Abstract
Mid-life obesity is associated with increased risk of Alzheimer's disease (AD). Amyloid precursor protein (APP) and its processing are centrally involved in the etiology of AD. Our previous studies demonstrated that human APP is expressed in adipocytes, up-regulated with obesity, and correlated with adipokine expression. This study was to determine whether APP expression is also dysregulated in mouse models of obesity and diabetes. Six-week-old C57BL/6 mice were fed normal or high-fat diets (HFD) until 16, 26, 36, 47, or 77 weeks of age. We measured gene expression of APP and adipokines, levels of glucose and insulin, and area under the glucose curve (AUCglucose) during the glucose tolerance test. Using quantitative real-time polymerase chain reaction (PCR), we demonstrated that APP expression in subcutaneous adipose tissue (SAT) significantly increased in all HFD mice groups, and that it correlated with the levels of AUCglucose, insulin, and expression of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and chemokine (C-C motif) ligand 2 (CCL2) genes. Thiazolidinedione treatment significantly reduced plasma insulin levels and APP expression in SAT and epididymal adipose tissue. APP expression in the SAT and brain also decreased significantly in streptozotocin-induced diabetic mice, indicating an important role of insulin in the regulation of APP gene expression. These results demonstrate that adipose tissue APP expression is increased with obesity and regulated by insulin levels, suggesting that the regulation and role of APP are similar in humans and mice. Insights into APP regulation and processing in the adipose tissue may improve our understanding of obesity-related adipose tissue inflammation and the increased risk of AD in obese individuals.
Introduction
Obesity increases the risk for a large number of chronic diseases, including insulin resistance, metabolic syndrome, Type 2 diabetes mellitus (T2DM), hypertension, dyslipidemia, and cardiovascular disease (Pi-Sunyer Citation2002; Lee & Pratley Citation2007). Mid-life obesity is also associated with risk of late-life dementia and Alzheimer's disease (AD; Kivipelto et al. Citation2005; Whitmer et al. Citation2005). Patients with AD, like patients with T2DM, have been reported to be insulin resistant and hyperinsulinemic compared with nondemented controls (Craft et al. Citation1998). Several studies have also demonstrated that hyperinsulinemia and T2DM appear to be risk factors for developing AD (Luchsinger et al. Citation2004; Craft Citation2007; Luchsinger & Gustafson Citation2009). Thus, insulin resistance in obesity and T2DM may predispose to AD.
Overexpression of amyloid precursor protein (APP) and abnormal APP enzymatic processing both play key roles, resulting in the production of amyloid beta (Aβ) fragments that are neurotoxic and proinflammatory. The deposition of Aβ peptides, including Aβ40 and Aβ42 is an early and consistent finding in AD (Hardy & Selkoe Citation2002). These peptides aggregate to form an insoluble extracellular deposit constituting the core of the neuritic plaques pathognomonic of AD (Butterfield et al. Citation2002).
In previous studies, we demonstrated that APP mRNA was highly expressed in adipose tissue/adipocytes and was up-regulated in obesity, and this correlated with insulin resistance, hyperinsulinemia, and a proinflammatory gene expression profile in adipose tissue/adipocytes (Lee et al. Citation2005, Citation2008; Nair et al. Citation2005). APP gene expression levels in subcutaneous adipocytes of obese individuals correlated with plasma Aβ40 levels and significantly decreased with weight loss (Lee et al. Citation2009). These novel observations suggest that plasma Aβ40 may be at least partly derived from adipose tissue and that APP expression in adipocytes may be related to insulin resistance. It is therefore possible that, in the adipose tissue of obese adults, up-regulated expression of APP and production of Aβ peptides could alter circulating levels and central nervous system clearance of Aβ peptides, thereby directly enhancing the risk of AD in later life (Lee et al. Citation2009).
Puig et al. (Citation2012) demonstrated that adipose tissue APP and tumor necrosis factor-α (TNF-α) levels were increased in mice fed high-fat diets (HFD) for 22 weeks, and Sommer et al. (Citation2009) showed that APP gene expression was increased by TNF-α in 3T3-L1 adipocytes. Currently, however, there are few studies on APP expression in adipose cells.
Dietary fat is considered an important factor in the development of human obesity. C57BL/6 mice are known to carry a genetic predisposition to developing obesity and T2DM when exposed to an HFD (Surwit et al. Citation1988). HFD-induced obese (DIO) C57BL/6 mice, therefore, may well exhibit characteristics of human obesity and have been used to study obesity, impaired glucose tolerance, and T2DM (Prpic et al. Citation2003; Winzell et al. Citation2003; Singhal et al. Citation2013).
In this study, using C57BL/6 mice fed an HFD for long periods (up to one-and-a-half years), we investigated whether the dysregulation of APP gene expression in SAT is associated with obesity, impaired glucose tolerance, and insulin resistance, as we previously demonstrated in human subjects. In addition, we investigated the regulation of APP gene expression in calorie-restricted, thiazolidinedione (TZD)-treated, and streptozotocin (STZ)-induced diabetic mice, and studied the effects of increased insulin sensitivity and induced diabetes to determine the major factors affecting APP gene expression in obesity.
Materials and methods
Experimental animals and study design
The protocols used in this study were reviewed and approved by the Animal Experimentation and Ethics Committee of the Catholic University of Daegu. Ninety-five 4-week-old C57BL/6 male mice were purchased from Daehan Biolink (Eumseong, Korea) and housed 3–4 per cage in a temperature-controlled room with a 12-h light/12-h dark cycle. For acclimatization, the mice were fed a normal diet (ND) containing 4.0% (wt/wt) total fat (Rodent NIH-31 Open Formula Auto; Zeigler Bros., Inc., Gardners, PA) and water ad libitum for 2 weeks. From 6 weeks of age, the mice were divided into several groups and maintained until 16, 26, 36, 47, or 77 weeks of age on a ND or an HFD (; please also see Supplemental Data Table S1). The mice were allowed free access to diet and water throughout the study period. The mice received continuous feeding of a ND or an HFD (containing 45% fat on a caloric basis; Feedlab Korea Co., Ltd., Korea) until an intraperitoneal (IP) glucose tolerance test (IPGTT) was performed at 16, 26, 36, 47, or 77 weeks of age. Except for measurements of body weight, animals were not disturbed or stressed during the designated experimental periods by any experimental procedures. The single IPGTT was performed 1 week before sacrifice.
Table 1. Body weights of C57BL/6 mice fed an ND or HFD from 6 until 16, 26, 36, 47, or 77 weeks of age, and subjected to TZD treatment, calorie restriction, STZ-induced diabetes, or no additional treatment (control mice).
TZD treatment and caloric-restriction intervention
Two groups of 47-week-old mice that had been given an HFD from 6 weeks of age underwent either a caloric-restriction (CR) intervention or TZD treatment. The CR group (n = 10) was maintained for 6 weeks on a diet of calorically restricted chow that permitted daily access to 70% of the intake of a control group (n = 10). Mice in the TZD (n = 10) and vehicle (n = 8) groups had free access to HFD and water. Rosiglitazone (10 mg/kg) or vehicle (0.5% carboxymethyl cellulose) was orally administered to the mice once daily for 14 consecutive days (Higuchi et al. Citation2010).
STZ-induced diabetic mouse models
To obtain mouse models mimicking Type 1 (T1DM) and T2DM without or with increased adiposity, we induced diabetes in ND and HFD mice, using STZ, respectively (Kusakabe et al. Citation2009; Poucher et al. Citation2012). Diabetic mice were generated by following the multiple low-dose STZ induction protocol of the Animal Models of Diabetic Complications Consortium (Hsueh et al. Citation2007). STZ (Sigma-Aldrich, St. Louis, MO) was dissolved in 0.1 M sodium citrate buffer (pH 4.5) at a concentration of 0.75%. IP injection of STZ at 50 mg/kg was applied to mice that had been fed either a ND (n = 15) or an HFD (n = 15) from 6 until 11 weeks of age. Injections were administered for 5 consecutive days. Animals continued on the same ND or HFD during the induction of diabetes with STZ and then until they were sacrificed at 16 weeks.
Intraperitoneal glucose tolerance test
IPGTT was performed to determine the metabolic characteristics of the animals one week before sacrifice. Mice were fasted for 6 h to achieve baseline blood glucose levels. During the fast, a glucometer (CareSens N; i-SENS, Korea) was used to measure glucose levels in blood collected from the tail vein; subsequently, the mice were intraperitoneally injected with 1.5 g/kg body weight of glucose (Sobel et al. Citation2006). Blood glucose levels were determined after 30, 60, 90, and 120 min. To assess glucose tolerance, the area under the glucose curve from 0 to 120 min (AUCglucose) was also calculated. Lower AUCglucose values reflect more efficient glucose clearance.
Biochemical analyses
One week after the IPGTT, the nonfasting mice were anesthetized by IP injection of sodium pentobarbital, and blood samples for biochemical analysis were collected by cardiac puncture. Blood was collected in ethylenediaminetetraacetic acid (EDTA)-containing blood-collection tubes. Blood samples were centrifuged at 2400× g for 15 min at 4°C, and the plasma obtained was additionally centrifuged at 12,500× g for 15 min. Nonfasting plasma concentrations of glucose and insulin were measured by glucometer and enzyme-linked immunosorbent assay (ALPCO Diagnostic, Salem, NH), respectively.
Quantitative real-time PCR
Quantitative real-time polymerase chain reaction (QPCR) was performed using complementary DNA (cDNA) samples from SAT, epididymal adipose tissue (EAT), and brain to assess expression levels of APP, TNF-α, interleukin-6 (IL-6), and chemokine (C-C motif) ligand 2 (CCL2; monocyte chemoattractant protein-1, MCP-1). Total RNA was extracted from tissue samples, using an RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA). During the extraction, RNA was treated with DNase I (Qiagen) to minimize potentially contaminating genomic DNA. QPCR was carried out using mouse TaqMan Gene Expression Assay (assay IDs: APP, Mm00431827_m1; TNF-α, Mm00443258_m1; IL-6, Mm00446190_m1; CCL2, Mm00441242_m1; Applied Biosystems, Foster City, CA) and a 7500 Real-Time PCR System (Applied Biosystems). Standard curves for each gene were generated by serial dilution of a cDNA mixture made using equal amounts of cDNA from SAT, EAT, and brain samples. The transcript levels of the target genes were normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH, TaqMan Mouse Endogenous Control; Applied Biosystems).
Statistical data analysis
Results were expressed as mean ± standard deviation (SD) or standard error of the mean (SEM), as specified. Data were analyzed using Student's t-test to determine the statistical significance of differences. General linear regression models were used to assess the association between APP gene expression and measures of glucose metabolism. Plasma insulin concentrations and cytokine expression levels were log-transformed when necessary to achieve a normal distribution (Lee et al. Citation2008). P values < 0.05 were considered significant.
Results
Effects of long-term HFD, TZD, CR, or STZ-induced diabetes on glucose metabolism in C57BL/6 mice
The mean body weights of mice fed the HFD until 16, 26, 36, 47, or 77 weeks of age were significantly higher than those of ND control mice (p < 0.001; ). AUCglucose during IPGTT and nonfasting plasma insulin levels were significantly higher in HFD mice than in ND mice at all ages (p < 0.05 or even more significant; Table S1), demonstrating that mice fed the HFD developed obesity, impaired glucose tolerance, and insulin resistance.
In addition, fasting blood glucose and AUCglucose during IPGTT significantly decreased in the TZD and CR groups compared with control groups (p < 0.001; Table S2). Nonfasting plasma insulin levels significantly decreased in TZD-treated mice and marginally significantly decreased in the CR mice (p < 0.05 and p = 0.078, respectively; Table S2).
Induction of diabetes by STZ treatment was associated with development of hyperglycemia, impaired-insulin secretion, and loss of body weight (Table S3). STZ-induced diabetic mice fed a ND or an HFD had significantly increased nonfasting plasma glucose levels (p < 0.01 and p < 0.001, respectively) and significantly decreased levels of nonfasting plasma insulin compared with nondiabetic diet-matched control mice (p < 0.01 and p < 0.01, respectively, Table S3).
APP gene expression in mice after long-term high-fat feeding
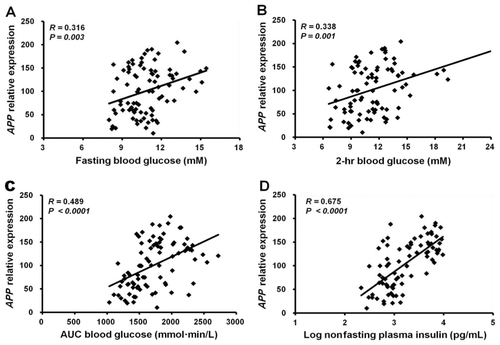
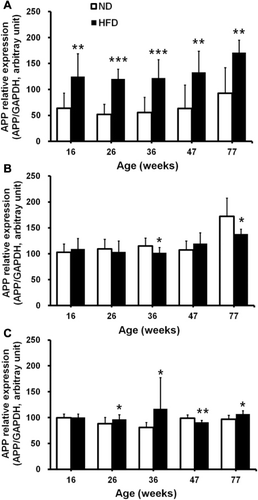
Using QPCR, expression levels of APP mRNA were measured in SAT, EAT, and brain samples obtained from mice fed either an HFD or a ND from age 6 weeks until 16, 26, 36, 47, or 77 weeks of age. APP gene expression in SAT was up-regulated by about twofold in all the HFD-induced obese mice groups (16–77 weeks of age) compared with that in the ND mice groups (p < 0.01 or p < 0.001; ). In EAT, APP gene expression was down-regulated in the HFD mice at 36 and 77 weeks of age compared with the age-matched ND mice controls (p < 0.05; ). The brains of HFD mice demonstrated increased APP gene expression at 26, 36, and 77 weeks of age (p < 0.05), whereas the brains of 47-week-old HFD mice showed reduced APP gene expression (p < 0.01, ). Further analyses demonstrated that APP gene expression in SAT significantly correlated with the levels of fasting blood glucose (R = 0.32, p = 0.003; ), 2-h blood glucose (R = 0.34, p = 0.001; ), and AUCglucose (R = 0.49, p < 0.0001; ) during IPGTT and nonfasting plasma insulin (R = 0.68, p < 0.0001; ).
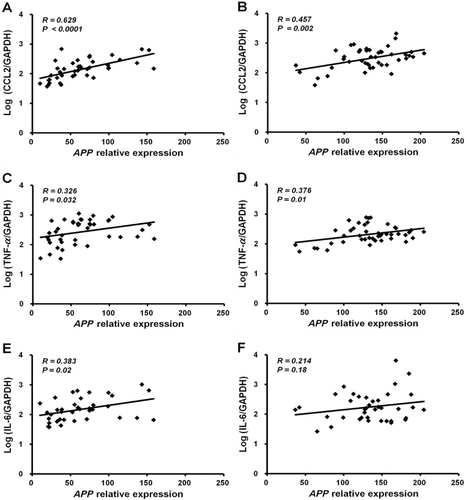
APP gene expression correlates with inflammatory adipokines
QPCR analyses of SAT demonstrated that APP expression levels correlate with those of representative proinflammatory adipokines and genes involved in macrophage activation. APP gene expression in ND or HFD mice significantly correlated with the expression of CCL2 (R = 0.63, p < 0.0001 and R = 0.46, p = 0.002, respectively; and ) and TNF-α (R = 0.33, p = 0.03 and R = 0.38, p = 0.01, respectively; and ), while it correlated with IL-6 gene expression in ND mice (R = 0.38, p = 0.02; ) but not in HFD mice (R = 0.21, p = 0.18; ). Nevertheless, APP expression levels were significantly correlated with IL-6 expression levels when ND and HFD mice were analyzed together (p = 0.02; data not shown).
Changes in APP gene expression in TZD-treated or calorie-restricted mice, and in those with STZ-induced diabetes
In the TZD-treated mice, APP gene expression significantly decreased in SAT and EAT compared with control mice treated with vehicle only (p < 0.001), but this expression remained unchanged in the brain (). CR reduced APP gene expression levels in SAT by approximately 11% compared with control mice, but this difference was not statistically significant (). APP expression in EAT was unchanged between CR and control mice but significantly increased in the brain (p < 0.05; ).
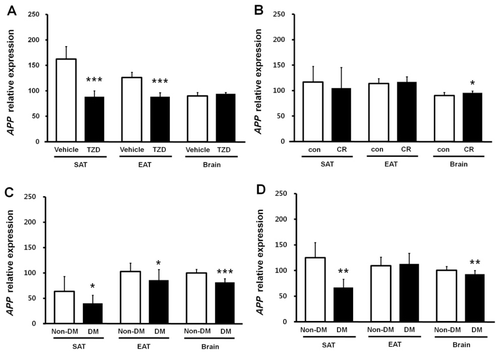
A low-dose STZ injection protocol was applied to ND or HFD mice from ages 6 to 11 weeks. Animals were sacrificed at 16 weeks after overt diabetes had been confirmed. Induction of diabetes by STZ resulted in significantly decreased APP gene expression in the SAT of both ND and HFD mice (ND diabetic vs. ND nondiabetic, p < 0.05; HFD diabetic vs. HFD nondiabetic, p < 0.01; and ). Brain APP gene expression also decreased in ND- and HFD-diabetic mice compared with age- and diet-matched nondiabetic control mice (p < 0.001 and p < 0.01, respectively). In EAT, however, APP expression decreased in ND-diabetic mice (p < 0.05) but not in HFD-diabetic mice compared with nondiabetic control mice ( and ).
Discussion
C57BL/6 mice were used in this study to examine the effects of diet-induced obesity and changes in insulin sensitivity, glycemia, and insulinemia on APP gene expression in SAT, EAT, and brain. The mice were subjected to long-term HFD feeding, TZD treatment, calorie restriction, or STZ-induced diabetes. The HFD diet was given from age 6 weeks up to as long as 77 weeks of age, representing an almost lifelong exposure. To the authors' knowledge, this is the longest HFD feeding experiment performed to investigate APP expression in C57BL/6 mice.
Compared with ND mice, the HFD-induced hyperinsulinemia impaired glucose tolerance (Table S1) and up-regulated (1.8- to2.3-fold) APP gene expression in the SAT () corroborated our previous findings that APP gene expression in subcutaneous abdominal adipocytes (SAC) increases in obese individuals (Lee et al. Citation2008).
APP gene expression in EAT was down-regulated in HFD mice only at 36 and 77 weeks old compared with age-matched ND mice controls. APP gene expression in the brain was up-regulated in HFD mice aged 26, 36, and 77 weeks but down-regulated at 47 weeks. There was, therefore, no consistent expression pattern of APP mRNA expression in the EAT or brain, whereas in SAT, it was continuously up-regulated in all HFD-induced obese mice groups from ages 16 to 77 weeks, suggesting there is depot- and tissue-specific regulation of APP.
We previously demonstrated that human APP mRNA is highly expressed in SAC and visceral adipose tissue, and the levels in SAC are up-regulated in obesity and correlate with in vivo measures of insulin resistance (Lee et al. Citation2008). We also found that APP gene expression in SAC from 19 obese human subjects increased approximately 2.5-fold compared with those from 20 nonobese subjects in a gene-chip microarray expression-profiling study. The APP gene-chip signal from SAC was highly correlated with the % body fat, fasting and 2-hr glucose levels, insulin concentration, and homeostasis model assessment of insulin resistance (HOMA-IR; Lee et al. Citation2005, Citation2008). The association between APP gene expression in SAT and metabolic parameters of insulin resistance or impaired glucose tolerance (such as but not limited to, fasting glucose levels, 2-h glucose, AUCglucose during IPGTT, and nonfasting plasma insulin levels) was also found in HFD-induced obese mice in the current study (). The results of the current and previous studies suggest that there might be common regulatory mechanisms or pathways for APP gene expression in adipose tissue of both human and mouse.
Puig et al. (Citation2012) demonstrated that both brain and adipose tissue in mice fed an HFD for 22 weeks have increased APP expression levels that are localized to neurons and macrophage/adipocytes, supporting the hypothesis that the proinflammatory state in brain and adipose tissue in diet-induced obesity is characterized partly by increased levels of APP. The results in the current study are consistent with those of Puig et al. Our previous studies and the current one show the relationship between APP expression levels and inflammatory cytokines (), suggesting that up-regulated expression of APP in adipose tissue/adipocytes from obese humans and animals may lead to inflammation in adipose tissue by direct as well as indirect pathways.
To investigate the direct effects of circulating insulin and glucose levels, insulin sensitivity, and induced diabetes on APP gene expression, we used QPCR to measure APP mRNA levels in SAT, EAT, and brain from TZD-treated, calorie-restricted, and STZ-induced diabetic mice. The correlations between glucose levels and APP expression in SAT (, , and ) suggest that the APP level may be affected by glucose concentration. However, the strong correlation between SAT APP expression and plasma insulin levels (), and the decreased APP expression in SAT and brain from hypoinsulinemic, STZ-induced diabetic mice ( and ) in which glucose levels were extremely high, demonstrate that APP expression is most likely to be regulated by insulin levels in both SAT and brain. In previous studies, we also showed that APP gene expression levels in SAC correlate with insulin levels, both of which significantly decrease with weight loss (Lee et al. Citation2008, Citation2009). These results suggest that insulin is the key regulator of APP gene expression in both human and mouse and that APP expression in the SAT responds to levels of glucose metabolism. These findings further support the proposed potential mechanisms linking obesity and insulin resistance to AD (Craft Citation2007; Luchsinger & Gustafson Citation2009) and the hypothesis that dysregulation of APP expression in adipose tissue might be linked with an increased risk of AD in obese individuals (Lee et al. Citation2008).
In summary, this study demonstrates that APP expression is significantly up-regulated in the SAT of HFD-induced obese mice, is significantly down-regulated in the SAT and brain in mice with STZ-induced diabetes, and is down-regulated in the SAT after TZD treatment administered to increase insulin sensitivity. Down-regulation is most likely due to decreased plasma insulin levels in the treated mice. The molecular mechanism by which insulin levels regulate APP expression in the SAT and brain needs to be further investigated. These results confirm previous findings in human studies, suggesting common regulatory mechanisms for APP gene expression in the adipose tissue of both human and mouse. Detailed insights into APP regulation and processing in adipose tissue may improve our understanding of the increased risk of AD in obese individuals.
Supplementary data
Supplementary data for this article can be found by clicking on the Supplementary Content tab at http://dx.doi.org/10.1080/19768354.2014.940383.
Metabolic data of diabetic mice
Download MS Word (47.5 KB)Metabolic data of TZD and CR mice
Download MS Word (46 KB)Metabolic data of DIO mice
Download MS Word (54 KB)Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology [NRF-2009-0069167], and the “Advanced Medical Material (Fiber) Development Program” through the Ministry of Knowledge Economy (MKE) and the Korea Institute for Advancement of Technology (KIAT).
Additional information
Funding
References
- Butterfield DA, Griffin S, Munch G, Pasinetti GM. 2002. Amyloid beta-peptide and amyloid pathology are central to the oxidative stress and inflammatory cascades under which Alzheimer's disease brain exists. J Alzheimers Dis. 4:193–201.
- Craft S. 2007. Insulin resistance and Alzheimer's disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 4:147–152.10.2174/156720507780362137
- Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D, Jr. 1998. Cerebrospinal fluid and plasma insulin levels in Alzheimer's disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 50:164–168.10.1212/WNL.50.1.164
- Hardy J, Selkoe DJ. 2002. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 297:353–356.10.1126/science.1072994
- Higuchi A, Ohashi K, Shibata R, Sono-Romanelli S, Walsh K, Ouchi N. 2010. Thiazolidinediones reduce pathological neovascularization in ischemic retina via an adiponectin-dependent mechanism. Arterioscler Thromb Vasc Biol. 30:46–53.10.1161/ATVBAHA.109.198465
- Hsueh W, Abel ED, Breslow JL, Maeda N, Davis RC, Fisher EA, Dansky H, McClain DA, McIndoe R, Wassef MK, et al. 2007. Recipes for creating animal models of diabetic cardiovascular disease. Circ Res. 100:1415–1427.10.1161/01.RES.0000266449.37396.1f
- Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A. 2005. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 62:1556–1560.10.1001/archneur.62.10.1556
- Kusakabe T, Tanioka H, Ebihara K, Hirata M, Miyamoto L, Miyanaga F, Hige H, Aotani D, Fujisawa T, Masuzaki H, et al. 2009. Beneficial effects of leptin on glycaemic and lipid control in a mouse model of type 2 diabetes with increased adiposity induced by streptozotocin and a high-fat diet. Diabetologia. 52:675–683.10.1007/s00125-009-1258-2
- Lee YH, Martin JM, Maple RL, Tharp WG, Pratley RE. 2009. Plasma amyloid-beta peptide levels correlate with adipocyte amyloid precursor protein gene expression in obese individuals. Neuroendocrinology. 90:383–390.10.1159/000235555
- Lee YH, Nair S, Rousseau E, Allison DB, Page GP, Tataranni PA, Bogardus C, Permana PA. 2005. Microarray profiling of isolated abdominal subcutaneous adipocytes from obese vs non-obese Pima Indians: increased expression of inflammation-related genes. Diabetologia. 48:1776–1783.10.1007/s00125-005-1867-3
- Lee YH, Pratley RE. 2007. Abdominal obesity and cardiovascular disease risk: the emerging role of the adipocyte. J Cardiopulm Rehabil. 27:2–10.10.1097/01.HCR.0000265014.36587.dd
- Lee YH, Tharp WG, Maple RL, Nair S, Permana PA, Pratley RE. 2008. Amyloid precursor protein expression is upregulated in adipocytes in obesity. Obesity (Silver Spring). 16:1493–1500.10.1038/oby.2008.267
- Luchsinger JA, Gustafson DR. 2009. Adiposity, type 2 diabetes, and Alzheimer's disease. J Alzheimers Dis. 16:693–704.
- Luchsinger JA, Tang MX, Shea S, Mayeux R. 2004. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 63:1187–1192.10.1212/01.WNL.0000140292.04932.87
- Nair S, Lee YH, Rousseau E, Cam M, Tataranni PA, Baier LJ, Bogardus C, Permana PA. 2005. Increased expression of inflammation-related genes in cultured preadipocytes/stromal vascular cells from obese compared with non-obese Pima Indians. Diabetologia. 48:1784–1788.10.1007/s00125-005-1868-2
- Pi-Sunyer FX. 2002. The obesity epidemic: pathophysiology and consequences of obesity. Obes Res. 10:97S–104S.10.1038/oby.2002.202
- Poucher SM, Cheetham S, Francis J, Zinker B, Kirby M, Vickers SP. 2012. Effects of saxagliptin and sitagliptin on glycaemic control and pancreatic beta-cell mass in a streptozotocin-induced mouse model of type 2 diabetes. Diabetes Obes Metab. 14:918–926.10.1111/j.1463-1326.2012.01619.x
- Prpic V, Watson PM, Frampton IC, Sabol MA, Jezek GE, Gettys TW. 2003. Differential mechanisms and development of leptin resistance in A/J versus C57BL/6J mice during diet-induced obesity. Endocrinology. 144:1155–1163.10.1210/en.2002-220835
- Puig KL, Floden AM, Adhikari R, Golovko MY, Combs CK. 2012. Amyloid precursor protein and proinflammatory changes are regulated in brain and adipose tissue in a murine model of high fat diet-induced obesity. PloS one. 7:e30378.10.1371/journal.pone.0030378
- Singhal SS, Figarola J, Singhal J, Reddy MA, Liu X, Berz D, Natarajan R, Awasthi S. 2013. RLIP76 protein knockdown attenuates obesity due to a high-fat diet. J Biol Chem. 288:23394–23406.10.1074/jbc.M113.480194
- Sobel BE, Lee YH, Pratley RE, Schneider DJ. 2006. Increased plasminogen activator inhibitor type-1 (PAI-1) in the heart as a function of age. Life Sci. 79:1600–1605.10.1016/j.lfs.2006.05.011
- Sommer G, Kralisch S, Lipfert J, Weise S, Krause K, Jessnitzer B, Lossner U, Bluher M, Stumvoll M, Fasshauer M. 2009. Amyloid precursor protein expression is induced by tumor necrosis factor alpha in 3T3-L1 adipocytes. J Cell Biochem. 108:1418–1422.10.1002/jcb.22382
- Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. 1988. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 37:1163–1167.10.2337/diab.37.9.1163
- Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr., Yaffe K. 2005. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 330:1360.10.1136/bmj.38446.466238.E0
- Winzell MS, Holm C, Ahren B. 2003. Downregulation of islet hormone-sensitive lipase during long-term high-fat feeding. Biochem Biophys Res Commun. 304:273–278.10.1016/S0006-291X(03)00552-7