitro. We also detected colocalization of CRM1 and 14-3-3 proteins using confocal microscopy. Nuclear pore localization of CRM1 was disrupted by treatment with a PKA activator or inhibitor or by a S1055D/S1055A mutation in the CRM1 14-3-3-binding motif. Transient transfection assays showed that the apoptosis rate of cells with the S1055D construct was twice that of cells with wild type (WT) or S1055A construct. Our observations indicated that phosphorylation on the serine 1055 residue of CRM1 by PKA promoted 14-3-3 binding and cytoplasmic localization, resulting in enhancement of cell apoptosis.
Introduction
Yeast genetic data using temperature-sensitive mutants showed that export of marker proteins containing nuclear export signals (NESs) is disrupted in yeast strains with conditional chromosome maintenance 1 (CRM1) mutations (Toda et al. Citation1992; Turi et al. Citation1994; Shimanuki et al. Citation1995). In Xenopus oocytes, overexpression of human CRM1 increases the export of nuclear-injected Rev protein (Fornerod et al. Citation1997a; Neville et al. Citation1997). Moreover, the cytotoxin leptomycin B (LMB) inhibited export of Rev protein in both mammalian cells and in Xenopus oocytes (Kudo et al. Citation1999; Fasken et al. Citation2000). These studies show that CRM1 is involved in the nuclear export of NES-containing proteins (Fornerod et al. Citation1997a; Yoneda et al. Citation1999).
The LMB effect is probably direct, because LMB binds to in vitro-translated CRM1 and in Schizosaccharomyces pombe, resistance to LMB maps to the CRM1 gene (Hamamoto et al. Citation1985; Kudo et al. Citation1999; Fasken et al. Citation2000). This places CRM1 in the family of RanGTP-binding proteins that includes other known and putative import and export receptors. Further supporting CRM1 as an export receptor for NES-containing proteins is the observation that the N-terminal region of CRM1 is homologous to the RanGTP-binding domain of importin β (Nishi et al. Citation1994; Fukuda et al. Citation1997). CRM1 has been identified with at least two proteins associated with the human nuclear pore complex, namely the nucleoporins CAN/Nup214 and Nup88. Two-hybrid assays in Saccharomyces cerevisiae show interactions between CRM1 and several nucleoporins, as well as Rev and Ran (Fornerod et al. Citation1997b; Askjaer et al. Citation1999; Kehlenbach et al. Citation1999).
The leucine-rich NES recognized by CRM1 was first identified in protein A phosphorylation inhibitor (PKI) and the viral HIV-1 Rev protein. Both sequences contain four regularly spaced leucines (Fornerod et al. Citation1997a; Neville et al. Citation1997; Kehlenbach & Gerace Citation2000). Numerous studies have contributed to the definition of the leucine-rich NES consensus sequence as Φ-X2–3-Φ-X2–3-Φ-X-Φ (Φ: L, I, F, V, M; X: any amino acid) (Fukuda et al. Citation1997; Jensen et al. Citation2000). The presence of leucine residues is not a prerequisite for NESs and several NESs have been identified that diverge from this postulated consensus sequence (Neumann et al. Citation2000; la Cour et al. Citation2003). Using the currently ill-defined NES consensus sequence, most proteins are predicted to harbor NES consensus sequences. This hampers the annotation of valid export signals and their characterization in vivo (Neumann et al. Citation2000; la Cour et al. Citation2003).
Recent progress in structural characterization of cyclic AMP-dependent protein kinase (Protein Kinase A, PKA) has expanded our knowledge of kinase signaling (Kuehn Citation1972; Makman & Klein Citation1972; Kleppe et al. Citation2011; Chan et al. Citation2012). The PKA holoenzyme is a heterotetramer of two catalytic (C) subunits held in an inactive state by association with a regulatory (R) subunit dimer. cAMP binds cooperatively to two sites termed A and B on each R subunit (Li Citation2011; Luconi et al. Citation2011). In the inactive holoenzyme, only the B site is exposed and available for cAMP binding. When occupied, this enhances the binding of cAMP to the A site by an intramolecular steric change. Binding of four cAMP molecules, two per R subunit, leads to a conformational change and dissociation into an R subunit dimer bound to four cAMP molecules, and two C monomers (Schillace & Carr Citation2006; Li Citation2011; Luconi et al. Citation2011). The C subunits become catalytically active and phosphorylate nearby target substrates on serine or threonine residues in the context of Arg-Arg-X-Ser/Thr, Arg-Lys-X-Ser/Thr, Lys-Arg-X-Ser/Thr, or Lys-Lys-X-Ser/Thr. Each R subunit of PKA contains an N-terminal docking and dimerization (D/D) domain, a PKA inhibitor site, and two tandem cAMP-binding domains. The D/D domain is connected to the cAMP-binding domain A by an extended, highly disordered linker that contains an autoinhibitory sequence and several putative phosphorylation sites (Pidoux & Tasken Citation2010; Pidoux et al. Citation2011).
The 14-3-3 proteins are intracellular, dimeric, phosphoserine-binding proteins that have been identified in eukaryotic organisms and are found primarily in the cytoplasm (Aitken et al. Citation1992; Yaffe et al. Citation1997). The eight mammalian members of the 14-3-3 family are encoded by β, γ, ϵ, η, σ, θ, τ, and ζ genes. Mammalian 14-3-3 proteins regulate tyrosine and tryptophan hydroxylases in neurotransmitter synthetic pathways. The 14-3-3 proteins bind and inhibit PKC, PDK1, and Ask1 (Shibuya Citation2003; Obsilova et al. Citation2008). Binding of 14-3-3 proteins to the apoptosis-promoting protein BAD prevents its binding to Bcl-XL (Muslin & Xing Citation2000; Tzivion et al. Citation2001). Generally, the binding of 14-3-3 proteins to partners depends on serine (Ser) or threonine (Thr) phosphorylation in the specific binding motif (RX1–2S/T*X2–3S/T or RX2–3S/T*XP, where * indicates the phosphorylated residue) (Aitken et al. Citation1992; Yaffe et al. Citation1997; Muslin & Xing Citation2000; Tzivion et al. Citation2001; Shibuya Citation2003; Chun et al. Citation2004; Obsilova et al. Citation2008).
Upon visual inspection of CRM1 amino acid sequence with the 14-3-3 binding motif and PKA target substrate information, we noticed the presence of a potential 14-3-3 binding motifs (1049KHKRQMSVPG1058) in its C-terminal domain (Fornerod et al. Citation1997b). Our results suggested that 14-3-3 proteins interact with wild type (WT) CRM1 through phosphorylation of the 1055 Ser residue in the CRM1 C-terminal domain by PKA. In addition, we present evidence suggesting that interaction with 14-3-3 proteins mediates the subcellular localization of CRM1, and leads to a decrease in cell survival. Thus, our observations shed light on the molecular mechanism(s) underlying CRM1 regulation, localization, and signaling that involve binding to 14-3-3 proteins.
Materials and methods
Antibodies
Antibodies against green fluorescent protein (GFP), glutathionine-S-transferase (GST), CRM1 and 14-3-3β were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Phosphor-Ser/Thr antibody was from Cell Signaling Technology Inc. (Boston, MA, USA).
Cell culture
Human embryonic kidney (HEK 293) cells were from ATCC (Manassas, VA, USA). Media and supplements were from GIBCO (Grand Island, NY, USA). Cells were maintained in Dulbecco's Modified Essential Medium containing 10% fetal bovine serum (FBS) heat-inactivated for 30 min at 56°C, 100 U potassium penicillin/ml, 100 mg streptomycin/ml, 2 mM glutamine and 20 mM sodium bicarbonate. Incubation was in 5% CO2, 95% humidity at 37°C.
Cell treatment
Cells were treated with 60 µM forskolin (Cell Signaling) or 10 µM H89 (Sigma Aldrich) for 24 h, starting 24 h after medium replacement.
Double-immunofluorescence microscopy
HEK 293 cells were plated to about 30% confluence on microscope cover glasses (Fisher, USA) in 4-well plates (SPL, Korea), and transiently transfected with enhanced green fluorescent protein (EGFP)-CRM1 WT or EGFP-CRM1 mutant plasmids using the lipofectamine (Life Technologies Corporation, USA) procedure. Cells were serum-starved for 6 h and subsequently treated with 10% FBS for 42 h. Cell confluence did not exceed 70%. Cells were blocked in 5% BSA in Phosphate Buffered Saline (PBS) for 1 h and incubated with a 1:100 dilution of anti-14-3-3 or anti-CRM1 (Santa Cruz Biotechnology), for 2 h at room temperature. For indirect immunofluorescence microscopy, washed slides were incubated for 1 h at room temperature with a 1:200 dilution of goat anti-rabbit Alexa Fluor 568 or goat anti-mouse Alexa Fluor 594 (Life Technologies Corporation). Slides were washed and mounted with Dako fluorescent mounting medium (Dako Co., USA), and examined using an LSM710 confocal microscope (ZEISS, Germany) in the Core Facility of Chungbuk National University (Chun et al. Citation2004).
Expression and purification of recombinant proteins
WT C-terminal fragment CRM1 (amino acids 960–1120) and CRM1 mutant (S1055A) tagged with GST were expressed in Escherichia coli BL21 and purified with GST-agarose beads according to the manufacturer's instruction (Amersham Biosciences Co.). Purified proteins were used as bait protein for pull-down assays or as substrates in PKA assays.
Fluorescence-activated cell sorting (FACS)
EGFP-CRM1 WT and EGFP-CRM1 mutants were transfected and the rate of apoptosis was measured by FACS Calibur (BD Bioscience, USA). Cells were trypsinized in 2-ml Petri plates with 70 µl of 1×trypsin. Transfected cells were washed twice in cold PBS, and 2 ml of 70% cold EtOH was added while vortexing gently. Cells were left in EtOH at −20°C overnight for fixing. Cells were spun at 1500 rpm for 5 min, resuspended in 2 ml PBS and spun again at 1500 rpm for 5 min. After adding 500 ml FACS buffer (PBS plus PI 4 mg/ml plus RNase 30 mg/ml), cells were incubated at 4°C for 1 h, and analyzed immediately to prevent clumping. The FACS Calibur was equipped with a gated amplifier and upgraded for enhanced system performance at The Core Facility of Chungbuk National University (Shin et al. Citation2012).
Site-directed mutagenesis
In order to obtain the mutants, amino acid changes were introduced using mutated oligonucleotides for S1055A (up 5′-cat aaa cgt caa atg Gct gtc cct ggc atc-3′, down 5′-aaa gat gcc agg gac agC cat ttg acg ttt-3′) or S1055D (up 5′-cat aaa cgt caa atg GAt gtc cct ggc atc-3′, down 5′-aaa gat gcc agg gac aTC cat ttg acg ttt-3′), and WT CRM1 as a template. The CRM1 mutant constructs were prepared using a QuickChange® Multi Site-Directed agenesis Kit (Stratagene). The C-terminal CRM1 960–1120 aa fragment was obtained using oligonucleotides (up 5′-ggtt agg atc caa aca tca tta aat cct gga aat cca-3′, down 5′-ggtt ctc gag tta atc aca cat ttc ttc tgg aat-3″), and wild type CRM1 as a template. The polymerase chain reaction (PCR) product was cloned in pGEX-1 vector BamH1 and Xho1 site. All CRM1 constructs were confirmed via DNA sequencing.
PKA assay
Assay kits and active PKA were from Promega. After PKA reaction with CRM1 C-terminal recombinant protein (960–1120 fragment), reactants were analyzed by western blotting with anti-phospho-Ser/Thr (Cell Signaling Technology Inc.).
Results
Interaction between CRM1 and 14-3-3 proteins
In the most well-characterized nuclear export mechanism for proteins, the nuclear export receptor CRM1 binds directly to leucine-rich NESs to translocate cargo proteins through the nuclear pore from the nucleus to the cytoplasm (Toda et al. Citation1992; Turi et al. Citation1994; Shimanuki et al. Citation1995; Fornerod et al. Citation1997a; Neville et al. Citation1997). CRM1 is the major nuclear export receptor (A). The CRIME domain (which stands for CRM1, importin β, etc.) shares homology with importin β (Hutten & Kehlenbach Citation2007; Fox et al. Citation2011). In the domain, 19 Huntingtin, elongation factor 3, protein phosphatase 2A, and the yeast kinase TOR1 (HEAT) repeat motifs have been have been defined by homology modeling. The HEAT helices 11A and 12A form a cargo-binding hydrophobic cleft. Leptomycin B (LMB) modifies Cys528 in the NES-binding region (Nishi et al. Citation1994; Kudo et al. Citation1999; Fasken et al. Citation2000). The acidic loop in the eighth HEAT repeat motifs is involved in RanGTP binding (Askjaer et al. Citation1999; Yoneda et al. Citation1999; Hutten & Kehlenbach Citation2007; Fox et al. Citation2011).
Figure 1. Functional domains and mutants of chromosome maintenance1 (CRM1). (A) Schematic structure of CRM1. Boxes 1–19 represent the HEAT repeat motifs, as defined by homology modeling. The CRIME domain (CRM1, importin β, etc.), which shares homology with importin β, and the acidic loop are involved in RanGTP binding. RanBP3-binding domain is indicated by green color. Modification of Cys528 by LMB targets the region involved in NES binding (Hamamoto et al. 1985; Nishi et al. 1994; Kudo et al. 1999). The C-terminal fragment corresponding to residues 707–1034 (CTR) is indicated by blue color. The consensus motif of Protein Kinase A (PKA) phpsphorylation site (Ser 1055) in the C-terminal domain is indicated above. The C-terminal GST fusion protein fragment region (960–1120 aa) is shown below. (B and C) Reciprocal Immunoprecipitation (IP) and Immunoblotting (IB) from HEK 293 cells. CRM1 and 14-3-3 immunoprecipitates were analyzed using anti-14-3-3 or anti-CRM1 antibody. Negative IP control was normal rabbit antibody. (D) Confocal microscopy. Endogenous CRM1 (red) or 14-3-3 (green) in HEK 293 cells was visualized using appropriate primary antibodies, and Alexa Fluor 568-conjugated secondary antibodies. Merged image (yellow) shows coincident distribution of wild type CRM1 and 14-3-3 proteins. Figures represent three or more independent experiments.
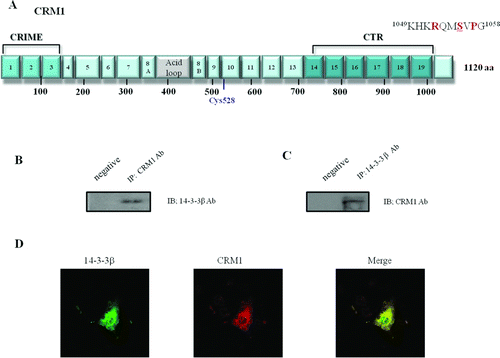
We noticed consensus 14-3-3-binding motifs in the C-terminus of CRM1 (1049KHKRQMSVPG1058) (A), suggesting that CRM1 was a 14-3-3-binding protein. To investigate formation of endogenous CRM1-14-3-3 complexes in cells, CRM1 and 14-3-3 were immunoprecipitated with anti-14-3-3 or anti-CRM1, using normal rabbit antibody as a negative control. Reciprocal immunoprecipitation (IP) and immunoblotting (IB) of HEK 293 cells with CRM1 or 14-3-3 antibody suggested that CRM1 formed a complex with a 14-3-3 protein (B and C). Confocal microscopy was used to visualize complexes of endogenous 14-3-3 proteins binding to CRM1. Merged images show coincident distribution of WT CRM1 and 14-3-3 proteins (D). These results demonstrated that CRM1 interacted with 14-3-3 proteins in HEK 293 cells.
Formation of CRM1 and 14-3-3 protein-protein complexes requires Ser 1055 in the CRM1 C-terminal domain
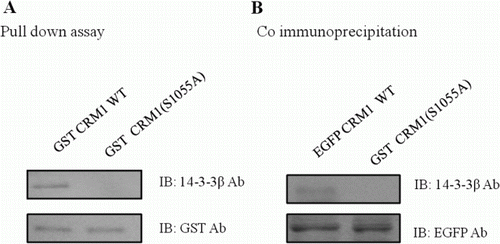
Since CRM1, which contained a conserved 14-3-3 binding motif, pulled down a 14-3-3 protein, we investigated whether the 14-3-3 binding motif was required for the association. To determine whether CRM1 interacted with 14-3-3 through the motif, we constructed the CRM1 S1055A point mutant, which affects amino acids 960–1120 in the C-terminal region. GST-CRM1 fusion proteins were purified and incubated with HEK 293 cell lysates to determine binding to 14-3-3 proteins. The WT C-terminus of CRM1 precipitated large amounts of 14-3-3 proteins from HEK 293 cell lysates, while the CRM1 S1055A mutant protein did not (A). Co-IP was used to confirm the cellular association between CRM1 and 14-3-3 proteins. As shown in B, EGFP-CRM1 WT immunoprecipitates contained 14-3-3 proteins. Antibodies against EGFP also captured both CRM1 and 14-3-3 proteins from the same lysates, supporting the hypothesis that the two proteins were physically associated (B). However, antibodies against EGFP did not precipitate 14-3-3 proteins from lysates of cells with EGFP-CRM1 S1055A (B). These results suggested that CRM1 interacted with 14-3-3 proteins through the 14-3-3 binding motif, and that the motif was required for the interaction. Among the eight mammalian members of the 14-3-3 family (β, γ, ϵ, η, σ, θ, τ, and ζ), 14-3-3θ showed the strongest signal (data not shown).
Figure 2. Formation of CRM1 and 14-3-3 protein–protein complexes. CRM1 and 14-3-3 immunoprecipitates were analyzed using anti-14-3-3 and anti-CRM1. CRM1 precipitated from HEK 293 cells (A). GST-tagged CRM1 mutants were expressed in E. coli and prebound to GST-agarose beads that were incubated with HEK 293 cell lysates and analyzed using anti-14-3-3 (B).
Phosphorylation of Ser 1055 in CRM1
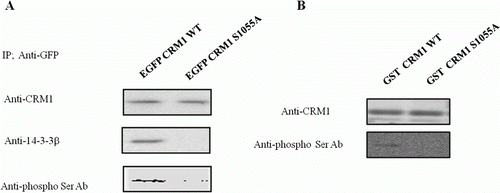
To verify the phosphorylation of CRM1, EGFP-CRM1 WT and EGFP-CRM1 S1055A were transfected and immunopurified CRM1 protein with EGFP antibody. The western blot was performed with CRM1, 14-3-3, or an anti-phosphor Thr/Ser residue antibody. In stark contrast to the results obtained with EGFP-CRM1, EGFP-CRM1 S1055A utterly failed to interact with 14-3-3 (B), indicating that the serine residue is the crucial factor with regard to the interaction between CRM1 and 14-3-3. Furthermore, anti-phosphor Thr/Ser residue antibody did not recognize EGFP-CRM1 S1055A, suggesting that 1055 serine residue is one of major phosphorylation sites in CRM1 (A).
Figure 3. Phosphorylation of Ser 1055 in CRM1 by PKA was required for 14-3-3 binding. Plasmids with EGFP-CRM1 (WT) and EGFP-CRM1 S1055A were transfected into HEK 293 cells and CRM1 protein was immunopurified with GFP antibody. (A) Western blot with CRM1, 14-3-3, or phospho-Thr/Ser antibodies. CRM1 S1055A (lacking the PKA phosphorylation site) did not form a 14-3-3 protein complex or react with anti-phospho-Thr/Ser. (B) PKA assay with GST-CRM1 WT C-terminal fragment (amino acids 960–1120) or GST-CRM1 S1055A C-terminal fragment. GST-CRM1 WT, S1055A were expressed in E. coli and prebound to agarose beads that were incubated with PKA in assay buffer and analyzed using anti-phospho-Ser/Thr. Figures represent three independent experiments.
In order to gain a better understanding of the phosphorylation of 1055 serine residue, PKA was performed in vitro with C-terminal of CRM1 WT and S1055A fusion protein (960–1120 aa), which was purified from E. coli. Similar to the results of A, CRM1 S1055A fusion protein was not phosphorylated by the active PKA, while CRM1 WT was well phosphorylated by it (B). These results suggested that PKA phosphorylated Ser 1055 of CRM1.
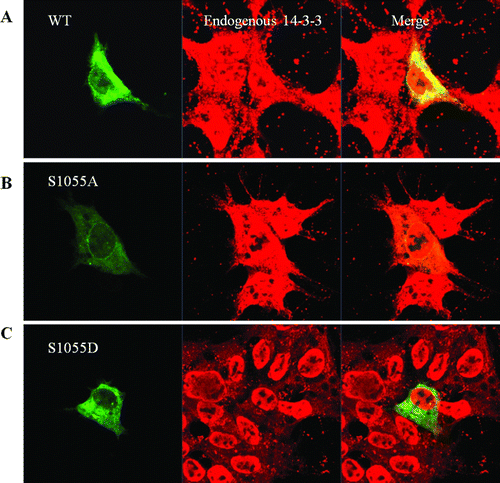
Figure 4. Sublocalization of CRM1 WT, S1055A, and S1055D with confocal microscopy. HEK 293 cells were transfected with plasmids for EGFP-CRM1, S1055A, or S1055D (green). Endogenous 14-3-3 proteins (red) were visualized using appropriate primary antibodies, and Alexa Fluor 568-conjugated secondary antibodies. Merged image (yellow) shows coincident distribution of wild type CRM1 and 14-3-3 proteins (A). No merged image (yellow) was seen with EGFP-CRM1 S1055A (B) or S1055D and 14-3-3 proteins (C). Figures represent three or more independent experiments.
Colocalization of CRM1 WT with 14-3-3 proteins by confocal microscopy
To verify phosphorylation of CRM1, EGFP-CRM1 WT and EGFP-CRM1 S1055A were transfected. In HEK 293 cell, the transfected EGFP-CRM1 or -CRM1 S1055A or -CRM1 S1055D (green) was shown directly. Endogenous 14-3-3 proteins (red) were visualized using their appropriate primary antibodies, and Alexa Flour 568-conjugated secondary antibodies. Merged images (yellow) show coincident distribution of WT CRM1 and 14-3-3 only (A). No coincident distribution with 14-3-3 proteins was seen on merged images with EGFP-CRM1 S1055A (B) or S1055D (C).
Thus, similar to the results in D, EGFP-CRM1 colocalized with endogenous 14-3-3 proteins in the cytoplasm. These results also supported the requirement for Ser 1055 for colocalization of CRM1 with 14-3-3 proteins.
Regulation of CRM1 subcellular localization depends on phosphorylation of Ser 1055
To define the role of CRM1 Ser 1055 phosphorylation, we compared the subcellular localization of EGFP-CRM1 WT, S1055A, or S1055D from plasmids transfected into HEK 293 cells. The phosphorylated CRM1 analog S1055D was not localized at the nuclear membrane but in the cytoplasm, whereas the unphosphorylatable CRM1 S1055A was predominantly localized in the nuclear membrane (A). Confocal microscopy scanning determined relative protein amounts. Scanning results are shown with micrographs. Although EGFP-CRM1 WT was in the cytoplasm, nucleus, and nuclear membrane, nuclear membrane localization was clear (A). These results suggested that phosphorylation of CRM1 serine 1055 was crucial for CRM1 subcellular localization.
Figure 5. Subcellular localization of CRM1 phosphorylated by PKA. (A) HEK 293 cells were transfected with plasmids for EGFP-CRM1 WT, S1055A, or S1055D (green). CRM1 S1055D was detected in the cytoplasm as a large dot (right) and not on the nuclear rim. Figures represent three independent experiments. Confocal microscopic pictures were scanned using profile in the ZEN program. (B) Subcellular localization in HEK 293 cells of EGFP-CRM1 WT was examined after treatment with forskolin or H89 for 24 h. EGFP-CRM1 WT (green) was observed by fluorescence microscopy.
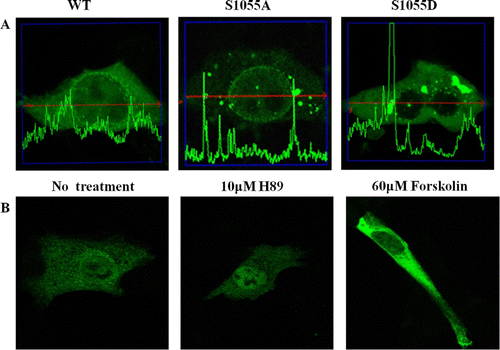
To further investigate the effect of CRM1 Ser 1055 phosphorylation, we determined the subcellular localization of EGFP-CRM1 WT in HEK 293 cells after 24 h of treatment with a PKA activator (60 µM forskolin) or inhibitor (10 µM H89) (Chijiwa et al. Citation1990; Geilen et al. Citation1992). After transfection with plasmids expressing EGFP-CRM1 WT, the subcellular localization was determined after treatment with forskolin or H89. Similar to the subcellular localization of CRM1 S1055D in A, forskolin treatment enhanced the cytoplasmic localization. Treatment with the PKA inhibitor H89 promoted nuclear localization (B). These results suggested that phosphorylation of Ser 1055 of CRM1 regulated subcellular localization. Furthermore, similar to the results in , Ser 1055 phosphorylation appeared to be by PKA, because a specific PKA activator or inhibitor modulated the CRM1 subcellular localization (similar to A).
Ser 1055 phosphorylation promoted cell apoptosis
To investigate the effect of the CRM1 phosphorylation on cell survival, we measured the apoptosis rate of cells with CRM1 mutant constructs (). HEK 293 cells were split and transfected at day 3 with control vector or CRM1 WT, S1055A, or S1055D constructs, and harvested as indicated for FACS. The apoptosis rate of cells with the S1055D construct was twice that of cells with WT or S1055A constructs. Thus, the disruption of nuclear pore integrity by overexpression of CRM1 S1055D () might negatively affect cell survival. Therefore, 1055 Ser phosphorylation by PKA (or another protein kinase) appeared to enhance cell apoptosis ().
Table 1. Serine 1055 phosphorylation of CRM1 promoted cell apoptosis.
Interaction between CRM1 and 14-3-3 proteins through Ser1055 phosphorylation
The 14-3-3 proteins formed a complex with CRM1 in the cytoplasm that depended on PKA phosphorylation. The C-terminal domain of CRM1 is involved in the targeting of the export complex to the nuclear pore complex (NPC), facilitating CRM1-dependent translocation of NES-containing proteins through the NPC. CRM1 phosphorylated by PKA bound to 14-3-3 proteins as new partner proteins through the CRM1 C-terminal domain that contained a conserved 14-3-3 binding motif (1049KHKRQMSVPG1058). Binding with 14-3-3 proteins resulted in the cytoplasm localization of CRM1. Unphosphorylated CRM1 was localized in the nucleus, crossing the nuclear pore. Phosphorylation of CRM1 on serine 1055 by PKA induced binding of 14-3-3 proteins to the 14-3-3 binding motif, and inhibited CRM1 shuttle function by releasing CRM1 from the nuclear pore. Thus, interaction with 14-3-3 proteins depended on PKA phosphorylation, which regulated CRM1 localization and function in vivo and in vitro.
Discussion
CRM1 appears to form a specific complex with the NES of proteins that is necessary for NES-mediated nuclear protein export (Fornerod et al. 1997a, 1997b; Fukuda et al. Citation1997; Yoneda et al. Citation1999). By analogy with nuclear import, Ran or a Ran-binding protein might regulate the interaction of complexes of CRM1 and NES-containing proteins with the nuclear pore complex before translocation out of the nucleus (Askjaer et al. Citation1999; Kehlenbach et al. Citation1999; Yoneda et al. Citation1999). We showed that CRM1 activity is cell cycle-regulated, and were interested in determining whether CRM1-14-3-3 interaction is critical for entry, progression, or exit from mitosis.
The 14-3-3 binding motif (1049KHKRQMSVPG1058) in CRM1 seems to be overlapped with PKA phosphorylation site (1055 serine residue), which is not perfectly matched with the best PKA substrate consensus sequence (R-R-X-S/T) (Pidoux & Tasken Citation2010; Li Citation2011). However, we demonstrated here that CRM1 forms a protein complex with 14-3-3, and the PKA phosphorylation on the 1055 serine residue contributes both the protein complex formation and CRM1 subcellular localization (Figures ).
In this study, we found that the CRM1-14-3-3 interaction induced by PKA caused the cell apoptosis (). As the expression of PKA induced mitotic arrest and apoptosis, we wished to determine whether there was the phosphorylated CRM1 protein analog (S1055D) effect in cells. Apoptotic cell populations were quantitated in parallel by annexin V-fluorescein isothiocyanate staining and by propidium iodide staining (). The data clearly show that the phosphorylated CRM1 from the cells induces apoptosis. Thus it seems to be that the PKA catalytic subunit promotes cell death through the phosphorylation on 1055 Ser residue of CRM1. Alternatively, because the activity of PKA is dependent on the cell cycle, PKA phosphorylates on Ser 1055 residue of CRM1 and prompt the binding with 14-3-3, and the disassembly of nuclear pore or membrane. And then, during the M phase, the unphosphorylated CRM1 which is disassociated with 14-3-3, again reforms the nuclear pore or membrane (). The functional role of the phosphorylation on Ser 1055 residue of CRM1 remains to be characterized.
Figure 6. Schematic diagram of interaction between CRM1 and 14-3-3 proteins, with PKA. The nuclear pore export complex facilitates CRM1-dependent translocation of NES-containing proteins. Phosphorylation of CRM1 by PKA results in binding to 14-3-3 proteins as a new partner protein through the CRM1 C-terminal domain, which contains a conserved 14-3-3-binding motif (1049KHKRQMSVPG1058). Interaction regulates CRM1 nuclear pore localization and NES function in vivo and in vitro. Phosphorylated CRM1 seems to be dephosphorylated by a phosphoprotein phosphatase (PPA).
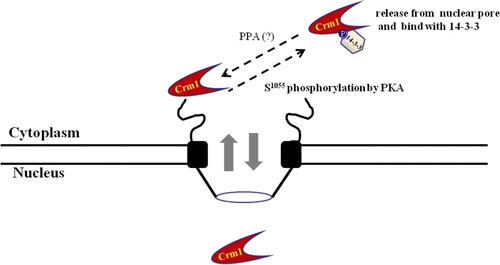
Although our data suggests that the interaction of CRM1 and 14-3-3 controls its subcellular localization, our findings also raise several questions regarding the interaction of CRM1 and 14-3-3. It remains for researchers to characterize the fashion and mechanisms by which the interactions between CRM1 and 14-3-3 are controlled, depending on physiological conditions. In addition, it remains to be determined whether CRM1 mutation (1049KHKRQMSVPG1058) itself affects the shuttle activity, regardless of protein-protein interactions with 14-3-3. It is also necessary to ascertain whether the phosphorylation of 14-3-3 is required for the activation and/or regulation of CRM1, or for the interaction between CRM1 and 14-3-3, because the bacterial expressed CRM1 C-terminal fragment, which is an unphosphorylated CRM1 form, also slightly pull-down 14-3-3 (A). Therefore, it seems that this difference reflects the affinity between the CRM1 and 14-3-3, and the cytoplasm localization of CRM1 and 14-3-3. Thus, it seems that 14-3-3 preferentially associates with the inactive conformations of the signaling molecules. Nevertheless, the high affinity of 14-3-3 for the inactive conformation of CRM1 interacting proteins is not reflected in CRM1, since the constitutively active CRM1 mutant binds readily to 14-3-3 (data not shown). Importantly, the phosphorylation and activity of CRM1 were neither necessary nor required for its functional interaction with 14-3-3. The 1055 Ser residue in motifs (1049KHKRQMSVPG1058) of CRM1 seems be one of the regulation points for the cell cycle. During the M period in cell mitotic division, the disassembly of nuclear membrane is triggered by the phosphorylation on 1055 Ser residue of CRM1.
The leucine-rich NES recognized by CRM1 was also identified in 14-3-3 proteins (Aitken et al. Citation1992; Yaffe et al. Citation1997; Obsilova et al. Citation2008). However, we do not know how the 14-3-3 NES motif contributes to binding with CRM1. Although association of 14-3-3 proteins with CRM1 contributes to CRM1 function by mediating its subcellular localization, whether differences in CRM1 subcellular localization are due to differences in cell lines or differences in CRM1 function remains to be seen. The subcellular localization of CRM1 was unaffected by treatment with growth factors and chemokines (28–30).
Molecular details of the CRM1 interaction with RanGTP have been solved (Hutten & Kehlenbach Citation2007; Fox et al. Citation2011). The model suggests that the HEAT helices 11A and 12A form a cargo-binding hydrophobic cleft (Hutten & Kehlenbach Citation2007; Fox et al. Citation2011). RanGTP contact areas are on the CRM1 C-terminus. Further, CRM1 is hypothesized to switch between a relaxed cytoplasmic and a strained nuclear conformation, depending on RanGTP binding (). In the hypothetical cytoplasmic conformation, the contact sites for RanGTP inside the CRM1 toroid are too far apart to bind Ran with high affinity (Hutten & Kehlenbach Citation2007; Fox et al. Citation2011). Also, the hydrophobic cleft on the outer side of the toroid is closed. Rigid body movements allow transition to the nuclear conformation. Accordingly, in the model, the conformational change also alters the curvature of the toroid near the cargo-binding site, opens the hydrophobic cleft, and allows the export cargo to dock. Thus, during CRM1 shuttling between the cytoplasm and nuclear across the pore, phosphorylation on Ser 1055 in the CRM1 C-terminal domain by PKA contributes to the conformational change to alter the curvature of the toroid near the cargo-binding site, closing the hydrophobic cleft to release the export cargo. Phosphorylation on Ser 1055 by PKA seems to inhibit or block RanGTP inside the CRM1 toroid (). We are determining whether the binding affinity of RanGTP to CRM1 is changed by phosphorylation.
During the consensus motif database search, we also noticed that RSK (90 kDa ribosomal S6 kinase) might also phosphorylate CRM1 Ser 1055 (Roux et al. Citation2003; Romeo et al. Citation2012). RSK is characterized as a hub kinase that regulates diverse cellular processes including cell growth, proliferation, survival and motility (Roux et al. Citation2003; Romeo et al. Citation2012). Phosphorylation by RSK might induce CRM1 to bind 14-3-3 proteins, similar to PKA ().
Phosphorylation on serine 1055 of CRM1 appeared to contribute to cell apoptosis (). We are pursuing whether RSK also phosphorylates CRM1. Regardless of the kinases that phosphorylate Ser 1055, the residue appears to be an important regulation site for CRM1 function.
In conclusion, this study identified 14-3-3 proteins as new binding partners for CRM1 through the motif 1049KHKRQMSVPG1058 in the CRM1 C-terminal domain. We demonstrated that CRM1 is a PKA substrate. Although the functional significance of this interaction remains poorly understood, the regulation of CRM1 nuclear pore localization by 14-3-3 proteins could be a relevant consequence of different signaling pathways involving CRM1. Results on both CRM1 localization and PKA activity revealed that these are changed by 14-3-3 protein binding. The 14-3-3 proteins appeared to be antagonistic to the CRM1 nuclear pore localization. Our results suggested the interaction of CRM1 with 14-3-3 proteins and 14-3-3 proteins function as negative regulators of CRM1 signaling. However, the precise control mechanisms underlying the subcellular localization of CRM1 by 14-3-3 proteins requires further characterization to determine the overall function of 14-3-3 proteins in CRM1 signal transduction pathways.
Future studies are needed to probe the molecular mechanisms modulating the association of CRM1 with 14-3-3 proteins, the dephosphorylation of CRM1, and the effect of PKA activity on CRM1 in and out of the nuclear pore.
Acknowledgments
This work was supported by Chungbuk National University Research Grant (2011) to S S Kang. We also appreciated The Core Facility of Chungbuk National University for their excellent skills.
References
- Aitken A , Collinge DB , van Heusden BP , Isobe T , Roseboom PH , Rosenfeld G , Soll J. 1992 . 14-3-3 proteins: a highly conserved, widespread family of eukaryotic proteins . Trends Biochem Sci . 17 12 : 498 – 501 . doi: 10.1016/0968-0004(92)90339-B
- Askjaer P , Bachi A , Wilm M , Bischoff FR , Weeks DL , Ogniewski V , Ohno M , Niehrs C , Kjems J , Mattaj IW , Fornerod M. 1999 . RanGTP-regulated interactions of CRM1 with nucleoporins and a shuttling DEAD-box helicase . Mol Cell Biol . 19 : 6276 – 6285 .
- Chan TO , Pascal JM , Armen RS , Rodeck U. 2012 . Autoregulation of kinase dephosphorylation by ATP binding in AGC protein kinases . Cell Cycle . 11 3 : 475 – 478 . doi: 10.4161/cc.11.3.19059
- Chijiwa T , Mishima A , Hagiwara M , Sano M , Hayashi K , Inoue T , Naito K , Toshioka T , Hidaka H. 1990 . Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells . J Biol Chem . 265 : 5267 – 5272 .
- Chun J , Kwon T , Lee EJ , Kim CH , Han YS , Hong SK , Hyun S , Kang SS. 2004 . 14-3-3 Protein mediates phosphorylation of microtubule-associated protein tau by serum- and glucocorticoid-induced protein kinase 1 . Mol Cells . 18 : 360 – 368 .
- Fasken MB , Saunders R , Rosenberg M , Brighty DW. 2000 . A leptomycin B-sensitive homologue of human CRM1 promotes nuclear export of nuclear export sequence-containing proteins in drosophila cells . J Biol Chem . 275 3 : 1878 – 1886 . doi: 10.1074/jbc.275.3.1878
- Fornerod M , Ohno M , Yoshida M , Mattaj IW. 1997a . CRM1 is an export receptor for leucine-rich nuclear export signals . Cell . 90 6 : 1051 – 1060 . doi: 10.1016/S0092-8674(00)80371-2
- Fornerod M , van Deursen J , van Baal S , Reynolds A , Davis D , Murti KG , Fransen J , Grosveld G. 1997b . The human homologue of yeast CRM1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component Nup88 . EMBO J . 16 4 : 807 – 816 . doi: 10.1093/emboj/16.4.807
- Fox AM , Ciziene D , McLaughlin SH , Stewart M. 2011 . Electrostatic interactions involving the extreme C terminus of nuclear export factor CRM1 modulate its affinity for cargo . J Biol Chem . 286 33 : 29325 – 29335 . doi: 10.1074/jbc.M111.245092
- Fukuda M , Asano S , Nakamura T , Adachi M , Yoshida M , Yanagida M , Nishida E. 1997 . CRM1 is responsible for intracellular transport mediated by the nuclear export signal . Nature . 390 6657 : 308 – 311 . doi: 10.1038/36894
- Geilen CC , Wieprecht M , Wieder T , Reutter W. 1992 . A selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-bromocinnamyl(amino)ethyl]-5-isoquinolinesulfonamide (H-89), inhibits phosphatidylcholine biosynthesis in HeLa cells . FEBS Lett . 309 3 : 381 – 384 . doi: 10.1016/0014-5793(92)80811-T
- Hamamoto T , Uozumi T , Beppu T. 1985 . Leptomycins A and B, new antifungal antibiotics. III. Mode of action of leptomycin B on Schizosaccharomyces pombe . J Antibiot . 38 11 : 1573 – 1580 . doi: 10.7164/antibiotics.38.1573
- Hutten S , Kehlenbach RH. 2007 . CRM1-mediated nuclear export: to the pore and beyond . Trends Cell Biol . 17 4 : 193 – 201 . doi: 10.1016/j.tcb.2007.02.003
- Jensen TH , Neville M , Rain JC , McCarthy T , Legrain P , Rosbash M. 2000 . Identification of novel Saccharomyces cerevisiae proteins with nuclear export activity: cell cycle-regulated transcription factor ace2p shows cell cycle-independent nucleocytoplasmic shuttling . Mol Cell Biol . 20 21 : 8047 – 8058 . doi: 10.1128/MCB.20.21.8047-8058.2000
- Kehlenbach RH , Dickmanns A , Kehlenbach A , Guan T , Gerace L. 1999 . A role for RanBP1 in the release of CRM1 from the nuclear pore complex in a terminal step of nuclear export . J Cell Biol . 145 4 : 645 – 657 . doi: 10.1083/jcb.145.4.645
- Kehlenbach RH , Gerace L. 2000 . Phosphorylation of the nuclear transport machinery down-regulates nuclear protein import in vitro . J Biol Chem . 275 23 : 17848 – 17856 . doi: 10.1074/jbc.M001455200
- Kleppe R , Krakstad C , Selheim F , Kopperud R , Doskeland SO. 2011 . The cAMP-dependent protein kinase pathway as therapeutic target: possibilities and pitfalls . Curr Top Med Chem . 11 : 1393 – 1405 . doi: 10.2174/156802611795589629
- Kudo N , Matsumori N , Taoka H , Fujiwara D , Schreiner EP , Wolff B , Yoshida M , Horinouchi S. 1999 . Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region . Proc Natl Acad Sci USA . 96 16 : 9112 – 9117 . doi: 10.1073/pnas.96.16.9112
- Kuehn GD. 1972 . Cell cycle variation in cyclic adenosine 3′,5′-monophosphate-dependent inhibition of protein kinase from Physarum polycephalum . Biochem Biophys Res Commun . 49 2 : 414 – 419 . doi: 10.1016/0006-291X(72)90426-3
- la Cour T , Gupta R , Rapacki K , Skriver K , Poulsen FM , Brunak S. 2003 . NESbase version 1.0: a database of nuclear export signals . Nucleic Acids Res . 31 1 : 393 – 396 . doi: 10.1093/nar/gkg101
- Li X. 2011 . Phosphorylation, protein kinases and ADPKD . Biochim Biophys Acta . 1812 10 : 1219 – 1224 . doi: 10.1016/j.bbadis.2011.03.001
- Luconi M , Cantini G , Baldi E , Forti G. 2011 . Role of a-kinase anchoring proteins (AKAPs) in reproduction . Front Biosci . 16 1 : 1315 – 1330 . doi: 10.2741/3791
- Makman MH , Klein MI. 1972 . Expression of adenylate cyclase, catecholamine receptor, and cyclic adenosine monophosphate-dependent protein kinase in synchronized culture of Chang's liver cells (S phase-membrane receptors-NaF stimulation) . Proc Natl Acad Sci USA . 69 2 : 456 – 458 . doi: 10.1073/pnas.69.2.456
- Muslin AJ , Xing H. 2000 . 14-3-3 proteins: regulation of subcellular localization by molecular interference . Cell Signal . 12 11–12 : 703 – 709 . doi: 10.1016/S0898-6568(00)00131-5
- Neumann G , Hughes MT , Kawaoka Y. 2000 . Influenza A virus NS2 protein mediates vRNP nuclear export through NES-independent interaction with hCRM1 . EMBO J . 19 24 : 6751 – 6758 . doi: 10.1093/emboj/19.24.6751
- Neville M , Stutz F , Lee L , Davis LI , Rosbash M. 1997 . The importin-beta family member Crm1p bridges the interaction between Rev and the nuclear pore complex during nuclear export . Curr Biol . 7 10 : 767 – 775 . doi: 10.1016/S0960-9822(06)00335-6
- Nishi K , Yoshida M , Fujiwara D , Nishikawa M , Horinouchi S , Beppu T. 1994 . Leptomycin B targets a regulatory cascade of crm1, a fission yeast nuclear protein, involved in control of higher order chromosome structure and gene expression . J Biol Chem . 269 : 6320 – 6324 .
- Obsilova V , Silhan J , Boura E , Teisinger J , Obsil T. 2008 . 14-3-3 proteins: a family of versatile molecular regulators . Physiol Res . 57 : 11 – 21 .
- Pidoux G , Tasken K. 2010 . Specificity and spatial dynamics of protein kinase A signaling organized by A-kinase-anchoring proteins . J Mol Endocrinol . 44 5 : 271 – 284 . doi: 10.1677/JME-10-0010
- Pidoux G , Witczak O , Jarnaess E , Myrvold L , Urlaub H , Stokka AJ , Kuntziger T , Tasken K. 2011 . Optic atrophy 1 is an A-kinase anchoring protein on lipid droplets that mediates adrenergic control of lipolysis . EMBO J . 30 21 : 4371 – 4386 . doi: 10.1038/emboj.2011.365
- Romeo Y , Zhang X , Roux PP. 2012 . Regulation and function of the RSK family of protein kinases . Biochem J . 441 2 : 553 – 569 . doi: 10.1042/BJ20110289
- Roux PP , Richards SA , Blenis J. 2003 . Phosphorylation of p90 ribosomal S6 kinase (RSK) regulates extracellular signal-regulated kinase docking and RSK activity . Mol Cell Biol . 23 14 : 4796 – 4804 .
- Schillace RV , Carr DW. 2006 . The role of protein kinase A and A-kinase anchoring proteins in modulating T-cell activation: progress and future directions . Crit Rev Immunol . 26 2 : 113 – 132 . doi: 10.1615/CritRevImmunol.v26.i2.20
- Shibuya EK. 2003 . G2 cell cycle arrest – a direct link between PKA and Cdc25C . Cell Cycle . 2 1 : 39 – 41 . doi: 10.4161/cc.2.1.291
- Shimanuki M , Saka Y , Yanagida M , Toda T. 1995 . A novel essential fission yeast gene pad1 +positively regulates pap1(+)-dependent transcription and is implicated in the maintenance of chromosome structure . J Cell Sci . 108 : 569 – 579 .
- Shin SH , Lee EJ , Chun J , Hyun S , Kim YI , Kang SS. 2012 . The nuclear localization of glycogen synthase kinase 3beta is required its putative PY-nuclear localization sequences . Mol Cells . 34 4 : 375 – 382 . doi: 10.1007/s10059-012-0167-2
- Toda T , Shimanuki M , Saka Y , Yamano H , Adachi Y , Shirakawa M , Kyogoku Y , Yanagida M. 1992 . Fission yeast pap1-dependent transcription is negatively regulated by an essential nuclear protein, crm1 . Mol Cell Biol . 12 : 5474 – 5484 .
- Turi TG , Webster P , Rose JK. 1994 . Brefeldin A sensitivity and resistance in Schizosaccharomyces pombe. Isolation of multiple genes conferring resistance . J Biol Chem . 269 : 24229 – 24236 .
- Tzivion G , Shen YH , Zhu J. 2001 . 14-3-3 proteins; bringing new definitions to scaffolding . Oncogene . 20 44 : 6331 – 6338 . doi: 10.1038/sj.onc.1204777
- Yaffe MB , Rittinger K , Volinia S , Caron PR , Aitken A , Leffers H , Gamblin SJ , Smerdon SJ , Cantley LC. 1997 . The structural basis for 14-3-3:phosphopeptide binding specificity . Cell . 91 7 : 961 – 971 .
- Yoneda Y , Hieda M , Nagoshi E , Miyamoto Y. 1999 . Nucleocytoplasmic protein transport and recycling of Ran . Cell Struct Funct . 24 6 : 425 – 433 .