Abstract
Distribution patterns and the relative frequency of different types of endocrine cells were demonstrated in the alimentary tract of the Korean golden frog (Rana plancyi chosenica Okada), which is known as a Korean endemic species, for the first time. The alimentary tract of the frog was divided into six portions from the esophagus to the rectum. Most endocrine cells were found in the epithelial lining and were generally spindle-shaped with cytoplasmic processes ending in the lumen (open-typed cell), whereas cells that were spherical in shape (close-typed cell) were occasionally found in mucosal glands. Endocrine cells were stained for the following regulatory peptides: Sp-1/chromogranin A (Cg A), serotonin, somatostatin, gastrin, cholecystokinin (CCK)-8, bombesin, pancreatic polypeptide (PP), and glucagon. Cells stained for Cg A and serotonin were present throughout the entire alimentary tract. Somatostatin-immunoreactive (IR) cells were detected throughout the entire alimentary tract except the rectum. Cells stained for gastrin and CCK-8 were restricted to the pylorus, duodenum, and ileum, whereas bombesin-IR cells were restricted to the esophagus, antrum, and pylorus. Glucagon-containing cells were located in the esophagus, antrum, pylorus, and duodenum. Most of the IR cells occurred with the highest frequency in the pylorus except for cells stained for somatostatin and bombesin, which showed the highest frequencies in the antrum and serotonin-IR cells, which showed in the duodenum, respectively. No PP-stained cells were detected in this study. In conclusion, distribution patterns and the frequency of these endocrine cells not only correspond well with other Salienta species but some deviating patterns were also observed.
1. Introduction
The Korean golden frog, Rana plancyi chosenica Okada, belonging to the Ranidae in the order of Salienta, is known as a Korean endemic species. They have distinct two golden dorsal bands as peculiar characteristics distinguished from the other true frog species. In Korea, numbers and habitats of this frog have dramatically decreased because of pollution and immigration of foreign species of frogs having similar feeding habits, especially bullfrogs. Once Korean golden frogs were widely distributed throughout the Korea, they were listed as a vulnerable species by International Union for Conservation of Nature (IUCN) red list and also listed as an endangered species, Grade II in South Korea.
Endocrine cells are dispersed in epithelia and glands of the gastrointestinal (GI) tract and synthesize various types of hormones and play an important role in physiological functions of the GI tract (Bell Citation1978). The study of GI endocrine cells is considered to be important for phylogenetic purpose (D'Este et al. Citation1994). In addition, distribution patterns and the relative frequency of these endocrine cells vary with animal species and feeding habits (Solcia et al. Citation1975).
There has been a surge of interest in the endocrine cells of the alimentary tract in recent years (Ku et al. Citation2003). This is remarkable, considering the fact that so many GI neuropeptides have been isolated from the amphibian skin (Nakajima et al. Citation1979; Van Noorden and Polak Citation1979). The GI endocrine cells in the various amphibians have been extensively studied by histochemical (Kim & Chung Citation1973; Lee et al. Citation1993), electron microscopical (Geuze Citation1971; Lee & Lee Citation1990), and immunohistochemical (Buchan Citation1986; Ku et al. Citation2003) methods. Although the distributions and the frequency of about 17 types of endocrine cells including serotonin, somatostatin, glucagon, cholecystokinin (CCK)-8, chromogranin, pancreatic polypeptide (PP), bombesin, neurotensin, gastrin-releasing peptide, substance P, polypeptide YY, secretin, gastrin, vasointestinal polypeptide, motilin, met-enkephalin, and β-enkephalin, have been elucidated in the alimentary tract of Rana dybowskii (Lee & Lee Citation1996), Rana temporaria (Bodegas et al. Citation1997), Rana pipiens (Lechago et al. Citation1978), Xenopus laevis (Lechago et al. Citation1978; Lee & Lee Citation1992, Citation1997), Rana esculenta (Trandaburu & Nürnberger Citation1995), Bufo regularis (El-Salhy et al. Citation1981, Citation1982), Rana catesbeiana (Lee et al. Citation1998b, Citation1999), Rana nigromaculata (Lee et al. Citation1998a), Bombina orientalis (Ku et al. Citation2000a), Hyla arborea japonica (Ku et al. Citation2000b), and Rana rugosa (Ku et al. Citation2003) by immunohistochemical methods, the endocrine cells in the alimentary tract of the endemic Korean golden frog, R. plancyi chosenica Okada, have not been studied yet. Therefore, we monitored these cells in the grass lizard using specific immunohistochemical methods and different antibodies raised against Sp-1/chromogranin A (Cg A), serotonin, somatostatin, gastrin, CCK-8, bombesin, PP, and glucagon, and the results were compared with those obtained in other Salienta species.
2. Materials and methods
Six adult Korean golden frogs (40–60 mm in length) of the Salienta, R. plancyi chosenica Okada, were captured around Buyeo, South Korea. After phlebotomy from the head, samples of six parts of the GI tract (from proximal to distal: esophagus, antrum, pylorus, duodenum, ileum, and rectum) were fixed in Bouin's solution, according to Ku et al. (Citation2003). After paraffin embedding, serial sections (3–4 µm thick) were prepared. Sections were deparaffinized, rehydrated, and stained with hematoxylin and eosin for light microscopic examination of the normal alimentary architecture. Other sections were used for immunostaining using the peroxidase anti-peroxidase (PAP) method (Sternberger Citation1986). Blocking of nonspecific peroxidase reactions was performed with normal goat serum prior to incubation with the specific antibodies (). After rinsing in phosphate buffered saline (PBS; 0.01 M, pH 7.4), sections were incubated with secondary antibodies (goat anti-rabbit IgG, dilution, 1:200; Sigma, St. Louis, MO, USA). Sections were then washed in PBS buffer and finally incubated with PAP complex (dilution, 1:200; Sigma). The peroxidase reaction was carried out using a solution 3,3′-diaminobenzidine tetrahydrochloride containing 0.01% H2O2 in Tris-HCl buffer (0.05 M, pH 7.6). After immunostaining, sections were analyzed with the use of a light microscope.
Table 1. Antisera used in this study.
Specificity of the immunohistochemical staining methods was determined as recommended by Sternberger (Citation1986), including preincubation of the antibodies with their corresponding antigens. In the restricted view fields, 10 mm2 of mucosa regions in computer monitor using automated image analysis process (DMI, Daegu, Korea) attached to light microscopy, each immunostained cells were counted according to the previous reports (Cho et al. Citation2006, Citation2008). The frequencies of IR cells were calculated as mean ± standard deviation (SD) of six frogs (one filed in each animal) of each alimentary tract.
3. Results
Eight types of endocrine cells were detected with the antibodies against Cg A, serotonin, somatostatin, gastrin, CCK-8, bombesin, PP, and glucagon. The distribution patterns and frequencies of these endocrine cells in the alimentary tract of the Korean golden frog are shown in . In addition, most of the endocrine cells were spindle-shaped (open-typed cells), whereas cells that were spherical in shape (closed-typed cell) were occasionally found in the mucosal glands including esophagus.
Table 2. Regional distributions and frequencies of the endocrine cells in the alimentary tract of the Korean golden frog, R. plancyi chosenica.
3.1. Cells stained for Cg A
Cg A-positive cells were observed throughout the entire alimentary tract from with variable frequencies in each portion of the alimentary tract and showed the highest frequencies in the pylorus as 55.33 ± 6.31 cells/10 mm2 of mucosa (). Open-typed Cg A-IR cells were dispersed in the epithelium or mucosal glands of entire alimentary tract with rare close-typed cells, occasionally with various frequencies (; ).
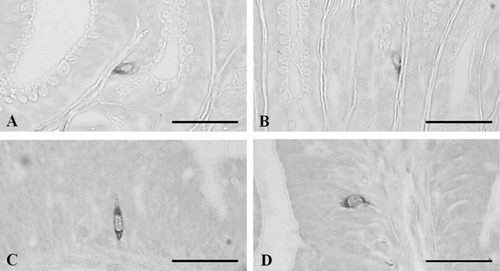
3.2. Cells stained for serotonin
Serotonin-contained cells were demonstrated throughout the whole alimentary tract and showed the highest frequency in the duodenum as 101.33 ± 21.66 cells/10 mm2 of mucosa (). They were the most predominant cell types found in this study. Most of the open-typed serotonin-positive cells were mainly situated in the epithelium of the esophagus, while close-typed cells were restricted to the mucosal gland regions (). In the antrum and pylorus, open- and close-typed cells stained for serotonin were dispersed between acinar cells of gastric glands (). In the duodenum, ileum, and rectum, serotonin-IR cells were mainly located in the epithelium and open-typed cells were predominantly observed as compared with close-typed cells in the epithelium of intestine ().
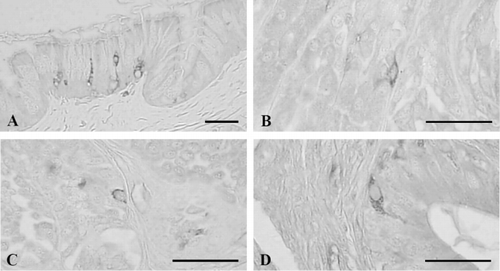
3.3. Cells stained for somatostatin
Somatostatin-contained cells were detected throughout the entire alimentary tract except for the rectum with the highest frequencies in the antrum as 46.83 ± 9.70 cells/10 mm2 of mucosa (). Although only close-typed cells were restrictively observed in the mucosal gland of esophagus ( and ), open- and close-typed cells were dispersed in the gastric glands, between chief or parietal cells in the antrum () and pylorus (). In the duodenum and ileum, only open-typed somatostatin-positive cells were located in the epithelium ( and ). No somatostatin-IR cells were demonstrated in the rectum of this frog.
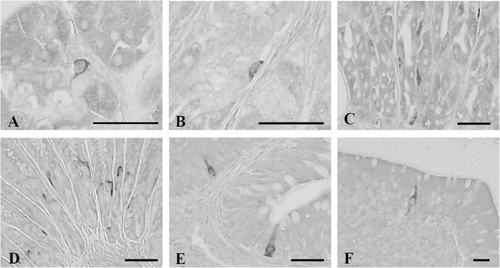
3.4. Cells stained for gastrin
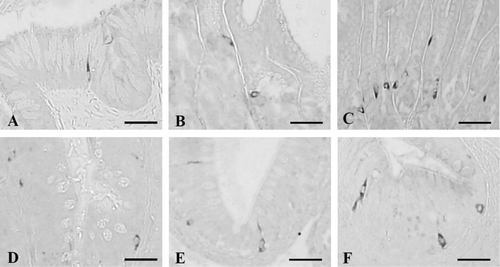
Gastrin-IR cells were restricted to the pylorus, duodenum, and ileum with the frequencies of 5.50 ± 1.05, 3.00 ± 0.89 and 1.67 ± 0.82 cells/10 mm2 of mucosa, respectively (). Only open-typed gastrin-positive cells were exclusively located in the epithelium of the pylorus, duodenum, and ileum ().
3.5. Cells stained for CCK-8
CCK-8-contained cells were detected in the pylorus, duodenum, and ileum with the frequencies of 45.67 ± 7.76, 6.00 ± 1.79 and 3.83 ± 1.17 cells/10 mm2 of mucosa, respectively (). In the pylorus, open- and close-typed CCK-8-positive cells were dispersed in the gastric glands ( and ), while only open-typed cells having long cytoplasmic process were demonstrated in the epithelial regions of the duodenum () and ileum ().
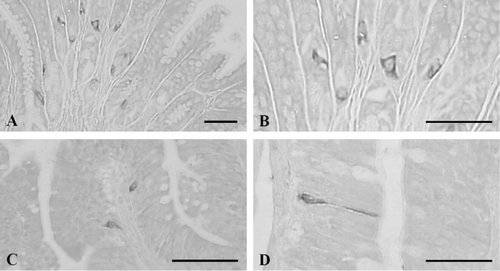
3.6. Cells stained for bombesin
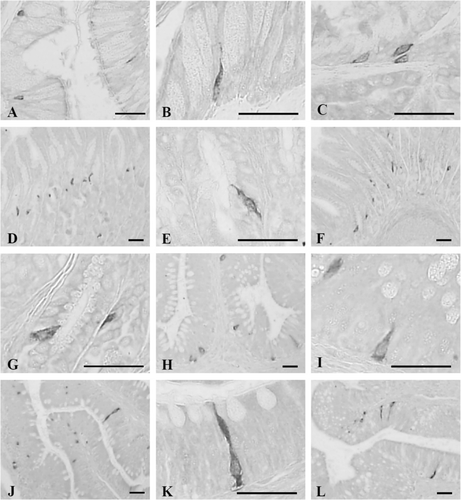
Bombesin-positive cells were demonstrated in the basal portion of the epithelium or mucosal glands of the esophagus with a frequency of 9.33 ± 1.17 cells/10 mm2 of mucosa (; and ) and they were dispersed in the gastric glands of the antrum and pylorus with the frequencies of 75.67 ± 11.17 and 50.33 ± 9.48 cells/10 mm2 of mucosa, respectively (; and ). Most of the cells stained for bombesin showed round to spherical shape, a classically close-typed cell shape ().
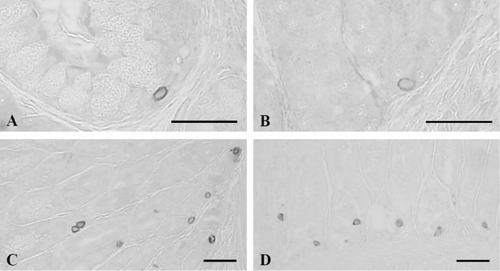
3.7. Cells stained for PP
No PP-IR cells were demonstrated throughout all six portions of alimentary tract in the Korean golden frog.
3.8. Cells stained for glucagon
Glucagon-positive cells were observed throughout the entire alimentary tract except for the ileum and rectum with the highest frequencies in the pylorus as 16.00 ± 3.03 cells/10 mm2 of mucosa (). In the esophagus, open-typed glucagon-contained cells were situated in the epithelium with a frequency of 8.67 ± 1.21 cells/10 mm2 of mucosa (). Open-typed glucagon-IR cells were found in the basal portions of the gastric glands of the antrum with a frequency of 11.50 ± 1.87 cells/10 mm2 of mucosa () and in the pylorus where they showed most predominant frequency, 16.00 ± 3.03 cells/10 mm2 of mucosa; most of the cells were also located in the gastric gland regions (). In the duodenum, open-typed glucagon-contained cells having cytoplasmic process were observed in the epithelium with a frequency of 5.83 ± 1.47 cells/10 mm2 of mucosa (). No glucagon-IR cells were demonstrated in the ileum and rectum of this frog, respectively.
4. Discussion
It is generally accepted that the endocrine cells in the alimentary tracts appeared remarkably different depending on the regional distribution, relative frequency, and cell types with animal species and each regional part of the alimentary tract (Alumets et al. Citation1977; D'Este et al. Citation1994). In addition, many studies have elucidated the regional distribution and frequency of different endocrine cells in the alimentary tract of the various Salienta, and also the researches or data processing about alimentary endocrine cells in frogs has been widely executed and the distribution and frequency of endocrine cells in the alimentary tract of frogs showed species-dependent differences (Lechago et al. Citation1978; El-Salhy et al. Citation1981, Citation1982; Lee & Lee Citation1992, Citation1996, Citation1997; Trandaburu & Nürnberger Citation1995; Bodegas et al. Citation1997; Lee et al. Citation1998a, Citation1998b, Citation1999; Ku et al. Citation2000a, Citation2000b, Citation2003). The GI endocrine cells were generally divided into two types: one was round- to spherical-shaped close-typed cells which were mainly located in the mucosal gland regions, and the other was spherical- to spindle-shaped open-typed cells which were situated in the epithelium (Ku et al. Citation2006). In this study, close-typed cells were mainly located in the mucosal gland regions, whereas most of the open-typed cells were found in the epithelial regions of the Korean golden frog.
Throughout the alimentary tract of Korean golden frog, different regional distributions and frequencies of neuropeptide-producing endocrine cells were demonstrated and their patterns were quite similar to those of other Salienta species except for some unique distributional patterns and frequencies observed in each part of alimentary tract. Although the appearance of serotonin- and somatostatin-IR cells in the esophageal mucosa was previously reported for bullfrog (Nada et al. Citation1984), red-bellied frog (Ku et al. Citation2000a), tree frog (Ku et al. Citation2000b), and wrinkled frog (Ku et al. Citation2003), and even if esophagus of the frogs was different from those of mammals, lined by pseudostratified columnar ciliated epithelium and contained numerous goblet cells that were scattered throughout the epithelium (El-Salhy et al. Citation1981), the demonstration of other types of neuropeptide-producing endocrine cells in the esophagus of the Salienta species was rare; only bombesin- and glucagon-contained cells were demonstrated in the esophagus of the wrinkled frog with very rare frequencies (Ku et al. Citation2003). In this study, serotonin-, somatostatin-, bombesin-, and glucagon-IR cells were observed in the esophagus of Korean golden frog as comparable frequencies with other parts of alimentary tract, and these are considered as a unique distributional pattern.
Cg belongs to a family of large anionic proteins (Cg A, B, and secretogranin II), the members of which are known to be present in the secretory granules of a broad spectrum of amine- and peptide-producing cells of adrenal medulla and GI endocrine system, as well as in some neurons of the peptidergic and catecholaminergic nervous system of several mammals (Grube & Yoshie Citation1989; Reinecke et al. Citation1991). Cg has been found to occur in a large variety of endocrine organs and cells outside the adrenal medulla, and it has been claimed as a common marker of all neuroendocrine cells (Lloyd & Wilson Citation1983; Cohn et al. Citation1984) including green frog (D'Este et al. Citation1994). However, the heterogeneous distributions of Cg-contained cells were reported in some species of Amphibia (Trandaburu & Ali Citation1998) and demonstrated Cg-positive cells were restricted to the stomach regions in various Salienta species (Lee & Lee Citation1992; Lee et al. Citation1998a; Ku et al. Citation2000a, Citation2000b, Citation2003) with relatively lower frequencies as compared with other endocrine cells. Cells stained for Cg A were observed throughout the entire alimentary tract of the Korean golden frog in this study, somewhat different from other Salienta species previously reported (Lee & Lee Citation1992; Lee et al. Citation1998a; Ku et al. Citation2000a, Citation2000b, Citation2003). However, Cg A may not be a suitable marker of endocrine cells because the relative frequency of Cg A-positive cells was lower than serotonin-positive cells and cells positive for other regulatory peptides in some regions of the alimentary tract of the Korean golden frog. To observe the possibility that used as endocrine marker of Cgs, mixed or concomitantly immunostained with other types of Cgs should be tested.
Serotonin is widely distributed in the nervous system and in GI endocrine cells (El-Salhy et al. Citation1985). Main functions of serotonin are inhibition of gastric acid secretion and contraction of smooth muscle in the GI tract (Guyton Citation1988). El-Salhy et al. (Citation1985) reported that serotonin-IR cells were detected throughout the whole GI tract of all species and established in the GI tract at the early stage of vertebrate evolution. The regional distributions and relative frequencies of cells stained for serotonin were detected in whole GI tract of various Salienta species (Lee & Lee Citation1992, Citation1996; Lee et al. Citation1998a, Citation1999; Ku et al. Citation2000a, Citation2000b, Citation2003). According to these previous reports, serotonin-positive cells were most predominant in antrum except for R. nigromaculata (Lee et al. Citation1998a) that were most predominant in the pylorus. It is reported that this variance of the relative frequencies in the anuran species might be due to sampling time or season (Lee & Lee Citation1996). In the present study, these cells were observed throughout the entire alimentary tract as like previous reports but the highest frequencies are detected in the duodenum quite different from other Salienta species as a unique distribution pattern in this species of frog.
Somatostatin consisting of 14 amino acids was isolated from hypothalamus of sheep for the first time and it could be divided into a straight form and a cyclic form (Brazeau et al. Citation1973). This substance inhibits the secretion of the other neuroendocrine hormones (Kitamura et al. Citation1984). In the anuran species, somatostatin-positive cells were also have been demonstrated throughout the entire alimentary tracts (El-Salhy et al. Citation1981; Lee & Lee Citation1990, Citation1992; Trandaburu & Nürnberger Citation1995; Lee et al. Citation1998a, Citation1999; Ku et al. Citation2000a, Citation2000b, Citation2003) and they were the most predominant in pylorus but decreased distally along the alimentary tract in adult anuran. In the present study, somatostatin-contained cells were detected throughout the whole alimentary tract except for the rectum with the highest frequency in the antrum, quite differed to those of other Salienta species.
Gastrin secreted by intestinal cells promotes gastric acid secretion, and CCK stimulates the pancreatic enzyme secretion. In Salienta species, gastrin-, CCK-8-, or gastrin/CCK-positive cells were restricted to the pylorus, duodenum, and ileum (Buchan Citation1986; Rajjo et al. Citation1988; Lee & Lee Citation1992, Citation1996; Ku et al. Citation2000a, Citation2000b, Citation2003). In X. laevis, cells stained for CCK were first detected in the gut shortly before food was first observed in the lumen of the intestine (Scalise & Vigna Citation1988). These distributions of gastrin and/or CCK-8-IR cells in the present study correspond well to those of previous reports. In the present study, bombesin-contained cells were restricted to the stomach regions and esophagus. These findings are coincident with those of previous studies (El-Salhy et al. Citation1981; Lee et al. Citation1998a, Citation1998b; Ku et al. Citation2000a, Citation2000b, Citation2003) except for esophagus; appearance of bombesin-positive cells in esophagus was only reported in the wrinkled frog, R. rugosa (Ku et al. Citation2003). In addition, the relative frequency of bombesin-IR cells in this study was somewhat more numerous than those of other Salienta species.
Since PP was isolated from insulin extraction of pancreas at 1961, the regional distribution of PP-IR cells in the GI tracts was relatively well-known, and PP-positive cells were found in antrum and small intestine of the Salienta species (Falkmer & Stefan Citation1978; El-Salhy et al. Citation1981; Larhammar Citation1996; Ku et al. 2000, Citation2003). No cells positively stained for PP were detected in the alimentary tract of the Korean golden frog as species-dependent characteristic distribution patterns.
Glucagon is synthesized in the A cells of the pancreas and regulates serum glucose levels. According to the anuran species, the variable distributional patterns of glucagon-IR cells in the GIT were reported (El-Salhy et al. Citation1981; Lee & Lee Citation1992; Lee et al. Citation1998a; Ku et al. 2000). Especially, Lee and Lee (Citation1992) reported that these cells were restricted to the fundus of X. laevis. However, El-Salhy et al. (Citation1981) and Lee et al. (Citation1998a) reported that they were found in the whole GI tract of B. regularis and R. nigromaculata except for the rectum, respectively. In addition, they were observed in the duodenum and ileum of B. orientalis (Ku et al. 2000) and from the esophagus to the ileum with the highest frequency in the antrum of R. rugosa (Ku et al. Citation2003). In the present study, glucagon-positive cells were demonstrated from the esophagus to the duodenum of the Korean golden frog with the highest frequency in the pylorus.
In conclusion, distribution patterns and the frequency of neuropeptide-producing endocrine cells of the Korean golden frog, R. plancyi chosenica, not only correspond well with other Salienta species but some deviating patterns were also observed.
Acknowledgment
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST) [grant number 2011-0030124].
References
- Alumets J, Sundler F, Hakanson R. 1977. Distribution, ontogeny and ultrastructure of somatostatin immunoreactive cells in the pancreas and gut. Cell Tissue Res. 186:467–479.
- Bell FR. 1978. The relevance of the new knowledge of gastrointestinal hormones to veterinary science. Vet Sci Commun. 2:305–314.10.1007/BF02291460
- Bodegas ME, Villaro AC, Burrell MA, Rovira J, Valverde E, Ortiz De Zarate A, Sesma P. 1997. An immunocytochemical and ultrastructural study of the larval anterior intestine of the frog Rana temporaria, with especial reference to endocrine cells. Tissue Cell. 29:549–559.10.1016/S0040-8166(97)80055-9
- Brazeau P, Vale W, Burgurs R, Ling N, Butcher M, Rivier J, Guillermin R. 1973. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 179:77–79.10.1126/science.179.4068.77
- Buchan AMJ. 1986. An immunocytochemical study of regulatory peptides in the amphibian gastrointestinal tract. Can J Zool. 64:1–7.10.1139/z86-001
- Cho KH, Lee HS, Ku SK. 2006. Changes in gastric endocrine cells in Balb/c mice bearing CT-26 carcinoma cells: an immunohistochemical study. Eur J Histochem. 50:293–300.
- Cho KH, Lee HS, Ku SK. 2008. Decrease in intestinal endocrine cells in Balb/c mice with CT-26 carcinoma cells. J Vet Sci. 9:9–14.10.4142/jvs.2008.9.1.9
- Cohn DV, Elting JJ, Frick M, Elde R. 1984. Selective localization of the parathyroid secretory Protein-I/Adrenal Medulla chromogranin a protein family in a wide variety of endocrine cells of the Rat*. Endocrinology. 144:1963–1974.10.1210/endo-114-6-1963
- D'Este L, Buffa R, Pelagi M, Siccardi AG, Renda T. 1994. Immunohistochemical localization of chromogranin A and B in the endocrine cells of the alimentary tract of the green frog, Rana esculenta. Cell Tissue Res. 277:341–349.10.1007/BF00327782
- El-Salhy M, Winder E, Lundqvist M. 1985. Comparative studies of serotonin-like immunoreactive cells in the digestive tract of vertebrates. Biomed Res. 6:371–375.
- El-Salhy M, Grimelius L, Wilander E, Abu-Sinna G, Lundqvist G. 1981. Histological and immunohistochemical studies of the endocrine cells of the gastrointestinal mucosa of the toad (Bufo regularis). Histochemistry. 71:53–65.10.1007/BF00592570
- El-Salhy M, Grimelius L, Lundberg JM, Tatemoto K, Terenius L. 1982. Immunocytochemical evidence for occurrence of PYY, a newly isolated gut polypeptide in endocrine cells in the gut of amphibians and reptiles. Biomed Res. 3:303–306.
- Falkmer S, Stefan Y. 1978. Pancreatic polypeptide (PP): phylogenetic aspects in gastrointestinal mucosa and endocrine pancreas. Scan J Gastroenterol. 13:49–59.10.3109/00365527809179805
- Geuze JJ. 1971. Light and electron microscope observations on the gastric mucosa of the frog (Rana esculenta). II. Structural alternations during hibernation. Z Zellforsch. 117:103–117.10.1007/BF00331105
- Grube D, Yoshie S. 1989. Immunohistochemistry of chromogranin A and B, and secretogranin II in the canine endocrine pancreas. Arch Histol Cytol. 52:287–298.10.1679/aohc.52.287
- Guyton AC. 1988. Textbook of medical physiology: secretory functions of the alimentary tract. Philadelphia: W.B. Saunders.
- Kim CW, Chung YW. 1973. A study on the enterochromaffin cells in the gastrointestinal mucosa of Rana amurensis during prehibernating, hibernating, post-hibernating and active period. Korean J Zool. 2:109–118.
- Kitamura N, Yamada J, Calingasan NY, Yamashita T. 1984. Immunocytochemical distribution of endocrine cells in the gastro-intestinal tract of the horse. Equine Vet J. 16:103–107.10.1111/j.2042-3306.1984.tb01870.x
- Ku SK, Lee HS, Lee JH. 2000a. An immunohistochemical study of endocrine cells in the alimentary tract of the Red-bellied frog, Bombina orientalis. J Vet Med Sci. 62:589–594.10.1292/jvms.62.589
- Ku SK, Lee HS, Lee JH. 2000b. Immunohistochemistry of endocrine cells in the alimentary tract of the tree frog, Hyla arborea japonica. Korean J Biol Sci. 4:95–100.10.1080/12265071.2000.9647530
- Ku SK, Lee HS, Lee JH. 2006. The regional distribution and relative frequency of gastrointestinal endocrine cells in the nude mice, Balb/c-nu/nu: an immunohistochemical study. Anat Histol Embryol. 35:104–110.10.1111/j.1439-0264.2005.00645.x
- Ku SK, Lee HS, Koh JK, Lee JH. 2003. An immunohistochemical study on the neuropeptide-producing endocrine cells in the alimentary tract of wrinkled frog, Rana rugosa (Ranidae). Gen Comp Endocrinol. 131:1–8.10.1016/S0016-6480(02)00642-1
- Larhammar D. 1996. Structural diversity of receptors for neuropeptide Y, peptide YY and pancreatic polypeptide. Regul Pept. 53:165–174.10.1016/0167-0115(96)00110-3
- Lechago J, Holmquist AL, Rosenquist GL, Walsh JH. 1978. Localization of bombesin-like peptides in the frog gastric mucosa. Gen Com Endocrinol. 36:553–558.10.1016/0016-6480(78)90095-3
- Lee HS, Lee JH. 1990. The ultrastructure of the gastro-endocrine cells in the gastric mucosa of the frog, Rana rugosa. Korean J Zool. 33:22–27.
- Lee HS, Lee JH. 1992. An immunohistochemical study of the endocrine cells on the gastro-entero-pancreatic system of the african clawed toad, Xenopus laevis. Korean J Vet Res. 32:523–529.
- Lee HS, Lee JH. 1996. Seasonal variations of the gastro-entero-pancreatic endocrine cells of the frog, Rana dybowskii. Korean J Vet Res. 36:11–21.
- Lee HS, Lee JH. 1997. Immunohistochemical study of the endocrine cells of the gastrointestinal mucosa of the African clawed toad, Xenopus laevis. Korean J Vet Res. 37:9–13.
- Lee HS, Lee JH, Lee CE. 1993. Histological study of the gastrointestinal endocrine cells of the frog, Rana rugosa (Amphibia, Anura). Nat Life. 23:121–125.
- Lee HS, Ku SK, Lee JH. 1998a. Immunohistochemical study of the gastrointestinal endocrine cells of the frog, Rana nigromaculata. Korean J Lab Anim Sci. 14:157–162.
- Lee HS, Ku SK, Lee JH. 1998b. Ontogeny of gastrointestinal endocrine cells in the bullfrog, Rana catesbeiana during developmental stages. Korean J Lab Anim Sci. 14:163–168.
- Lee HS, Ku SK, Lee JH. 1999. Changes in the serotonin-, somatostatin- and motilin-immunoreactive cells in the gastrointestinal tract of the bullfrog, Rana catesbeiana, at various developmental stages. Korean J Biol Sci. 3:97–102.10.1080/12265071.1999.9647471
- Lloyd RV, Wilson BS. 1983. Specific endocrine tissue marker defined by a monoclonal antibody. Science. 222:628–630.10.1126/science.6635661
- Nada A, Hiratsuka T, Komatsu K. 1984. The occurrence of serotonin-containing cells in the esophageal epithelium of the bullfrog, Rana catesbeiana: a fluorescence histochemical and immunohistochemical study. Histohemistry. 81:115–118.10.1007/BF00490103
- Nakajima T, Sasuhara T, Tshikawa O. 1979. Gut peptides, secretion, function and clinical aspects: new frog skin peptides homologous to the ranatensin or bombesin family. Amsterdam: Elsevier/North Holland.
- Rajjo IM, Vigna SR, Crim JW. 1988. Cholecystokinin immunoreactivity in the digestive tract of bowfin (Amia calva), bluegill (Lepomis macrochirus), and bullfrog (Rana catesbeiana). Gen Comp Endocrinol. 70:133–144.10.1016/0016-6480(88)90102-5
- Reinecke M, Hoog A, Ostenson CG, Efendic S, Grimelius L, Falkmer S. 1991. Phylogenetic aspects of pancreastatin- and chromogranin-like immunoreactive cells in the gastro-entero-pancreatic neuroendocrine system of vertebrates. Cen Com Endocrinol. 83:167–182.10.1016/0016-6480(91)90021-W
- Scalise FW, Vigna SR. 1988. Temporal pattern of appearance and distribution of cholecystokinim-like peptides during development in Xenopus laevis. Gen Comp Endocrinol. 112:303–311.10.1016/0016-6480(88)90213-4
- Solcia E, Capella C, Vassallo G, Buffa R. 1975. Endocrine cells of the gastric mucosa. Int Rev Cytol. 42:223–286.
- Sternberger LA. 1986. Immunocytochemistry. 3rd ed. New York: Wiley.
- Trandaburu T, Nürnberger F. 1995. Somatostatin-immunoreactive cells in the gastrointestinal tract of the frog, Rana esculenta. Cell Tissue Res. 279:437–440.10.1007/BF00318502
- Trandaburu T, Ali SS. 1998. Granin proteins (chromogranin A and secretogranin II C23–3 and C26–3) in the intestine of amphibians. Anat Anz. 180:523–528.10.1016/S0940-9602(98)80059-9
- Van Noorden S, Polak JM. 1979. Hormones of the alimentary tract. Hormones and evolution. New York: Academic Press.