Abstract
We isolated a metalloprotease (MP) homologue from an abalone muscle cDNA library. A 3284-kb full-length cDNA encoding a predicted polypeptide of 667 amino acids was sequenced. The abalone MP Haliotis discus hannai (HdMP) exhibited a domain structure typical of the peptidase M4 family, a 21-amino acid N-terminal hydrophobic signal sequence followed by a long propeptide sequence of 347 amino acids and the mature protease domain comprising 299 amino acids. The mature region contains features characteristic of a zinc protease, including a zinc-binding motif (HEXXH) and an active site. The protein showed 32–38% amino acid sequence identity with other known MP sequences and with a hypothetical protein from owl limpet. The mRNA transcript is expressed in almost all tissues, with high expression in the mantle and adductor muscle of healthy abalones, and is expressed constitutively during the early developmental stages after fertilization. Lipopolysaccharide or poly I:C stimulation induced the expression of the HdMP transcript in the digestive track and gills of abalones. Collectively, these results suggest that HdMP could play multiple roles in defense, the immune response, growth, and regulation of reproduction.
Introduction
Metalloproteases (MPs) are important for many physiological processes in mammals, such as cell migration, tissue remodeling, and processing of growth factors, and have been identified as important factors in the pathophysiology of several human diseases including cancer and hypertension. Many bacterial pathogens rely on proteases to infect the host. Several classes of MPs have been described in humans, bacteria, snake venoms, and insects (Rosendahl et al. Citation1997; Millichip et al. Citation1998; Kiyama et al. Citation1999; Ramos & Selistre-de-Araujo Citation2001; Vincent et al. Citation2001). These enzymes have the ability to cleave, activate, and solubilize growth factors as well as components of the basement membrane and extracellular matrix (ECM; Matrisian Citation1990; Matrisian Citation1992; Hooper Citation1994; Stöcker & Bode Citation1995; Roghani et al. Citation1999). MPs are usually characterized by a catalytic zinc ion in the active site (Matrisian Citation1990). Many MPs have a conserved consensus amino acid sequence, HEXXH (where X represents any amino acid residue), involved in metal ligation, while others including the matrix metalloproteases (MMPs) and snake venom MPs have an extended zinc-binding motif (HEXXHXXGXXH; Stöcker & Bode Citation1995). The HEXXH pentapeptide is very common among protein sequences and important for electron transfer with zinc for peptide bond hydrolysis. According to the general classification of zinc MPs, there are five groups (Hooper Citation1994), three of which possess this zinc-binding motif, while the remaining possess metal ligands. MMPs are a family of zinc-dependent endopeptidases that degrade ECM components such as collagens, fibronectin, and proteoglycans (Matrisian Citation1992; Massova et al. Citation1998; Visse & Nagase Citation2003). Consequently, MMPs are involved in various processes of ECM metabolism such as embryonic development, morphogenesis, and tissue remodeling, as well as inflammation and wound healing (Woessner Citation1991; Chambers & Matrisian Citation1997; Nagase & Woessner Citation1999).
Shellfish farming represents an important economic activity worldwide. Abalone is an important shellfish and industrial resource in Asia, Africa, and the USA (Mateos et al. Citation2012; Qian et al. Citation2012). In Asia, abalone has been cultivated since the 1980s with increasing annual production; however, mass mortality of abalone caused by parasites, super saturation of dissolved gas, fungi, secondary infections, and Vibrio sp. has occurred in various areas of the world (Kamaishi et al. Citation2010).
In the present study, we cloned a MP from abalone Haliotis discus hannai (HdMP) and analyzed its primary structure. RT-PCR was used to evaluate tissue-specific mRNA expression in different adult abalone tissues, as well as in the whole body of larvae during early developmental stages. Furthermore, to elucidate their functional diversification, we examined the expression levels after injection of different immune modulators such as lipopolysaccharide (LPS) and poly I:C.
Materials and methods
Shellfish
Pacific abalones (Haliotis discus hannai) were obtained from Jeju Fisheries Research Institute (Jeju, Republic of Korea) and acclimatized to laboratory conditions for 10 days before injection with LPS and poly I:C. The shellfish were maintained in flat-bottomed rectangular tanks containing fresh seawater at 20–23°C and provided a seaweed diet.
Cloning of the full-length cDNA of HdMP by 5′-RACE
The HdMP cDNA was identified from a muscle cDNA library using expressed sequence tag (EST) analysis of abalone (Park et al. Citation2007). The EST clone (RM-172) contained a 2700-base pair (bp) insert. Sequence analysis indicated that this clone was incomplete at the 5′-end. The full-length cDNA was amplified from first-strand cDNA from abalone muscle using 5′-RACE and primers based on the RM-172 sequence. 5′-RACE was performed using the MMP-RACE primer (5′-CTTCCGTTCTTTGGTGGATCATCCAGATAG-3′) and the SMART RACE cDNA Amplification Kit (Clontech) with the universal primer supplied in the kit.
Sequence analysis
The nucleotide and deduced amino acid sequences were analyzed using GENETYX version 8.0 (SDS Software Development). Multiple alignments of the MP proteins were performed using CLUSTALW (Thompson et al. Citation1994) and visualized using MEGA 3.1 (Kumar et al. Citation2001). A phylogenetic tree based on the deduced amino acid sequences was constructed using the neighbor-joining (NJ) algorithm (Saitou & Nei Citation1987), and the confidence level of each branching node was determined by bootstrap resampling (1000 pseudo-replicates) using MEGA 3.1. Signal peptide and propeptide sequences were predicted using SignalP and MotifScan (http://www.cbs.dtu.dk/services/SignalP/ and http://myhits.isb-sib.ch/cgi-bin/motif_scan/, respectively). Potential N-linked glycosylation sites were predicted using the NetNGlyc 1.0 server (http://www.cbs.dtu.dk/services/NetNGlyc/).
Southern blot analysis
Purified genomic DNA was digested completely using the restriction enzymes DraI, PstI, and EcoRI. The DNA fragments were separated on a 1% agarose gel by pulse-field electrophoresis and then transferred to Hybond N+ nylon membranes (Amersham Biosciences) using 20× saline-sodium citrate (SSC). The membranes were subsequently hybridized at 43°C for 12 h in digoxigenin (DIG) Easy Hyb hybridization solution (Roche Diagnostics) containing a DIG-labeled DNA probe. The probe was labeled using a PCR DIG Probes Synthesis kit (Roche Diagnostics) and the protease cDNA. Washing of the hybridized membranes and detection of the probe were performed using the DIG Detection kit (Roche Diagnostics) according to the manufacturer's instructions.
LPS and poly I:C stimulation and tissue collection
For LPS induced mRNA expression analysis, the abalones were injected with 100 µl (100 µg/µl) LPS (Sigma) in phosphate-buffered saline (PBS). For viral mimic poly I:C induction, the abalones were injected with 100 µl (10 µg/ µl) Poly I:C (Sigma) in PBS. Untreated and PBS-injected shellfish were maintained separately for use as controls. The digestive track and gills were collected from three abalones at 0, 3, 6, 12, and 24 h postinjection of LPS and at 0, 24, 48, and 72 h postinjection of poly I:C. Healthy abalone gill, hepatopancreas, digestive track, mantle, adductor muscle, and hemolymph tissue samples were collected for tissue-specific mRNA expression analysis. The collected samples were ground immediately in liquid nitrogen for RNA extraction. Three independent experiments were conducted, and three independent tissue samples were prepared for each experiment (n = 3).
Semi quantitative RT-PCR analysis
Total RNA samples were extracted from tissues using TRIzol reagent (Invitrogen). Subsequently, first-strand cDNA synthesis was performed using the Advantage RT-for-PCR Kit (Clontech). HdMP expression levels were measured by RT-PCR using specific primers, forward 5′-ACCCAGTGTTTGCCATAGAG-3′ and reverse 5′-CAAAGGTCAATGCCCCAACA-3′, based on the nucleotide sequence of HdMP cDNA (). As an internal control, β-actin was amplified using the appropriate primers (β-actin-RT-Forward; 5′-GCCGCTTGACTCTTGTGTGC-3 and β-actin-RT-Reverse; 5′-CTCCTCTGGTGCAACGCGG-3′). The PCR conditions were as follows: 95°C for 5 min, followed by 30 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s, with a final step of 72°C for 10 min. The PCR products (10 µL) were analyzed on a 1.5% (w/v) agarose gel containing ethidium bromide (100 ng/mL) using a Mini-Gel Electrophoresis System (Cosmo bio). The agarose gel bands were scanned using computer-by-computer analysis, and the optical density was plotted as a histogram. The Bio-Rad Gel-Doc system was used to quantify the PCR data, which were normalized to the respective β-actin expression levels (Nam et al. Citation2009). All assays were performed in triplicate as independent experiments.
Results and discussion
HdMP cDNA cloning
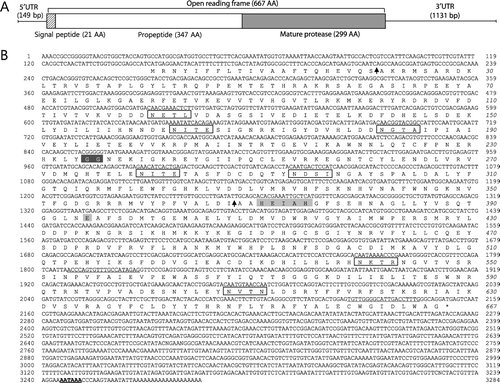
Previously, we constructed seven cDNA libraries from the gill, digestive gland, hepatopancreas, skin, muscle, testis, and ovary of adult abalone and analyzed 1393 EST clones (Park et al. Citation2007). Among the EST clones, RM-172 was homologous to known MP genes of other species based on a computer homology search of the public database. The HdMP cDNA sequence consists of 3284 nucleotides with a stop codon flanked by 149 bp of 5′-untranslated region (UTR) and 1131 bp of 3′-UTR region (). In the 3′-terminal region, a putative polyadenylation signal sequence AATAAA and a poly(A+) tail were found. These structural characteristics indicate that the HdMP cDNA is not derived from prokaryote-like intestinal bacteria. The deduced 667-amino acid polypeptide has a molecular weight of 76.87 kDa and a theoretical isoelectric point of 5.34. The N-terminal hydrophobic signal peptide and putative processing site between the propeptide and the mature enzyme were predicted using SignalP and MotifScan. A search of the BLASTP conserved domain database showed that the HdMP protein contains a domain structure (residues 269–525) typical of the peptidase M4 family (Marchler-Bauer et al. Citation2013), as evidenced by a 21-amino acid N-terminal hydrophobic signal sequence followed by a long propeptide sequence of 347 amino acids and the mature protease domain comprising 299 amino acids. This peptidase M4 family includes numerous zinc-dependent metallopeptidases that hydrolyze peptide bonds, such as thermolysin, pseudolysin, aureolysin, neutral protease, and bacillolysin, and contain the HEXXH motif as part of their active site (Rawlings & Barrett Citation1995). HEXXH is important in electron transfer with zinc for the hydrolysis of peptide bonds. The two histidine residues of the pentapeptide serve as zinc ligands, and the glutamic acid residue promotes the nucleophilic attack of a buried water molecule on the carbonyl carbon atom of the substrate peptide bond, forming a tetrahedral intermediate. The additional Glu residue is a zinc-binding ligand (Coleman Citation1998). The double glycine motif is critical for enzyme activity. The glycine residues are single-bonded, serving as hinge residues and having unique conformational degrees of freedom. Consequently, glycine is important for structural or protease dynamics that may be necessary for catalysis (Eckhard et al. Citation2009). Seven potential N-linked glycosylation sites have been predicted in positions 121–124 (NETL), 163–166 (NITE), 182–185 (NGTA), 280–283 (NITE), 294–297 (NDSI), 540–543 (NKTR), and 608–611 (NVTN). Oligosaccharides attached to proteins allow protein–protein interactions, stabilize the protein and play a role in the recognition of other ligands (Imperiali & O'Connor Citation1999). The HdMP cDNA sequence was submitted to the NCBI GenBank under accession number KJ599465.
Sequence alignment and phylogenetic analysis
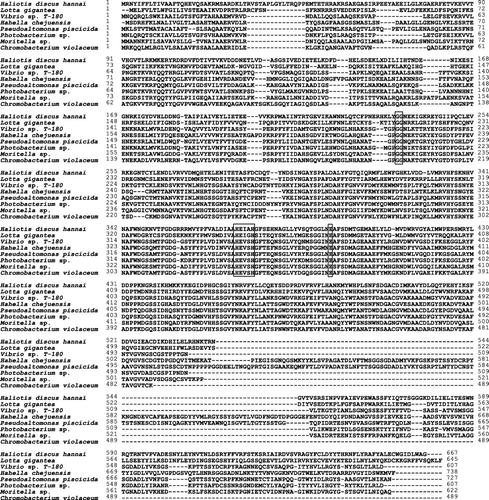
The HdMP amino acid sequences were aligned with the corresponding known MP sequences from NCBI GenBank and a hypothetical protein sequence from owl limpet, Lottia gigantea, with currently unknown function (Simakov et al. Citation2013), using the CLUSTALW program. The HdMP showed 32–38% identity with MP derived from Vibrio sp. T-1800 (38%, BAC87681), Hahella chejuensis (33%, YP433709), Pseudoalteromonas piscicida (33%, BAB79615), Photobacterium sp. (35%, ZP01158654), Moritella sp. (32%, ZP01898184), and Chromobacterium violaceum (36%, AAN78225), as well as a hypothetical protein from L. gigantea (36%, ES085431). Alignment of HdMP amino acid residues with other sequences indicated the presence of a conserved zinc-binding motif (HEXXH) and active site (). Interestingly, the HdMP gene possessed the zinc-binding motif HEIAH, differing from the HEVSH sequence of the other bacterial MP.
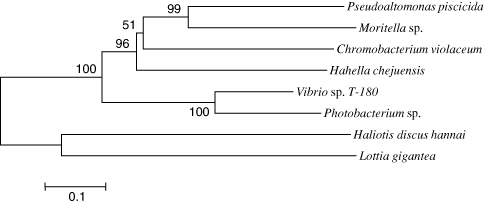
A phylogenetic tree was generated using MEGA (). The overall tree topology showed that HdMP was grouped with a hypothetical protein from L. gigantea, despite low identity (36% amino acid identity), and the bacterial MPs were grouped into another cluster. This result indicates a common origin of the MP genes in eukaryotes, and HdMP and bacterial MPs were distinct from each other, despite showing 40–47% sequence identity in the catalytic domain.
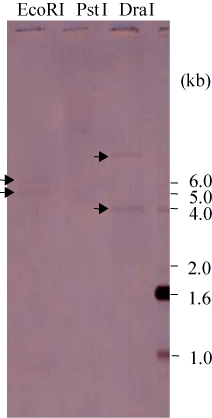
Southern blot analysis
Southern blot analysis was used to verify the presence or absence of a specific DNA nucleotide sequence and to identify the size of the restriction fragment containing the sequence. To determine the number of copies of the MP gene in the abalone genome, we performed Southern blot analysis (). After digestion with restriction enzymes, namely EcoRI, PstI, and DraI, different hybridization bands were detected, and no uncut products were observed. When DNA was digested with EcoRI, two hybridization bands (5 and 6 kb) appeared and with DraI two fragments (4 and 7 kb) were visible. These findings suggest two copies of the MP gene are present in the abalone genome.
HdMP gene expression based on RT-PCR
HdMP tissue expression was analyzed using RT-PCR (). Results showed ubiquitous expression of HdMP mRNA, predominantly in the mantle and adductor muscle and moderately in other tissues such as gill, digestive track, and hemolymph, but it was not detectable in the hepatopancreas. This result is similar to the expression pattern of MMPs in pufferfish, Japanese flounder, and the common carp. Fugu MMP-2 was highly expressed in the ordinary muscle, gill, brain, and skin and minimally expressed in tissues that contain blood cells such as whole blood, kidney body, and spleen (Tsukamoto et al. Citation2007). Japanese flounder MMP-2 was strongly expressed in the brain, gill, heart, and testis, but no signal was observed in the liver or gallbladder (Kinoshita et al. Citation2002). MMPs are enzymes that degrade proteins in tissue ECMs and are associated with numerous physiological and pathological processes. Because MMPs are expressed in most normal tissues, this suggests a putative general role of these enzymes in all cell types. High MP expression levels in muscle and the digestive tract of fish and shellfish are associated with muscle softening (Ishida et al. Citation2003; Lødemel et al. Citation2004).
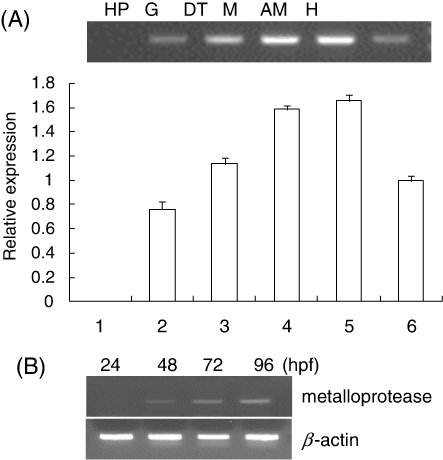
To determine the developmental stage during which HdMP transcription occurs, we analyzed the expression levels at 24, 48, 72, and 96 h postfertilization (hpf; ). During the trochophore stage (24 hpf), the HdMP expression level was barely or not detected at all. HdMP mRNA was detected at 48 hpf and gradually increased by 96 hpf, indicating developmentally regulated expression. This suggests that HdMP is involved in cell proliferation and differentiation during planktonic larval stages.
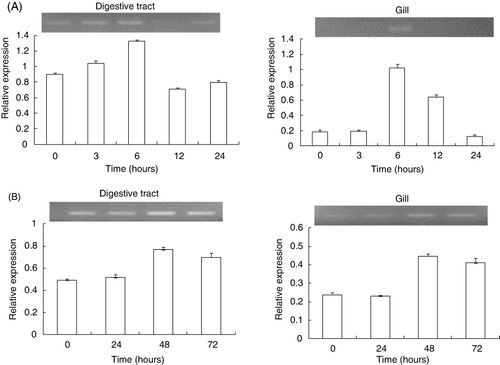
The HdMP mRNA expression in the digestive track and gills after injection of LPS or Poly I:C was also monitored. LPS treatment-enhanced HdMP mRNA expression was clearly upregulated after injection, especially at 6 h postinjection when the expression level was highest, as compared with the controls; it then subsequently decreased at 12 h postinjection (). When Poly I:C was injected into abalone, HdMP mRNA expression increased significantly at 48 h postinjection compared with the controls. An increasing trend was observed up to 72 h postinjection (). MMP-2 of grass carp is involved in the immune response against bacterial infection, showing increased expression in the spleen and kidney head at 4 h and then significantly downregulated one day after Aeromonas hydrophila infection (Xu et al. Citation2012). Although the details of the molecular mechanisms of HdMP are unclear, we speculate that HdMP plays an important role in the host response against pathogens.
In summary, we report the cloning, characterization, and in vivo expression of HdMP. The information obtained here may be useful for the study of MPs in other marine invertebrates. Further studies are necessary to elucidate MP gene regulation and function in the abalone immune defense mechanism and developmental processes.
Acknowledgment
This work was supported by a grant from the National Fisheries Research and Development Institute (NFRDI), Republic of Korea.
References
- Chambers AF, Matrisian LM. 1997. Changing views of the role of matrix metalloproteases. J Natl Cancer Inst. 89:1260–1270.10.1093/jnci/89.17.1260
- Coleman JE. 1998. Zinc enzymes. Curr Opin Chem Biol. 2:222–234.10.1016/S1367-5931(98)80064-1
- Eckhard U, Schönauer E, Ducka P, Briza P, Nüss D, Brandstetter H. 2009. Biochemical characterization of the catalytic domains of three different Clostridial collagenases. Biol Chem. 390:11–18.10.1515/BC.2009.004
- Hooper NM. 1994. Families of zinc metalloproteases. FEBS Lett. 354:1–6.10.1016/0014-5793(94)01079-X
- Imperiali B, O'Connor SE. 1999. Effect of N-linked glycosylation on glycopeptide and glycoprotein structure. Curr Opin Chem Biol. 3:643–649.10.1016/S1367-5931(99)00021-6
- Ishida N, Yamashita M, Koizumi N, Terayama M, Ineno T, Minami T. 2003. Inhibition of post-mortem muscle softening following in situ perfusion of protease inhibitors in tilapia. Fish Sci. 69:632–638.10.1046/j.1444-2906.2003.00666.x
- Kamaishi T, Miwa S, Goto E, Matsuyama T, Oseko N. 2010. Mass mortality of giant abalone Haliotis gigantea caused by a Francisella sp. bacterium. Dis Aquat Organ. 89:145–154.10.3354/dao02188
- Kinoshita M, Yabe T, Kubota M, Takeuchi K, Kubota S, Toyohara H, Sakaguchi M. 2002. cDNA cloning and characterization of two gelatinases from Japanese flounder. Fish Sci. 68:618–626.10.1046/j.1444-2906.2002.00469.x
- Kiyama R, Tamura Y, Watanabe F, Tsuzuki H, Ohtani M, Yodo M. 1999. Homology modeling of gelatinase catalytic domains and docking simulations of novel sulfonamide inhibitors. Med Chem. 42:1723–1738.10.1021/jm980514x
- Kumar S, Tamura K, Jakobsen IB, Nei M. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics. 12:1244–1245.10.1093/bioinformatics/17.12.1244
- Lødemel JB, Mæhre HK, Winberg J-O, Olsen RL. 2004. Tissue distribution, inhibition and activation of gelatinolytic activities in Atlantic cod (Gadus morhua). Comp Biochem Physiol B Biochem Mol Biol. 137:363–371.10.1016/j.cbpc.2003.12.007
- Marchler-Bauer A, Zheng C, Chitsaz F, Derbyshire MK, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Lanczycki CJ, et al. 2013. CDD: conserved domains and protein three-dimensional structure. Nucleic Acids Res. 41:348–352.10.1093/nar/gks1243
- Massova I, Kotra LP, Fridman R, Mobashery S. 1998. Matrix metalloproteinases: structures, evolution, and diversification. FASEB J. 12:1075–1095.
- Mateos HT, Lewandowski PA, Su XQ. 2012. The effect of replacing dietary fish oil with canola oil on fatty acid composition and expression of desaturase and elongase genes in Jade Tiger hybride abalone. Food Chem. 131:1217–1222.10.1016/j.foodchem.2011.09.107
- Matrisian LM. 1990. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 6:121–125.10.1016/0168-9525(90)90126-Q
- Matrisian LM. 1992. The matrix-degrading metalloproteinases. Bioassays. 14:455–463.10.1002/bies.950140705
- Millichip MI, Dallas DJ, Wu E, Dale S, McKie N. 1998. The metallo-disintegrin ADAM10 (MADM) from bovine kidney has type IV collagenase activity in vitro. Biochem Biophys Res Commun. 245:594–598.10.1006/bbrc.1998.8485
- Nagas H, Woessner Jr JF. 1999. Matrix metalloproteases. J Biol Chem. 274:21491–21494.10.1074/jbc.274.31.21491
- Nam BH, Park EM, Kim YO, Kong HJ, Kim WJ, Kang JH, Ji YJ, Lee SJ, Choi TJ. 2009. Identification of two differentially expressed forms of progranulin mRNA in the teleost fish Paralichthys olivaceus. Fish Shellfish Immunol. 26:177–182.10.1016/j.fsi.2008.09.009
- Park EM, Nam BH, Kim YO, Kong HJ, Kim WJ, Lee SJ, Kong IS, Choi TJ. 2007. EST-based survey of gene expression in seven tissue types from the abalone Haliotis discus hannai. J Fish Sci Technol. 10:119–126.
- Qian ZJ, Kim SA, Lee JS, Kim HJ, Choi IW, Jung WK. 2012. Antioxidant and anti-inflammatory effects of abalone intestine digest, Haliotis discus hannai in RAW 264.7 macrophages. Biotechnol Bioprocess Eng. 17:475–484.10.1007/s12257-011-0544-2
- Ramos OHP, Selistre-de-Araujo HS. 2001. Identification of metalloprotease gene families in sugarcane. Genet Mol Biol. 24:285–290.10.1590/S1415-47572001000100037
- Rawlings ND, Barrett AJ. 1995. Evolutionary families of metallopeptidases. Meth Enzymol. 248:183–228.
- Roghani M, Becherer JD, Moss ML, Atherton RE, Erdjument-Bromage H, Arribas J, Blackburn RK, Weskamp G, Tempst P, Blobel CP. 1999. Metalloprotease-disintegrin MDC9: intracellular maturation and catalytic activity. J Biol Chem. 274:3531–3540.10.1074/jbc.274.6.3531
- Rosendahl MS, Ko SC, Long DL, Brewer MT, Rosenzweig B, Hedl E, Anderson L, Pyle SM, Moreland J, Meyers MA, et al. 1997. Identification and characterization of a pro-tumor necrosis factor-processing enzyme from the ADAM family of zinc metalloproteases. J Biol Chem. 272:24588–24593.10.1074/jbc.272.39.24588
- Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 4:406–425.
- Simakov O, Ferdinand Marletaz F, Cho SJ, Edsinger-Gonzales E, Havlak P, Hellsten U, Kuo DH, Larsson T, Lv J, Arendt D, et al. 2013. Insights into bilaterian evolution from three spiralian genomes. Nature. 493:526–531.10.1038/nature11696
- Stöcker W, Bode W. 1995. Structural features of a superfamily of zinc-endopeptidases: the metzincins. Curr Op Struct Biol. 5:383–390.10.1016/0959-440X(95)80101-4
- Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acid Res. 22:4673–4680.10.1093/nar/22.22.4673
- Tsukamoto H, Yokoyama Y, Suzuki T, Mizuta S, Yoshinaka R. 2007. Molecular cloning and expression of gelatinases (MMP-2 and MMP-9) in the pufferfish Takifugu rubripes. Comp Biochem Physiol B Biochem Mol Biol. 148:295–302.10.1016/j.cbpb.2007.06.007
- Vincent B, Paitel E, Saftig P, Frobert Y, Hartmann D, De Strooper B, Grassi J, Lopez-Perez E, Checler F. 2001. The disintegrins ADAM10 and TACE contribute to the constitutive and phorbol ester-regulated normal cleavage of the cellular prion protein. J Biol Chem. 276:37743–37746.10.1074/jbc.M003965200
- Visse R, Nagase H. 2003. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 92:827–839.10.1161/01.RES.0000070112.80711.3D
- Woessner Jr JF. 1991. Matrix metalloproteases and their inhibitors in connective tissue remodeling. FASEB J. 5:2145–2154.
- Xu XY, Shen YB, Fu JJ, Liu F, Guo SZ, Yang XM, Li JL. 2012. Matrix metalloproteinase 2 of grass carp Ctenopharyngodon idella (CiMMP2) is involved in the immune response against bacterial infection. Fish Shellfish Immunol. 33:251–257.10.1016/j.fsi.2012.05.015