Abstract
We have previously shown that root extracts from Anthriscus sylvestris (AS) can stimulate antigen-presenting cells such as dendritic cells and macrophages, which implies that AS extract can be useful as an immune adjuvant to promote the immune response. Here, we investigated the in vivo effect of AS extract on CD4+ T cell differentiation using the DO11.10 ovalbumin (OVA)-specific TCR transgenic mouse model. We found that the repeated injection of AS extract plus OVA antigens resulted in increases in both the Th1-polarizing IL12 and Th17-polarizing IL23 cytokines on macrophages. Consistent with the upregulation of IL12 and IL23, both IFNγ-producing Th1 and IL17-producing Th17 cells specific for OVA antigens were significantly induced upon AS stimulation. In addition, natural killer T (NKT) cells and neutrophils were activated to produce IL17 in AS extract-treated mice. Furthermore, in vivo treatment with AS extract significantly decreased the frequency of Foxp3+CD25+ inducible Treg cells compared to the vehicle-treated control group. Taken together, our findings suggest that in vivo treatment with AS extract induces proinflammatory cytokine production by macrophages, leading to the activation of IL17-producing innate immune cells such as NKT cells and neutrophils, presumably at early time points and later, as well as the induction of IFNγ- or IL17-producing adaptive immune cells such as Th1 and Th17 cells with the concomitant downregulation of Treg cells. These findings imply that AS-derived extract might be useful as an adjuvant to treat Th2-biased immune diseases such as atopic dermatitis.
Introduction
The dried root of Anthriscus sylvestris (AS), a herbaceous biennial plant, has been used as a traditional medicine with antipyretic and analgesic activity to treat the common cold. Deoxypodophyllotoxin (DPT), which has antiproliferative (Ikeda et al. Citation1998), antitumor (Kim et al. Citation2002), and antiviral (Masuda et al. Citation2002) activities, is a major pharmacological agent in this plant (Jin et al. Citation2008). DPT has been reported to stimulate innate immune cells such as macrophages to induce adaptive immune responses (Lee et al. Citation2011). Macrophages are strategically located throughout the body tissues, where they ingest and process foreign materials, dead cells, and debris (Murray & Wynn Citation2011). Macrophages are equipped with a broad range of pathogen-recognition receptors that detect danger signals and make the macrophages efficiently induce the production of inflammatory cytokines (Geissmann et al. Citation2010). Similar to macrophages, dendritic cells (DCs) are antigen-presenting cells that are specialized for the capture, processing, and presentation of antigens to T cells (Shortman & Naik Citation2007). After sensing any danger(s) to the host via innate immune receptors such as Toll-like receptors, DCs mature and subsequently present antigens to CD4+ T cells (Gi et al. Citation2009).
CD4+ T cells can be differentiated into distinct types of T helper cells depending on the cytokine microenvironment during the initial immune responses to invading pathogens (O'Garra et al. Citation2011). For example, interleukin 12 (IL12) from macrophages and DCs induces the development of Th1-type cells to produce large amounts of interferon-γ (IFNγ) (O'Garra Citation1998). The development of IL4-producing Th2 cells can be dependent on early IL4 produced by innate immune cells such as mast cells (Zhu et al. Citation2010). In addition, IL17-producing helper T cells (Th17 cells) initially develop in the presence of IL6 and TGFβ and require IL23 cytokines for their maintenance and survival (Gutcher et al. Citation2011). The development of regulatory T cells (Treg cells), as critical mediators of self-tolerance and immune homeostasis, is known to be critically dependent on Foxp3 expression induced by cytokines IL2 and TGFβ (Sakaguchi et al. Citation2008).
The present study was carried out to determine whether AS extract enables the regulation of the developmental pathway of T helper effector subsets. The development profiles were evaluated in the terms of cytokine production and subset-specific master gene expression. We demonstrated that in vivo treatment with AS extracts regulated Th1/Th17 cell development by enhancing IL12/IL23 expression by macrophages. Taken together, our results provide evidence that AS extract can be useful as a potential therapeutic to treat Th2-associated diseases such as atopic dermatitis.
Materials and methods
Mice
Six- to eight-week-old Balb/c mice were purchased from Jung Ang Lab Animal Inc. (Seoul, Korea). The DO11.10 OVA-specific TCR transgenic (tg) mice used in this study were of the Balb/c genetic background. Mice were bred and maintained at Sejong University and were fed a γ-irradiated, sterile diet and autoclaved distilled water. The animal experiments were approved by the Institutional Animal Care and Use Committee at Sejong University (SJ-20100401009).
Cell isolation and culture
Splenocytes were cultured in RPMI 1640 (Gibco BRL, USA) culture media supplemented with 10% fetal bovine serum (FBS), 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 2 mM L-glutamine, 100 units/mL penicillin-streptomycin, and 5 mM 2-mercaptoethanol (referred to as RPMI complete medium). CD4+ T cells were enriched using CD4 magnetic-activated cell sorting (MACS) beads (Miltenyi Biotec, Germany) according to the manufacturer's instructions. The purity of the CD4+ T cell population was more than 95% after MACS.
Reagents and antibodies
OVA peptide323–339 (ISQAVHAAHAEINEAGR) was synthesized by Peptron Inc. (Daejeon, Korea). The following mAbs from BD Bioscience were used: fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, or PE-Cy7-conjugated anti-TCRβ (clone H57-597); PE-, PE-Cy7, or allophycocyanin (APC)-conjugated anti-CD4 (clone RM4-5); APC-conjugated anti-CD25 (clone PC61); PE-Cy7-conjugated-DO11.10 clonotypic TCR (clone KJ1-26); FITC-conjugated anti-CD11c (clone HL3); PE-Cy7-conjugated anti-CD11b (clone M1/70); APC-conjugated anti-F4/80 (clone BM8); PE-conjugated-MHC II (clone M5/114.15.2); biotin-conjugated anti-CD49b (clone DX5); PE-conjugated anti-streptavidin; FITC-conjugated anti-IL4 (clone BVD6-24G2); PE-conjugated anti-IL12p40/p70 (clone C15.6); PE-conjugated-IFNγ (clone XMG1.2); FITC-conjugated IgG2b (κ isotype control; clone A95-1); and PE-conjugated IgG1 (κ isotype control; clone R3-34). The following mAbs from eBioscience were used: PE-conjugated-Foxp3 (clone NRRF-30) and FITC-conjugated-IL17 (clone eBio17B7). The PE-conjugated anti-IL23p19 (clone 320244) mAb was obtained from R&D Systems. All flow cytometric data were acquired using a FACSCalibur (Becton Dickinson, USA) and analyzed with the FlowJo software (Tree Star, USA).
Surface marker and intracellular cytokine staining
For surface marker staining, splenocytes were prepared and washed twice with FACS buffer (cold PBS containing 1% FBS). The cells were then stained with fluorescence-labeled mAbs specific for surface marker molecules for 45 min followed by incubation with anti-CD16/CD32 mAbs on ice for 10 min to block nonspecific binding of the mAbs to the Fc receptor. For intracellular cytokine staining, splenocytes were restimulated with phorbol myristate acetate (PMA) (50 ng/ml) and ionomycin (1 µg/ml) for 2 hrs in the presence of brefeldin A (10 µg/ml), an intracellular protein transport inhibitor, in RPMI complete medium at 37°C. Subsequently, the cells were stained with mAbs specific for cell surface markers for 45 min at 4°C, fixed with 4% paraformaldehyde in PBS, washed once with FACS buffer, and subsequently permeabilized with permeabilizing solution containing 1% bovine serum albumin (BSA) and 0.1% saponin. Permeabilized cells were further stained for an additional 30 min at 4°C with the following cytokine-specific mAbs: FITC- or PE-conjugated anti-IL4, PE-conjugated anti-IFNγ, PE-conjugated anti-IL12p40/p70, PE-conjugated anti-Foxp3, FITC-conjugated anti-IL17, PE-conjugated anti-IL23, and FITC- or PE-conjugated isotype control rat IgG mAbs.
RT-PCR
Total RNAs were isolated from CD4+ T cells with Easy Red reagent (Intron, Korea) and were reverse transcribed into cDNA using oligo(dT) priming and M-MLV RT (Invitrogen Life Technologies, USA) in accordance with the manufacturer's instructions. The following primers were used in this study: for T-bet, 5′-AAC CAG TAT CCT GTT CCC AGC-3′ (forward) and 5′-TGT CGC CAC TGG AAG GAT AG-3′ (reverse); for GATA3, 5′-CTC CTT TTT GCT CTC CTT TTC-3′ (forward) and 5′ -AAG AGA TGA GGA CTG GAG TG-3′ (reverse); for RORγt, 5′-ACT TTT CCC A CT TCC TCA GCG-3′ (forward) and 5′-AGA GTT CCT TAT AGA GTG GCG GG-3′ (reverse); and for β-actin, 5′-GTA TGG AAT CCT GTG GCA TC-3′ (forward) and 5′-AAG CAC TTG CGG TGC ACG AT-3′ (reverse). PCR was set up according to the instructions for the PCR amplification kit using 1 µg of cDNA, 5 pmol of each primer, and HiPi PCR Premix (ELPis Biotech, Korea) in a 20 µl reaction volume. The reaction was performed in an XP Thermal Cycler (Bioer Technology, Hangzhou, China) with thermal cycling parameters as follows: 94°C for 5 min; 35 cycles of 94°C for 45 sec, 61°C for 45 sec, 72°C for 1 min; and 72°C for 5 min for T-bet, GATA3, and RORγt and 94°C for 5 min; 30 cycles of 94°C for 30 sec, 54°C for 30 sec, 72°C for 35 sec; and 72°C for 5 min for β-actin. Amplified PCR products were loaded on a 1.5% agarose gel, electrophoresed, and then stained with ethidium bromide for visualization. The intensity of the bands was measured using the Gel Logic100 imaging system (Kodak, NY, USA) and analyzed using the ImageJ software program (National Institutes of Health software).
Statistical analysis
Statistical significance was determined using the Excel software (Microsoft, USA). To compare two groups, Student's t test was performed. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 were considered significant.
Results and discussion
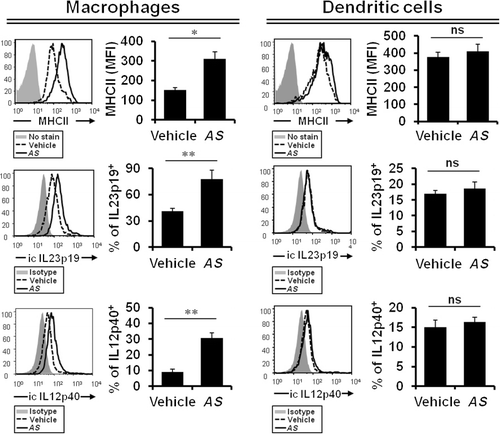
AS extract induces the activation of macrophages rather than peripheral DCs
Previously, we have reported that in vitro treatment with AS extract induces IL12/23p40 gene expression in bone marrow-derived dendritic cells (BMDCs) (Im et al. Citation2005). In addition, one study has recently reported that DPT, an active component and major lignin of the traditional plant AS, promotes the activation of CD8+ T cells and Th1 immune responses by inducing IL12 production in BMDCs (Lee et al. Citation2011). Based on these previous findings, we decided to investigate the in vivo effects of AS extract on innate immune cells such as peripheral macrophages and DCs. To do this, splenocytes were harvested from either vehicle- or AS extract-treated mice and subsequently stained with anti-CD11b and anti-CD11c mAbs to gate the macrophage and DC populations for the subsequent FACS analysis. To measure the status of activation in macrophages and DCs after AS extract treatment, we examined the expression levels of MHC II, IL12, and IL23 and found that AS extract significantly increased the expression of these cytokines in macrophages, but, unexpectedly, not in DCs (). Therefore, these results show that the AS extract treatment preferentially upregulated the activity of macrophages rather than of DCs. Notably, our findings are somewhat different from others in that in vivo AS extract treatment had little influence on the stimulation of DCs in our experimental settings. We speculate that this difference might be attributed to the following several factors: (1) source of reagents (extract vs. purified compound), (2) source of DCs (primary DCs vs. BMDCs), or (3) protocol for immunization (repeated injection vs. single injection), etc.
In vivo treatment of AS extracts induces Th1 and Th17 differentiation
Naive CD4+ T cells are primed upon recognizing antigens presented by antigen-presenting cells and consequently begin to differentiate into distinct subsets of effector cells depending on the cytokine microenvironment (Murphy & Reiner Citation2002). Thus, we decided to investigate whether cytokines induced by AS extracts could influence the polarization of CD4+ T cells. To this purpose, DO11.10 ovalbumin (OVA)-specific TCR tg mice were employed to monitor the antigen-specific immune responses. Purified CD4+ splenic T cells from DO11.10 OVA-specific TCR tg mice were cultured with anti-CD3 and anti-CD28 mAbs for 16 hrs, and we analyzed the secretion of intracellular IFNγ, IL4, or IL17 in OVA-specific CD4+ T cells. As shown in , we observed an increase in the IFNγ and IL17 levels in AS extract-treated mice ( and ). However, although there was no significant difference in the IL4 production of CD4+ T cells between the vehicle- and AS extract-treated mice, IL4-producing Th2 cells were reduced (). Consistent with the previous study on DPT purified from AS (Lee et al. Citation2011), in vivo AS extract treatment induced Th1 immune responses in mice. Therefore, these results clearly show that the AS extract treatment switched the adaptive immune response toward the Th17 as well as Th1 phenotypes by inducing the production of Th1- and Th17-associated cytokines such as IL12 and IL23.
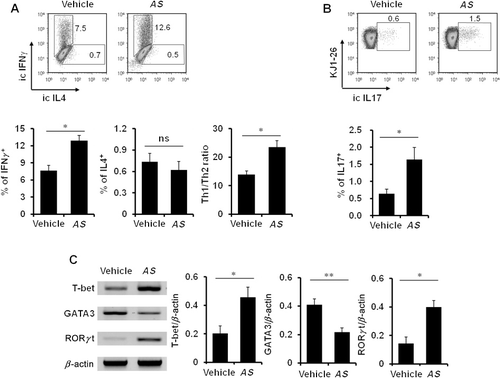
The following transcription factors are known to be involved in the differentiation of T helper subsets: T-bet (Th1), GATA3 (Th2), and RORγt (Th17) (Szabo et al. Citation2000; Ouyang et al. Citation2008; Ho et al. Citation2009). Thus, we next examined whether AS extract treatment changed the expression patterns of transcription factors essential for T helper development. To do this, we measured the mRNA levels of transcription factors required for helper T cell differentiation in AS extract-treated mice. As shown in , our results showed that not only higher levels of T-bet and RORγt but also reduced levels of GATA3 were present in CD4+ T cells from AS extract-treated mice.
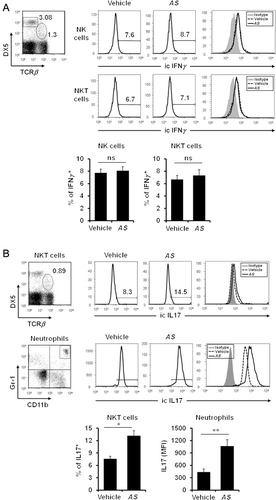
In vivo treatment of AS extracts induces IL17 production by innate immune cells such as natural killer T (NKT) cells and neutrophils
Because AS extract induces the differentiation of IFNγ producing Th1 cells, it may also influence IFNγ production by innate immune cells such as NK and NKT cells, which are known to possess cytotoxic capacity (Lee et al. Citation2013). It has been known that cytokine IL17 mediates protection against bacterial and fungal infections (Iwakura et al. Citation2011). Other than Th17 cells, innate immune cells, such as NKT cells and neutrophils, can produce IL17 and do so even more rapidly than adaptive immune cells such as Th17 cells (Ferretti et al. Citation2003; Rachitskaya et al. Citation2008). Moreover, neutrophils have been reported to be a main source of IL17 production in human psoriatic lesions and mouse models of infectious and autoimmune inflammation (Hoshino et al. Citation2008; Werner et al. Citation2011). Thus, we decided to examine whether in vivo treatment with AS extract can induce cytokine production by innate immune cells such as NK, NKT cells, and neutrophils. By measuring the intracellular cytokine level upon in vivo AS extract stimulation, we analyzed the cytokine production profile of these cells in AS extract-treated mice. We found that in vivo AS extract stimulation did not result in the production of IFNγ by NK and NKT cells () but could induce NKT cells and neutrophils to upregulate IL17 production ().
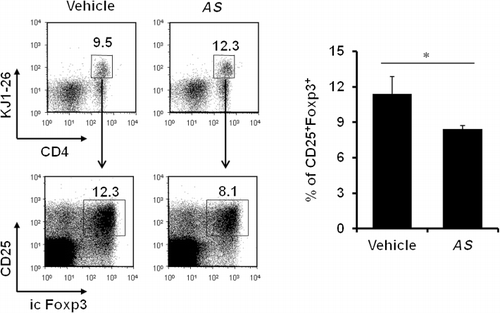
In vivo AS extract treatment downregulates antigen-specific Foxp3+regulatory T cells
Treg cells expressing the transcription factor Foxp3 are known to be central in establishing and maintaining immunological self-tolerance and homeostasis (Sakaguchi et al. Citation2008). Thus, we investigated whether AS extract treatment could influence Treg cell populations such as Foxp3+ Treg cells. We analyzed the percentage of antigen-specific, inducible Treg cells in the AS extract-treated mice by employing the DO11.10 OVA-specific TCR tg mouse system. When KJ1-26 positive T cells were gated (stained with DO11.10 TCR-specific mAb), the frequency of Foxp3+CD25+ inducible Treg cells was significantly decreased by approximately 30% in AS extract-treated mice compared to the vehicle-treated control group (). These results therefore indicate that AS extract treatment simultaneously facilitates the Th1/Th17 responses through downregulating Foxp3-expressing Treg cells that are known to antagonize Th1/Th17 activities.
Taken together, our data strongly suggest that in vivo treatment with AS extract induces proinflammatory cytokine production by macrophages, leading to the activation of IL17-producing innate immune cells such as NKT cells and neutrophils, presumably at the early time point and later, to induce IFNγ- or IL17-producing adaptive immune cells such as Th1 and Th17 cells with the concomitant downregulation of Treg cells. Based on our findings, AS extracts might be useful as potential therapeutics to treat Th2-associated diseases such as atopic dermatitis.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education [grant number 2010-0023683].
References
- Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. 2003. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 170:2106–2112.10.4049/jimmunol.170.4.2106
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. 2010. Development of monocytes, macrophages, and dendritic cells. Science. 327:656–661.10.1126/science.1178331
- Gi M, Im W, Hong S. 2009. Dendritic cells as danger-recognizing biosensors. Sensors (Basel). 9:6730–6751.10.3390/s90906730
- Gutcher I, Donkor MK, Ma Q, Rudensky AY, Flavell RA, Li MO. 2011. Autocrine transforming growth factor-beta1 promotes in vivo Th17 cell differentiation. Immunity. 34:396–408.10.1016/j.immuni.2011.03.005
- Ho IC, Tai TS, Pai SY. 2009. GATA3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nat Rev Immunol. 9:125–135.10.1038/nri2476
- Hoshino A, Nagao T, Nagi-Miura N, Ohno N, Yasuhara M, Yamamoto K, Nakayama T, Suzuki K. 2008. MPO-ANCA induces IL-17 production by activated neutrophils in vitro via classical complement pathway-dependent manner. J Autoimmun. 31:79–89.10.1016/j.jaut.2008.03.006
- Ikeda R, Nagao T, Okabe H, Nakano Y, Matsunaga H, Katano M, Mori M. 1998. Antiproliferative constituents in umbelliferae plants. IV. Constituents in the fruits of Anthriscus sylvestris Hoffm. Chem Pharm Bull (Tokyo). 46:875–878.10.1248/cpb.46.875
- Im W, Kim H, Yun D, Seo SY, Park SH, Locksley RM, Hong S. 2005. Cytokine reporter mouse system for screening novel IL12/23 p40-inducing compounds. Mol Cells. 20:288–296.
- Iwakura Y, Ishigame H, Saijo S, Nakae S. 2011. Functional specialization of interleukin-17 family members. Immunity. 34:149–162.10.1016/j.immuni.2011.02.012
- Jin M, Moon TC, Quan Z, Lee E, Kim YK, Yang JH, Suh SJ, Jeong TC, Lee SH, Kim CH, Chang HW. 2008. The naturally occurring flavolignan, deoxypodophyllotoxin, inhibits lipopolysaccharide-induced iNOS expression through the NF-kappaB activation in RAW264.7 macrophage cells. Biol Pharm Bull. 31:1312–1315.10.1248/bpb.31.1312
- Kim Y, Kim SB, You YJ, Ahn BZ. 2002. Deoxypodophyllotoxin; the cytotoxic and antiangiogenic component from Pulsatilla koreana. Planta Med. 68:271–274.10.1055/s-2002-23140
- Lee JS, Kim DH, Lee CM, Ha TK, Noh KT, Park JW, Heo DR, Son KH, Jung ID, Lee EK, et al. 2011. Deoxypodophyllotoxin induces a Th1 response and enhances the antitumor efficacy of a dendritic cell-based vaccine. Immune Netw. 11:79–94.10.4110/in.2011.11.1.79
- Lee SW, Park HJ, Kim N, Hong S. 2013. Natural killer dendritic cells enhance immune responses elicited by alpha -galactosylceramide-stimulated natural killer T cells. Biomed Res Int. 2013:460706.
- Masuda T, Oyama Y, Yonemori S, Takeda Y, Yamazaki Y, Mizuguchi S, Nakata M, Tanaka T, Chikahisa L, Inaba Y, Okada Y. 2002. Flow cytometric estimation on cytotoxic activity of leaf extracts from seashore plants in subtropical Japan: isolation, quantification and cytotoxic action of (-)-deoxypodophyllotoxin. Phytother Res. 16:353–358.10.1002/ptr.902
- Murphy KM, Reiner SL. 2002. The lineage decisions of helper T cells. Nat Rev Immunol. 2:933–944.10.1038/nri954
- Murray PJ, Wynn TA. 2011. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 11:723–737.10.1038/nri3073
- O'Garra A. 1998. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 8:275–283.10.1016/S1074-7613(00)80533-6
- O'Garra A, Gabrysova L, Spits H. 2011. Quantitative events determine the differentiation and function of helper T cells. Nat Immunol. 12:288–294.10.1038/ni.2003
- Ouyang W, Kolls JK, Zheng Y. 2008. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 28:454–467.10.1016/j.immuni.2008.03.004
- Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR. 2008. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 180:5167–5171.10.4049/jimmunol.180.8.5167
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. 2008. Regulatory T cells and immune tolerance. Cell. 133:775–787.10.1016/j.cell.2008.05.009
- Shortman K, Naik SH. 2007. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 7:19–30.10.1038/nri1996
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 100:655–669.10.1016/S0092-8674(00)80702-3
- Werner JL, Gessner MA, Lilly LM, Nelson MP, Metz AE, Horn D, Dunaway CW, Deshane J, Chaplin DD, Weaver CT, et al. 2011. Neutrophils produce interleukin 17A (IL-17A) in a dectin-1- and IL-23-dependent manner during invasive fungal infection. Infect Immun. 79:3966–3977.10.1128/IAI.05493-11
- Zhu J, Yamane H, Paul WE. 2010. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol. 28:445–489.10.1146/annurev-immunol-030409-101212
