Abstract
Dioscorea nipponica, a perennial herb growing in mountainous areas, has been used as a folk medicine for asthma, rheumatoid arthritis, bronchitis, and other disease in Korea, whereas the effect of this plant on cancer cells has not been clearly clarified. Cellular cytotoxicity was examined by cell viability assay and DNA fragmentation by DNA ladder assay. Activation of different protein expression was detected by western blot analyses. We first demonstrated that D. nipponica extract (DNE) reducing the SH-SY5Y cell viability with IC50 of 27.57 µg/ml at 24 h. However, DNE downregulated the expression of antiapoptotic protein includes B-cell lymphoma-2 (Bcl-2), B-cell lymphoma-extra large (Bcl-xL), and myeloid cell leukemia 1 (Mcl-1) which also activated cleaved caspase-9 and caspase-3 in a dose- and time-dependent manner. Consequently, DNE-induced cytotoxicity was not mediated by the Fas/FasL, phosphatidylinositide-3 kinase/AKT/glycogen synthase kinase-3β (PI3K/AKT/GSK-3β), and mitogen activated protein (MAP) kinases signaling pathway. In addition, DNE-induced cells shown damage morphology which had become cell rounding, neurite retraction, membrane blebbing, is right spell blebbing, and shrunken in a dose- and time-dependent manner that clearly indicate this morphological change might be due to the process of apoptosis which shown fragmented DNA. These results indicate that apoptotic effects of DNE on SH-SY5Y cells are mediated by intrinsic mitochondrial caspases signaling pathway, suggesting that DNE might be effective as an anticancer agent for neuroblastoma malignancies.
Introduction
Neuroblastoma, one of the few human malignancies known to demonstrate spontaneous regression from an undifferentiated state to a completely benign cellular appearance originating from the sympathetic nervous system, is the most frequently occurring extra-cranial malignancy in childhood (Maris et al. Citation2007; Bénard et al. Citation2008). However, despite many advances in diagnosis and standard interventions in the past three decades, neuroblastoma has remained a formidable challenge to clinical and basic scientists (Castel et al. Citation2007). Despite multimodal therapeutic approaches including chemotherapy, radio therapy, and immunotherapy, the survival rate for patients with malignant neuroblastoma remains poor. Newer therapeutic strategies are urgently warranted for successful treatment of this devastating childhood malignancy. Therefore, the need exists to develop novel agents to improve treatment outcomes in this high-risk group. In particular, we would search for novel therapeutic strategies for neuroblastoma; natural product has attracted interest to achieve a new approach for chemotherapy.
In recent years, naturally occurring plant products have gained increasing attention for potential use in intervention against malignant invasive progression in late-stage neoplastic diseases (Shankar et al. Citation2008; Ravindranath et al. Citation2009). Previous research has demonstrated that certain foods, including many vegetables, fruits, and grains, as well as phytochemicals of diversified pharmacological efficacies, offer significant protection against various cancers (Chen et al. Citation2005; Vieira et al. Citation2007; Huang et al. Citation2008). There is increasing focus on providing a scientific basis for the use of these agents as a preventive strategy for people with high risk of cancers. Dioscorea plants are important agricultural crops in tropical regions, grown for their large tubers in parts of Africa, Asia, and Oceania (Oke Citation1972). The rhizome of Dioscorea nipponica Makino has high saponin content and use in folk medicine as treatment for coronary heart disease, asthma, rheumatoid arthritis, and hyperlipidemia, as well as a source of hormonal sterol synthesis precursors (Yoshikawa et al. Citation2007). Recently, studies revealed that Dioscorea plant extracts exert antiobesity effects (Kwon et al. Citation2003; Xiao et al. Citation2010). However, limited studies exist concerning the anticancer effects of D. nipponica.
The precise molecular mechanism(s) of D. nipponica extract (DNE)-induced apoptosis in human neuroblastoma cells and its anticancer effect has not yet been elucidated. A new dimension in the management of neoplasia was the increasing awareness that chemoprevention, which refers to the administration of chemical agents to prevent the events associated with carcinogenesis (Hong & Sporn Citation1997). A large number of chemopreventive and chemotherapeutic agents have been discovered from natural products and these provide a promising strategy to fight against cancer by inducing apoptosis in malignant cells (Kelloff et al. Citation2000; Sporn & Suh Citation2000). Traditionally, many species of Dioscorea have been used as folk medicine for syndromes related to metabolic disorders. The extracts of Dioscorea species have been reported to have a hypoglycemic effect (Hikino et al. Citation1986), immunostimulatory effect (Zhao et al. Citation2005), antiinflammatory effects (Kim et al. Citation2004), antitumor activity (Hu & Yao Citation2003), and antiosteoporotic activity (Yin et al. Citation2004). D. nipponica Makino has been used to cure coronary heart disease in China and its methanol extract has shown a potential antiobesity effect (Lin et al. Citation2007). Rhizome of Dioscorea species has been used for treatment of arthritis, muscular pain, and urinary disease in oriental medicine. Therefore, the present study aimed to investigate the effects of D. nipponica root extracts on cellular cytotoxicity and apoptosis in cultured SH-SY5Y and to study the possible underlying mechanisms. However, our study emphasizes that DNE inhibited the viability of neuroblastoma cells by dysfunction of mitochondrial pathway in apoptotic cell death via the downregulation of B-cell lymphoma-2 (Bcl-2) family proteins as well as caspases activation.
Materials and methods
Materials
Roots of D. nipponica Makino (Dioscoreaceae) were purchased from DeaGuang, apothecary's shop, in Chuncheon, South Korea. A voucher specimen (HRIC-1034) was deposited at the Regional Innovation Center, Hallym University, Chuncheon, South Korea. Roots of D. nipponica (1000 g) were chopped and blended using a Waring blender and then boiled with 2 L of 80% ethanol at 80°C for 2 h. The insoluble materials were removed through centrifugation at 10,000× g for 30 min, and the resulting supernatant was concentrated and freeze-dried to yield a brown powder (Yield: 23.5%). The chemical was dissolved in dimethyl sulfoxide (DMSO) at a stock solution of 10 mg/ml and then it was diluted with medium to obtain the working concentration. Dulbecco's Modified Eagle's Medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco/BRL (Grand Island, NY, USA). Antibodies against Fas, FasL, Bcl-2, B-cell lymphoma-extra large (Bcl-xL), and myeloid cell leukemia 1 (Mcl-1) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Phospho-extracellular-signal-regulated kinases (ERK1/2), phospho-MAP kinase kinase (MKK3/6), phospho-p38, phospho-activating transcription factor (ATF)-2, phospho-MEK1/2, phospho-ERK1/2, phospho-Elk-1, phospho-stress-activated protein kinase (SAPK), c-JUN, cleaved caspase-3, caspase-9, caspase-8, β-actin, phospho-glycogen synthase kinase-3β (GSK-3α/β), phospho-GSK-3α, phospho-GSK-3β, GSK-3β, p-AKT, AKT, and U0126 were obtained from Cell Signaling Technology (Beverly, MA). DEVD-FMK (Asp-Glu-Val-Asp-O-Methyl-fluormethylketon) was obtained from BD Biosciences. PD98059, SP600125, and SB203580 were purchased from Tocris (Bristol, UK). All other reagents were of analytical grade or of the highest purity available.
Cell culture
Human SH-SY5Y neuroblastoma cells were grown at 37°C under a humidified atmosphere of 5% CO2. The cells were cultured in DMEM plus 10%FBS, 50 U/ml penicillin, and 50 µg/ml streptomycin. Cells were starved with DMEM containing 0.5% FBS. After 24 h starvation, the cells were treated with various concentrations of DNE as described in the figure legends.
Cell viability assay
Cell viability was determined using a cytotoxicity assay kit, the CCK-8 (Dojindo Lab, Japan) according to the manufacturer's protocol. The cells were plated into 96-wells to a density of 50–60% confluence. After 24-h incubation in starvation media, the cells were treated with various concentrations of DNE. After treatment of 24 h, the CCK-8 (10 µl) was added to each wells of the plates and incubated the plate for 3 h. A 96-well microtitre plate reader (molecular devices) was used to determine the absorbance at 450 nm for the CCK-8. The mean concentrations in each set of three wells were measured.
Cell morphology
The cells were plated into 24-wells plates at 37°C under a humidified atmosphere of 5% CO2. After 24 h of starvation, the cells were treated with various concentrations of DNE. The culture plates were examined at the Korea Basic Science Institute Chuncheon Center for microscopy technical assistance and photographed for the cell morphology experiment.
Detection of DNA fragmentation
For the detection of apoptotic DNA cleavage, the DNA fragmentation assay was performed using ladder DNA fragmentation assay. In brief, cells were collected after treatment at a various concentrations of DNE as described in the figure legends and washed in phosphate buffered saline (PBS). The cells were then lysed with 500 µl of genomic DNA extraction buffer (0.1 M NaCl, 10 mM ethylenediaminetetraacetic acid (EDTA), 0.3 M Tris–HCl, 0.2 M sucrose, and pH 8.0). The lysate was incubated with 20 µl of 10% sodium dodecyl sulfate (SDS) solution and incubated at 65°C for 30 min. Then 120 µl potassium acetate (pH 5.3) was added and stored on ice for 1 h after that centrifuged for 10 min at 4°C 12,000 rpm. Next 2 µl (10 mg/ml) RNase was added to supernatant, and incubated for 30 min at room temperature. The DNA was extracted by washing the resultant pellet in phenol/chloroform extraction and precipitation by ethanol and then dissolved pellet with distilled water. DNA fragmentation was visualized by electrophoresis in a 0.8% agarose gel containing ethidium bromide.
Western blot analysis
SH-SY5Y cells were starved on 60-mm culture dishes in DMEM with 0.5% FBS for 24 h. Cells were pretreated with various concentration of DNE as indicated in each figure legend and then washed twice with ice-cold PBS. Cells were lysed in lysis buffer (2% SDS, Na3VO4, and protease inhibitor cocktail). After incubation on ice for 10 min sonicated 10 sec in 10% amplitude, the lysates were centrifuged (13,000× g, 20 min). Supernatants were collected and protein concentrations were determined by Bradford assay (Bio-Rad, Richmond, CA, USA). Equal amounts of protein were separated by SDS-PAGE (8–15% reducing gels), transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA), and blocked with 5% nonfat milk. Membranes were incubated in primary antibody overnight at 4°C. Membranes were then washed in Tris-Buffered Saline and Tween 20 (TBST) (10 mM Tris–HCl, 140 mM NaCl, 0.1% Tween-20, pH 7.6), incubated with appropriate secondary antibody, and washed again in TBST. Bands were visualized by enhanced chemiluminescence (ECL) and exposed to X-ray film.
Statistical analysis
Results are expressed as mean ± standard error. Statistical significance was assessed by one-way analysis of variance (ANOVA) followed by Dunnett's test or the paired t-test using Prism 4 (GradPad Software, La Jolla, CA, USA). A p < 0.05 was considered significant.
Results
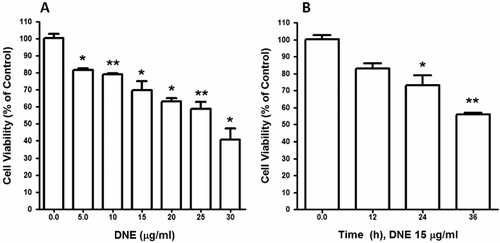
DNE-induced cellular cytotoxicity of human SH-SY5Y neuroblastoma cells in a dose- and time-dependent manner
To characterize the effect of DNE on neuroblastoma cell viability, human neuroblastoma cells were treated with various concentrations of DNE for 24 h and viability was assessed by cell viability assay. We used 0–30 µg of DNE to determine its effect on cell viability. With the increasing DNE concentrations, the percentage of cell viability was decreased and IC50 was 27.57 µg/ml at 24 h. However, DNE dose dependently and significantly decreased cellular viability as compared to control (). Furthermore, a time exposure result was decreased in the viable number of cells. The viability was decreased significantly in response to 15 µg of DNE at 24 h incubation. When the experimental time period was increased to 36 h, a significantly greater amount of cell death was observed at the same concentration compared to that in the control (). Taken together, these data demonstrate that DNE increased cellular cytotoxicity in a dose- and time-dependent manner.
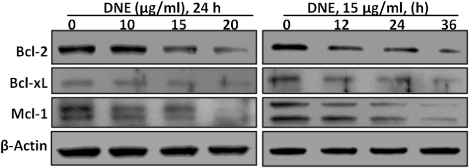
DNE treatment affects the members of Bcl-2 family proteins in human SH-SY5Y neuroblastoma cells
To understand the molecular mechanisms involved in the activation of cell death induced by DNE, we first evaluated whether this natural compound regulates antiapoptotic Bcl-2 family proteins expression in SH-SY5Y cells. Western blotting analysis demonstrated that treatment of SH-SY5Y cells with DNE changed in a dose- (10–20 µg/ml for 24 h) – and time- (15 µg/ml for 12–36 h) – dependent reduction in the levels of the antiapoptotic protein Bcl-2, Bcl-xL, and Mcl-1 compare to the control (). The results shown here demonstrate that DNE-induced cell death occurred via the mitochondrial apoptotic pathway in SH-SY5Y cells which favors an increase in mitochondrial dysfunction.
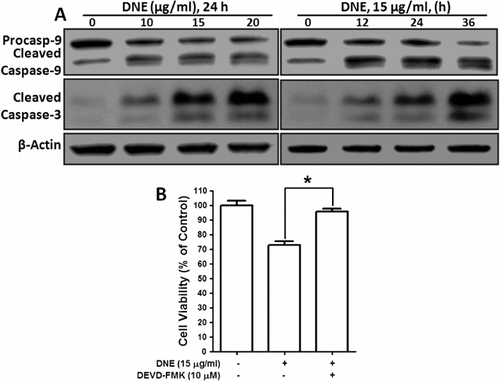
Activation of caspase-3 and caspase-9 is an essential event for DNE-induced apoptosis in human SH-SY5Y neuroblastoma cells
To further determine whether DNE activates the caspase pathway, we incubated SH-SY5Y cells in the absence or presence of DNE and then harvested the cells for western blot analysis. Because caspase-9 appears to be involved in the induction of apoptosis, we measured the levels of procaspase-9, cleaved caspase-9, and caspase-3. Incubation of SH-SY5Y cells with DNE dose- and time-dependent reduced the levels of procaspase-9 and upregulated the levels of the biologically active cleaved caspase-9 and caspase-3 thereby activating the apoptotic cascade pathway (). These results demonstrate that DNE dose- and time-dependently decreased levels of procaspase-9 and increased cleaved caspases-9 and -3, indicating that DNE-induced apoptosis is mediated by activating the intrinsic caspase pathway. To ensure this effect was a direct activation of the effector caspase-3 activation, we used caspase-3 inhibitor in DNE-induced SH-SY5Y cells. Interestingly, we found that DNE-induced cellular cytotoxicity was almost significantly decreased by the caspase-3 inhibitor, DEVD-FMK (Asp-Glu-Val-Asp-O-Methyl-fluormethylketon), (). Therefore, it is clear that DNE selectively induced apoptosis via the activation of caspase-9- and caspase-3-mediated pathway in SH-SY5Y neuroblastoma cells.
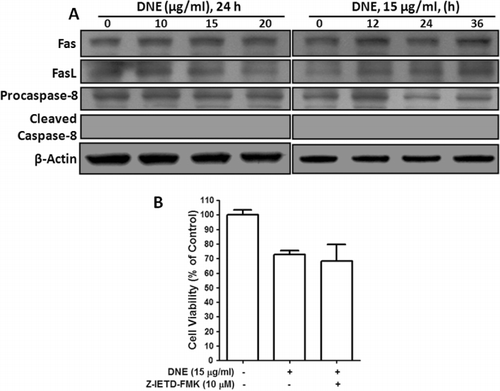
DNE-induced apoptosis is not Fas/FasL receptor-mediated caspase-8 dependent in human SH-SY5Y neuroblastoma cells
To ensure DNE-induced cytotoxicity, we examined the activity of the death receptor-mediated apoptotic pathway, specifically the Fas receptor, Fas ligand, and caspase-8 by western blot and also caspase-8 inhibitor by cell viability. No major changes were observed in the expression of either Fas receptor or Fas ligand and caspase-8 proteins in a dose- and time-dependent treatment of DNE in SH-SY5Y cells (). Consequently, we found that DNE-induced cellular cytotoxicity was not prevented by the caspase-8 inhibitor, Z-IETD-FMK (). This explanation implies that DNE-induced apoptosis does not involve Fas receptor or Fas ligand mediator activation of caspase-8 in SH-SY5Y cells.
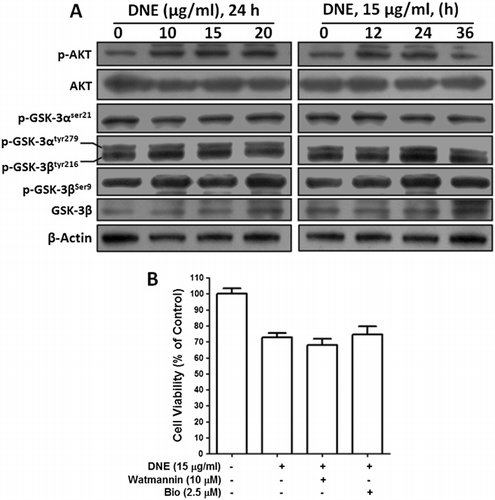
Effects of DNE on PI3K/AKT/GSK-3β signaling pathways in human SH-SY5Y neuroblastoma cells
To characterize the role of PI3K/AKT/GSK-3β-induced apoptosis, we investigated the phosphorylation of AKT, GSK-3αSer21, GSK-3βSer9 and GSK-3αtyr279/βtyr216, and also their inhibitors in SH-SY5Y cells. As shown in , AKT was slightly phosphorylated in dose- and time-dependent experiment; however, DNE had no effect on the level of expression of the total AKT protein. We then investigated the effect of DNE on the proteins downstream (GSK-3) of the AKT signaling pathway. We found that DNE did not remarkably change the phosphorylation of GSK-3αSer21, GSK-3βSer9, and GSK-3αtyr279/βtyr216, as shown in , without having any affect on the expression of the nonphosphorylated GSK-3β protein. Furthermore, we employed inhibitors of GSK-3 (Bio, which inhibit GSK-3α and GSK-3β) and PI3K (Watmannin) by cell viability assay. Indeed, we observed that all inhibitors did not prevent cellular cytotoxicity induced by 15 µg/ml of DNE at 24 h () which suggests that the PI3K/AKT/GSK-3β pathway did not involve and decrease Mcl-1 levels during this apoptosis.
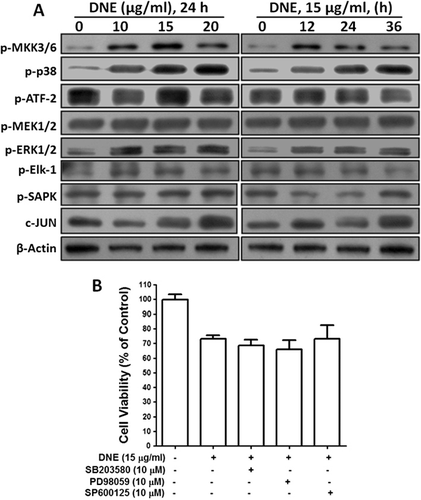
Effects of DNE on MAP kinases signaling pathways in human SH-SY5Y neuroblastoma cells
To examine whether DNE induced the activation of mitogen activated protein (MAP) kinases during the apoptosis, we employed the activation of the major MAP kinases such as phospho-p38, ERK1/2, phospho-SAPK/Junamino-terminal kinase (JNK), and their upstream and downstream protein expressions in SH-SY5Y cells. However, p38 and its upstream MKK3/6 protein were upregulated, but its downstream ATF-2 protein has unchanged in dose- and time-dependent DNE-induced cells (). Subsequently, upstream MAPK or Erk kinases (MEK1/2) and downstream (Elk-1) ERK1/2 kinase proteins had no effect in DNE-induced apoptosis (). Moreover, SAPK kinase and its downstream regulator (c-JUN, member of the Jun family, slightly increased) did not show any remarkable changes during DNE-induced apoptosis of SH-SY5Y cells (). In addition, to further confirm the involvement of MAP kinase in DNE-induced apoptosis, the p38 MAPK inhibitor, SB203580, the ERK1/2 inhibitor, PD98059, and the JNK inhibitor, SP600125 were also used. The result obtained by cellular viability suggests that all MAP kinase inhibitors did not prevent cellular toxicity induced by DNE in SH-SY5Y cells (). Taken together, these findings suggest that MAP kinases signaling pathway did not take part during DNE-induced apoptosis in SH-SY5Y cells.
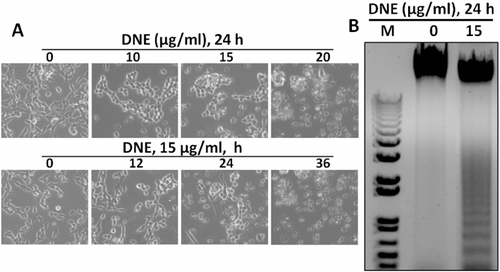
DNE-changed cellular morphology and DNE-induced apoptosis by DNA fragmentation
To observe the morphological effects of SH-SY5Y cell at 24 h incubation of DNE was examined under a Bright-field microscope and photographed. It showed that damage cells which had become round, neurite retraction, membrane blebbing, and shrunken in a dose- and time-dependent exposure of DNE, while the untreated cells were well spread (). To further confirm these morphological effects, we examined internucleosomal DNA fragmentation by DNA gel electrophoresis. No DNA fragment was found in untreated cells but DNA fragments were observed in cells treated with 15 µg/ml of DNE, indicating that the cells underwent apoptosis (). These results clearly indicate that the morphological changes of SH-SY5Y cell by DNE were due to apoptosis which shown fragmented DNA.
Discussion
The present study was designed to define the mechanism(s) of the cellular cytotoxic properties of DNE root extracts because it causes dose- and time-dependent reduction of human SH-SY5Y neuroblastoma cell viability by the process of apoptosis which result in the design of novel approaches for the management of cancer cells. However, the restraining effects and involved mechanisms of DNE on cancer metastasis have not yet to be elucidated. DNE has reported antiobesity and antiinflammation effects in both in vivo and in vitro experimental models (Kwon et al. Citation2003; Xiao et al. Citation2010). To date, no studies describe the anticancer effects of DNE on neuroblastoma cancer cells. The purpose of this study was to investigate whether the ethanol fraction of DNE affects the apoptosis of SH-SY5Y cells through the activation of caspases, which might explain mechanisms underlying the cytotoxicity of cancer cells.
Apoptosis, or programmed cell death, occurs as a physiological phenomenon during normal embryonal development and is involved in cell turnover throughout life. In cancer biology, it is now evident that many cancer cells circumvent normal apoptotic mechanisms to prevent their self-destruction. Biochemically, apoptotic cells are characterized by reduction in mitochondrial transmembrane potential, intracellular acidification, production of reactive oxygen species, externalization of phosphatidylserine residues in membrane bilayers, selective proteolysis of cellular proteins, and degradation of DNA into internucleosomal fragments (Cryns & Yuan Citation1998; Green & Reed Citation1998; Chen et al. Citation2003; Bouchier-Hayes et al. Citation2005). However, caspase is one of the key executioners of apoptosis and is triggered via endoplasmic reticulum stress, extracellular stimuli, or mitochondrial damage (Budihardjo et al. Citation1999; Cecconi & Gruss, Citation2001; Wang Citation2001). In particular, caspase-3 plays a pivotal role in the terminal and execution phases of apoptosis induced by diverse stimuli (Thornberry & Lazebnik Citation1998). Upon activation, initiator caspase-9 triggers the proteolytic activation of executioner caspase-3, caspase-7, and caspase-8 in a process that results in the cleavage of poly(ADP-ribose) polymerase (PARP) and subsequent DNA degradation and apoptotic death (Kuida Citation2000; Cecconi & Gruss Citation2001; Cain et al. Citation2002). In the present study, exposing SH-SY5Y cells to DNE resulted in the proteolytic activation of caspase-3, which is the main executioner of apoptosis. This study examined whether caspase-3, which is a substrate of caspase-9, is cleaved in cells exposed to DNE (). As expected, caspase-3 degraded clearly in a concentration- and time-dependent manner, which correlated with caspase signaling pathway and apoptosis. Under the same experimental conditions, z-DEVD-FMK prevented DNE-induced apoptosis by blocking caspase activation. Therefore, DNE might be used as a potential apoptosis-inducing agent in neuroblastoma cancer cells for the development of anticancer drugs.
A number of studies have shown that the balance between pro-apoptotic and antiapoptotic Bcl-2 protein family members controls the mitochondrial apoptotic pathway (Strasser Citation2005; Adams & Cory Citation2007; Youle & Strasser Citation2008). A potential concern with the permeability of the mitochondrial membrane is precisely regulated by the Bcl-2 family proteins (Cory & Adams Citation2002; Breckenridge & Xue Citation2004). The Bcl-2 family plays an important regulatory role in apoptosis, either as an inhibitor or activator (Adams & Cory Citation2001). In particular, Bcl-2 inhibits members of the caspase family directly, including caspase-3 and caspase-9 (Ekert et al. Citation1999). Thus, the balance of Bcl-2 family members is crucial to the apoptotic trigger. In this study, DNE dose- and time-dependent downregulated the protein level of Bcl-2, Bcl-xL, and Mcl-1 (). Therefore, decreasing Bcl-2 family protein expression might be indicated that DNE-induced apoptosis strongly correlates with the downregulation of mitochondrial apoptotic pathway. The pathways and mechanisms of induction of DNE in SH-SY5Y neuroblastoma cells warrant further research.
Most previous studies have shown that caspase activation is triggered primarily via two distinct interconnected pathways, namely the death receptor and the mitochondria-mediated pathways (Khosravi-Far & Esposti Citation2004). In the death receptor-mediated pathway, the binding of death receptor ligands (e.g., FasL and TRAIL) to their specific death receptors (e.g., Fas, DR4, and DR5) located on the plasma membrane induces the activation of caspase-8 which directly triggers the activation of downstream caspase-3 and/or cleaves Bid, a BH3-only pro-apoptotic Bcl-2 family protein (Jin & El-Deiry Citation2005). Thereafter, we determined that DNE treatment did not induce an increase in the levels of Fas/FasL activation and caspase-8 and found that caspase-8 inhibitor did not prevent cellular viability (). This finding demonstrates that Fas/FasL-mediated caspase-8 did not contribute to the activation of caspase-3 via direct activation of this enzyme in DNE-treated SH-SY5Y cells.
Moreover, inhibition of the interaction of FasL with the Fas receptor leads to a reduction in apoptosis in response to genotoxic stress and growth factor withdrawal (Faris et al. Citation1998; Kasibhatla et al. Citation1998; Le-Niculescu et al. Citation1999; Kolbus et al. Citation2000). Consistent with the idea that FasL is a target in the MAP kinases signaling pathway that induces apoptosis is the presence of AP-1-binding sites in the human FasL promoter region, which presumably contribute to the dependence of Fas/FasL interactions on MAP kinases phosphorylation (Kasibhatla et al. Citation1998; Faris et al. Citation1998; Le-Niculescu et al. Citation1999; Kolbus et al. Citation2000). However, in this study, p38, ERK1/2, and JNK MAP kinases in SH-SY5Y cells were not involved by the induction of FasL and apoptosis (). According to this observation, we presume that DNE-induced apoptosis was not involved in the Fas/FasL interactions in MAPK signaling pathway. In addition, one of the direct downstream targets for AKT is GSK-3β. Phosphorylation of GSK-3β by AKT inhibits its own enzymatic activity as a serine/threonine kinase (Brady et al. Citation1998; van Weeren et al. Citation1998). As a consequence of the activation of PI-3-K signaling, GSK-3β activity is attenuated by activated AKT. When active, GSK-3β can phosphorylate a number of protein targets. One of the primary targets for GSK-3β is β-catenin, in which phosphorylation by GSK-3β leads to its proteasome degradation (Takahashi-Yanaga & Sasaguri Citation2009). Similarly, one of the Bcl-2 family members of antiapoptotic factor Mcl-1 is phosphorylated by GSK-3β and is subsequently degraded (Maurer et al. Citation2006). Activity of AKT blocks the ability of GSK-3β to phosphorylate and degrade Mcl-1. Through this regulation on Mcl-1 by inhibiting GSK-3β, AKT also indirectly affects the balance of Bcl-2 family of proapoptotic and antiapoptotic factors. In this study, we found that DNE did not affect the phosphorylation of AKT, which is closely linked with cell survival. Phosphorylation of the upstream kinase PI3K was also not affected by DNE (). Moreover, inhibition of AKT (Watmannin) and GSK-3 (Bio) signaling did not prevent cellular viability of DNE-induced SH-SY5Y cells (). Thus, it is suggested that cytotoxicity of tumor cell viability and induction of apoptosis by DNE did not take part through the activation of the PI3K/AKT/GSK-3β pathway. Therefore, results from the present study suggest that DNE might possess potential anticancer activity in SH-SY5Y cells via the reduction of antiapoptotic protein as well as induction of caspase pathway which followed DNA fragmentation (), rather than the Fas/FasL, PI3K/AKT/GSK-3β, and MAP kinase signaling pathway. Recently, Ho et al. (Citation2011) demonstrated DNE's antimetastatic effects on melanoma tumor in vivo, and evaluated the toxicity of DNE in vivo. Future in vivo efficacy studies of DNE's effects on neuroblastoma are warranted. Thus, cytotoxicity of SH-SY5Y cells by DNE could provide important preventive and therapeutic benefits against neuroblastoma cancer.
Conclusion
In the present study, we examined apoptosis as the mechanism underlying DNE-induced cellular cytotoxicity in human SH-SY5Y neuroblastoma cell. DNE was dose- and time-dependent activated caspase signaling triggered by the modulation of Bcl-2 family proteins which results in the accumulation of fragmented DNA. However, the effective components of this extracted compound were still unclear and need to be further clarified. Several reports have demonstrated that D. nipponica Makino was rich in steroidal saponins such as diosgenin, dioscin, pseuodoprotodiscin, protodioscin, and methyl protodioscin (Kwon et al. Citation2003; Lin et al. Citation2007; Liu et al. Citation2007; Kang et al. Citation2011). Accordingly, we suggest that steroidal saponins might be the potential components of DNE responsible for its anticancer activity. Therefore, these results warrant further investigation of D. nipponica Makino as a source of pharmacologically active agents, the identification and purification of the bioactive compound(s) in the present ethanol extract and the assessment of their antitumor therapeutic efficacy in vivo in experimental brain tumor models.
Acknowledgments
This research was supported by Hallym University Research Fund (HRF-201209-028) and Basic Science Research Program through the National Research Foundation of Korea (NRF) and funded by the Ministry of Education (NRF-2009-0094071), the Republic of Korea.
References
- Adams JM, Cory S. 2001. Life-or-death decisions by the Bcl-2 protein family. Trends Biochem Sci. 26:61–66. 10.1016/S0968-0004(00)01740-0
- Adams JM, Cory S. 2007. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 19:488–496. 10.1016/j.coi.2007.05.004
- Bénard J, Raguénez G, Kauffmann A, Valent A, Ripoche H, Joulin V, Job B, Danglot G, Cantais S, Robert T, et al. 2008. MYCN-non-amplified metastatic neuroblastoma with good prognosis and spontaneous regression: a molecular portrait of stage 4S. Mol Oncol. 2:261–271. 10.1016/j.molonc.2008.07.002
- Bouchier-Hayes L, Lartigue L, Newmeyer DD. 2005. Mitochondria: pharmacological manipulation of cell death. J Clin Invest. 115:2640–2647. 10.1172/JCI26274
- Brady MJ, Bourbonais FJ, Saltiel AR. 1998. The activation of glycogen synthase by insulin switches from kinase inhibition to phosphatase activation during adipogenesis in 3 T3–L1cells. J Biol Chem. 273:14063–14066. 10.1074/jbc.273.23.14063
- Breckenridge DG, Xue D. 2004. Regulation of mitochondrial membrane permeabilization by BCL-2 family proteins and caspases. Curr Opin Cell Biol. 16:647–652. 10.1016/j.ceb.2004.09.009
- Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. 1999. Biochemical pathways of caspase activation during apoptosis. Annual Rev Cell Dev Biol. 15:269–290. 10.1146/annurev.cellbio.15.1.269
- Cain K, Bratton SB, Cohen GM. 2002. The Apaf-1 apoptosome: a large caspase-activating complex. Biochimie. 84:203–214. 10.1016/S0300-9084(02)01376-7
- Castel V, Grau E, Noguera R, Martinez F. 2007. Molecular biology of neuroblastoma. Clin Transl Oncol. 9:478–483. 10.1007/s12094-007-0091-7
- Cecconi F, Gruss P. 2001. Apaf1 in developmental apoptosis and cancer: how many ways to die? Cell Mol Life Sci. 58:1688–1697. 10.1007/PL00000806
- Chen Q, Crosby M, Almasan A. 2003. Redox regulation of apoptosis before and after cytochrome c release. Korean J Biol Sci. 7:1–9. 10.1080/12265071.2003.9647675
- Chen PN, Hsieh YS, Chiou HL, Chu SC. 2005. Silibinin inhibits cell invasion through inactivation of both PI3K-Akt and MAPK signaling pathways. Chem Biol Interact. 156:141–150. 10.1016/j.cbi.2005.08.005
- Cory S, Adams JM. 2002. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2:647–656. 10.1038/nrc883
- Cryns V, Yuan J. 1998. Proteases to die for. Genes Dev. 12:1551–1570. 10.1101/gad.12.11.1551
- Ekert PG, Silke J, Vaux DL. 1999. Caspase inhibitors. Cell Death Differ. 6:1081–1086. 10.1038/sj.cdd.4400594
- Faris M, Kokot N, Latinis K, Kasibhatla S, Green DR, Koretzky GA, Nel A. 1998. The c-Jun N-terminal kinase cascade plays a role in stress-induced apoptosis in Jurkat cells by up-regulating Fas ligand expression. J Immunol. 160:134–144.
- Green DR, Reed, JC. 1998. Mitochondria and apoptosis. Science. 281:1309–1312. 10.1126/science.281.5381.1309
- Hikino H, Konno C, Takahashi M, Murakami M, Kato Y, Karikura M, Hayashi T. 1986. Isolation and hypoglycemic activity of dioscorans A, B, C, D, E, and F; glycans of Dioscorea japonica rhizophors. Planta Med. 52:168–171. 10.1055/s-2007-969112
- Ho ML, Hsieh YS, Chen JY, Chen KS, Chen JJ, Kuo WH, Lin SJ, Chen PN. 2011. Antimetastatic potentials of Dioscorea nipponica on melanoma in vitro and in vivo. Evid Based Complement Altern Med. 2011:1–13.
- Hong WK, Sporn MB. 1997. Recent advances in chemoprevention of cancer. Science. 278:1073–1077. 10.1126/science.278.5340.1073
- Hu K, Yao X. 2003. The cytotoxicity of Methyl Protodioscin against human cancer cell lines in vitro. Cancer Invest. 21:389–393. 10.1081/CNV-120018230
- Huang HP, Shih YW, Chang YC, Hung CN, Wang CJ. 2008. Chemoinhibitory effect of mulberry anthocyanins on melanoma metastasis involved in the Ras/PI3K pathway. J Agric Food Chem. 56:9286–9293. 10.1021/jf8013102
- Jin Z, El-Deiry WS. 2005. Overview of cell death signaling pathways. Cancer Biol Ther. 4:139–163. 10.4161/cbt.4.2.1508
- Kang TH, Moon E, Hong BN, Choi SZ, Son M, Park JH, Kim SY. 2011. Diosgenin from Dioscorea nipponica ameliorates diabetic neuropathy by inducing nerve growth factor. Biol Pharm Bull. 34:1493–1498. 10.1248/bpb.34.1493
- Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green DR. 1998. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappa B and AP-1. Mol Cell. 1:543–551. 10.1016/S1097-2765(00)80054-4
- Kelloff GJ, Crowell JA, Steele VE, Lubet RA, Malone WA, Boone CW, Kopelovich L, Hawk ET, Lieberman R, Lawrence JA, et al. 2000. Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J Nutr. 130:467S–471S.
- Kim MJ, Kim HN, Kang KS, Baek NI, Kim DK, Kim YS, Kim SH, Jean BH. 2004. Methanol extract of Dioscoreae rhizoma inhibits pro-inflammatory cytokines and mediators in the synoviocytes of rheumatoid arthritis. Int Immunopharmacol. 4:1489–1497. 10.1016/j.intimp.2004.07.001
- Khosravi-Far R, Esposti MD. 2004. Death receptor signals to mitochondria. Cancer Biol Ther. 3:1051–1057. 10.4161/cbt.3.11.1173
- Kolbus A, Herr I, Schreiber M, Debatin KM, Wagner EF, Angel P. 2000. c-Jun- dependent CD95-L expression is a rate-limiting step in the induction of apoptosis by alkylating agents. Mol Cell Biol. 20:575–582. 10.1128/MCB.20.2.575-582.2000
- Kuida, K. 2000. Caspase-9. Int J Biochem Cell B. 32:121–124. 10.1016/S1357-2725(99)00024-2
- Kwon CS, Sohn HY, Kim SH, Kim JH, Son KH, Lee JS, Lim JK, Kim JS. 2003. Anti-obesity effect of Dioscorea nipponica Makino with lipase-inhibitory activity in rodents. Biosci Biotechnol Biochem. 67:1451–1456. 10.1271/bbb.67.1451
- Le-Niculescu H, Bonfoco E, Kasuya Y, Claret FX, Green DR, Karin M. 1999. Withdrawal of survival factors results in activation of the JNK pathway in neuronal cells leading to Fas ligand induction and cell death. Mol Cell Biol. 19:751–763.
- Lin S, Wang D, Yang D, Yao J, Tong Y, Chen J. 2007. Characterization of steroidal saponins in crude extract from Dioscorea nipponica Makino by liquid chromatography tandem multi-stage mass spectrometry. Anal Chim Acta. 599:98–106. 10.1016/j.aca.2007.07.070
- Liu CZ, Zhou HY, Yan Q. 2007. Fingerprint analysis of Dioscorea nipponica by high-performance liquid chromatography with evaporative light scattering detection. Anal Chim Acta. 582:61–68. 10.1016/j.aca.2006.08.057
- Maris JM, Hogarty MD, Bagatell R, Cohn SL. 2007. Neuroblastoma. Lancet. 369:2106–2120. 10.1016/S0140-6736(07)60983-0
- Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. 2006. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 21:749–760. 10.1016/j.molcel.2006.02.009
- Oke OL. 1972. Yam – a valuable source of food and drugs. World Rev Nutr Diet. 15:156–184.
- Ravindranath MH, Ramasamy V, Moon S, Ruiz C, Muthugounder S. 2009. Differential growth suppression of human melanoma cells by tea (Camellia sinensis) epicatechins (ECG, EGC and EGCG). Evid Based Complement Altern Med. 6:523–530. 10.1093/ecam/nem140
- Shankar S, Ganapathy S, Hingorani SR, Srivastava RK. 2008. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front Biosci. 13:440–452. 10.2741/2691
- Sporn MB, Suh N. 2000. Chemoprevention of cancer. Carcinogenesis. 21:525–530. 10.1093/carcin/21.3.525
- Strasser A. 2005. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 5:189–200. 10.1038/nri1568
- Takahashi-Yanaga F, Sasagur T. 2009. Drug development targeting the glycogen synthase kinase-3beta (GSK-3beta)-mediated signal transduction pathway: inhibitors of the Wnt/beta-catenin signaling pathway as novel anticancer drugs. J Pharmacol. 109:179–183.
- Thornberry NA, Lazebnik Y. 1998. Caspases: enemies within. Science. 281:1312–1316. 10.1126/science.281.5381.1312
- van Weeren, PC, de Bruyn, KM, de Vries-Smits, AM, van Lint, J, Burgering, BM. 1998. Essential role for protein kinase B (PKB) in insulin-induced glycogen synthase kinase 3 inactivation characterization of dominant-negative mutant of PKB. J Biol Chem. 273:13150–13156. 10.1074/jbc.273.21.13150
- Vieira JR, de Souza IA, do Nascimento SC, Leite SP. 2007. Indigofera suffruticosa: an alternative anticancer therapy. Evid Based Complement Altern Med. 4:355–359. 10.1093/ecam/nel102
- Wang X. 2001. The expanding role of mitochondria in apoptosis. Genes Dev. 15:2922–2933.
- Xiao J, Wang NL, Sun B, Cai GP. 2010. Estrogen receptor mediates the effects of pseudoprotodiocsin on adipogenesis in 3T3–L1 cells. Am J Physiol Cell Physiol. 299:128–138. 10.1152/ajpcell.00538.2009
- Yin J, Tezuka Y, Kouda K, Tran QL, Miyahara T, Chen Y, Kadota S. 2004. Antiosteoporotic activity of the water extract of Dioscorea spongiosa. Biol Pharm Bull. 27:583–586. 10.1248/bpb.27.583
- Yoshikawa M, Xu F, Morikawa T, Pongpiriyadacha Y, Nakamura S, Asao Y, Kumahara A, Matsuda H. 2007. Medicinal flowers. XII. (1) New spirostane-type steroid saponins with antidiabetogenic activity from Borassus flabellifer. Chem Pharm Bull (Tokyo). 55:308–316. 10.1248/cpb.55.308
- Youle RJ, Strasser A. 2008. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 9:47–59. 10.1038/nrm2308
- Zhao G, Kan J, Li Z, Chen Z. 2005. Structural features and immunological activity of a polysaccharide from Dioscorea opposita Thunb roots. Carbohydr Polym. 61:125–131. 10.1016/j.carbpol.2005.04.020
